Abstract
Dbs is a Rho-specific guanine nucleotide exchange factor (RhoGEF) that regulates neurotrophin-3-induced cell migration in Schwann cells. Here we report that Dbs regulates cell motility in tumor-derived, human breast epithelial cells through activation of Cdc42 and Rac1. Cdc42 and Rac1 are activated in T47D cells that stably express onco- or proto-Dbs, and activation is dependent upon growth of the cells on collagen I. Transient suppression of expression of Cdc42 or Rac1 by small interfering RNAs attenuates Dbs-enhanced motility. Both onco- and proto-Dbs-enhanced motility correlates with an increase in tyrosine phosphorylation of focal adhesion kinase on Tyr-397 and p130Cas on Tyr-410 and an increase in the abundance of the Crk·p130Cas complex. Suppression of expression of Cdc42 or its effector, Ack1, reduces tyrosine phosphorylation of focal adhesion kinase and p130Cas and disrupts the Crk·p130Cas complex. We further determined that suppression of expression of Cdc42, Ack1, p130Cas, or Crk reduces Rac1 activation and cell motility in Dbs-expressing cells to a level comparable with that in vector cells. Therefore, a cascade of activation of Cdc42 and Rac1 by Dbs through the Cdc42 effector Ack1 and the Crk·p130Cas complex is established. Suppression of the expression of endogenous Dbs reduces cell motility in both T47D cells and MDA-MB-231 cells, which correlates with the down-regulation of Cdc42 activity. This suggests that Dbs activates Cdc42 in these two human breast cancer cell lines and that the normal function of Dbs may be required to support cell movement.
Rho GTPases are a subfamily of the Ras superfamily of small signaling molecules that are widely expressed in mammalian cells (1). RhoA, Cdc42, and Rac1 are the most extensively studied members of the Rho GTPase family, and each plays a prominent and discrete role in cell migration (2, 3). Cdc42 promotes the formation of filopodia and is required to establish cell polarity (3–5); Rac1 promotes the formation of lamellipodia at the leading edge of motile cells (6), and RhoA promotes the formation of stress fibers which generate the traction forces needed to retract the cell tail and move the cell body beyond the leading edge (7, 8). Consistent with this important role in cell motility, RhoA, Cdc42, and Rac1 are often overexpressed in human tumors including breast, lung, and colon (9), and overexpression of constitutively active RhoA, Cdc42, or Rac1 increases cell migration and invasion (2, 10, 11).
The spatiotemporal regulation of Rho GTPase activity is tightly controlled by three classes of proteins. Rho-specific guanine nucleotide exchange factors (RhoGEFs)2 activate Rho proteins by facilitating the exchange of GDP for GTP; Rho GTPase-activating proteins (RhoGAPs) stimulate the intrinsic rate of hydrolysis of Rho proteins, thus converting them into their inactive state; Rho-specific guanine nucleotide dissociation inhibitors (RhoGDIs) compete with RhoGEFs for binding to GDP-bound Rho proteins and sequester Rho in the inactive state (12).
Dbs was identified in the screen for proteins whose overexpression cause malignant growth in murine fibroblasts (13, 14). The full-length Dbs protein (proto-Dbs) is a RhoGEF family member which contains multiple recognizable domains (Fig. 1A) including a Sec14-like domain, spectrin-like repeats, a RhoGEF domain (includes a DH and PH domain), and an SH3 domain (13). The original oncogenic version of Dbs that was identified (amino acid residues 525–1097; designated onco-Dbs) contains the RhoGEF domain alone. When expressed in murine fibroblasts, the transforming and catalytic activity of Dbs is subject to autoinhibition that is mediated by the NH2-terminal Sec14 domain (15). Although the endogenous function of Dbs is not known, recent studies suggest that Dbs and the Rac-specific exchange factor Tiam1 regulate neurotrophin-stimulated cell migration in Schwann cells through activation of Cdc42 and Rac1, respectively (16, 17).
FIGURE 1.
Onco-Dbs and proto-Dbs induce cell migration in tumor-derived breast epithelial cells. A, domain structure of the onco-Dbs and proto-Dbs proteins (Sec14 = Sec14-like domain; Spec = Spectrin-like repeats; DH = Dbl homology domain; PH = pleckstrin homology domain; SH3 = Src homology 3 domain). B, stable expression of HA-epitope-tagged onco-Dbs (Mr = 65) and proto-Dbs (Mr = 129 kDa) was confirmed by Western blot using an anti-HA antibody. Three independent sets of cell lines were generated. C, T47D cells stably expressing vector (Vec), onco-Dbs, or proto-Dbs were compared in a transwell motility assay on filters pre-coated with collagen I. The motility of cells stably expressing onco-Dbs or proto-Dbs is expressed relative to that of cells stably expressing vector. Data are represented as the mean ± S.D. of three independent experiments performed in triplicate. D, T47D cells stably expressing vector, onco-Dbs, or proto-Dbs were cultured to monolayer on dishes coated with poly-l-lysine or collagen I, as indicated. Cells were serum-starved overnight, and then the surface of the plate was scraped. Migration of cells at the wound edge was monitored and photographed at 18 h. Representative images are shown. E, growth curves of T47D cells stably expressing vector, onco-Dbs, or proto-Dbs. Cells were cultured in triplicate on poly-l-lysine (filled symbols) or on dishes pre-coated with collagen I (open symbols) and counted on the indicated days. Data shown are representative of three independent experiments.
Conversion of Rho proteins to their active GTP-bound state allows them to interact with effector signaling molecules. Ack1 is a nonreceptor-tyrosine kinase that binds to active Cdc42 but not Rac1 or RhoA (18, 19). Activated Ack1 is overexpressed in primary tumors and cancer cell lines and has been implicated in cancer metastasis (20). Recent studies have identified a signaling complex that regulates the motility of human breast epithelial cells that contains Cdc42, Ack1, p130Cas, and Crk (21). Ack1 and p130Cas interact through their respective SH3 domains, and Ack1 phosphorylates p130Cas in a collagen I-dependent manner. p130Cas was first identified as a hyperphosphorylated adapter protein in cells transformed by v-Src and v-Crk (22, 23). Further studies showed that p130Cas is associated with both cellular Src and Crk in a tyrosine phosphorylation-dependent manner (24, 25). Focal adhesion kinase (FAK) binds to the NH2 terminus of p130Cas and phosphorylates the COOH terminus in a region that is involved in p130Cas binding to Src (26). The binding of Crk to p130Cas recruits binding partners to the SH3 domain of Crk, including C3G and DOCK180, which activate Rap1 and Rac1, respectively (27–31). Thus, formation of the Crk·p130Cas complex is considered to be a molecular switch that can induce cell migration by activating Rac1 (32).
Here we show that both proto-Dbs and onco-Dbs increase cell migration in human breast adenocarcinoma cells in a collagen I-dependent manner. Increased motility is dependent upon the activation of Rac1 and Cdc42 and is mediated by the assembly of Crk·p130Cas complexes. Suppression of endogenous Dbs expression in human tumor-derived breast epithelial cells limits cell motility, suggesting that Dbs may be a critical regulator of cell behavior in breast cancer.
EXPERIMENTAL PROCEDURES
Molecular Constructs
The pCTV3H mammalian expression vector has been described previously (13). pCTV3H-dbs-HA6 and pCTV3H-dbs-FL contain cDNA fragments that encode a transforming derivative of Dbs (onco-Dbs) and full-length Dbs (proto-Dbs), respectively (33). Both proteins are fused to a hemagglutinin (HA) epitope tag.
Cell Culture and Transfection
T47D, MDA-MB-231, M14, MDA-MB-453, and ZR-57–1 cells were cultured at 37 °C in 5% CO2 in RPMI 1640 medium supplemented with 10% fetal bovine serum (Benchmark). MCF-10A cells were cultured at 37 °C in 5% CO2 in Dulbecco's modified Eagle's medium/F-12 supplemented with 5% horse serum (Invitrogen), 20 ng/ml epidermal growth factor (Sigma), 0.5 mg/ml hydrocortisone (Sigma), and 10 μg/ml insulin (Invitrogen). All media were supplemented with 100 unit/ml penicillin and 100 μg/ml streptomycin. T47D cells stably expressing proto-Dbs, onco-Dbs, or their cognate vector were generated as described previously (13). Briefly, cells were transfected by Lipofectamine reagent (Invitrogen) according to the manufacturer's recommended protocol. Cells were maintained in growth medium supplemented with hygromycin B (200 μg/ml, Invitrogen) for 14 days. For each individual stable cell line, more than 200 drug-resistant colonies were combined to generate a polyclonal stable cell line. Three independent cell lines were generated and tested for each construct.
Cell Growth Assays
T47D cells stably expressing onco-Dbs, proto-Dbs, or cognate vector were resuspended in growth medium. 5 × 103 cells were seeded into each well of a 48-well tissue culture dish. Cells were cultured either on plastic or plastic precoated with collagen I (50 μg/ml). Cells were trypsinized (3 wells per day) and counted using a hemocytometer for 10 consecutive days.
RhoA, Cdc42, and Rac1 Activation Assay
Affinity precipitation assays to measure the levels of endogenous GTP-bound RhoA, Cdc42, and Rac1 were performed using the Rho binding domain of Rhotekin (GST-C21) and the Cdc42/Rac1 binding domain of PAK (GST-PAK) as previously described (34). 2 × 106 cells were cultured on 10-cm tissue culture dishes precoated with collagen I (50 μg/ml; BD Biosciences) or poly-l-lysine (0.01%; Sigma). Cells were maintained in growth medium for 48 h and then serum-starved for 18 h in RPMI 1640 medium supplemented with 0.5% fetal bovine serum. Cells were subconfluent when cell lysates were prepared.
Transwell Motility Assays
Assays were performed as previously described (11). Subconfluent cells were detached by 0.05% trypsin, and equal numbers were loaded into the top chamber of transwells coated from the underside with collagen I and allowed to migrate for 16 h. Where indicated, cell assays were performed 48 h after transfection with siRNAs. In all cases sufficient numbers of cells were loaded such that we observed greater than 50 cells/field passing through the filter in the vector controls. Cells that migrated to the underside of the membranes were fixed and stained with Diff-Quik (Dade Behring) and counted. All experiments were performed in triplicate, and 8–12 fields of view for each transwell were counted. The average amount of cells per field was then calculated. Results were expressed as -fold changes relative to controls. Where indicated, the Rac-specific inhibitor, NSC23766 (C24H38Cl3N7, 50 μm, Calbiochem), was added to the medium in the bottom chamber and the medium that was used to resuspend the cells 30 min before loading the transwell.
In Vitro Wound Healing Assay
Assays were performed as previously described by others (35). T47D cells stably expressing proto-Dbs, onco-Dbs, or their cognate vector were cultured on 35-mm tissue culture dishes. Where indicated, dishes were precoated with poly-l-lysine (0.01%) or collagen I (50 μg/ml). Confluent monolayers of cells were starved in RPMI 1640 media supplemented with 0.5% fetal bovine serum overnight. The surface of the monolayer was scraped using a pipette tip, and cells alongside the wound were allowed to migrate toward the opening for 18 h under serum-deprived conditions (0.5% fetal bovine serum). Closure was monitored and photographed at multiple sites, and representative images are provided. Where indicated, cells were transiently transfected with siRNAs 48 h before serum starvation.
siRNAs
Cells at 70% confluence were transfected by siRNAs from different manufacturers with SiLentFectTM lipid reagent (Bio-Rad) according to the manufacturer's protocols. Minimal concentrations of siRNAs that can reduce the expression of the target proteins by 70% was determined by Western blot and an Odyssey IR Imager. Concentrations of individual siRNAs that were used are 100 nm Cdc42 (Santa Cruz sc-29256 or Ambion s2765), 150 nm Rac1 (Santa Cruz sc-36351 or Ambion s11713), 150 nm p130Cas (Santa Cruz sc-44317 or Ambion s225161), 100 nm Crk (Santa Cruz sc-37072 or Ambion s3521), 150 nm Ack (Santa Cruz sc-29632 or Ambion s19851), 150 nm Ost (Santa Cruz sc-41728 or Dharmacon DR3773961), 150 nm Bcr (Santa Cruz sc-29795 or Dharmacon l-003875-00), and 150 nm Tiam1 (Santa Cruz sc-36669 or Ambion s14138). Scrambled siRNAs (Santa Cruz sc-37007 or Ambion AM4611) were included in all experiments as negative controls. Scrambled siRNAs were also used to bring the total amount of siRNAs up to the same concentration when needed. The viability of cells transfected by siRNAs was determined by the trypan blue exclusion method as previously described (36).
Immunoprecipitation and Western Blot Analysis
Immunoprecipitation and Western blot analysis were performed as previously described (37). Antibodies used for Western blots include: anti-HA (Berkeley Antibody Co., Inc.), anti-Crk (BD Transduction Laboratories), anti-p-Crk (pY207, Cell Signaling), anti-p130Cas (BD Transduction), anti-phospho-p130Cas (pY410, Cell Signaling), anti-FAK (Santa Cruz, sc-557), anti-phospho-FAK (pY397, BIOSOURCE), anti-Ack1 (Santa Cruz, sc-323), anti-Cdc42 (Santa Cruz, sc-8401), anti-Rac1 (BD Transduction Laboratories), anti-RhoA (Santa Cruz, sc-418), anti-Src (Santa Cruz, sc-18), anti-phospho-Src (pY418, Calbiochem), anti-Bcr (Cell Signaling), anti-Tiam1 (Santa Cruz, sc-872), anti-Ost (Santa Cruz, sc-901), and anti-actin (Santa Cruz, sc-8432).
Statistics
Statistical analyses were performed by one-way analysis of variance followed by the Tukey HSD (honestly significant difference) test for multiple comparisons using JMP statistical software. p values of <0.05 were considered significant. Once the statistical analysis was complete, the data were converted to -fold activations to facilitate ease of comparison between the figures.
RESULTS
Increased Cell Motility in T47D Cells That Stably Express Dbs
T47D is a well differentiated human breast carcinoma cell line that has been previously established as a useful system to study the effects of deregulated Rho protein signaling on cell migration and invasion (11, 38, 39). To examine the role of Dbs in tumor cell motility, we generated T47D cell lines that stably express HA-tagged onco-Dbs, proto-Dbs, and cognate vector (Fig. 1A). To control for clonal differences in cell behavior, three independent sets of cell lines were generated and compared. Expression of the HA-tagged proteins was confirmed by Western blot (Fig. 1B). When examined in a transwell motility assay, the cell lines expressing onco-Dbs or proto-Dbs showed increased cell motility (∼2- and 3-fold, respectively) when compared with vector control cells (Fig. 1C). To confirm the increased motility, the cell lines were also compared in an in vitro wound healing assay. Because transwell motility assays were performed on filters coated with collagen I, the wound healing assays were performed on plates coated with either poly-l-lysine or collagen I. When cultured on plates pre-coated with collagen I, cells stably expressing onco-Dbs or proto-Dbs both showed enhanced cell motility in the wound healing assay (Fig. 1D). However, when cultured on tissue culture dishes pre-coated with poly-l-lysine, no differences in motility were observed. No significant enhancement of motility was observed when cells were cultured on uncoated plastic, collagen IV, fibronectin, or laminin (not shown). To confirm that the increased migration could not be attributed to an accelerated rate of cell division, the growth rates of all cell lines was compared on both collagen I and poly-l-lysine (Fig. 1E). Although cells grown on collagen I grew faster than those grown on poly-l-lysine, no differences were observed between vector and Dbs-expressing cells under either culture condition.
Dbs Activates Endogenous Cdc42 and Rac1 in T47D Cells in a Collagen I-dependent Manner
Because Dbs regulates motility in Schwann cells through activation of Cdc42, we determined if Dbs induces T47D cell motility by activating this GTPase. Rac1 and RhoA were included in the analysis for comparison. When plated on polyl-lysine, no differences were observed in RhoA-GTP, Cdc42-GTP, or Rac1-GTP levels in the cell lines that stably express onco-Dbs, proto-Dbs, or vector (Fig. 2, A and B). However, when cells were cultured on dishes pre-coated with collagen I, elevated levels of Cdc42-GTP and Rac1-GTP were observed in the cells that express onco-Dbs or proto-Dbs (Fig. 2, C and D). Consistent activation of RhoA was also observed in cells that express onco-Dbs but not proto-Dbs. This suggests that Cdc42 and Rac1, but not RhoA, may contribute to proto-Dbs-mediated cell motility.
FIGURE 2.
Dbs activates Rac1 and Cdc42 in a collagen I-dependent manner. T47D cells stably expressing vector, onco-Dbs, or proto-Dbs were cultured on tissue culture dishes pre-coated with poly-l-lysine (A and B) or collagen I (C and D). A and C, cell lysates were collected and examined by Western blot for expression of total Cdc42, RhoA, and Rac1. Lysates were subjected to affinity purification with immobilized GST-PAK or GST-Rhotekin, and active GTP-bound Cdc42, RhoA, or Rac1 was visualized by Western blot. B and D, quantifications were performed using an Odyssey IR imager. Levels of active Rho were first normalized against total Rho and then expressed as -fold activation relative to vector controls. Data are represented as the mean ± S.D. of three independent experiments.
Endogenous Cdc42 and Rac1 Are Required for Dbs-induced Motility in T47D Cells
Next we determined whether Rac1 or Cdc42 activation is required to support Dbs-mediated motility. Using siRNAs, we suppressed the expression of Cdc42 and Rac1 in T47D cells stably expressing onco-Dbs, proto-Dbs, or cognate vector. Scrambled siRNAs were included in the analysis as negative controls. For comparison, Rac was also inhibited with a specific pharmacological inhibitor (NSC23766). The siRNAs targeting Cdc42 (Fig. 3A) or Rac1 (Fig. 3B) suppressed the expression level of their target proteins significantly, which reduced the levels of endogenous Cdc42-GTP and Rac-GTP to a level that was lower than cells transfected with scrambled controls. In parallel transfections, siRNAs targeting Cdc42 and Rac1 reduced the motility of cells stably expressing onco-Dbs or proto-Dbs to a level equivalent to that of cells stably expressing cognate vector (Fig. 3, C and D). A similar reduction in motility was observed in cells treated with the Rac-specific inhibitor (Fig. 3, E–G). In all cases the reduced motility could not be attributed to increased cell death, as more than 95% of treated cells were viable as determined by trypan blue exclusion (data not shown). Collectively, these results suggest that Dbs regulates cell motility in T47D cells in a Cdc42- and Rac1-dependent manner.
FIGURE 3.
Endogenous Cdc42 and Rac1 are required for Dbs-induced motility in T47D cells. T47D cells stably expressing vector, onco-Dbs, or proto-Dbs were cultured on dishes pre-coated with collagen I and then transfected by siRNAs (si) targeting Cdc42 (A) and Rac1 (B). Scrambled siRNAs (Ctrl) were included as negative controls. A, total expression of Cdc42 (top panel) was determined by Western blot using an anti-Cdc42 antibody. The active level of Cdc42 (middle panel) was determined by affinity purification with immobilized GST-PAK. Vec, vector. B, total expression of Rac1 (top panel) was determined by Western blot using an anti-Rac1 antibody. The active level of Rac1 (middle panel) was determined by affinity purification with immobilized GST-PAK. In parallel experiments the cells were examined in transwell motility (C) or wound healing (D) assays performed on collagen I. All experiments were performed in triplicate, and data are presented as in Fig. 1. Rho activation (E), transwell motility (F), and wound healing (G) assays were also performed in the presence (NSC23766) or absence (mock) of a Rac specific inhibitor. E, the active level of RhoA was determined by affinity purification with immobilized GST-C21.
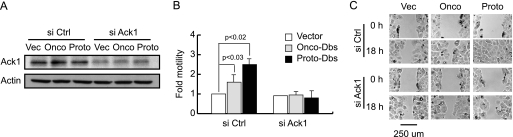
Ack1 Is Required for Dbs-mediated Motility in T47D Cells
Ack1 is a non-receptor tyrosine kinase that is an effector for Cdc42 (40). To determine whetherAck1 mediates Dbs-induced motility, we transiently suppressed the expression of Ack1 in T47D cells that stably express onco-Dbs, proto-Dbs, or cognate vector. siRNAs targeting Ack1 significantly suppressed the expression of Ack1 (70%; Fig. 4A) in all three cell lines and reduced the motility of cells stably expressing onco-Dbs or proto-Dbs to a level similar to that seen in cells stably expressing vector (Fig. 4, B and C). The reduced cell motility was not attributable to increased cell death as measured by trypan blue exclusion (data not shown).
FIGURE 4.
Ack1 is required for Dbs-mediated motility in T47D cells. T47D cells stably expressing vector (Vec) or onco- or proto-Dbs were cultured on dishes pre-coated with collagen I and transfected by siRNAs targeting Ack1 (si Ack1). Scrambled siRNAs (si Ctrl) were included as negative controls. A, lysates were collected, and the expression level of Ack1 was determined by Western blot using an anti-Ack1 antibody. In parallel transfections the cells were examined in transwell motility (B) and wound healing (C) assays. Assays were performed and quantitated as described in Fig. 1. B, data are represented as the mean ± S.D. of three independent experiments.
Dbs Increases the Phosphorylation of p130Cas and Its Association with Crk in a Cdc42- and Ack1-dependent Manner
Previous studies have demonstrated that phosphorylation of p130Cas on Tyr-410 creates a docking site for Crk and supports the formation of the Crk·p130Cas complex (41). Although the association between p130Cas and Crk can be regulated by Cdc42 through Ack1, it is unclear whether Ack1 phosphorylates p130Cas directly or through other kinases that phosphorylate p130Cas, such as Src and FAK (21). We determined whether Dbs regulates motility in T47D cells by promoting the association of p130Cas and Crk. Although the overall expression level of p130Cas in T47D cells that stably express onco-Dbs or proto-Dbs was not significantly different from vector control cells, the level of Tyr-410 phosphorylation was increased by 2–3-fold (Fig. 5A). Consistent with this, a 2–3-fold increase in the amount of Crk that co-immunoprecipitates with p130Cas was also observed in the cells that stably express onco- or proto-Dbs (Fig. 5A).
FIGURE 5.
Dbs increases the phosphorylation of p130Cas and its association with Crk in a Cdc42- and Ack1-dependent manner. A, T47D cells stably expressing onco-Dbs, proto-Dbs, or cognate vector were cultured on collagen I. Lysates were prepared and separated into two aliquots. The first aliquot was subject to immunoprecipitation (IP) using an anti-p130Cas antibody. The immunoprecipitate was then examined by Western blot (WB) for the presence of p130Cas and Crk. The second aliquot was then examined by Western blot for levels of p130Cas phosphorylated on Tyr-410 (p-p130Cas), total p130Cas, Crk phosphorylated on Tyr-221 (p-Crk), and total Crk, as indicated. B and C, the stable cell lines were cultured on collagen I and transfected with siRNAs (si) targeting Cdc42 and Ack1. Scrambled siRNAs were included as controls (si Ctrl). Lysates were collected, and one aliquot was examined by Western blot for expression of Cdc42 and Ack1 (B). C, an additional aliquot of the lysate was then examined for levels of phosphorylated (p-p130Cas) and total p130Cas. A third aliquot was subject to immunoprecipitation, and the immunoprecipitate was examined by Western blot with an anti-Crk antibody.
The association of Crk with its binding partners is negatively regulated by an intramolecular interaction between its SH2 domain and phosphorylated Tyr-221 (42). To determine whether the increased association of Crk with p130Cas could also be attributed to reduced phosphorylation of Crk on Tyr-221, we also examined the levels of total and Tyr-221-phosphorylated Crk in T47D cells that stably express onco-Dbs or proto-Dbs (Fig. 5A). No differences were observed in the levels of phosphorylated or total Crk when compared with vector control cells, suggesting that Dbs does not regulate the phosphorylation of Crk.
To determine whether the phosphorylation of p130Cas and its association with Crk is mediated by Cdc42 and Ack1, we transiently suppressed the expression of Cdc42 or Ack1 in T47D cells stably expressing onco-Dbs, proto-Dbs, or cognate vector (Fig. 5B). Although reduced expression of Cdc42 and Ack1 does not change the overall expression level of p130Cas (Fig. 5C) in both the onco- and proto-Dbs-expressing cells, the level of p130Cas that was phosphorylated on Tyr-410 was either equivalent to or below the levels in the vector control cells. Similarly, the increased association of p130Cas with Crk in the cells expressing onco-Dbs and proto-Dbs was also found to be dependent upon the expression of Cdc42 and Ack1. Therefore, the phosphorylation of p130Cas and its association with Crk is regulated by Dbs in a Cdc42- and Ack1-dependent manner.
Dbs Increases FAK Phosphorylation in a Cdc42- and Ack1-dependent Manner
FAK and Src have been shown to phosphorylate the substrate domain of p130Cas (26, 43–46), and both are themselves activated by phosphorylation on tyrosine within their respective activation loops (Tyr-397 for FAK and Tyr-418 for Src) (47). To determine whether Src or FAK are activated in T47D cells that stably express onco-Dbs or proto-Dbs, we utilized antibodies that specifically recognize the activated versions of both kinases. No differences were observed in the overall level of total and activated Src in the onco-Dbs- or proto-Dbs-expressing cells relative to the vector control cells (Fig. 6A). However, elevated levels of activated FAK were detected in cells that express onco- and proto-Dbs relative to vector control cells.
FIGURE 6.
Dbs increases FAK phosphorylation in a Cdc42- and Ack1-dependent manner. A, T47D cells that stably express vector (Vec), onco-Dbs, or proto-Dbs were cultured on collagen I. Lysates were prepared and examined by Western blot for levels of total Src, Src phosphorylated on Tyr-418 (p-Src), total FAK, and FAK phosphorylated on Tyr-397 (p-FAK). B, the cells were also transfected with siRNAs (si) targeting Cdc42 and Ack1 as indicated. Cell lysates were then collected and examined by Western blot for levels of total and phosphorylated (Tyr-397; p-FAK) FAK.
To determine whether FAK phosphorylation is dependent on Cdc42 or Ack1, levels of total and activated FAK were measured in the lysates in which the expression of Cdc42 or Ack1 was suppressed (Fig. 5B). Although the siRNAs that target Cdc42 or Ack1 did not change the overall expression level of FAK, the level of activated FAK in the cells that express onco-Dbs or proto-Dbs was reduced to the levels seen in the vector controls (Fig. 6B). This suggests that Dbs activates FAK in an Ack1-dependent manner and provides a mechanism through which Ack can promote the assembly of the Crk·p130Cas complexes.
p130Cas and Crk Are Required for Dbs-induced Cell Migration and Rac Activation
Next, we determined if p130Cas and Crk are required for Dbs-mediated motility in T47D cells. Using siRNAs, we transiently suppressed expression of p130Cas, Crk, Ack1, or Cdc42 in T47D cells stably expressing onco-Dbs, proto-Dbs, or cognate vector (Figs. 5B and 7A). siRNAs that individually target p130Cas or Crk reduced the motility of cells stably expressing onco-Dbs or proto-Dbs to a level equivalent to that seen in vector control cells (Fig. 7, B and C). Because others have shown that the p130Cas·Crk complex promotes motility by stimulating Rac activation, we also performed affinity precipitation assays to determine whether Rac activation in cells that stably express onco- or proto-Dbs is dependent upon expression of p130Cas, Crk, Ack1, or Cdc42. Reduced expression of p130Cas, Crk, Ack1, or Cdc42 reduced the level of Rac activation in both the onco-Dbs- and proto-Dbs-expressing cells to the levels seen in the vector control cells (Fig. 7D). The latter observation suggests that Dbs activation of Rac1 in T47D cells is indirect and mediated by Cdc42.
FIGURE 7.
p130Cas and Crk are required for Dbs-induced cell migration and Rac activation. A, T47D cells stably expressing vector (Vec), onco-Dbs, or proto-Dbs were cultured on collagen I and then transfected with siRNAs (si) targeting p130Cas, Crk, or Ack1. Scrambled siRNAs (si Ctrl) were included as a negative control. Cell lysates were collected and examined by Western blot for expression of Crk, p130Cas, and Ack1. Cells transfected with siRNAs targeting p130Cas and Crk were also examined in transwell motility (B) and wound healing (C) assays. Data for both assays was collected, quantitated, and represented as described in Fig. 1. D, lysates from all transfections was also examined by affinity purification assays for levels of active Rac1 (Rac1 GTP).
Endogenous Dbs Supports Cell Motility in Human Breast Epithelial Cells
Because overexpression of proto-Dbs increases the motility of T47D cells, we determined whether endogenous Dbs supports cell motility in this same cell type. To examine this, we transiently suppressed the expression of endogenous Dbs as well as two additional RhoGEF family members, Tiam1 and Bcr. Bcr is a RhoGEF that is active toward RhoA, Cdc42, and Rac1 (48) and is the fusion partner for Abl in chronic myelogenous leukemia (49). Tiam1 is a Rac-specific exchange factor that is mutated in human renal cancers (50) and cooperates with Dbs to regulate motility in Schwann cells (17). siRNAs targeting Dbs, Bcr, and Tiam1 significantly reduced the expression of their respective target proteins (Fig. 8A). The identity of endogenous Dbs was confirmed using two independent commercially available siRNA mixes (not shown). Lysates were also examined for levels of activated Cdc42 and Rac1 (Fig. 8B). The reduced expression of Dbs (but not Tiam1 or Bcr) was associated with reduced levels of activated Cdc42 in T47D cells, indicating that Dbs normally regulates Cdc42 in this cell type. Reduced expression of Dbs had no effect on RhoA-GTP levels, indicating that RhoA is not a normal substrate for Dbs in this cell type. Activated Rac levels were reduced substantially when Tiam1 expression was suppressed, to a more limited extent when Dbs expression was suppressed and not at all when Bcr expression was suppressed. In parallel experiments, the transfected cells were examined in transwell motility (Fig. 8C) and in vitro wound healing (Fig. 8D) assays. In the transwell motility assays, siRNAs targeting Dbs and Tiam1 reduced cell motility by about 50 and 90%, respectively (Fig. 8C), whereas siRNAs targeting Bcr had no effect. Similarly, cell motility is dependent upon Tiam1 and Dbs expression, but not Bcr expression, in the wound healing assay (Fig. 8D). When tissue culture dishes were pre-coated with poly-l-lysine, siRNAs targeting Bcr, Dbs, or Tiam1 had no significant effect on T47D cell migration (not shown).
FIGURE 8.
Endogenous Dbs and Tiam1 support cell motility in T47D cells. T47D cells were cultured on collagen I and transfected with siRNAs (si) targeting Dbs, Bcr, and Tiam1. Scrambled siRNAs (si Ctrl) were included as controls. A, cell lysates were prepared and examined by Western blot for expression of Dbs, Bcr, and Tiam1 as indicated. B, lysates were also examined by affinity precipitation assays for levels of activated Cdc42 (Cdc42 GTP), Rac1 (Rac1 GTP), and RhoA (RhoA GTP). Cells transfected with siRNAs for Dbs, Bcr, and Tiam1 were also examined in transwell motility (C) and wound healing (D) assays. Both assays were performed on collagen I, and data were collected and presented as in Fig. 1.
To confirm that Dbs-mediated motility is not restricted to T47D cells, we examined a panel of human breast tumor-derived cell lines for Dbs expression (Fig. 9A). For comparison, the panel also included the highly motile M14 melanoma cell line and MCF10A, which are derived from benign breast tissue. Dbs expression was observed in all cell lines examined, with the lowest expression in the MCF10A and T47D cells. The identity of Dbs was confirmed using a second commercially available antibody (not shown). MDA-MB-231 is the most highly motile and invasive human breast cancer epithelial cell line on our panel. We suppressed the expression of endogenous Dbs in this cell line (Fig. 9B) and examined the levels of Cdc42 activity and cell motility. Similar to what we observed in the T47D cells, the reduced expression of Dbs correlated with the reduced levels of activated Cdc42 (Fig. 9B) and cell motility (Fig. 9C) in a dose-dependent manner.
FIGURE 9.
Endogenous Dbs supports cell motility in MBA-MD-231 cells. A, cell lysates were collected from the indicated cell lines and examined by Western blot for expression of endogenous Dbs. Tubulin expression was also examined as a control for equivalent loading. B, MBA-MD-231 cells were cultured on collagen I and transfected with siRNAs (si) targeting Dbs at the indicated concentrations. Scrambled siRNAs (si Ctrl) were included as controls. Cell lysates were examined by Western blot for expression of Dbs as indicated. Lysates were also examined by affinity precipitation assays for levels of activated Cdc42 (Cdc42 GTP). C, MBA-MD-231 cells transfected with siRNAs for Dbs were also examined in transwell motility assays. Assays were performed on collagen I, and data were collected and presented as in Fig. 1.
DISCUSSION
In the current study we have determined that stable expression of both onco- and proto-Dbs enhances the motility of human tumor-derived breast epithelial cells. Although Dbs enhances motility when cultured on collagen I, no enhancement was observed when cells were cultured on plastic, poly-l-lysine, collagen IV, fibronectin, or laminin. Activation of Cdc42 and Rac1 was also only observed when cells were cultured on collagen I, and siRNAs targeting endogenous Dbs in T47D and MDA-MB-231 cells only suppress motility if cells are cultured on collagen I. This suggests that endogenous and overexpressed Dbs may regulate motility through similar mechanisms. This specificity for collagen I may have particular significance for breast cancer progression. During metastasis, breast cancer cells must transverse the stromal extracellular matrix, which contains collagen I (51), and excessive expression of collagen I is a predisposing factor in breast and pancreatic cancer (52–54). In addition, culturing on collagen I can trigger epithelial-to-mesenchymal transition in pancreatic cancer cells, lung cancer cells, and mouse mammary epithelial cells (55–57).
Although several reports have indicated that specific members of the RhoGEF family may be mutated in human tumors, it is not clear that overexpression of a RhoGEF is sufficient to stimulate Rho activation or transformation (58). RhoGEFs normally exist in the cell in an inactive state where they are subject to tight autoinhibitory regulation (58). In NIH 3T3 cells the catalytic and transforming activity of Dbs is autoinhibited by the NH2-terminal Sec14 domain (15). However, in T47D cells we observed that proto-Dbs stimulates motility to a similar extent as onco-Dbs, suggesting that proto-Dbs may not be subject to autoinhibition in this cell type. It also suggests that simply increasing the level of expression of a RhoGEF in a tumor cell line can be sufficient to increase the motile phenotype. Although it is possible that collagen I supports the effects of Dbs on motility by relieving the autoinhibitory mechanism, this seems unlikely, as onco-Dbs, which lacks the Sec14 domain, also requires collagen I to support motility.
Both onco- and proto-Dbs activate Cdc42 and Rac1 in T47D cells, and siRNAs or pharmacological inhibitors that target these GTPases block cell motility. Because previous in vitro and in vivo nucleotide exchange analyses have shown that Dbs is active toward RhoA and Cdc42, but not Rac1 (14, 59), we presume that Dbs activates Rac1 indirectly in T47D cells. In a previous study it has been shown that collagen I promotes the association of Cdc42 with a complex that contains Ack1, p130Cas, and Crk and that this complex in turn promotes the motility of breast cancer cell lines (21). Because the Crk·p130Cas complex has been shown to promote Rac activation, this provides a mechanism through which Dbs could activate Rac1 in a Cdc42-dependent manner. In the current study we have shown that onco-Dbs and proto-Dbs can activate Cdc42, increase the phosphorylation of p130Cas, and increase its association with Crk in a Cdc42- and Ack1-dependent manner. Additionally, we have shown that siRNAs targeting p130Cas, Crk, or Cdc42 reduce Rac1 activation. Thus, in our model we propose that collagen I stimulates the association of p130Cas with Cdc42 as previously demonstrated (21). Activation of Cdc42 by Dbs would then recruit Ack1 and promote the assembly and stabilization of Crk·p130Cas complexes, which would then recruit and activate exchange factors for Rac. Our previous observation that Rac binds directly to the PH domain of Dbs also provides a mechanism for recruiting Rac to the complex and would allow Cdc42 and Rac to be assembled into a regulatory cascade (59).
Although it is not clear how Ack1 promotes the assembly of Crk·p130Cas complexes, both FAK and Src can phosphorylate p130Cas, and both are known to regulate cell motility (60). Phosphorylation on Tyr-397 in the activation loop of FAK and Tyr-418 in the activation loop of Src activate their respective kinase activities (61, 62). By using antibodies that recognize the phosphorylated state of these residues, we discovered that the level of phospho-Src is unchanged, but the level of phospho-FAK is increased in T47D cells that express Dbs. Furthermore, siRNAs targeting Cdc42 or Ack1 reduce the level of phosphorylated FAK to control levels, suggesting that FAK is downstream of Cdc42 and Ack1. Therefore, Ack1 may phosphorylate and activate FAK, which in turn may phosphorylate p130Cas. However, we cannot exclude the possibility that Ack1 may phosphorylate p130Cas directly.
Endogenous Dbs was detected in all breast epithelial cell lines examined, and suppression of Dbs expression reduces the motility of T47D and MDA-MB-231 cells, which correlates with a reduction in the level of active Cdc42. These results imply that endogenous Dbs utilizes Cdc42 as a substrate in both of these cell types and is required for their motility. The specificity of this observation was confirmed in parallel experiments in which we showed that a second known Cdc42 activator, Bcr, does not control Cdc42 activation or motility in these cell lines. Our observations are consistent with a previous study in which siRNA-mediated suppression of endogenous Dbs reduced Cdc42-GTP levels and neurotrophin-3-mediated cell migration in Schwann cells (16).
Our previous studies have shown that onco-Dbs mediates transformation in fibroblasts through regulation of MLC2 in a RhoA/ROCK-dependent manner (37). Consistent with this, onco-Dbs activates RhoA when overexpressed in T47D cells. However, proto-Dbs does not activate RhoA in this cell type, and suppression of endogenous Dbs expression does not alter RhoA-GTP levels. Thus, proto-Dbs does not appear to be coupled to RhoA activation in T47D cells.
Dbs was also detected in MCF10A cells, suggesting that it may have other functions in breast epithelial cells and that its presence is not sufficient to induce a motile phenotype. Interestingly, the Rac1-specific exchange factor Tiam1 is also required for Rac activation and cell motility in both Schwann cells (17) and breast epithelial cells (current study), and we observe a precise positive correlation between Tiam1 expression and motility in tumor-derived breast epithelial cells.3 This supports our model that Dbs and Tiam1 may in fact be key regulatory components of a signaling complex that is required to support normal cell motility during development and aberrant motility during tumorigenesis. Our results suggest that in the absence of either exchange factor, motility is impaired.
This work was supported, in whole or in part, by National Institutes of Health Grant CA97066 (NCI, to the United States Public Health Service). This work was also supported by American Cancer Society Research Scholar Grant RGS-04-199-01 (to I. P. W.).
H. Adams, unpublished observations.
- RhoGEF
- Rho-specific guanine nucleotide exchange factor
- FAK
- focal adhesion kinase
- HA
- hemagglutinin
- GST
- glutathione S-transferase
- siRNA
- small interfering RNA
- PAK
- p21-activated kinase.
REFERENCES
- 1.Ridley A. J., Hall A. ( 1992) Cell 70, 389– 399 [DOI] [PubMed] [Google Scholar]
- 2.Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. ( 2003) Science 302, 1704– 1709 [DOI] [PubMed] [Google Scholar]
- 3.Nobes C. D., Hall A. ( 1995) Biochem. Soc. Trans. 23, 456– 459 [DOI] [PubMed] [Google Scholar]
- 4.Kozma R., Ahmed S., Best A., Lim L. ( 1995) Mol. Cell. Biol. 15, 1942– 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown J. L., Jaquenoud M., Gulli M. P., Chant J., Peter M. ( 1997) Genes Dev. 11, 2972– 2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. ( 1992) Cell 70, 401– 410 [DOI] [PubMed] [Google Scholar]
- 7.Worthylake R. A., Lemoine S., Watson J. M., Burridge K. ( 2001) J. Cell Biol. 154, 147– 160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Worthylake R. A., Burridge K. ( 2003) J. Biol. Chem. 278, 13578– 13584 [DOI] [PubMed] [Google Scholar]
- 9.Sahai E., Marshall C. J. ( 2002) Nat. Rev. Cancer 2, 133– 142 [DOI] [PubMed] [Google Scholar]
- 10.Keely P. J., Westwick J. K., Whitehead I. P., Der C. J., Parise L. V. ( 1997) Nature 390, 632– 636 [DOI] [PubMed] [Google Scholar]
- 11.Keely P. J. ( 2001) Methods Enzymol. 333, 256– 266 [DOI] [PubMed] [Google Scholar]
- 12.Whitehead I. P., Campbell S., Rossman K. L., Der C. J. ( 1997) Biochim. Biophys. Acta 1332, F1– 23 [DOI] [PubMed] [Google Scholar]
- 13.Whitehead I., Kirk H., Kay R. ( 1995) Oncogene 10, 713– 721 [PubMed] [Google Scholar]
- 14.Horii Y., Beeler J. F., Sakaguchi K., Tachibana M., Miki T. ( 1994) EMBO J. 13, 4776– 4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kostenko E. V., Mahon G. M., Cheng L., Whitehead I. P. ( 2005) J. Biol. Chem. 280, 2807– 2817 [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi J., Chan J. R., Miyamoto Y., Tsujimoto G., Shooter E. M. ( 2005) Proc. Natl. Acad. Sci. U. S. A. 102, 5198– 5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamauchi J., Miyamoto Y., Tanoue A., Shooter E. M., Chan J. R. ( 2005) Proc. Natl. Acad. Sci. U. S. A. 102, 14889– 14894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manser E., Leung T., Salihuddin H., Tan L., Lim L. ( 1993) Nature 363, 364– 367 [DOI] [PubMed] [Google Scholar]
- 19.Yang W., Cerione R. A. ( 1997) J. Biol. Chem. 272, 24819– 24824 [DOI] [PubMed] [Google Scholar]
- 20.van der Horst E. H., Degenhardt Y. Y., Strelow A., Slavin A., Chinn L., Orf J., Rong M., Li S., See L. H., Nguyen K. Q., Hoey T., Wesche H., Powers S. ( 2005) Proc. Natl. Acad. Sci. U. S. A. 102, 15901– 15906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modzelewska K., Newman L. P., Desai R., Keely P. J. ( 2006) J. Biol. Chem. 281, 37527– 37535 [DOI] [PubMed] [Google Scholar]
- 22.Birge R. B., Fajardo J. E., Reichman C., Shoelson S. E., Songyang Z., Cantley L. C., Hanafusa H. ( 1993) Mol. Cell. Biol. 13, 4648– 4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda M., Tanaka S., Nagata S., Kojima A., Kurata T., Shibuya M. ( 1992) Mol. Cell. Biol. 12, 3482– 3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakai R., Iwamatsu A., Hirano N., Ogawa S., Tanaka T., Mano H., Yazaki Y., Hirai H. ( 1994) EMBO J. 13, 3748– 3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamoto T., Sakai R., Ozawa K., Yazaki Y., Hirai H. ( 1996) J. Biol. Chem. 271, 8959– 8965 [DOI] [PubMed] [Google Scholar]
- 26.Tachibana K., Urano T., Fujita H., Ohashi Y., Kamiguchi K., Iwata S., Hirai H., Morimoto C. ( 1997) J. Biol. Chem. 272, 29083– 29090 [DOI] [PubMed] [Google Scholar]
- 27.Gotoh T., Hattori S., Nakamura S., Kitayama H., Noda M., Takai Y., Kaibuchi K., Matsui H., Hatase O., Takahashi H. ( 1995) Mol. Cell. Biol. 15, 6746– 6753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasegawa H., Kiyokawa E., Tanaka S., Nagashima K., Gotoh N., Shibuya M., Kurata T., Matsuda M. ( 1996) Mol. Cell. Biol. 16, 1770– 1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiyokawa E., Hashimoto Y., Kobayashi S., Sugimura H., Kurata T., Matsuda M. ( 1998) Genes Dev. 12, 3331– 3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knudsen B. S., Feller S. M., Hanafusa H. ( 1994) J. Biol. Chem. 269, 32781– 32787 [PubMed] [Google Scholar]
- 31.Tanaka S., Morishita T., Hashimoto Y., Hattori S., Nakamura S., Shibuya M., Matuoka K., Takenawa T., Kurata T., Nagashima K., Matsuda M. ( 1994) Proc. Natl. Acad. Sci. U. S. A. 91, 3443– 3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klemke R. L., Leng J., Molander R., Brooks P. C., Vuori K., Cheresh D. A. ( 1998) J. Cell Biol. 140, 961– 972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitehead I. P., Lambert Q. T., Glaven J. A., Abe K., Rossman K. L., Mahon G. M., Trzaskos J. M., Kay R., Campbell S. L., Der C. J. ( 1999) Mol. Cell. Biol. 19, 7759– 7770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olabisi O. O., Mahon G. M., Kostenko E. V., Liu Z., Ozer H. L., Whitehead I. P. ( 2006) Cancer Res. 66, 6250– 6257 [DOI] [PubMed] [Google Scholar]
- 35.Nobes C. D. ( 2000) Methods Enzymol. 325, 441– 449 [DOI] [PubMed] [Google Scholar]
- 36.Tolnai S. ( 1975) Methods Cell Sci. 1, 37– 38 [Google Scholar]
- 37.Liu Z., Kostenko E. V., Mahon G. M., Olabisi O. O., Whitehead I. P. ( 2006) J. Biol. Chem. 281, 16043– 16051 [DOI] [PubMed] [Google Scholar]
- 38.Keydar I., Chen L., Karby S, Weiss F. R., Delarea J., Radu M., Chaitcik S., Brenner H. J. ( 1979) Eur. J. Cancer 15, 659– 670 [DOI] [PubMed] [Google Scholar]
- 39.Keely P. J., Fong A. M., Zutter M. M., Santoro S. A. ( 1995) J. Cell Sci. 108, 595– 607 [DOI] [PubMed] [Google Scholar]
- 40.Shen F., Lin Q., Gu Y., Childress C., Yang W. ( 2007) Mol. Biol. Cell 18, 732– 742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin N. Y., Dise R. S., Schneider-Mergener J., Ritchie M. D., Kilkenny D. M., Hanks S. K. ( 2004) J. Biol. Chem. 279, 38331– 38337 [DOI] [PubMed] [Google Scholar]
- 42.Rosen M. K., Yamazaki T., Gish G. D., Kay C. M., Pawson T., Kay L. E. ( 1995) Nature 374, 477– 479 [DOI] [PubMed] [Google Scholar]
- 43.Hamasaki K., Mimura T., Morino N., Furuya H., Nakamoto T., Aizawa S., Morimoto C., Yazaki Y., Hirai H., Nojima Y. ( 1996) Biochem. Biophys. Res. Commun. 222, 338– 343 [DOI] [PubMed] [Google Scholar]
- 44.Schlaepfer D. D., Broome M. A., Hunter T. ( 1997) Mol. Cell. Biol. 17, 1702– 1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ueki K., Mimura T., Nakamoto T., Sasaki T., Aizawa S., Hirai H., Yano S., Naruse T., Nojima Y. ( 1998) FEBS Lett. 432, 197– 201 [DOI] [PubMed] [Google Scholar]
- 46.Vuori K., Hirai H., Aizawa S., Ruoslahti E. ( 1996) Mol. Cell. Biol. 16, 2606– 2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wozniak M. A., Modzelewska K., Kwong L., Keely P. J. ( 2004) Biochim. Biophys. Acta 1692, 103– 119 [DOI] [PubMed] [Google Scholar]
- 48.Chuang T. H., Xu X., Kaartinen V., Heisterkamp N., Groffen J., Bokoch G. M. ( 1995) Proc. Natl. Acad. Sci. U. S. A. 92, 10282– 10286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowley J. D. ( 1973) Nature 243, 290– 293 [DOI] [PubMed] [Google Scholar]
- 50.Minard M. E., Herynk M. H., Collard J. G., Gallick G. E. ( 2005) Oncogene 24, 2568– 2573 [DOI] [PubMed] [Google Scholar]
- 51.McSherry E. A., Donatello S., Hopkins A. M., McDonnell S. ( 2007) Cell. Mol. Life Sci. 64, 3201– 3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alowami S., Troup S., Al-Haddad S., Kirkpatrick I., Watson P. ( 2003) Breast Cancer Res. 5, 129– 135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shintani Y., Hollingsworth M. A., Wheelock M. J., Johnson K. R. ( 2006) Cancer Res. 66, 11745– 11753 [DOI] [PubMed] [Google Scholar]
- 54.Zeisberg M., Bonner G., Maeshima Y., Colorado P., Müller G. A., Strutz F., Kalluri R. ( 2001) Am. J. Pathol. 159, 1313– 1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shintani Y., Fukumoto Y., Chaika N., Svoboda R., Wheelock M. J., Johnson K. R. ( 2008) J. Cell Biol. 180, 1277– 1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shintani Y., Maeda M., Chaika N., Johnson K. R., Wheelock M. J. ( 2008) Am. J. Respir. Cell Mol. Biol. 38, 95– 104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shintani Y., Wheelock M. J., Johnson K. R. ( 2006) Mol. Biol. Cell 17, 2963– 2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rossman K. L., Der C. J., Sondek J. ( 2005) Nat. Rev. Mol. Cell Biol. 6, 167– 180 [DOI] [PubMed] [Google Scholar]
- 59.Cheng L., Mahon G. M., Kostenko E. V., Whitehead I. P. ( 2004) J. Biol. Chem. 279, 12786– 12793 [DOI] [PubMed] [Google Scholar]
- 60.Chodniewicz D., Klemke R. L. ( 2004) Biochim Biophys Acta 1692, 63– 76 [DOI] [PubMed] [Google Scholar]
- 61.Kmiecik T. E., Shalloway D. ( 1987) Cell 49, 65– 73 [DOI] [PubMed] [Google Scholar]
- 62.Schaller M. D., Hildebrand J. D., Shannon J. D., Fox J. W., Vines R. R., Parsons J. T. ( 1994) Mol. Cell. Biol. 14, 1680– 1688 [DOI] [PMC free article] [PubMed] [Google Scholar]