Abstract
Cholesterol oxides, in particular 7-ketocholesterol, are proatherogenic compounds that induce cell death in the vascular wall when localized in lipid raft domains of the cell membrane. Deleterious effects of 7-ketocholesterol can be prevented by vitamin E, but the molecular mechanism involved is unclear. In this study, unlike γ-tocopherol, the α-tocopherol vitamin E form was found to prevent 7-ketocholesterol-mediated apoptosis of A7R5 smooth muscle cells. To be operative, α-tocopherol needed to be added to the cells before 7-ketocholesterol, and its anti-apoptotic effect was reduced and even suppressed when added together or after 7-ketocholesterol, respectively. Both pre- and co-treatment of the cells with α-tocopherol resulted in the redistribution of 7-ketocholesterol out of the sphingolipid/cholesterol-enriched (lipid raft) domains. In turn, fewer amounts of α-tocopherol associated with lipid rafts on 7-ketocholesterol-pretreated cells compared with untreated cells, with no prevention of cell death in this case. In further support of the implication of lipid raft domains, the dephosphorylation/inactivation of Akt-PKB was involved in the 7-ketocholesterol-induced apoptosis. Akt-PKB dephosphorylation was prevented by α-tocopherol, but not γ-tocopherol pretreatment.
It has been suggested that cholesterol oxide-induced apoptosis is a key event in the initiation and progression of atherosclerosis lesions (1, 2). In the initial step of the disease, cholesterol oxides in modified low density lipoproteins were found to promote the death of endothelial cells lining the intravascular lumen (1, 2). In more advanced stages and as the atherosclerotic lesion progresses, cholesterol oxides could also contribute to the destruction of foam cells and vascular smooth muscle cells, to the formation of the lipid core, to the reduction of cell proliferation, and eventually to plaque destabilization (1, 2). Among cholesterol oxides that are mainly synthesized during oxidation of low density lipoproteins, 7-ketocholesterol is one of the most abundant in plasma and atherosclerotic lesions (3). Moreover, in a number of cell models, it has been established that 7-ketocholesterol is one of the cholesterol oxide derivatives with the highest pro-apoptotic potential (4, 5). The 7-ketocholesterol derivative associates preferentially with membrane lipid raft domains (6), which are characterized by the lateral packing of glycosphingolipids, sphingolipids, and cholesterol. Because of their insolubility in cold non-ionic detergents, rafts are also called detergent-resistant membranes (7). These structures are thought to be involved in cellular signaling mechanisms (8, 9). It is worthy of note that 7-ketocholesterol has been shown to induce cell death through inactivation of the phosphatidylinositol 3-kinase/Akt signaling pathway (10), which is known to be highly specific to lipid raft domains (9).
Vitamin E is composed of closely related molecules, i.e. tocopherols and tocotrienols, which are each composed of four α, β, γ, and δ analogues. Although vitamin E was widely studied for its ability to prevent cellular damage by reactive oxygen species, it has recently been the subject of intense research for its putative, non-antioxidant functions (11, 12). Among the various forms of vitamin E, α-tocopherol is most abundant in the body as it is specifically recognized by the liver α-tocopherol transfer protein. Although several studies have shown that vitamin E has the ability to counteract the pro-apoptotic effect of 7-ketocholesterol in cultured cells (10, 13), the underlying molecular mechanism is unclear.
In the present study the molecular mechanism involved in the vitamin E-mediated protection against apoptosis induced by 7-ketocholesterol was investigated on the well known A7R5 aortic smooth muscle cell model. It is reported here that α-tocopherol, but not γ-tocopherol, effectively protects the cells against 7-ketocholesterol-induced apoptosis when applied as a pretreatment before the addition of 7-ketocholesterol. Unlike γ-tocopherol, α-tocopherol was able to activate the Akt-PKB anti-apoptotic signaling pathway in the lipid raft domains (14), leading to phosphorylation and, thus, inactivation of Bad (15). Most importantly, the protective effect of α-tocopherol is shown to operate through its prior incorporation into the lipid raft domains of the plasma membrane, which leads to the subsequent exclusion and, thus, inactivation of 7-ketocholesterol.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
The A7R5 aortic smooth muscle cell line was purchased from the American Tissue and Culture Collection (Manassas, VA). 3,3′-Dihexyloxacarbocyanine iodide (DIOC6(3))3 was purchased from Molecular Probes, Inc. (Eugene, OR). 7-Ketocholesterol, cycloheximide, staurosporine, Hoechst 33342, propidium iodide (PI), Fluo-3/AM, pluronic F-127, probenicid, IgePal, and Triton X-100 were purchased from Sigma (Sigma-Aldrich). The α- and γ-tocopherol were purchased from SUPELCO (Sigma). The protease Inhibitor Mixture Tablets, Complete Mini were purchased from Roche Applied Science. The anti-Bad polyclonal antibody, the anti-Bad phospho-Ser-136 polyclonal antibody, the anti-Akt/PKB polyclonal antibody, the anti-Akt/PKB phospho-Thr-308 polyclonal antibody, and the anti-Akt/PKB phospho-Ser-473 monoclonal antibody were purchased from Cell Signaling Technology (Hitchin, UK). The total mouse IgG-horseradish peroxidase (HRP) and total rabbit IgG-HRP were purchased from DAKO (Glostrup, Denmark).
Cells and Cell Treatments
A7R5 cells were grown in Dulbecco's modified Eagle's medium, 4.5 g/liter glucose (Invitrogen), and antibiotics (100 units/ml penicillin, 100 μg/ml streptomycin) (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum. α- or γ-tocopherol (Sigma, Supelco) were used at a final concentration of 100 μm. 7-Ketocholesterol was used at 20 μg/ml. 7-ketocholesterol was introduced into the culture medium 1 h before, together with or 1 h after the tocopherols. Cycloheximide and staurosporine were introduced into the culture medium 1 h after tocopherols and used at a concentration of 50 and 0.5 μm, respectively.
Determination of Cell Permeability with Propidium Iodide
Cell permeability was determined with PI (λExmax, 540 nm; λEmmax, 625 nm), which only stains dead cells (16), after 24 h of treatment. The red fluorescence was immediately measured on a Galaxy flow cytometer (Partec, Münster, Germany) at excitation and emission wavelengths of 488 and 585/42 nm, respectively. For each sample, 10,000 cells were acquired, and the data were analyzed with Flomax software (Partec).
Flow Cytometric Measurement of Mitochondrial Transmembrane Potential (ΔΨm) with the Dye DIOC6(3)
Variations in mitochondrial transmembrane potential (ΔΨm) were measured with DIOC6(3) (λExmax, 484 nm; λEmmax, 501 nm) used at a final concentration of 40 nmol/liter (17) after 24 h of treatment. The flow cytometric analyses were performed on a Galaxy flow cytometer (Partec), and the green fluorescence was collected through a 524/44-nm band pass filter. Fluorescent signals were measured on a logarithmic scale of four decades of log. For each sample, 10,000 cells were acquired, and the data were analyzed with Flomax software (Partec).
Characterization of Nuclear Morphology by Staining with Hoechst 33342
Nuclear morphology of control and treated cells was studied by fluorescence microscopy after staining with Hoechst 33342 (λExmax, 346 nm; λEmmax, 420 nm) used at 10 μg/ml after 24 h of treatment. The morphological aspect of cell deposits, applied to glass slides by cytocentrifugation with a cytospin 4 centrifuge (Shandon, Cheshire, UK), was observed with an Axioskop light microscope (Zeiss, Jena, Germany) by using UV light excitation. Three hundred cells were examined for each experiment.
Quantification of Cellular Hydroxyperoxides
Cellular hydroperoxide levels were evaluated by measuring the oxidation rate of the oxidant-sensitive dye 2′,7′-dichlorofluorescein diacetate to the fluorescent product dichlorofluorescein. A7R5 cells were plated in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and penicillin-streptomycin at a cell density of 1 × 104 cells per well. After 6 h of treatment, adherent cells were washed twice with Hanks' balanced salt solution, and the medium was replaced with 10 μg/ml 2′,7′-dichlorofluorescein diacetate in Dulbecco's modified Eagle's medium culture media without phenol red. The formation of dichlorofluorescein was monitored at excitation/emission wavelengths of 485/538 nm over 30 min using a VICTOR 1420 multilabel counter.
Flow Cytometric Measurement of Cytosolic Calcium with the Dye Fluo-3
After 6 h of treatment, A7R5 cells were detached by trypsinization and washed 2 times at room temperature in Ca2+ flux assay buffer (Hanks' balanced salt solution containing 20 mm HEPES and 0.5% bovine serum albumin, pH 7.4). The cells were then incubated with Fluo-3/AM (Molecular Probes; 6 μmol/liter; λExmax, 506 nm; λEmmax, 526 nm) for 30 min at 30 °C in Hanks' balanced salt solution, pH 7.4, with Pluronic F-127 (Sigma; 0.02%). After loading, the cells were suspended in Hanks' balanced salt solution, pH 7.4, supplemented with probenecid (Sigma; 5 mmol/liter) to prevent leakage of the dye (18). Fluorescence was measured by flow cytometry with a Galaxy flow cytometer (Partec) using a 524/44-nm band pass filter. For each sample, events were acquired for 60 s, and the data were analyzed with Flomax software (Partec).
Western Blotting
The phosphorylation of Bad and Akt-PKB was investigated by Western blot analysis of A7R5 cells incubated for 24 h with 7-ketocholesterol alone or in association with α- or γ-tocopherol. The cells were resuspended in radioimmune precipitation assay lysis buffer containing a mixture of protease inhibitors (Complete Mini, Roche Applied Science). After 30 min of incubation at 4 °C, the cell debris were eliminated by centrifugation for 20 min at 14,000 × g, and the supernatants were collected. The protein concentrations were measured using bicinchoninic acid reagent (Pierce) according to the method of Smith et al. (19). 50 μg of protein were incubated in loading buffer (125 mmol/liter Tris-HCl, pH 6.8, 10% β-mercaptoethanol, 4.6% SDS, 20% glycerol, and 0.003% bromphenol blue), boiled for 3 min, separated by SDS-polyacrylamide gel electrophoresis, and electroblotted onto a polyvinylidene difluoride membrane (Bio-Rad). After blocking nonspecific binding sites for 2 h at room temperature in TPBS (phosphate-buffered saline, 0.1% Tween 20), the membranes were incubated overnight at 4 °C with the primary antibody diluted in TPBS. After three 15-min washes with TPBS, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody at a dilution of 1:2500 for 1 h at room temperature and washed 3 times in TPBS for 15 min. Autoradiography of the immunoblots was performed using an enhanced chemiluminescence detection kit (Amersham Biosciences). Each experiment was repeated three times with identical results.
Isolation and Analysis of Sphingolipid/Cholesterol-enriched Lipid Raft Domains
Sphingolipid/cholesterol-enriched, lipid raft domains were isolated as previously described (20). Briefly, after 6 h of treatment, 1 × 106 A7R5 cells were washed with ice-cold phosphate-buffered saline followed by the addition of 1 ml of 25 mm MES buffer, pH 6.5, 150 mm NaCl containing 1% (w/v) Triton X-100 and a protease inhibitor tablet (Roche Applied Science). After 30 min at 4 °C, the cells were further homogenized on ice using a Dounce glass homogenizer. The lysates were then mixed with ice-cold MES buffer (1 ml) and 2 ml of 80% (w/v) sucrose in MES buffer and layered at the bottom of ultracentrifuge tubes. Samples were then overlaid with 5 ml of 30% and 3 ml of 5% sucrose in MES buffer. The samples were centrifuged at 39,000 rpm for 20 h at 4 °C using a Beckman swinging bucket rotor (SW41). 1.2-ml fractions were collected from the top to the bottom, vortexed, and stored at −20 °C before analysis. The cholesterol and sphingomyelin composition of the fractions were used as markers of rafts. Cholesterol, oxysterol, sphingomyelin, phosphatidylserine, phosphatidylcholine, and lysophosphatidylcholine were assayed using gas chromatography coupled to mass spectrometry as previously described (21–23).
α- and γ-Tocopherol Assay
Each subcellular fraction (200 μl) was vortexed-mixed with 200 μl of water, 400 μl of internal standard (100 ng/ml tocol (Spiral, Dijon, France) in ethanol), and 1 ml of hexane in 2 ml glass amber vials for 1 min. Extracts were centrifuged at 550 × g for 10 min at 4 °C. The upper layers (800 μl) were transferred into new vials and evaporated to dryness under nitrogen. The dried extracts were resuspended with 100 μl of HPLC grade methanol, and 27 μl were further analyzed with a linear 100% methanol gradient on a Beckman GOLD high performance liquid chromatography system equipped with a 508 autosampler. Fluorescence was detected at λExmax 292 nm and λEmmax 325 nm with a Shimadzu fluorescence detector. Concentrations of α- and γ-tocopherol were determined from the ratio of the peak area corresponding to one given molecule to the peak area corresponding to the internal standard. Levels were determined by comparison of this ratio with a standard curve of known amounts of α- or γ-tocopherol.
Statistical Analyses
Results are expressed as the mean ± S.E. The statistical significance of differences between groups was determined with Statview software (Cary, NC) using two-way analysis of variance.
RESULTS
α-Tocopherol Protects A7R5 Cells against 7-Ketocholesterol-induced Apoptosis
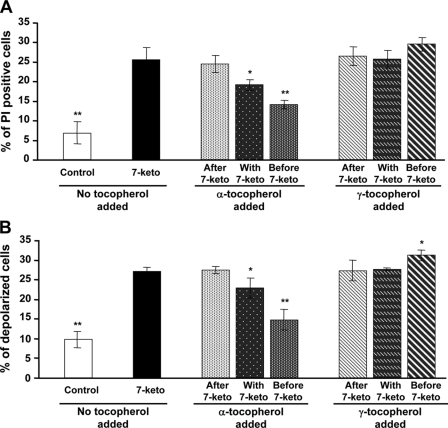
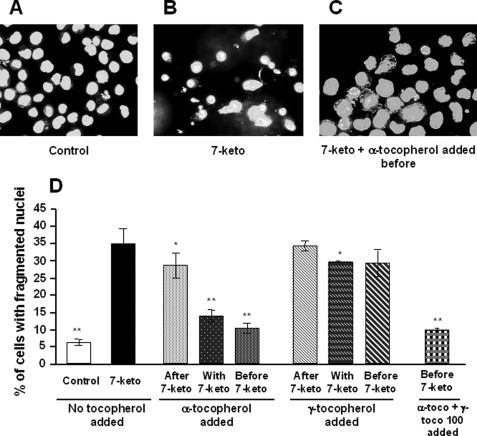
Treatment of A7R5 cells with 7-ketocholesterol (20 μg/ml) was cytotoxic, as shown by a significant increase in cell permeability to propidium iodide, with 26% of positive cells after 24 h of treatment versus 7% in control cells (p < 0.001) (Fig. 1A). Concomitantly, treatment with 7-ketocholesterol produced a significant decrease in mitochondrial transmembrane potential. As measured with the cationic lipophilic dye DIOC6(3), up to 27% of the A7R5 cells were depolarized after 24 h of treatment with 20 μg/ml 7-ketocholesterol versus 10% in control cells (p < 0.001) (Fig. 1B). As shown in Fig. 2D, 35% of the 7-ketocholesterol-treated cells showed abnormalities in nuclear morphology, which was characterized by fragmented nuclei (known to occur during apoptosis), revealed by staining with Hoechst 33342 versus 6% in control cells (p < 0.001).
FIGURE 1.
α-Tocopherol, but not γ-tocopherol protects A7R5 cells against 7-ketocholesterol-induced cell death. A7R5 cells were either untreated (Control) or incubated for 24 h with 7-ketocholesterol (7-keto, 20 μg/ml) alone or in combination with α- or γ-tocopherol (100 μm). 7-Ketocholesterol was added either 1 h before, at the same time, or 1 h after treatment with tocopherols. A, cell permeability to PI was measured by flow cytometry. After treatment, cells were incubated with PI, and 10,000 cells were subjected to flow cytometry analysis. Results are presented as the mean values ± S.D. (n = 6). p < 0.05 (*) and p < 0.001 (**) versus 7-ketocholesterol. B, transmembrane mitochondrial potential was measured by flow cytometry using the DIOC6(3) dye. After treatment, fluorescence associated with DIOC6(3) was measured by flow cytometry, and 10,000 cells were analyzed for each assay. Results are presented as the means ± S.D. (n = 6). p < 0.05 (*) and p < 0.001 (**) versus 7-ketocholesterol.
FIGURE 2.
α-Tocopherol inhibits 7-ketocholesterol-induced A7R5 apoptosis. A7R5 cells were untreated (Control), incubated for 24 h with 7-ketocholesterol (7-keto, 20 μg/ml) alone, or incubated for 24 h with 7-ketocholesterol in combination with α- or γ-tocopherol or both (100 μm each). 7-Ketocholesterol was added either 1 h after, at the same time, or 1 h before treatment with tocopherols. Subsequently, A7R5 cells were observed by fluorescence microscopy after nuclei staining with Hoechst 33342. A, untreated A7R5 cells. B, 7-ketocholesterol-treated cells. C, cells treated by α-tocopherol added 1 h before 7-ketocholesterol. D, percentages of apoptotic cells. Results are presented as the means ± S.D. (n = 6). p < 0.05 (*); p < 0.001 (**) versus 7-ketocholesterol.
7-Ketocholesterol-induced cell death was significantly reduced when A7R5 cells were incubated in the presence of α-tocopherol. The effect of α-tocopherol was strongly dependent on whether α-tocopherol was added before or after the cholesterol oxide. It was most effective when added 1 h before 7-ketocholesterol, of intermediate magnitude when added together with 7-ketocholesterol, and ineffective when added 1 h after 7-ketocholesterol (Fig. 1, middle panels). Concordant conclusions were drawn when cell death was assessed by measuring the proportion of either PI-positive cells, depolarized cells, or cells with fragmented nuclei (Figs. 1 and 2).
γ-Tocopherol Does Not Protect A7R5 Cells against 7-Ketocholesterol-induced Apoptosis
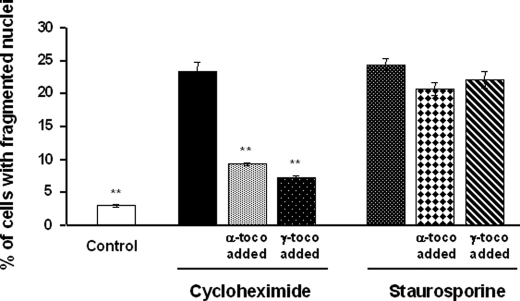
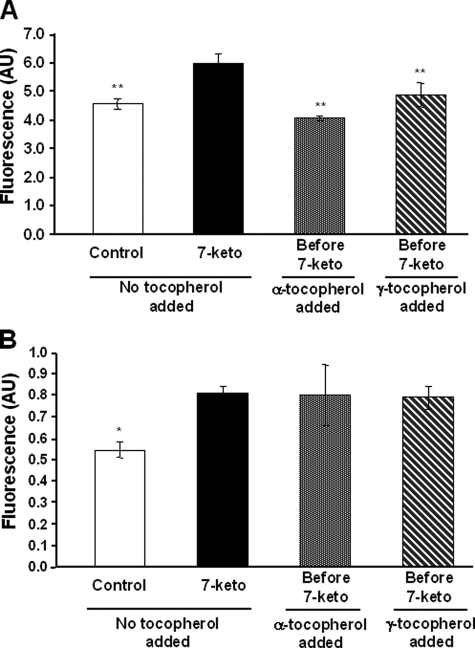
γ-Tocopherol is known to display antioxidant properties similar to those of α-tocopherol (24). In contrast to α-tocopherol, γ-tocopherol did not prevent the 7-ketocholesterol-mediated changes in the relative proportions of PI-positive cells, depolarized cells, or fragmented nucleus-containing cells. In this respect, γ-tocopherol was consistently ineffective even when added as a pretreatment, 1 h before 7-ketocholesterol (Figs. 1 and 2). γ-Tocopherol was mainly neutral as it did not modify the antiapoptotic property of α-tocopherol when both isomers were added together before 7-ketocholesterol (Fig. 2D). The difference between the α- and γ-tocopherol isomers appeared to be restricted to the 7-ketocholesterol-induced apoptosis. Both α- and γ-tocopherol significantly reduced the impact of cycloheximide on apoptosis in terms of fragmented nuclei but with no difference between the two isomers (Fig. 3). In addition, when apoptosis was induced by staurosporine, neither α- nor γ-tocopherol modified cell death (Fig. 3). In the present study the 7-ketocholesterol-induced rise in intracellular oxidative stress (as assessed by 2′,7′-dichlorofluorescein oxidation rate) was brought back to control/untreated values when either α- or γ-tocopherol was added before 7-ketocholesterol (Fig. 4A). As previously reported, 7-ketocholesterol induced a significant increase in cytosolic free Ca2+, which is known to produce calcineurine activation and dephosphorylation of the proapoptotic protein Bad (6). Neither α- nor γ-tocopherol could normalize the free intracellular Ca2+ pool in A7R5 cells (Fig. 4B).
FIGURE 3.
Effects of α- and γ-tocopherol on cycloheximide- and staurosporine-induced A7R5 apoptosis. A7R5 cells were either untreated (Control) or incubated for 24 h with cycloheximide (50 μm) or staurosporine (0.5 μm) alone or after 1 h of treatment with α- or γ-tocopherol (toco, 100 μm). After treatment, A7R5 cells were observed by fluorescence microscopy after nuclei staining with Hoechst 33342, and the percentage of apoptotic cells was determined. Results are presented as the means ± S.D. (n = 3). **, p < 0.001 versus cycloheximide.
FIGURE 4.
Effects of α- and γ-tocopherol on reactive oxygen species production and free Ca2+ content of cytosol in the presence of 7-ketocholesterol. A, reactive oxygen species production was measured in A7R5 cells which were either untreated (Control) or incubated with 7-ketocholesterol (7-keto, 20 μg/ml) for 6 h alone or after 1 h of treatment with α- or γ-tocopherol (100 μm). Dichlorofluorescein fluorescence was monitored at excitation/emission wavelengths of 485/538 nm over 30 min. Results are presented as the means ± S.D. (n = 5). **, p < 0.001 versus 7-ketocholesterol. B, cytosolic free Ca2+ was measured in A7R5 cells which were either untreated (Control) or incubated with 7-ketocholesterol (7-keto, 20 μg/ml) alone or after 1 h of treatment with α- or γ-tocopherol (100 μm). After 6 h of incubation, cells were loaded with Fluo-3/AM, and dye fluorescence was measured by flow cytometry. Results are presented as the means ± S.D. (n = 3). *, p < 0.05 versus 7-ketocholesterol. AU, arbitrary units.
Taken together, these observations indicate that the anti-apoptotic effect of α-tocopherol is not shared by γ-tocopherol. The two “isomers” modify the oxidative stress in 7-ketocholesterol-treated cells in similar ways and have no significant effects on calcium fluxes.
α-Tocopherol but Not γ-Tocopherol Impairs the Incorporation of 7-Ketocholesterol into the Lipid Raft Domains of the Plasma Membrane
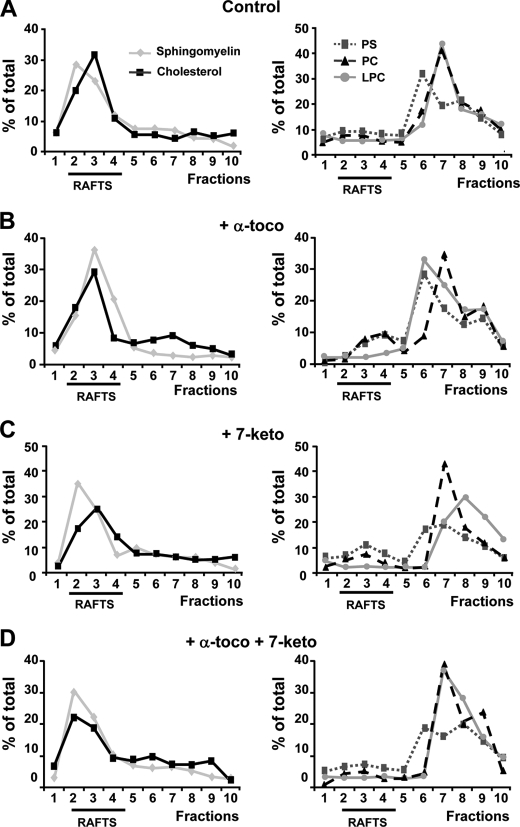
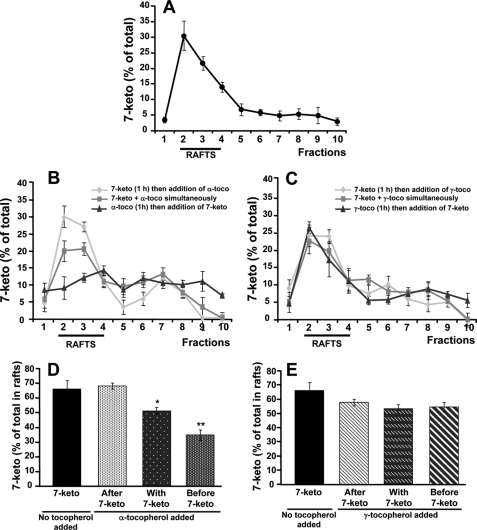
Tocopherols and cholesterol oxides are known to incorporate into plasma membranes. In the present study the sphingolipid/cholesterol-enriched, lipid raft domains corresponded constantly to fractions 2–4, with no changes in their distribution whether 7-ketocholesterol and/or tocopherols were added or not (Fig. 5). Phosphatidylserine, phosphatidylcholine, and lysophosphatidylcholine localized into the non-raft, detergent-soluble membranes (fractions 5–10). Again, no changes in the distribution of non-raft fractions were observed whether 7-ketocholesterol and/or tocopherols were added or not (Fig. 5). In agreement with previous studies in human pro-monocytic THP-1 cells (6), 7-ketocholesterol was found to incorporate mainly into lipid rafts (Fig. 6A). Interestingly, however, when α-tocopherol was added 1 h before the cholesterol oxide, it was able to produce a marked redistribution of 7-ketocholesterol outside the lipid rafts (Fig. 6, B and D). In contrast, α-tocopherol produced no effect when added after 7-ketocholesterol (Fig. 6, B and D). Under the same experimental conditions, the cholesterol oxide remained consistently associated with the lipid rafts in the absence or in the presence of γ-tocopherol whether γ-tocopherol was added before, with, or after 7-ketocholesterol (Fig. 6, C and E).
FIGURE 5.
7-Ketocholesterol and α-tocopherol produce no effect on sphingomyelin and cholesterol distribution into detergent-resistant membrane (lipid raft) domains. A7R5 cells were either untreated (Control), treated with α-tocopherol alone (α-toco, 100 μm), treated with 7-ketocholesterol alone (7-keto, 20 μg/ml), or treated with a combination of α-tocopherol (α-toco, 100 μm) plus 7-ketocholesterol (7-keto, 20 μg/ml added 1 h after α-toco). Incubations were conducted for 6 h. A7R5 cells were lysed in cold Triton X-100 and fractionated on a sucrose density gradient which allowed recovery of 10 distinct fractions from low (fraction 1) to high (fraction 10) density. Equal volume aliquots of the fractions were collected and analyzed by chromatography-mass spectroscopy for sphingomyelin, cholesterol, phosphatidylserine (PS), phosphatidylcholine (PC), and lysophosphatidylcholine (LPC). Sphingomyelin and cholesterol were mostly localized in the 2 to 4 fractions, whereas the bulk of phosphatidylserine, phosphatidylcholine, and lysophosphatidylcholine was recovered in the 5–10 fractions. Membrane lipid profiles were determined in cells which were untreated (A), treated with α-tocopherol (B), treated with 7-ketocholesterol (C), or treated with α-tocopherol plus 7-ketocholesterol (D). Values are representative of three distinct experiments.
FIGURE 6.
α-Tocopherol but not γ-tocopherol alters 7-ketocholesterol incorporation into plasma membrane. A7R5 cells were treated with 7-ketocholesterol (7-keto, 20 μg/ml) for 6 h alone or in combination with α- or γ-tocopherol (α-toco and γ-toco, 100 μm). 7-Ketocholesterol was added either 1 h before, together with, or 1 h after tocopherols. A7R5 cells were lysed in cold Triton X-100 and fractionated on a sucrose density gradient. Equal volume aliquots of the fractions were collected and analyzed by gas chromatography-mass spectroscopy for 7-ketocholesterol content in treated cells (A, B, and C). Percentages of 7-ketocholesterol in raft fractions were determined (D and E). Values are the means ± S.D. (n = 3). p < 0.05 (*) and p < 0.001 (**) versus 7-ketocholesterol.
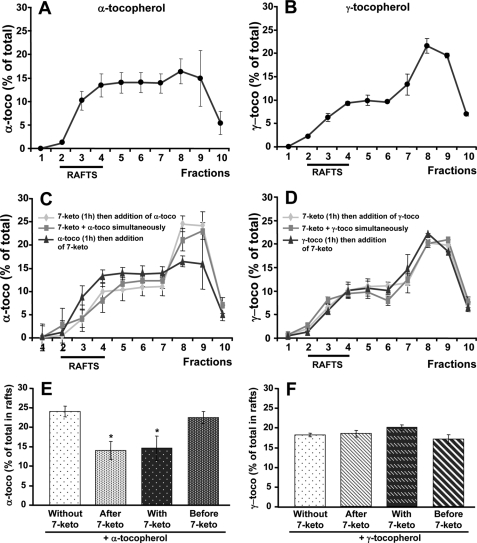
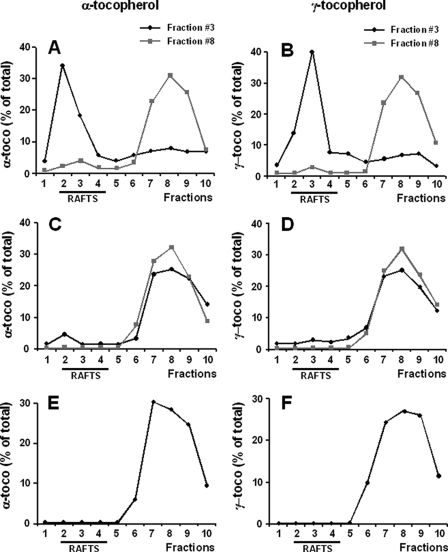
As shown in Figs. 7, A and E, about one-quarter of the α-tocopherol was localized in the lipid rafts of A7R5 cells, which were not treated with 7-ketocholesterol. In cells with no 7-ketocholesterol treatment, the amounts of α-tocopherol in the lipid rafts were higher than those of γ-tocopherol, which tended to localize preferentially in the non-lipid raft fractions (Fig. 7B). The relative proportion of α-tocopherol in the raft fractions decreased from 25% to ∼15% when cells were pre- or co-treated with 7-ketocholesterol (p < 0.05) (Fig. 7, C and E), whereas 7-ketocholesterol produced no detectable effect when added after α-tocopherol. These results indicate that a small, but significant fraction of α-tocopherol (approximating 10% of the total in cell membranes) can localize preferentially within the lipid rafts. As compared with 7-ketocholesterol-treated cells, no incremental association of γ-tocopherol with the lipid rafts was observed in control/untreated cells (Fig. 7, D and F), although γ-tocopherol and α-tocopherol incorporated in similar amounts to the cell membranes. Indeed, total amounts of either vitamin E isomer (as calculated by the cumulative addition of membrane fractions 1–10) were identical (α-tocopherol, 606 ± 115 ng per 106 cells; γ-tocopherol, 663 ± 274 ng per 106 cells; not significant). The selective association of α-tocopherol with lipid rafts was fully prevented by the incorporation of 7-ketocholesterol. In this case, lower/similar proportions (between 15 and 20%) of the amounts of either vitamin E isomer were found in the lipid rafts of 7-ketocholesterol-treated cells. In all cases, α- and γ-tocopherol were associated with membrane-derived structures as assessed by the lack of leakage of tocopherols from one fraction to another after a second ultracentrifugation step (Fig. 8, A and B). However, when the former ultracentrifugation fractions were replenished with Triton before the second ultracentrifugation step, most of the tocopherol material was recovered in the non-raft fractions (Fig. 8, C and D). It indicates that tocopherols are loosely bound to the membranes and can easily leak out of the rafts, with distribution profiles similar to these observed with tocopherols alone (Fig. 8, E and F).
FIGURE 7.
7-Ketocholesterol affects α-tocopherol but not γ-tocopherol incorporation into plasma membrane. A7R5 cells were treated with α- or γ-tocopherol (α-toco, and γ-toco, 100 μm) for 6 h alone or in combination with 7-ketocholesterol (7-keto, 20 μg/ml). In both cases, 7-ketocholesterol was added 1 h before, together with, or 1 h after tocopherols. A7R5 cells were lysed in cold Triton X-100 and fractionated on a sucrose density gradient. Equal volume aliquots of the fractions were collected and analyzed by HPLC. A and C, for α-tocopherol contents. B and D, for γ-tocopherol contents. Percentages of α-tocopherol (E) or of γ-tocopherol (F) in raft fractions were determined. Values are the means ± S.D. (n = 3). *, p < 0.05 versus 7-ketocholesterol.
FIGURE 8.
Cell-associated α- and γ-tocopherols are loosely bound to membrane fractions. After treatment with α- or γ-tocopherol (α-toco, γ-toco, 100 μm) for 6 h, A7R5 cells were recovered, lysed in cold Triton X-100, and fractionated on a sucrose density gradient. Equal volumes of fractions 1–10 were collected, and fractions 3 and 8 were immediately subjected to a second sucrose density gradient, which was replenished (C and D) or not (A and B) with 1% Triton (α-tocopherol, A and C; γ-tocopherol, B and D). In parallel experiments, tocopherols alone were subjected to the same gradient in the presence of 1% Triton (E and F). Tocopherol profiles were determined by HPLC analysis. Values are the means of two distinct experiments.
α-Tocopherol-induced Protection Is Associated with Activation of the Akt-PKB Signaling Pathway
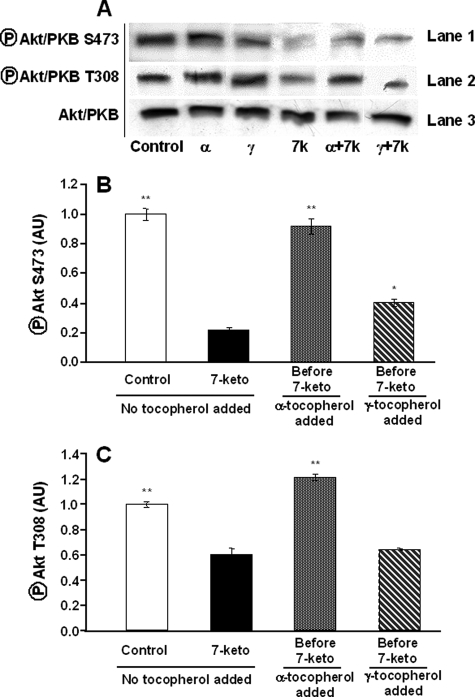
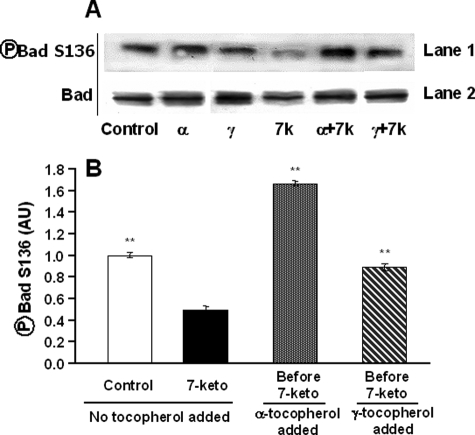
Akt-PKB is a kinase that is phosphorylated and then activated by PDK1 after interacting with phosphoinositides of the plasma membrane (25). Once activated, Akt-PkB is able to phosphorylate Bad on Ser-136 leading to its inactivation (26). In A7R5 cells treated with 7-ketocholesterol alone, Akt-PKB phosphorylation was decreased both on threonine 308 and serine 473 (Fig. 9A). Whereas γ-tocopherol did not modify the phosphorylation profile of Akt-PKB in 7-ketocholesterol-treated cells, α-tocopherol treatment was accompanied by Akt-PKB activation as demonstrated by sustained phosphorylation on threonine 308 and serine 473. α-Tocopherol, but not γ-tocopherol, was, thus, able to restore the control-like phosphorylation profile of Akt-PKB in 7-ketocholesterol-treated cells (Fig. 9). Accordingly, the 7-ketocholesterol-mediated decrease in Bad phosphorylation on serine 136 was fully reversed by α-tocopherol and even turned to an almost 4-fold increase as compared with the level observed in cells treated with 7-ketocholesterol alone (Fig. 10, A and B). Only a moderate, 2-fold increase in phosphorylation of serine 136 of Bad was produced in the presence of γ-tocopherol (Fig. 10B).
FIGURE 9.
Akt/PKB signaling pathway participates in the α-tocopherol-induced protection against 7-ketocholesterol-induced apoptosis. A7R5 cells were either untreated (Control) or incubated with α- or γ-tocopherol alone (α and γ, 100 μm) or in association with 7-ketocholesterol (7k, 7-keto, 20 μg/ml added 1 h after tocopherol) for 24 h. A, cell extracts were collected, subjected to SDS-PAGE, and immunoblotted with Akt/PKB antibody (lane 3), phospho-Akt/PKB Ser 473 antibody (lane 1), and phospho-Akt/PKB Thr-308 antibody (lane 2). Densitometry analysis (mean ± S.D. of triplicate determination) is presented in B and C. p < 0.05 (*); p < 0.001 (**) versus 7-ketocholesterol. AU, arbitrary units.
FIGURE 10.
Involvement of Bad in α-tocopherol-induced protection against 7-ketocholesterol-induced apoptosis. A7R5 cells were either untreated (Control) or incubated with α- or γ-tocopherol alone (α and γ, 100 μm) or in association with 7-ketocholesterol (7k, 7-keto, 20 μg/ml added 1 h after tocopherol) for 24 h. A, cell extracts were collected, subjected to SDS-PAGE, and immunoblotted with Bad antibody (lane 2) and phospho-Bad Ser-136 antibody (lane 1). B, densitometry analysis. Values are the means ± S.D. (n = 3). **, p < 0.001 versus 7-ketocholesterol. AU, arbitrary units.
DISCUSSION
In earlier studies, only α-tocopherol was found to exert a potent anti-proliferative effect in a variety of cell lines, including A7R5 cells, in which γ-, δ-, and β-tocopherol isomers were ineffective (27). A7R5 aortic smooth muscle cells are commonly used as a cell model to study the metabolism of vitamin E/tocopherols in relation with vascular biology. As far as cell death is concerned, it is demonstrated here that only α-tocopherol, and not γ-tocopherol, is able to block 7-ketocholesterol-mediated apoptosis. Concordant observations were made by measuring cell permeability to propidium iodide, mitochondrial transmembrane potential, and abnormalities in nuclear morphology. In addition, it is shown here that α-tocopherol, unlike γ-tocopherol, has the ability to impair the preferential incorporation of the cholesterol oxide into the sphingolipid/cholesterol-enriched, lipid raft domains of the plasma membrane. As in earlier studies (28), incubating A7R5 cells with the same amounts of either α- or γ-tocopherol resulted in similar cellular concentrations of either molecule. Overall, the present data come in new support of a specific property of α-tocopherol that is not shared by γ-tocopherol.
Earlier studies reported that α-tocopherol is more effective than γ-tocopherol in modulating apoptotic signaling in oxidized low density lipoprotein-treated human coronary smooth muscle cells (29). In addition, γ-tocopherol and α-tocopherol acetate, both of which share well recognized antioxidant properties with α-tocopherol, were mostly found to be ineffective in inhibiting cholesterol oxide-induced apoptosis in various cell models (29, 30). In the present study the difference between α- and γ-tocopherol appeared to be restricted to the 7-ketocholesterol-induced apoptosis. Indeed, the proportion of cycloheximide-induced cell death was reduced to the same extent by either isomer, and neither α-tocopherol nor γ-tocopherol could counteract the apoptotic potential of staurosporine (Fig. 3). It is known that α-tocopherol displays structure-specific properties that are not shared by other related isomers and which could then extend beyond its intrinsic antioxidative activity (12, 13, 30, 31). Although normalization of the level of hydroxyperoxides in 7-ketocholesterol-treated A7R5 cells (Fig. 4A) by either α- or γ-tocopherol seems to support the above hypothesis, the implication of the antioxidative properties of α-tocopherol in its anti-apoptotic effect cannot be fully excluded. Thus, the present study sheds new light on the molecular characteristics that might account for the clear difference between α-tocopherol and the other vitamin E forms; unlike γ-tocopherol, α-tocopherol displayed a specific distribution pattern in the plasma membrane, with a propensity to associate with the lipid raft domains. Because cholesterol oxides preferentially associate with the sphingolipid/cholesterol-enriched raft domains (Ref. 6 and this study), it is quite conceivable that the lipid raft-associated fraction of tocopherols would be the main, if not the only fraction that could exert a beneficial effect. It remains that α-tocopherol could have played a specific antioxidative function locally, within the A7R5 lipid raft domains to which γ-tocopherol poorly associates.
Tocopherols and cholesterol derivatives are known to incorporate into the plasma membrane, and 7-ketocholesterol-induced apoptosis is known to involve membrane signaling pathways leading to activation of the pro-apoptotic protein Bad through dephosphorylation of its serine 112 and/or 136 residues (6). As expected, α-tocopherol-mediated protection appeared in the present study mainly related to the inactivation of the pro-apoptotic protein Bad, which exhibited a high level of phosphorylation on serine 136, even greater than that found in non-treated cells. This increased phosphorylation appeared to be related to sustained Akt-PKB activity as demonstrated by the phosphorylation state of Akt-PKB at serine 473 and threonine 308. A direct relationship to the observed alterations in lipid raft domains is sustained by recent studies of Lasserre et al. (14) who reported that Akt-PKB is recruited and activated in these specialized microdomains. Unlike α-tocopherol, γ-tocopherol was unable to restore the phosphorylation profile of Akt-PKB in 7-ketocholesterol-treated A7R5 cells. Again, these observations come in support of marked differences in the effects of related forms of vitamin E on cell signaling.
Alterations in the incorporation of 7-ketocholesterol and related events occurred solely when α-tocopherol was preincorporated into lipid raft domains, before 7-ketocholesterol, and with no competitive effect of γ-tocopherol. Indeed, α-tocopherol was unable to reverse the deleterious effect of the cholesterol oxide already present in the lipid rafts. Through its early action when used as a pretreatment, α-tocopherol would then be able to prevent the damage caused by cholesterol derivatives involved in the initiation and development of atherosclerosis. To some extent, it might provide a clue to the controversial results from epidemiological studies dealing with the cardio-protective action of vitamin E supplementation. As suggested by Munteanu and Zingg (32), vitamin E supplementation would, thus, not be an effective therapy against existing cardiovascular disease but would rather play a preventive role.
In conclusion, the present study demonstrated that α-tocopherol can prevent the incorporation of 7-ketocholesterol into lipid raft domains where it can produce its anti-apoptotic effect. These observations give support to the recent hypothesis that if the localization/partition of α-tocopherol differs from that of non-α-tocopherol forms, then it should translate into specific biological actions (33). Accordingly, the effect of α-tocopherol, although not shared by γ-tocopherol in the present study, might still be due to the antioxidative protection of polyunsaturated fatty acids at specific sites.
Acknowledgment
We thank Philip Bastable for manuscript editing.
This work was supported by grants from the Université de Bourgogne, the Centre Hospitalier Universitaire de Dijon, the Conseil Régional de Bourgogne, INSERM, the Agence Nationale de la Recherche, and the Fondation de France.
- DIOC6(3)
- 3,3′-dihexyloxacarbocyanine iodide
- PI
- propidium iodide
- MES
- 2-(N-morpholino)-ethanesulfonic acid
- HPLC
- high performance liquid chromatography
- PKB
- protein kinase B.
REFERENCES
- 1.Lizard G., Monier S., Cordelet C., Gesquière L., Deckert V., Gueldry S., Lagrost L., Gambert P. ( 1999) Arterioscler. Thromb. Vasc. Biol. 19, 1190– 1200 [DOI] [PubMed] [Google Scholar]
- 2.Bauriedel G., Hutter R., Welsch U., Bach R., Sievert H., Lüderitz B. ( 1999) Cardiovasc. Res. 41, 480– 488 [DOI] [PubMed] [Google Scholar]
- 3.Jessup W., Brown A. J. ( 2005) Rejuvenation Res. 8, 9– 12 [DOI] [PubMed] [Google Scholar]
- 4.Terasaka N., Wang N., Yvan-Charvet L., Tall A. R. ( 2007) Proc. Natl. Acad. Sci. U. S. A. 104, 15093– 15098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthier A., Lemaire-Ewing S., Prunet C., Montange T., Vejux A., Pais de Barros J. P., Monier S., Gambert P., Lizard G., Néel D. ( 2005) FEBS J. 272, 3093– 3104 [DOI] [PubMed] [Google Scholar]
- 6.Berthier A., Lemaire-Ewing S., Prunet C., Monier S., Athias A., Bessède G., Pais de Barros J. P., Laubriet A., Gambert P., Lizard G., Néel D. ( 2004) Cell Death Differ. 11, 897– 905 [DOI] [PubMed] [Google Scholar]
- 7.Brown D. A., Rose J. K. ( 1992) Cell 68, 533– 544 [DOI] [PubMed] [Google Scholar]
- 8.Simons K., Toomre D. ( 2000) Nat. Rev. Mol. Cell Biol. 1, 31– 39 [DOI] [PubMed] [Google Scholar]
- 9.Foster L. J., de Hoog C. L., Mann M. ( 2003) Proc. Natl. Acad. Sci. U. S. A. 100, 5813– 5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vejux A., Guyot S., Montange T., Riedinger J. M., Kahn E., Lizard G. ( 2009) J. Nutr. Biochem. 20, 45– 61 [DOI] [PubMed] [Google Scholar]
- 11.Ricciarelli R., Tasinato A., Clément S., Ozer N. K., Boscoboinik D., Azzi A. ( 1998) Biochem. J. 334, 243– 249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azzi A., Stocker A. ( 2000) Prog. Lipid Res. 39, 231– 255 [DOI] [PubMed] [Google Scholar]
- 13.Uemura M., Manabe H., Yoshida N., Fujita N., Ochiai J., Matsumoto N., Takagi T., Naito Y., Yoshikawa T. ( 2002) Eur. J. Pharmacol. 456, 29– 37 [DOI] [PubMed] [Google Scholar]
- 14.Lasserre R., Guo X. J., Conchonaud F., Hamon Y., Hawchar O., Bernard A. M., Soudja S. M., Lenne P. F., Rigneault H., Olive D., Bismuth G., Nunès J. A., Payrastre B., Marguet D., He H. T. ( 2008) Nat. Chem. Biol. 4, 538– 547 [DOI] [PubMed] [Google Scholar]
- 15.del Peso L., González-García M., Page C., Herrera R., Nuñez G. ( 1997) Science 278, 687– 689 [DOI] [PubMed] [Google Scholar]
- 16.Yeh C. J., Hsi B. L., Faulk W. P. ( 1981) J. Immunol. Methods 43, 269– 275 [DOI] [PubMed] [Google Scholar]
- 17.Miguet C., Monier S., Bettaieb A., Athias A., Besséde G., Laubriet A., Lemaire S., Néel D., Gambert P., Lizard G. ( 2001) Cell Death Differ. 8, 83– 99 [DOI] [PubMed] [Google Scholar]
- 18.Princen K., Hatse S., Vermeire K., De Clercq E., Schols D. ( 2003) Cytometry A 51, 35– 45 [DOI] [PubMed] [Google Scholar]
- 19.Smith A. J., Cawston T. E., Hazleman B. L. ( 1985) J. Immunol. Methods 84, 125– 134 [DOI] [PubMed] [Google Scholar]
- 20.Grazide S., Maestre N., Veldman R. J., Bezombes C., Maddens S., Levade T., Laurent G., Jaffrézou J. P. ( 2002) FASEB J. 16, 1685– 1687 [DOI] [PubMed] [Google Scholar]
- 21.Becart J., Chevalier C., Biesse J. ( 1990) J. High Resolut. Chromatogr. 13, 126– 129 [Google Scholar]
- 22.Gambert P., Lallemant C., Archambault A., Maume B. F., Padieu P. ( 1979) J. Chromatogr. 162, 1– 6 [DOI] [PubMed] [Google Scholar]
- 23.Delmas D., Rébé C., Lacour S., Filomenko R., Athias A., Gambert P., Cherkaoui-Malki M., Jannin B., Dubrez-Daloz L., Latruffe N., Solary E. ( 2003) J. Biol. Chem. 278, 41482– 41490 [DOI] [PubMed] [Google Scholar]
- 24.Wang X., Quinn P. J. ( 1999) Prog. Lipid Res. 38, 309– 336 [DOI] [PubMed] [Google Scholar]
- 25.Scheid M. P., Woodgett J. R. ( 2003) FEBS Lett. 546, 108– 112 [DOI] [PubMed] [Google Scholar]
- 26.Datta S. R., Dudek H., Tao X., Masters S., Fu H., Gotoh Y., Greenberg M. E. ( 1997) Cell 91, 231– 241 [DOI] [PubMed] [Google Scholar]
- 27.Traber M. G., Packer L. ( 1995) Am. J. Clin. Nutr. 62, Suppl. 6, 1501– 1509 [DOI] [PubMed] [Google Scholar]
- 28.Chatelain E., Boscoboinik D. O., Bartoli G. M., Kagan V. E., Gey F. K., Packer L., Azzi A. ( 1993) Biochim. Biophys. Acta 1176, 83– 89 [DOI] [PubMed] [Google Scholar]
- 29.de Nigris F., Franconi F., Maida I., Palumbo G., Anania V., Napoli C. ( 2000) Biochem. Pharmacol. 59, 1477– 1487 [DOI] [PubMed] [Google Scholar]
- 30.Lyons N. M., Woods J. A., O'Brien N. M. ( 2001) Free Radic. Res. 35, 329– 339 [DOI] [PubMed] [Google Scholar]
- 31.Munteanu A., Zingg J. M., Azzi A. ( 2004) J. Cell Mol. Med. 8, 59– 76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munteanu A., Zingg J. M. ( 2007) Mol. Aspects Med. 28, 538– 590 [DOI] [PubMed] [Google Scholar]
- 33.Traber M. G., Atkinson J. ( 2007) Free Radic. Biol. Med. 43, 4– 15 [DOI] [PMC free article] [PubMed] [Google Scholar]