Abstract
To understand the mechanism for ion transport through the sodium/bicarbonate transporter SLC4A4 (NBCe1), we examined amino acid residues, within transmembrane domains, that are conserved among electrogenic Na/HCO3 transporters but are substituted with residues at the corresponding site of all electroneutral Na/HCO3 transporters. Point mutants were constructed and expressed in Xenopus oocytes to assess function using two-electrode voltage clamp. Among the mutants, D555E (charge-conserved substitution of the aspartate at position 555 with a glutamate) produced decreasing HCO3− currents at more positive membrane voltages. Immunohistochemistry showed D555E protein expression in oocyte membranes. D555E induced Na/HCO3-dependent pH recovery from a CO2-induced acidification. Current-voltage relationships revealed that D555E produced an outwardly rectifying current in the nominally CO2/HCO3−-free solution that was abolished by Cl− removal from the bath. In the presence of CO2/HCO3−, however, the outward current produced by D555E decreased only slightly after Cl− removal. Starting from a Cl−-free condition, D555E produced dose-dependent outward currents in response to a series of chloride additions. The D555E-mediated chloride current decreased by 70% in the presence of CO2/HCO3−. The substitution of Asp555 with an asparagine also produced a Cl− current. Anion selectivity experiments revealed that D555E was broadly permissive to other anions including NO3−. Fluorescence measurements of chloride transport were done with human embryonic kidney HEK 293 cells expressing NBCe1 and D555E. A marked increase in chloride transport was detected in cells expressing D555E. We conclude that Asp555 plays a role in HCO3− selectivity.
The electrogenic Na/HCO3 cotransporter NBCe1 (SLC4A4) is one of the SLC4A gene family members transporting HCO3− across the plasma membrane (1–3). NBCe1 plays a role in transepithelial HCO3− movement and pHi regulation in many tissues (4–6). NBCe1 is responsible for HCO3− reabsorption in the proximal tubules of the kidney (7). The proximal tubule cells reclaim HCO3− from the lumen through a series of reactions involving titration of HCO3− by H+ secretion via the apical Na/H exchanger, production of CO2, and regeneration of HCO3− and H+ in the tubule cells. HCO3− then moves to the interstitium via the basolateral NBCe1. The essential feature driving this basolateral Na+/HCO3− exit is the stoichiometry of 1:3 Na+:HCO3−, which makes the equilibrium potential for NBCe1 more positive than the resting membrane potential of the proximal tubule cells (8). The stoichiometry of 1Na+:1HCO3− or 1Na+:2HCO3− causes both ions to move into cells in other tissues such as pancreas, brain, and cardiovascular tissues (9, 10).
Despite the importance of NBCe1 for basolateral HCO3− reabsorption in the proximal tubules, the mechanism of electrogenic Na/HCO3 transport via the transporter is not well understood. Ion movement depends on loading ions at their translocation or binding sites that likely reside within the membrane field at some distance from the bath solution (11). This implies that the transmembrane domains (TMs)2 of NBCe1 and amino acid residues within TMs play critical roles in ion transport.
Sequence analysis of different SLC4A proteins shows similar hydropathy plots, predicting that these proteins share structural elements of transport function (12). Such similarities have facilitated structure/function studies to define molecular domains or motifs responsible for conferring Na/HCO3 transport of NBCe1. Abuladze et al. (13) performed a large scale mutagenesis on acidic and basic amino acids in non-TMs and found many residues affecting Na+-dependent base flux. McAlear et al. (14) identified amino acids in TM8 involving ion translocation. By a systematic approach of chimeric transporters between NBCe1 and the electroneutral Na/HCO3 cotransporter NBCn1 (SLC4A7) (15), we and our colleagues (16) demonstrated that electrogenic Na/HCO3 transport of NBCe1 requires interactions between the regions TM1–5 and TM6–13 of the protein. Zhu et al. (17) recently proposed TM1 as a domain lining the ion translocation pathway. On the other hand, Chang et al. (18) reported that the cytoplasmic N-terminal domain might contribute to HCO3− permeation.
In the present study, we searched amino acid residues that are highly conserved among electrogenic Na/HCO3 transporters but not among electroneutral Na/HCO3 transporters and examined their role in electrogenic Na/HCO3 transport. Nine candidate residues in human renal NBCe1-A (5, 19) were selected and mutated by replacement with the amino acids at the corresponding sites of NBCn1. Mutant transporters were expressed in Xenopus oocytes and assessed via two-electrode voltage clamp. Our data show that Asp555 of NBCe1 plays an important role in HCO3− selectivity.
EXPERIMENTAL PROCEDURES
Site-directed Mutagenesis
The plasmids containing human renal NBCe1-A (GenBankTM accession number: NM_003759) (19) and rat NBCn1 (GenBankTM accession number: NM_058211) (15) served as templates for site-directed mutagenesis. Mutagenic primers were designed to replace the codons for candidate amino acids (primer sequences are presented in the supplemental data). Mutations were made using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). PCR was carried out with 16 cycles of 95 °C for 1 min, 55 °C for 0.5 min, and 68 °C for 10 min. The products were sequenced.
Transporter Expression in Xenopus Oocytes and Two-electrode Voltage Clamp
Oocytes of Xenopus laevis (stages V and VI) were prepared as described (20). Defolliculated oocytes were injected with 15–20 ng of NBCe1 or mutant RNAs that were prepared by linearization with NheI and in vitro transcription with T7 RNA polymerase using the mMessage/mMachine transcription kit (Ambion, Austin, TX). Control oocytes were injected with 46 nl of sterile water. The oocytes were maintained for 3–5 days (18 °C) before use. For two-electrode voltage clamp, the oocytes were impaled with two microelectrodes filled with 3 m KCl (a resistance of <1.5 mΩ) and clamped at −60 mV using a voltage-clamp amplifier OC-725C (Warner, Hamden, CT). A staircase voltage command (−120 to +60 mV with 20-mV increments; 100 ms) was applied in ND96 solution (96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, 5 mm HEPES, pH 7.4) and then in a solution buffered with 5% CO2/25 mm HCO3− (pH 7.4). NaHCO3 was substituted for NaCl. The voltage command was applied before and 1 min after switching solutions. The difference between the current in CO2/HCO3− solution and the current in ND96 solution was calculated to obtain the HCO3−-dependent current and plotted as a function of voltage. The same voltage command was applied in ion depletion experiments. Na+-free solutions were made by substituting with N-methyl-d-glucammonium and Cl−-free solutions were made by substituting with gluconate. For anion substitution experiments, recordings were made in solutions that replace 25 mm Cl− with equimolar concentrations of Cl−, Br−, I−, SCN−, HCO3−, or NO3− at a constant pH of 7.4. Voltage signals were sampled by an A-D converter Digidata 1322 (Molecular Devices, Sunnyvale, CA). The data were acquired using pClamp 8 (Molecular Devices). Experiments were done at room temperature.
Na+ and HCO3− Dependence of Transporter Currents
The oocytes were superfused with Na+-free 5% CO2/25 mm HCO3− until CO2-induced acidification reached steady state (∼15 min). The oocytes were then exposed to a series of test solutions containing different concentrations of Na+ ([Na+]o). Each test solution was separated by the Na+-free CO2/HCO3− solution to maintain steady state between test solutions. Varying [Na+]o was accomplished by substitution with N-methyl-d-glucammonium or Li+ (at constant pH 7.4). All of the solutions contained 21 mm Cl−. For HCO3− dependence experiments, the oocytes were superfused with modified Cl−-free ND96 until the basal current reached steady state and then exposed to a series of Cl−-free solutions containing different concentrations of HCO3− ([HCO3−]o) at constant pH 7.4. The pH was maintained by varying PCO2 according to the Henderson-Hasselbalch equation. Each solution was bracketed with Cl−-free ND96.
Simultaneous Measurement of pHi and Current
The oocytes were impaled with three electrodes: one for measuring membrane potential, one for measuring current, and one for measuring pHi. The pH electrode was made with borosilicate fiber capillaries, silanized, filled with the proton ionophore 1 mixture B (Sigma-Aldrich), and back-filled with a pH 7.0 phosphate buffer. The current and voltage electrodes were filled with 3 m KCl (a resistance of <1.5 mΩ) and connected to the OC-725C clamp. The pH electrode was connected to a high impedance electrometer FD 223 (World Precision Instruments, Sarasota, FL). The voltage electrode signal was subtracted from the pH electrode signal using a subtraction amplifier (model V3.1, Yale University, New Haven) and amplified for input into a Digidata 1322. The data were acquired with customized software (Cell and Molecular Physiology, Yale University). The slope of pH to voltage was obtained by placing the three electrodes in the chamber filled with standards at pH 6.0 and 8.0. The measurements were done in ND96 and then in 5% CO2, 25 mm HCO3− at a holding potential of 0 mV.
Transfection of HEK 293 Cells
Cells (70–80% confluent) were plated on coverslips in a 35-mm dish and incubated overnight in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 50 units/ml of penicillin, and 50 μg/ml streptomycin. The cells were transfected with pcDNA3.1/NBCe1 or pcDNA3.1/D555E using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. The cells were incubated in the reduced serum medium Opti-MEM (Invitrogen) for 6 h and then in Dulbecco's modified Eagle's medium for 48 h before use.
Immunocytochemistry
Oocyte sections (1 μm in thickness) were prepared as described previously (21). The sections were treated with 1.2% H2O2 for 30 min to inhibit endogenous peroxidase activity. After washes with the phosphate-buffered saline (PBS), sections were preincubated with PBS containing 10% normal goat serum and then incubated for 1 h with the antibody (1:500) that recognizes the C-terminal end of rat renal NBCe1 (22). After washes, the sections were incubated with the horseradish peroxidase-conjugated secondary antibody (1:600) (Chemicon, Temecula, CA) for 1 h and with TSA-Plus-Tetramethylrhodamine (PerkinElmer Life Sciences) for 1 min. The sections were examined using a Zeiss Axiovert 135 microscope (Oberkochem, Germany) with a Plan-Neofluar 40× lens (numerical aperture of 0.75). For immunofluorescence of HEK 293 cells, the cells on coverslips were fixed with 4% paraformaldehyde, permeabilized with PBS containing 0.02% Triton X-100 (PBT), and blocked in PBT plus 10% normal goat serum for 1 h. The coverslips were then incubated with the NBCe1 antibody (1:500) overnight and treated with the goat Alexa 488 anti-rabbit IgG (Invitrogen).
Immunoblot
Transfected HEK 293 cells were collected and homogenized in an ice-cold buffer containing 300 mm mannitol, 0.1 mg/ml phenylmethanesulfonyl fluoride, 1× protease inhibitor mixture I (Calbiochem; San Diego, CA) and 5 mm HEPES (pH 7.2). The cells were centrifuged at 810 × g for 10 min, and the supernatants were collected and assayed to determine protein concentration using the Bradford reagents (Sigma-Aldrich). Equal amounts of total proteins from the samples were separated on a 7% SDS-polyacrylamide gel and blotted to a nitrocellulose membrane. The blot was incubated with the NBCe1 antibody (1:500) in PBS containing 0.05% Tween 20 and 5% nonfat dry milk for 2 h. The blot was washed for 40 min and then incubated with the horseradish peroxidase-conjugated goat anti-rabbit IgG (Chemicon) for 1 h. The blot was washed and visualized by ECL chemiluminescence (GE Healthcare).
Fluorescence Measurement of Chloride Transport
Chloride transport in HEK 293 cells expressing NBCe1 and D555E was measured using the protocol (23) with a slight modification. Briefly, 48 h after transfection, the cells on coverslips were incubated for 4 min in the 1:3 mixture of nitrate buffer (135 mm NaNO3, 2.4 mm K2HPO4, 0.6 mm KH2PO4, 1 mm CaSO4, 1 mm MgSO4, 10 mm HEPES, 10 d-glucose, pH 7.4) and water containing 50 mm 6-methoxyl-N-(3-sulfopropyl) quinolinium (SPQ). The coverslip was mounted to a chamber affixed on the stage of a Zeiss Axiovert 135 inverted microscope with a Plan-Neofluar 40× lens (numerical aperture of 0.75). The microscope was equipped with a Lambda 10-2 filter wheel controller (Sutter Instrument, Novato, CA) and a multi-wavelength filter set. Fluorescent dye was excited at 355 nm, and the emission light at 450 nm was captured. Excitation light was reflected by a 400-nm dichroic mirror. Images (exposure time of 200 ms) were acquired every 1 min with a digital camera Regita Exi (Q-Imaging) and analyzed by using MetaMorph software (Molecular Devices). At the end of the experiment, the cells were treated with 150 mm KSCN and 5 μm valinomycin to quench all the fluorescence. The relative fluorescence (F/Fo) was calculated from the observed fluorescence (F) and the fluorescence in the absence of Cl− (Fo). The rate of fluorescence change was computed from the initial rising phase of fluorescence after switching solutions from chloride buffer to nitrate buffer. The data were collected by fitting a linear least square equation over a period of 5 min.
Statistical Analysis
The data were reported as the means ± S.E. The level of significance was assessed using the unpaired, two-tailed Student t test for comparison between NBCe1 and point mutants and the paired, one-tailed test for comparison of single transporters in two different solutions. The one-way analysis of variance was used for comparison of currents in multiple solutions and currents among mutants. The p value of less than 0.05 was considered significant. The slope conductance and the rate of pHi change were fitted by a line using a least square method by OriginPro 8 (OriginLab, Northampton, MA).
RESULTS
Selection of Candidate Amino Acids
To identify the candidate residues responsible for electrogenic Na/HCO3 transport of NBCe1, we set three guidelines. First, amino acids in TMs of Na/HCO3 transporters are analyzed for sequence comparison. In defining TM boundaries, we used the 13-TM topology model (3) proposed based on extensive studies of the topology model for the Cl/HCO3 exchanger AE1 (SLC4A1) (24–27). Second, the candidate amino acids should be conserved in electrogenic Na/HCO3 cotransporters NBCe1 and NBCe2 (SLC4A5) but substituted with amino acids that are conserved in electroneutral Na/HCO3 cotransporters NBCn1 and NBCn2 (SLC4A10), the electroneutral Na+-driven Cl/HCO3 exchanger NDCBE1 (SLC4A8), and the Cl/HCO3 exchanger AE1. Third, the amino acid difference between electrogenic transporters and electroneutral transporters should be significant enough to predict a change in function.
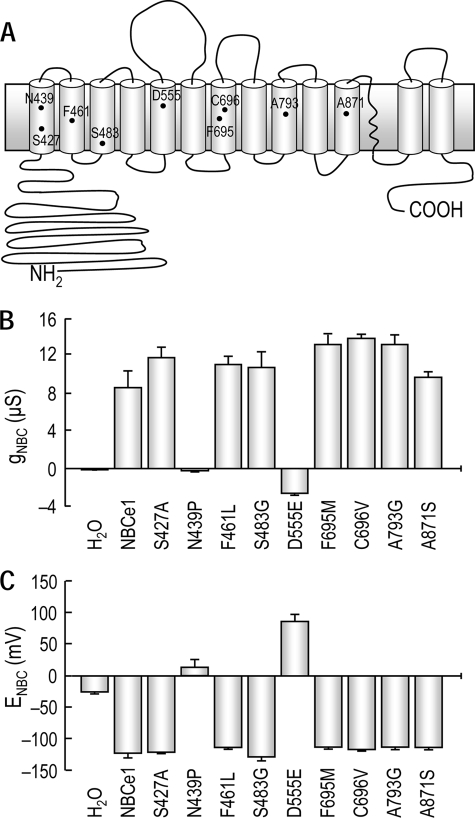
Fifteen amino acid residues of human renal NBCe1-A (19) were selected according to the above criteria (selected residues are available in supplemental data; NBCe1-A is referred to NBCe1 in this study). We examined nine of these residues (Fig. 1A): Ser427 and Asn439 (TM1), Phe461 (TM2), Ser483 (TM3), Asp555 (TM5), Phe695 and Cys696 (TM7), Ala793 (TM9), and Ala871 (TM11). Five of these residues are located in the region TM1–5, and four are in the region TM6–13. Point mutant transporters were constructed by substituting these residues with amino acids present at the corresponding sites of NBCn1.
FIGURE 1.
Electrogenic HCO3− currents produced by mutant transporters. A, candidate amino acid residues in NBCe1 selected based on sequence comparison. The residues are positioned in the 13-TM topology of Na/HCO3− transporters proposed by Romero et al. (3). B and C, electrophysiological measurements of the currents produced by mutant transporters. Xenopus oocytes expressing NBCe1 and mutant transporters were clamped at −60 mV, and a step voltage command (−120 to +60 mV, 20-mV increments, 100 ms) was applied before and after switching solutions from CO2/HCO3−-free ND96 (pH 7.4) to a comparable solution buffered with 5% CO2/25 mm HCO3− (pH 7.4). The electrogenic HCO3− current (INBC) was computed by subtracting the current in ND96 from the current in CO2/HCO3−. A current-voltage (I-V) plot was made, from which the slope conductance (gNBC) and reversal potential (ENBC) were computed at the zero current voltage. The numbers of oocytes/group were four to seven mutants, five NBCe1, and three water-injected controls.
Low HCO3− Conductance of D555E
Mutant transporters were expressed in Xenopus oocytes and assessed via two-electrode voltage clamp. The oocytes were clamped at −60 mV (close to the average resting membrane potential of −62 ± 4 mV for 8 uninjected oocytes), and a voltage command from −120 to +60 mV was applied in CO2/HCO3−-free ND96 solution (pH 7.4) and then again 1 min after switching to a solution buffered with 5% CO2, 25 mm HCO3− (pH 7.4). The difference between the current in CO2/HCO3− and the current in ND96 is a good estimate of the current mediated by the transporter (INBC). Slope conductance (gNBC) and reversal potential (ENBC) were calculated at zero current voltage or at voltages close to zero current, from INBC-V plots.
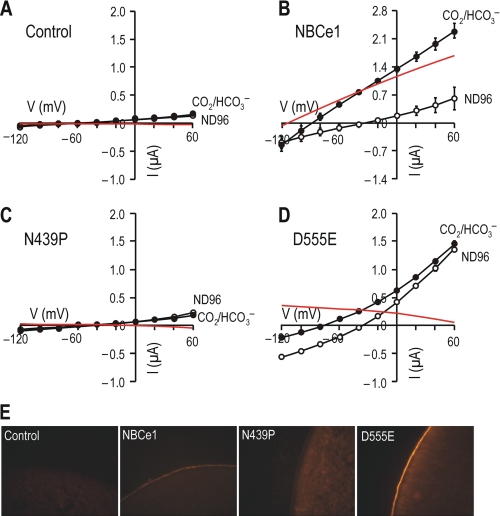
Most of the mutant transporters had gNBC and ENBC values comparable with those for wild type NBCe1, except N439P (substitution of Asn439 with a Pro) and D555E (substitution of Asp555 with a Glu) (Fig. 1, B and C). N439P had basal gNBC and ENBC values, whereas D555E had a negative gNBC and a positive ENBC, markedly distinct from values for NBCe1. Fig. 2 shows INBC-V plots for NBCe1, N439P, D555E, and controls. The application of 5% CO2, 25 mm HCO3− produced a large outward current in oocytes expressing NBCe1 (Fig. 2B). This change is due to the cotransport activity of NBCe1 because there is net movement of HCO3− ions across the membrane (1 Na+ and 2 HCO3−) (28). The slope in CO2/HCO3− (closed circles) was significantly larger than the slope in ND96 (open circles) (13.3 ± 2.5 μS versus 5.7 ± 1.3 μS, n = 5 for each, p < 0.01). Thus, INBC (red line) was larger at more positive voltages. The slope in ND96 was higher than that for water-injected controls (Fig. 2A), because of a HCO3−-independent basal current of NBCe1 as reported by Sciortino and Romero (28).
FIGURE 2.
I-V relationships for NBCe1, N439P, and D555E. I-V relationships were determined for oocytes injected with water (A) or RNAs for NBCe1 (B), N439P (C), or D555E (D) by using the voltage command as described in the legend to Fig. 1. The recordings were made first in ND96 (open circles) and then in 5% CO2/25 mm HCO3− (closed circles). The difference between two mean currents is INBC (red line). The numbers of oocytes per group are given in Fig. 1. In E, paraffin sections of oocytes were labeled with the antibody to the C-terminal domain of rat renal NBCe1 (22). Tetramethylrhodamine fluorescence was detected on the plasma membrane of oocytes expressing NBCe1 or D555E, but not N439P and water-injected controls.
Oocytes expressing N439P had negligible currents in either ND96 or CO2/HCO3−, thus producing no measurable INBC (Fig. 2C). In contrast, oocytes expressing D555E displayed outwardly rectifying currents in ND96 (Fig. 2D). This rectification is due to Cl−, as discussed later. The application of CO2/HCO3− produced the outward currents decidedly larger than the currents in ND96. However, the difference between those two currents became progressively smaller at positive voltages that should be favorable for electrogenic Na/HCO3 transport. Thus, INBC did not increase at positive voltages but instead decreased. ENBC was estimated to be 85 ± 10 mV and gNBC was −2.6 ± 0.2 μS (n = 7 for both). Immunochemistry with the anti-NBCe1 antibody (22) revealed prominent fluorescence on plasma membranes of oocytes expressing NBCe1 or D555E (Fig. 2E). For oocytes expressing N439P, immunofluorescence on membranes was negligible, indicating that N439P has a defect in targeting to membranes.
Decrease in Na/HCO3 Transport of D555E
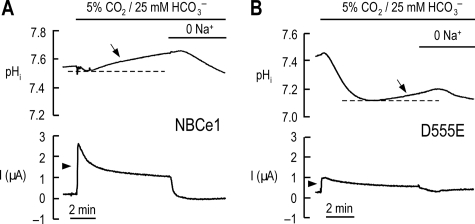
To assess the transport characteristics of D555E, we simultaneously measured the pHi and INBC values of oocytes under voltage clamp conditions. For this experiment, the oocytes were clamped at 0 mV, where electrogenic Na/HCO3 transport should favorably occur. Fig. 3 shows representative pHi and INBC traces of an oocyte expressing NBCe1 or D555E. Upon the application of 5% CO2, 25 mm HCO3−, the pHi in an oocyte expressing NBCe1 decreased as CO2 entered into the cytosol and generated H+ after its hydration (Fig. 3A; the immediate, short peak is a solution delivery artifact). The pHi then quickly recovered from a CO2-induced acidification (arrow) because HCO3− was transported into the oocyte via NBCe1. The mean rate of pHi change per time (dpHi/dt; computed at the initial rising phase of the recovery from acidification) was 48.4 ± 13.4 × 10−5 pH/s (n = 4). The application of CO2/HCO3− also produced an outward INBC (1.91 ± 0.01 μA, n = 4; arrowhead). Na+ removal in the continued presence of CO2/HCO3− reversed the direction of pHi recovery and INBC. In an oocyte expressing D555E (Fig. 3B), the application of CO2/HCO3− caused more robust acidification. This large acidification corresponds to a relatively small HCO3− influx via D555E. Consistent with this, the dpHi/dt was 17.0 ± 1.4 × 10−5 pH/sec (n = 6). The application of CO2/HCO3− also produced less INBC. The mean amplitude was 0.48 ± 0.07 μA, corresponding to ∼25% of INBC mediated by NBCe1. Na+ removal reversed the pHi recovery and decreased INBC.
FIGURE 3.
Simultaneous measurements of pHi and INBC in oocytes expressing NBCe1 or D555E under voltage clamp conditions. A, representative pHi and INBC traces of an oocyte expressing NBCe1. pHi recovery (arrow) from a CO2-induced acidification and an outward INBC (arrowhead) upon CO2/HCO3− application are hallmarks for electrogenic Na/HCO3 transport. The immediate short peak in pHi upon CO2/HCO3− application is a solution delivery artifact. The measurements were done at the holding potential of 0 mV. One of four experiments is shown. B, representative pHi and INBC traces of an oocyte expressing D555E. One of six experiments is shown.
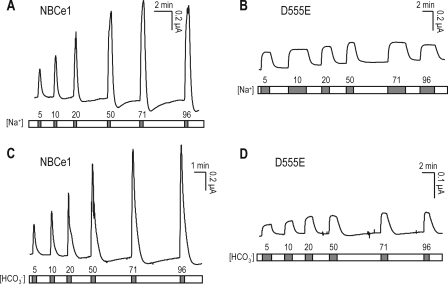
To examine the Na+ and HCO3− dependence of D555E, we then clamped oocytes at −60 mV, superfused with Na+-free 5% CO2, 25 mm HCO3− for ∼15 min to reach steady state and then exposed to 5–96 mm [Na+]o in the continued presence of CO2/HCO3−. NBCe1 produced a peak INBC with progressively larger amplitudes at higher [Na+]o (Fig. 4A). In contrast, D555E produced a steady state INBC with relatively small amplitudes at all [Na+]o (Fig. 4B). INBC mediated by D555E appeared to be nearly saturated at 5 mm [Na+]o. At 96 mm [Na+]o, the mean INBC mediated by D555E corresponded to 27% of the mean value for NBCe1. In other experiments, the oocytes were superfused with modified Cl−-free ND96 until the basal current became steady state (∼10 min) and then exposed to Cl−-free solutions buffered with 5–96 mm [HCO3−]o. Similar to the responses to [Na+]o, NBCe1 produced a peak INBC with progressively larger amplitudes at higher [HCO3−]o (Fig. 4C), whereas D555E produced a steady state INBC with relatively small amplitudes at all [HCO3−]o levels (Fig. 4D). INBC was nearly saturated at 5 mm [HCO3−]o, although it appeared to be slightly enhanced at higher [HCO3−]o.
FIGURE 4.
INBC responses to Na+ and HCO3−. A and B, representative INBC response to [Na+]o in an oocyte expressing NBCe1 or D555E. The oocyte was superfused with Na+-free 5% CO2/25 mm HCO3− until the basal current reached steady state (∼15 min) and then applied with 5–96 mm [Na+]o in the continued presence of CO2/HCO3−. For NBCe1, the delivery of Na+ was stopped after the peak response to minimize time to return to the basal level between test solutions. One of four experiments for NBCe1 and one of five experiments for D555E are shown. C and D, representative INBC response to [HCO3−]o in an oocyte expressing NBCe1 or D555E. The oocyte was superfused with modified Cl−-free ND96 until the basal current reached steady state and then applied with CO2/HCO3− solutions containing 5–96 mm [HCO3−]o. Gluconate replaced Cl−. One of four experiments for NBCe1 and one of five experiments for D555E are shown. All of the experiments were done at the holding potential of −60 mV.
The results obtained from the above two sets of experiments indicate that D555E produces a small INBC in response to [Na+]o and [HCO3−]o. The amplitude of the D555E-mediated INBC is not greatly enhanced at higher concentrations of those ion species.
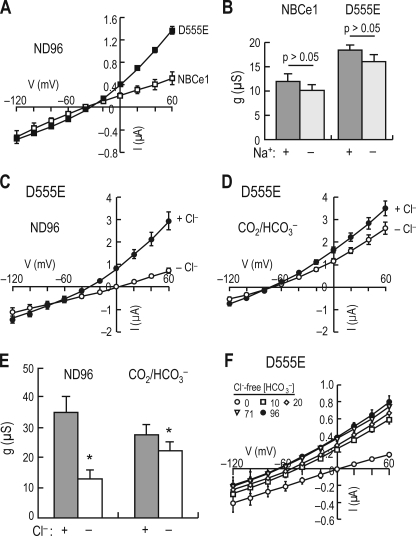
Cl− Current Mediated by D555E
Fig. 5A illustrates I-V relationships for NBCe1 and D555E in ND96. The current mediated by D555E was outwardly rectified at positive voltages. The zero current voltage was −36.3 ± 0.7 mV (n = 7). The major components of ND96 are 96 mm Na+ and 100.8 mm Cl−. To test whether Na+ was responsible for this outward rectification, we examined I-V relationships before and after Na+ removal in ND96 (Fig. 5B). Na+ removal had negligible effect on I-V relationships for both NBCe1 and D555E. The slope conductance remained unaffected (p > 0.05 for both; paired and two-tailed Student t test).
FIGURE 5.
Chloride current produced by D555E. A, I-V relationships for NBCe1 and D555E in ND96. I-V relationships for NBCe1 (n = 5; open squares) and D555E (n = 7; closed squares) were obtained using the voltage command as described in Fig. 1. B, effect of Na+ removal on the conductance associated with NBCe1 or D555E in ND96. The slope conductance was measured between 20 and 60 mV. For both NBCe1 and D555E, the conductances were similar in solutions with and without Na+ (p > 0.05, paired and one-tail Student t test). The oocytes expressing D555E are represented in C–F. C, I-V relationships in ND96 with and without Cl− (n = 6). The recordings were done before (closed circles) and after switching to Cl−-free solution (open circles). D, I-V relationships in CO2/HCO3− with and without Cl−. The oocytes in C were used to record I-V in 5% CO2/25 mm HCO3− (closed circles) and then 1 min after switching to Cl−-free CO2/HCO3− (open circles). E, summary of the conductance associated with D555E in the presence and absence of Cl−. The asterisk represents p < 0.05 (paired and one-tail Student t test). F, D555E-mediated INBC at different [HCO3−]o under Cl−-free conditions (n = 6). The recordings were made in modified Cl−-free ND96 before (open circles) and after switching to Cl−-free CO2/HCO3− solutions containing 10–96 mm [HCO3−]. Test solutions were bracketed with Cl−-free ND96.
We then tested whether Cl− was responsible for the outward rectification by analyzing I-V relationships before and after changing the solution from normal ND96 to Cl−-free ND96 (gluconate replaced Cl−). Cl− removal markedly reduced the outward rectification (Fig. 5C). The slope conductance (measured between 20 and 60 mV using the linear least square fitting) decreased from 35.2 ± 5.3 to 12.8 ± 2.3 μS (n = 6, p < 0.01). In the presence of 5% CO2, 25 mm HCO3− (Fig. 5D), Cl− removal slightly decreased the slope conductance (from 27.7 ± 3.5 to 22.3 ± 2.9 μS, n = 6, p < 0.01), but zero current voltages were similar (−67.1 ± 4.1 mV in normal CO2/HCO3− versus −65.6 ± 5.7 mV in Cl−-free CO2/HCO3−, p > 0.1). Fig. 5E summarizes the results. Cl− significantly contributes to the current mediated by D555E in the absence of HCO3− but contributes considerably less in the presence of HCO3−.
Fig. 5F shows I-V relationships under Cl−-free conditions. The oocytes were superfused with Cl−-free ND96 until the basal current reached steady state and then exposed to Cl−-free solutions containing 10–96 mm [HCO3−]o (∼1 min). Each test solution was bracketed with Cl−-free ND96. Outward currents were observed at positive voltages and progressively increased at higher [HCO3−]o. The slopes among different [HCO3−]o were relatively linear and parallel. Nevertheless, these plots did not cross the I-V plot for 0 mm [HCO3−]o and remained separated at negative voltages. This separation at negative voltages is most likely due to the combination of Cl− efflux when intracellular [HCO3−] is very low and HCO3− efflux when intracellular [HCO3−] is high.
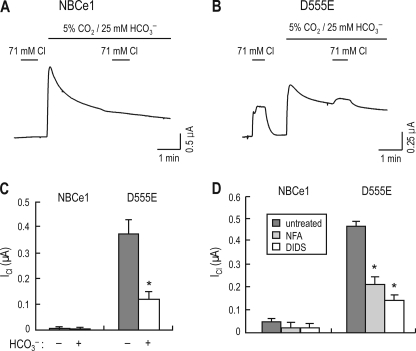
D555E-mediated Chloride Current (ICl) and INBC
To further characterize the relationship between ICl and INBC mediated by D555E, we clamped oocytes at 0 mV and recorded ICl by applying 71 mm [Cl−]o in ND96 versus CO2/HCO3− under Cl−-free conditions. Fig. 6 shows representative ICl and INBC of an oocyte expressing NBCe1 or D555E. NBCe1 produced no measurable response to Cl− in either ND96 or CO2/HCO3− (Fig. 6A). In contrast, D555E produced ICl in response to Cl− in ND96 (Fig. 6B; the immediate and short peak is a solution delivery artifact). The same application of chloride in CO2/HCO3− produced a smaller ICl. The reduction was 68% based on the calculation of the mean ICl (398.7 ± 69.2 nA in ND96 versus 127.8 ± 26.1 nA in CO2/HCO3−, n = 5) (Fig. 6C). ICl was inhibited by anion channel blockers niflumic acid and 4,4′-diisothiocyanato-2,2′-disulfonate stilbene (DIDS) (Fig. 6D). The inhibition was 55% by 100 μm niflumic acid (n = 4) and 70% by 100 μm DIDS (n = 4).
FIGURE 6.
Effect of HCO3− on D555E-mediated ICl. A and B, representative ICl of an oocyte expressing NBCe1 or D555E under Cl−-free conditions. The oocyte (clamped at 0 mV) expressing NBCe1 or D555E was exposed to 71 mm [Cl−]o before and after switching solutions to 5% CO2/25 mm HCO3−. The immediate, short peak in B is a solution delivery artifact. C, summary of ICl produced by NBCe1 and D555E. The data were obtained from four oocytes expressing NBCe1 and five oocytes expressing D555E. ICl was calculated after subtraction of the current in Cl−-free solutions from the current in Cl−-containing solutions. D, effect of niflumic acid (NFA) and DIDS on ICl. Oocytes (n = 5) were applied with 71 mm [Cl−]o in Cl−-free ND96 to induce ICl. The experiments were then repeated in the presence of 100 μm niflumic acid or 100 μm DIDS. The asterisk represents p < 0.05 (one-way analysis of variance).
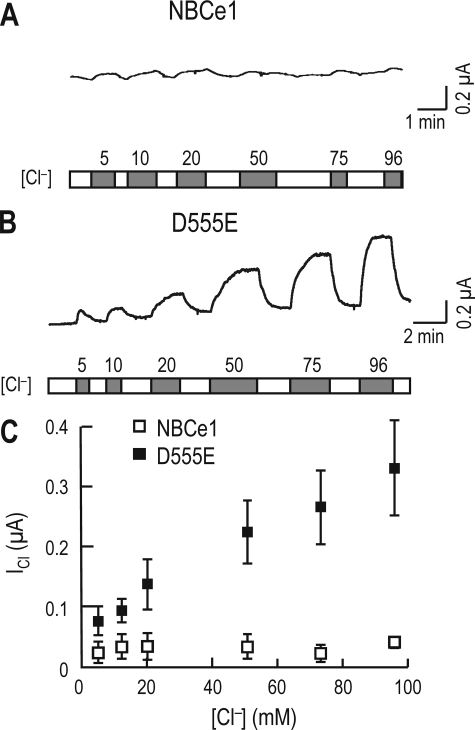
In other experiments in which oocytes were exposed to 5–96 mm [Cl−]o, NBCe1 produced negligible response at all [Cl−]o (Fig. 7A). However, D555E produced ICl with progressively larger amplitudes at higher [Cl−]o (Fig. 7B). The D555E-mediated ICl was plotted as a function of [Cl−]o (Fig. 7C). The data did not fit a Michaelis-Menten plot but appeared to include a Michaelis-Menten and a linear component comparable with the combined fit for the HCO3− current of rat NBCe1 (10, 29).
FIGURE 7.
Response of D555E-mediated ICl to Cl−. A and B, representative ICl of an oocyte expressing NBCe1 or D555E at different [Cl−]o. The oocyte expressing NBCe1 or D555E was superfused with modified Cl−-free ND96 until the basal current reached steady state and then applied with 5–96 mm [Cl−]o. One of four experiments for NBCe1 and one of five experiments for D555E are shown. C, effect of Cl− on ICl. ICl was plotted as a function of [Cl−]o. The data were obtained from the experiments in A and B.
Selectivity of D555E to Other Anions
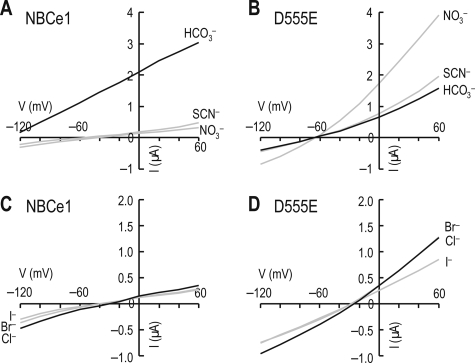
The above data demonstrate that the charge-conserved substitution of Asp555 with a Glu induces ICl. To examine other anion currents that might be mediated by D555E, we superfused oocytes with solutions replacing 25 mm Cl− with equimolar concentrations of HCO3−, NO3−, SCN−, Br−, I−, or Cl− (all solutions had pH 7.4). Recordings were done before and 1 min after switching solutions (Fig. 8). In oocytes expressing NBCe1, only HCO3− caused a large shift in the zero current voltage, proving that NBCe1 is highly selective to HCO3−. In oocytes expressing D555E, however, polyatomic anions HCO3−, NO3−, and SCN− caused negative shifts in zero current voltage with reference to Cl−, whereas elemental anions Br− and I− had negligible effect. The zero current voltages for HCO3−, NO3−, and SCN− were similar (p > 0.05) but significantly different from those for Br−, I−, and Cl− (p < 0.05). Thus, D555E was not restricted to HCO3− but had acquired selectivity to other anions, particularly NO3− and SCN−. Water-injected controls had no effect (data not shown).
FIGURE 8.
Selectivity of D555E to other anions. A and C, representative I-V relationships for different anions in an oocyte expressing NBCe1. The recordings were made in modified ND96 that replaced 25 mm Cl− with equimolar concentrations of HCO3−, NO3−, SCN−, Cl−, I−, or Br− (constant pH 7.4). One of six experiments is shown. B and D, representative I-V relationships for different anions in an oocyte expressing D555E. The recording protocol was identical to that in A and C. One of six experiments is shown.
Electrogenicity of D555E
Similar to NBCe1, D555E produced an outward current in CO2/HCO3− (Fig. 4B), thus indicating that D555E is electrogenic. To further examine the role of Asp555 in the electrogenicity of the transporter, we constructed additional mutant transporters: D555N (charge-neutralizing substitution of Asp555 with an asparagine), E742D (reciprocal substitution of Glu742 at the corresponding site of NBCn1 with an aspartate), and E742Q (charge-neutralizing and reciprocal substitution of Glu742 with a glutamine). The electrogenicity of these mutants was evaluated by analyzing their gNBC values.
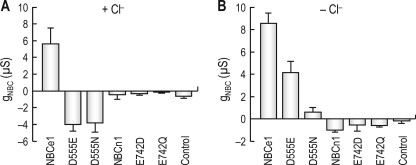
In the presence of Cl− (Fig. 9A), both D555E and D555N had negative gNBC (−4.0 ± 0.8 μS for D555E, n = 7 and −3.7 ± 1.1 μS for D555N, n = 9). These are different from a positive gNBC for NBCe1 (p < 0.01). On the other hand, NBCn1 and its mutants E742D and E742Q had negligible gNBC. No measurable INBC was produced by these mutants. In the absence of Cl− (Fig. 9B), whereas D555E had a positive gNBC (4.1 ± 1.1 μS, n = 4), D555N had only a small positive gNBC (0.6 ± 0.4 μS, n = 5). For D555N, the zero current voltage was negatively shifted by CO2/HCO3− (from −3.8 ± 2.1 mV to −22.9 ± 1.2 mV; figure not shown). Thus, a small positive gNBC represents a near parallel, negative shift in I-V plot. These results suggest that D555N is HCO3− permeable, but the HCO3− conductance is very small. A similar D555N mutation made by Abuladze et al. (13) reduces Na+-mediated base influx by 40% compared with NBCe1. The electroneutral transporter NBCn1 and its mutants E742D and E742Q had negligible conductance in the absence of Cl− (Fig. 9B). From these experiments, we conclude that, despite its unique presence in both NBCe1 and NBCe2, Asp555 is not associated with the electrogenicity of the transporters. The residue instead offers HCO3− selectivity.
FIGURE 9.
HCO3− conductance associated with Asp555-related mutants. A, gNBC in the presence of Cl−. D555N is the substitution of Asp555 with an asparagine, and E742D and E742Q are the substitutions of Glu742 with an aspartate and a glutamine, respectively, at the corresponding sites of NBCn1. The numbers of oocytes were eight control, three NBCe1, eight NBCn1, and seven to eleven mutants. B, gNBC in the absence of Cl−. The recording protocol was identical to that in A, except that experiments were done under Cl−-free conditions. The numbers of oocytes were four controls, three NBCe1, three NBCn1, and four to five mutants.
Chloride Transport of HEK 293 Cells Expressing D555E
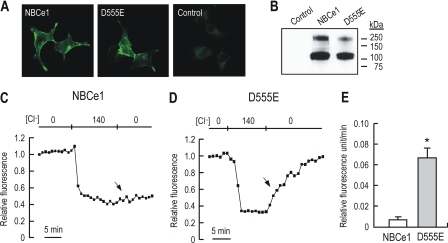
Despite the above results, it is possible that the chloride current we observed might not be intrinsic to the mutant transporter but rather derived from endogenous chloride channels in frog oocytes. To address this issue, we expressed NBCe1 and D555E in HEK 293 cells and measured chloride transport using the Cl−-sensitive fluorescence dye SPQ (23). The expression of the transporters were confirmed by immunocytochemistry and immunoblot (Fig. 10, A and B). Immunofluorescence of NBCe1 or D555E was detected on plasma membranes of transfected cells. An immunoreactive band with the expected size of 120 kDa was detected in both groups of transfected cells. An additional band of 250 kDa is likely a dimer of the transporter (30). In chloride transport assay (Fig. 10, C and D), cells loaded with SPQ were first superfused with nitrate buffer to allow depletion of intracellular Cl− and then switched to chloride buffer. Solutions were then switched to nitrate buffer to monitor Cl− efflux. Compared with NBCe1-expressing cells, D555E-expressing cells had a significant increase in fluorescence representing an increase in Cl− efflux (arrow). Fig. 10E summarizes the relative fluorescence unit/min for both groups of cells. The rate of fluorescence change was 9-fold higher in D555E-expressing cells than in NBCe1-expressing cells. These data support the generality of our findings and suggest that the observed chloride conductance is not due to the Xenopus oocyte expression system.
FIGURE 10.
Fluorescence measurement of Cl− transport mediated by D555E in HEK 293 cells. A, immunocytochemistry of HEK 293 cells transfected with NBCe1, D555E, or vector only. The cells were stained with the anti-NBCe1 antibody and the goat Alexa 488 anti-rabbit secondary antibody. B, immunoblot of crude membrane preparation from transfected cells. C and D, SPQ assay of chloride transport. The cells loaded with SPQ were perfused with nitrate buffer containing 135 mm NaNO3 and then with chloride buffer containing the equimolar concentration of NaCl. After fluorescence reached steady state, the cells were exposed to nitrate buffer to monitor Cl− efflux (arrows). At the end of the experiments, the cells were treated with 150 mm KSCN and 5 μm valinomycin to quench all the fluorescence. E, summary of the rate of fluorescence change. The rate (i.e. relative fluorescence unit/min) was computed from the initial rising phase of fluorescence after switching solutions from chloride buffer to nitrate buffer. Four NBCe1-expressing cells and five D555E-expressing cells were used from two experiments.
DISCUSSION
Overview
The major findings of the present study are the following. First, the mutation of Asp555 in NBCe1 to Glu reduces transport activity and produces a small INBC. Second, the mutation induces ICl in CO2/HCO3−-free ND96. Third, this Cl− current does not require Na+. Fourth, the mutation increases permeability for other anions in addition to HCO3−. Fifth, the mutation does not abolish the electrogenicity of the transporter. Our findings provide the first molecular evidence for an amino acid residue (Asp555) that is closely associated with HCO3− selectivity/translocation of the Na/HCO3 transporters.
Cl− Current Mediated by D555E
Oocytes expressing D555E produce ICl. This raises the question of whether D555E expression has caused an activation of endogenous chloride channels in Xenopus oocytes (31). However, it is highly unlikely that D555E-mediated ICl is due to endogenous channels because ICl is markedly reduced in CO2/HCO3− (Figs. 5D and 6B). Oocyte chloride channels are less sensitive to HCO3− (32). Furthermore, the chloride transport of D555E-expressing HEK 293 cells (Fig. 10) supports the conclusion that D555E-mediated ICl is not caused by endogenous oocyte channels.
Because of ICl produced in ND96, the INBC-V relationship for D555E shows a negative shift in gNBC and a positive shift in ENBC as compared with NBCe1 or other mutants (Fig. 1). Additional experiments reveal that these changes do not fully represent the properties of D555E. In different media, the currents are mediated by different ions: ICl in ND96, but mostly INBC in CO2/HCO3−. Therefore, a clearer estimation of INBC can be achieved in the absence of Cl−. Under Cl−-free conditions, D555E produces a positive gNBC (Fig. 9B). The positive gNBC would be expected to generate a negative shift in ENBC, similar to that seen in oocytes expressing NBCe1. We can predict a negative ENBC when a straight line is extrapolated from INBC-V plots at positive voltages (Fig. 5F). Nonetheless, we were unable to compute the exact ENBC because of the Cl− efflux at negative voltages. This difficulty in estimating ENBC hinders our attempt to calculate the Na+:HCO3− stoichiometry of D555E.
HCO3− versus Cl− Selectivity of Asp555
The electrophysiological properties of D555E provide valuable insight into the mechanism of Na/HCO3 transport via NBCe1. The fact that D555E changes to INBC from ICl when HCO3− is available suggests that HCO3− has more favorable access to its binding site in the mutant transporter than does Cl−. Cl− binds to this site when HCO3− is unavailable as ICl is produced in ND96. Thus, the presence or absence of ICl determines whether the site becomes occupied with HCO3−. The data in Fig. 5B illustrate that ICl is produced under Na+-free conditions. Our unpublished observation also shows that ICl can be produced even in Na+-free CO2/HCO3−, implying that the site is not occupied with HCO3− under Na+-free conditions.3 Thus, we speculate that HCO3− does not bind alone and that its binding to the transporter may require the precondition of Na+ binding.
The acquired ability of D555E to produce ICl and other ionic currents raises the strong possibility that Asp555 is part of the site for HCO3− selectivity. On the other hand, this brings a question of the role of Glu at the corresponding site in electroneutral Na/HCO3 transporters. If Asp555 is responsible for anion selectivity, does this mean that the electroneutral transporters (i.e. NBCn1, NBCn2, and NDCBE1) containing a Glu have broader anion selectivity? Obviously, addressing this question requires additional studies, but we note that among electroneutral Na/HCO3 transporters, NBCn2 and NDCBE1 have the ability to transport Cl− or have an associated Cl− conductance. Even for NBCn1, its Cl− dependence has been determined by measuring pHi recovery upon Cl− removal in CO2/HCO3− (15). The Glu residue is also found at the corresponding sites in Cl/HCO3 exchangers AE1–3. Thus, there is a possibility that the Glu residue in electroneutral transporters may play a central role in Cl− transport or conductance and/or ion selectivity. It will be interesting to test Cl− transport and anion selectivity of E742D and E742Q.
By what molecular mechanism does Asp555 contribute to HCO3− selectivity? D555E adds a single carbon to the side chain at the aspartate site, maintaining a net negative charge. Thus, charge alone at the site cannot account for the altered transport of Cl−. Our anion selectivity experiments suggest that steric properties of Asp555 are more important for distinguishing HCO3− from other anions. The effective radius of NO3− is 1.89 Å, slightly larger than the molecular radius of 1.81 Å for Cl−. Nevertheless, NO3− causes a negative shift in zero current voltage and produces a large conductance, comparable with the effect of HCO3−. NO3− and HCO3− have similar structures, consisting of one central nitrogen or carbon atom surrounded by three identical oxygen atoms in a trigonal planar arrangement, except that a hydrogen atom is attached to one of the oxygens in HCO3−. This implies that Asp555 may serve as an anion selectivity filter that distinguishes HCO3− from other polyatomic anions in a trigonal planar arrangement. The substitution of Asp555 with a Glu containing a longer carbon side chain may thus disrupt this steric arrangement, allowing other polyatomic anions with similar structures to translocate via the altered site. D555E is also permeable to SCN−, but this polyatomic anion is linear in a space-filling model and has a smaller effective radius than HCO3−.
Does the D555E mutation affect the electrogenicity of the transporter? D555E produces an outward current upon CO2/HCO3 application (Figs. 6B and 9B). INBC mediated by D555E is relatively small, corresponding to ∼27% of INBC mediated by NBCe1 at 96 mm [Na+]o (Fig. 4) despite the robust expression of D555E in oocyte membranes (Fig. 2). Thus, the D555E mutation does not abolish electrogenicity. There are two possible explanations for why INBC mediated by D555E is small. First, it may be due to weak electrogenicity. Weak electrogenicity may remain if ion translocation or binding sites are affected but not completely lost, giving rise to an altered stoichiometry of 1 Na+ versus <2 HCO3− (net charge between −1 and 0). This non-integer coupling ratio can occur when ion translocation involves cooperativity of multiple HCO3−-binding sites and the mutation affects one of those binding sites. Nonetheless, we do not think that weak electrogenicity is responsible for causing a decrease in INBC mediated by D555E. Lack of inducible INBC in oocytes expressing E742D and E742Q (Fig. 9) supports our interpretation. Second, the D555E mutation may have no effect on the coupling ratio but instead reduce the rate of HCO3− binding. It is possible that the D555E mutation not only affects anion selectivity, but it may also slow down the HCO3− binding rate. In this case, we expect that D555E has reduced transport activity without losing the 1Na+:2HCO3− coupling ratio. The exact stoichiometry of D555E warrants further investigation.
Asp555 as a Residue in the Entry of the Pore-forming Unit
Our finding of Asp555 as a site for HCO3− selectivity provides valuable insights into the pore-forming region of NBCe1. Structural biology predicts that a protein will provide a close general structural model for other proteins if the core regions of those proteins have over 50% sequence homology (33). This suggests a similar topology of TMs for both NBC and AE. The 13-TM topology model was thus proposed on the basis of long and extensive studies of the AE topology (24–27). Asp555 is 4 amino acids away from the DIDS-interacting motif Lys-Met-Ile-Lys (positions 559–602) (34), being located near Lys559 on the same side of the α-helical TM5 structure. The DIDS-interacting sites are suggested to be close to the entry of the pore-forming unit in bicarbonate transporters, although those sites do not directly participate in ion binding/translocation (35). In AE1, the residues between Ser852 and Leu857 (TM13) are proposed to serve as an anion selectivity filter (36). These residues are located close to Lys851, which is another DIDS-binding site (37). Based on these, we propose that Asp555 is located within the membrane field and near the entry of the pore region of the transporter.
Relations of Our Study to Natural NBCe1 Mutants
Among the mutant transporters we used in this study, it is worthwhile to briefly discuss S427A (substitution of Ser427 with an alanine in TM1). Ser427 is one of the sites for naturally occurring point mutations in NBCe1 (38). Functional characterization of S427L shows that it alters voltage- and Na+-dependent HCO3− movement, resulting in ∼10% of INBC compared with NBCe1. S427L has been proposed to contain a defect in the “voltage-sensing” processes of the transporter. In addition, S427L is targeted to the apical membrane of the polarized kidney cells, whereas NBCe1 is targeted to the basolateral membrane (39). All electroneutral Na/HCO3 transporters possess an alanine at the corresponding site of Ser427. Our data show that S427A has gNBC and ENBC values comparable with those for NBCe1 (Fig. 1). Thus, the substitution of Ser427 with a nonpolar residue leucine dramatically alters transporter function, whereas the substitution with another nonpolar residue alanine has no significant effect. This implies that the side chain polarity of Ser427 is less involved in the altered function of S427L. We speculate that the functional defect of S427L may involve a conformational change caused by a larger leucine (molecular weight, 113) replacing a smaller serine (molecular weight, 87). The substitution with an alanine (molecular weight, 89) may not cause severe conformational change, thus maintaining the protein structure for function.
Summary
By structure/function analyses of NBCe-specific residues in TMs, we identified Asp555 as a site playing a role in HCO3− selectivity. This finding supports the conclusion that the functional specificity of Na/HCO3 transporters is defined by amino acid residues within the transmembrane domains. The results obtained from this study are valuable for understanding the molecular mechanism of ion translocation or selectivity via Na/HCO3 transporters.
Supplementary Material
Acknowledgments
We thank Drs. Ron Abercrombie and Criss Hartzell for helpful discussion. We also thank Dr. John White for editing the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant GM078502. This work was also supported by a National Kidney Foundation Young Investigator Grant (to I. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Appendices 1 and 2.
H. S. Yang, unpublished observation.
- TM
- transmembrane domain
- PBS
- phosphate-buffered saline
- SPQ
- 6-methoxyl-N-(3-sulfopropyl) quinolinium
- DIDS
- 4,4′-diisothiocyanato-2,2′-disulfonate stilbene.
REFERENCES
- 1.Bernardo A. A., Bernardo C. M., Espiritu D. J., Arruda J. A. ( 2006) Semin. Nephrol. 26, 352– 360 [DOI] [PubMed] [Google Scholar]
- 2.Alper S. L. ( 2006) Exp. Physiol. 91, 153– 161 [DOI] [PubMed] [Google Scholar]
- 3.Romero M. F., Fulton C. M., Boron W. F. ( 2004) Pfluegers Arch. Eur. J. Physiol. 447, 495– 509 [DOI] [PubMed] [Google Scholar]
- 4.Boron W. F. ( 1989) in The Regulation of Acid-Base Balance ( Seldin D. W., Giebisch G. eds) pp. 33– 56, Raven Press, New York [Google Scholar]
- 5.Burnham C. E., Amlal H., Wang Z., Shull G. E., Soleimani M. ( 1997) J. Biol. Chem. 272, 19111– 19114 [DOI] [PubMed] [Google Scholar]
- 6.Gawenis L. R., Bradford E. M., Prasad V., Lorenz J. N., Simpson J. E., Clarke L. L., Woo A. L., Grisham C., Sanford L. P., Doetschman T., Miller M. L., Shull G. E. ( 2007) J. Biol. Chem. 282, 9042– 9052 [DOI] [PubMed] [Google Scholar]
- 7.Boron W. F., Fong P., Hediger M. A., Boulpaep E. L., Romero M. F. ( 1997) Weiner. Klin. Wochenschr. 109, 445– 456 [PubMed] [Google Scholar]
- 8.Soleimani M., Grassl S. M., Aronson P. S. ( 1987) J. Clin. Investig. 79, 1276– 1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shirakabe K., Priori G., Yamada H., Ando H., Horita S., Fujita T., Fujimoto I., Mizutani A., Seki G., Mikoshiba K. ( 2006) Proc. Natl. Acad. Sci. U. S. A. 103, 9542– 9547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAlear S. D., Liu X., Williams J. B., McNicholas-Bevensee C. M., Bevensee M. O. ( 2006) J. Gen. Physiol. 127, 639– 658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Läuger P. ( 1991) In Electrogenic Ion Pumps ( Läuger P. ed) pp. 61– 91, Sinauer Associates Press, MA [Google Scholar]
- 12.Pushkin A., Kurtz I. ( 2006) Am. J. Physiol. Renal Physiol. 290, F580– F599 [DOI] [PubMed] [Google Scholar]
- 13.Abuladze N., Azimov R., Newman D., Liu W., Tatishchev S., Pushkin A., Kurtz I. ( 2005) J. Physiol. 565, 717– 730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAlear S. D., Bevensee M. O. ( 2006) J. Biol. Chem. 281, 32417– 32427 [DOI] [PubMed] [Google Scholar]
- 15.Choi I., Aalkjaer C., Boulpaep E. L., Boron W. F. ( 2000) Nature 405, 571– 575 [DOI] [PubMed] [Google Scholar]
- 16.Choi I., Yang H. S., Boron W. F. ( 2007) J. Physiol. 578, 131– 142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Q., Azimov R., Kao L., Newman D., Liu W., Abuladze N., Pushkin A., Kurtz I. ( 2009) J. Biol. Chem. 284, 8919– 8929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang M. H., Dipiero J., Sonnichsen F. D., Romero M. F. ( 2008) J. Biol. Chem. 283, 18402– 18410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi I., Romero M. F., Khandoudi N., Bril A., Boron W. F. ( 1999) Am. J. Physiol. Cell Physiol. 276, C576– C584 [DOI] [PubMed] [Google Scholar]
- 20.Perry C., Blaine J., Le H., Grichtchenko I. I. ( 2006) Am. J. Physiol. Renal Physiol. 290, F417– F427 [DOI] [PubMed] [Google Scholar]
- 21.Choi I., Chiu S. Y. ( 1997) FEBS Lett. 405, 133– 136 [DOI] [PubMed] [Google Scholar]
- 22.Bevensee M. O., Schmitt B. M., Choi I., Romero M. F., Boron W. F. ( 2000) Am. J. Physiol. Cell Physiol. 278, C1200– C1211 [DOI] [PubMed] [Google Scholar]
- 23.Chao A. C., Dix J. A., Sellers M. C., Verkman A. S. ( 1989) Biophys J. 56, 1071– 1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujinaga J., Tang X. B., Casey J. R. ( 1999) J. Biol. Chem. 274, 6626– 6633 [DOI] [PubMed] [Google Scholar]
- 25.Kuma H., Shinde A. A., Howren T. R., Jennings M. L. ( 2002) Biochemistry 41, 3380– 3388 [DOI] [PubMed] [Google Scholar]
- 26.Tang X. B., Fujinaga J., Kopito R., Casey J. R. ( 1998) J. Biol. Chem. 273, 22545– 22553 [DOI] [PubMed] [Google Scholar]
- 27.Popov M., Li J., Reithmeier R. A. F. ( 1999) Biochem. J. 339, 269– 279 [PMC free article] [PubMed] [Google Scholar]
- 28.Sciortino C. M., Romero M. F. ( 1999) Am. J. Physiol. Renal Physiol. 277, F611– F623 [DOI] [PubMed] [Google Scholar]
- 29.Grichtchenko I. I., Romero M. F., Boron W. F. ( 2000) J. Gen. Physiol. 115, 533– 545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kao L., Sassani P., Azimov R., Pushkin A., Abuladze N., Peti-Peterdi J., Liu W., Newman D., Kurtz I. ( 2008) J. Biol. Chem. 283, 26782– 26794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber W.-M. ( 1999) J. Membr. Biol. 170, 1– 12 [DOI] [PubMed] [Google Scholar]
- 32.Qu Z., Hartzell H. C. ( 2000) J. Gen. Physiol. 116, 825– 844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chothia C., Lesk A. M. ( 1986) EMBO J. 5, 823– 826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu J., Daly C. M., Parker M. D., Gill H. S., Piermarini P. M., Pelletier M. F., Boron W. F. ( 2006) J. Biol. Chem. 281, 19241– 19250 [DOI] [PubMed] [Google Scholar]
- 35.Cabantchik Z. I., Rothstein A. ( 1974) J. Membr. Biol. 15, 207– 226 [DOI] [PubMed] [Google Scholar]
- 36.Zhu Q., Casey J. R. ( 2004) J. Biol. Chem. 279, 23565– 23573 [DOI] [PubMed] [Google Scholar]
- 37.Okubo K., Kang D., Hamasaki N., Jennings M. ( 1994) J. Biol. Chem. 269, 1918– 1926 [PubMed] [Google Scholar]
- 38.Dinour D., Chang M. H., Satoh J., Smith B. L., Angle N., Knecht A., Serban I., Holtzman E. J., Romero M. F. ( 2004) J. Biol. Chem. 279, 52238– 52246 [DOI] [PubMed] [Google Scholar]
- 39.Li H. C., Szigligeti P., Worrell R. T., Matthews J. B., Conforti L., Soleimani M. ( 2005) Am. J. Physiol. Renal Physiol. 289, F61– F71 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.