Abstract
Members of the vascular endothelial growth factor (VEGF) family play a pivotal role in angiogenesis and lymphangiogenesis. They are potential therapeutics to induce blood vessel formation in myocardium and skeletal muscle, when normal blood flow is compromised. Most members of the VEGF/platelet derived growth factor protein superfamily exist as covalently bound antiparallel dimers. However, the mature form of VEGF-D (VEGF-DΔNΔC) is predominantly a non-covalent dimer even though the cysteine residues (Cys-44 and Cys-53) forming the intersubunit disulfide bridges in the other members of the VEGF family are also conserved in VEGF-D. Moreover, VEGF-D bears an additional cysteine residue (Cys-25) at the subunit interface. Guided by our model of VEGF-DΔNΔC, the cysteines at the subunit interface were mutated to study the effect of these residues on the structural and functional properties of VEGF-DΔNΔC. The conserved cysteines Cys-44 and Cys-53 were found to be essential for the function of VEGF-DΔNΔC. More importantly, the substitution of the Cys-25 at the dimer interface by various amino acids improved the activity of the recombinant VEGF-DΔNΔC and increased the dimer to monomer ratio. Specifically, substitutions to hydrophobic amino acids Ile, Leu, and Val, equivalent to those found in other VEGFs, most favorably affected the activity of the recombinant VEGF-DΔNΔC. The increased activity of these mutants was mainly due to stabilization of the protein. This study enables us to better understand the structural determinants controlling the biological activity of VEGF-D. The novel variants of VEGF-DΔNΔC described here are potential agents for therapeutic applications, where induction of vascular formation is required.
Vascular endothelial growth factors (VEGFs)3 are considered as key growth factors inducing angiogenesis and lymphangiogenesis during embryogenesis as well as maintaining vasculature during adulthood. Their abnormal expression is found in pathological conditions such as cancer and retinopathies (1). Five mammalian VEGFs, VEGF-A, -B, -C, -D and placenta growth factor (PlGF), are known (2) as well as Orf virus-derived VEGF-E proteins (3) and multiple homologues from snake venoms (VEGF-Fs) (4). Several members of the VEGF family exist as different isoforms, either as a result of the alternative splicing of their mRNAs or due to proteolytic processing. These forms vary in their specificities and affinities to three main VEGF receptors, co-receptors such as neuropilins and heparan sulfate proteoglycans and other components of the extracellular matrix, translating into different biological effects (5).
VEGFR-2 is an important receptor regulating vasculogenesis and angiogenesis. It is mainly expressed on endothelial cells, but expression is also found in several other cell types (6). Mammalian VEGFR-2 ligands include VEGF-A (7), VEGF-C (8), and VEGF-D (9). In addition to VEGFR-2, VEGF-C and VEGF-D are ligands for VEGFR-3, which mainly mediates lymphangiogenesis in adults but also participates in the formation of blood vessels during embryogenesis (2).
Because of their importance in angiogenesis, VEGFs have been suggested as potential therapeutic agents in different pathological conditions to improve compromised blood flow. Studies aiming at inducing angiogenesis in vivo have been performed by introducing VEGFs to tissues either directly as recombinant proteins (10) or using gene therapy vectors (11). Findings from several laboratories have shown that VEGFs have strong angiogenic activity in vivo, and they could be used for the treatment of conditions like lower limb ischemia and ischemic coronary artery disease. The short in vivo half-life of these growth factors and the requirement for sustained angiogenic stimulus makes gene therapy a preferred option. Of the VEGFs, VEGF-A and the mature form of VEGF-D (VEGF-DΔNΔC, see below) are the strongest agents to induce vascular formation (12, 13).
VEGFs share structural similarity with platelet-derived growth factors. Together they are classified as the VEGF/platelet-derived growth factor family, belonging to a larger family of cystine knot growth factors. The members of this family share a cystine knot motif, which is found in many extracellular proteins and is conserved among numerous species (14). Characteristic of the cystine knot proteins is that they contain a conserved structure of antiparallel β-sheets connected by three disulfide bonds. Typically cystine knot growth factors form dimers, which within the VEGF/platelet-derived growth factor family are often linked by intersubunit disulfide bonds. The crystal structures have been solved for VEGF-A (15), PlGF (16), VEGF-B (17), VEGF-E (18), and two snake venom VEGF-Fs, vammin and VR-1 (19).
There are currently no published structures of VEGF-C or VEGF-D. They can be divided into their own subfamily based on sequence similarity and several characteristic features; 1) they are the only VEGFs that bind to VEGFR-3, 2) they are expressed as long precursor forms having poor receptor binding affinities, and 3) they require proteolytic processing at their N-terminal and C-terminal ends to become more active. In contrast to other members of the VEGF family, the mature, proteolytically processed ΔNΔC-forms of VEGF-C and VEGF-D exist predominantly as non-covalently bound dimers, even though they have the conserved cysteine residues that form the intersubunit disulfide bonds in other VEGFs (8, 20). However, both VEGF-C and VEGF-D also have an additional cysteine residue located at the dimer interface (8, 20). Mutation of this residue in VEGF-C only minimally altered the receptor binding affinity (21), but it stabilized the dimer structure (56).
In the current study we investigated the importance of residues at the subunit interface for the function of VEGF-DΔNΔC. We built homology models of VEGF-DΔNΔC and used alanine scanning and site-specific mutagenesis as well as tested the biological activity of various mutated forms of VEGF-DΔNΔC. Our study revealed that the conserved cysteine residues (Cys-44 and Cys-53), which are known to form intersubunit disulfide bridges in other VEGFs, were essential for the activity of the recombinant VEGF-DΔNΔC. Furthermore, the monomer to dimer ratio of VEGF-DΔNΔC could be regulated by mutagenesis. In addition, it was found that replacement of the “extra” cysteine (Cys-25) by various amino acids, preferably Ile, Leu, or Val, actually enhanced the activity of VEGF-DΔNΔC. This was at least partially due to increased stability of the protein.
EXPERIMENTAL PROCEDURES
Homology Modeling
Homology models of VEGF-DΔNΔC, consisting of residues 93–201 of the full-length VEGF-D, were constructed based on the crystal structures of VEGF-A (22) (PDB code 1FLT) and the VEGF-F VR-1 (19) (PDB code 1WQ9). The numbering of amino acids in the models is based on the VEGF-DΔNΔC sequence (1–109). To obtain a structure-based sequence alignment, the known crystal structures of VEGF-A, VR-1, PlGF (23) (PDB code 1RV6), VEGF-B (17) (PDB code 2C7W), and VEGF-E (18) (PDB code 2GNN) were superimposed using the program VERTAA within BODIL (24). The sequences of VEGF-D (NCBI accession number O43915) and VEGF-C (NCBI accession number O43915 P49767) were aligned to the structure-based sequence alignment using the program MALIGN within BODIL. The alignment was manually refined at residues 75–85 (loop 3) based on visual inspection of the known crystal structures. The pairwise alignment of VEGF-DΔNΔC (residues 5–102) with VEGF-A or with VR-1 was extracted from the structure-based sequence alignment and used for homology modeling of VEGF-D.
Two different models were constructed based on the VEGF-A structure (22); that is, a dimeric model of VEGF-D with 2-fold symmetry restraints and the conserved disulfide bonds and a model of VEGF-D monomer with a disulfide bond between Cys-25 and Cys-44, defined as an additional disulfide in MODELLER (25). Apart from VEGF-C, VEGF-F has the most similar amino acids at the dimer interface as VEGF-D. Therefore, an additional dimeric model of VEGF-D was constructed based on the VR-1 crystal structure (19). For each of the three VEGF-D models to be constructed, ensembles of 10 were generated, and the model with the lowest value of the MODELLER objective function was chosen for further analysis. The models were superimposed on the known crystal structures using the program VERTAA in BODIL. To obtain models of the different VEGF-D mutants, the amino acids in question of the VR-1-based VEGF-D model were mutated in BODIL.
Cloning of the Expression Vectors
All constructs were subcloned into pDonr201 vector (Invitrogen) using the Gateway® Cloning System (Invitrogen) to create Entry clones. The coding sequences were amplified using PCRs that introduced flanking attB sites to the inserts as follows. The coding sequence of N-terminal FLAG-tagged VEGF-DΔNΔC was amplified from pVDApexΔNΔC vector (received as a generous gift from Dr. Marc G. Achen) by PCR using primers that introduced a His tag to the 3′-end and flanking attB sites. The VEGF-A121 insert was cloned using a stepwise elongation of sequence-PCR (26) strategy from pCMVhVEGF165 plasmid. Briefly, the first PCR amplified the VEGF-A121 coding sequence and introduced a His tag to the 3′-end. This PCR product was used as the template for the second round that introduced the attB sites flanking the coding sequence. The insert encoding soluble VEGFR-2-human IgG Fc fragment fusion protein (sVEGFR2-Fc) was generated using the Gateway chimeragenesis method (27) by amplifying the VEGFR-2 extracellular domain from pBLAST45-hFlk1 (InvivoGen) and human IgG Fc-fragment from pAdCMV-Flt1(1–5)-Fc using PCRs which introduced the flanking attB sites to the 5′-end of sVEGFR2 and 3′-end of the Fc fragment and 84-bp-long homologous sequences to the 3′-end of VEGFR-2 and 5′-end of the Fc fragment. Plasmids encoding mutant VEGF-DΔNΔC proteins were generated by QuikChange® (Stratagene) using the VEGF-DΔNΔC Entry clone as a template. All Entry clones were completely sequenced to verify the sequences before cloning into a pBVboostFGII expression vector using the BVboost system LR-reaction (28). The recombinant baculoviruses were generated as described earlier (29).
Protein Expression and Purification
Human embryonic kidney cells (293T cells) on 6-well plates were transiently transfected using the FuGENE® HD Transfection Reagent (Roche Applied Science) and vectors encoding the VEGF-DΔNΔC variants. The transfected 293T cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin solution (Sigma) at 37 °C and a humidified atmosphere of 5% CO2, and the culture medium was collected at 72 h. VEGF-D concentrations were measured using a VEGF-D enzyme-linked immunosorbent assay kit (R&D Systems), and the expression of the VEGF-DΔNΔC variants was verified using immunoblotting with an anti-VEGF-D monoclonal antibody (MAB 286, R&D Systems). Alternatively, a horseradish peroxidase-conjugated secondary antibody (Pierce), a chemiluminescent-based detection system (SuperSignal West Dura Extended Duration Substrate, Pierce) and CL-XPosure films (Thermo Scientific), or an alkaline phosphatase-conjugated secondary antibody (Bio-Rad) and NBT/BCIP (Roche Applied Science) were used for detection.
Recombinant proteins were produced in recombinant baculovirus (multiplicity of infection 5)-infected High FiveTM cells (Invitrogen) in shaken cultures at 27 °C for 72 h. Proteins were purified from clarified culture media using BD Talon Metal Affinity Resin (Clontech). The resin was agitated in clarified medium for 2 h and packed into chromatography columns for washing and elution. The washing step was done with a buffer containing 50 mm NaH2PO4 and 300 mm NaCl, pH 7.0. Recombinant proteins were eluted using 50 mm HEPES, 20 mm NaCl, and 200 mm imidazole, pH 7.4, and dialyzed against 50 mm HEPES, 20 mm NaCl, pH 7.4. Protein concentrations were measured using the DC Protein Assay Kit (Bio-Rad), and purified proteins were analyzed both in reducing and non-reducing conditions using SDS-PAGE; the gels were stained with PAGE Blue Protein Staining solution (Fermentas). VEGF-C and sVEGFR3-Fc recombinant proteins were generous gifts from Dr. Kari Alitalo.
Pulldown Assay with Recombinant sVEGFR2-Fc
The media of transiently transfected 293T cells were precleared using Protein A-Sepharose (GE Healthcare). Supernatants were collected and supplied with 5 μg/ml sVEGFR2-Fc recombinant protein and 0.1 μg/ml heparin (Sigma) and incubated at 4 °C overnight. Controls were incubated without sVEGFR2-Fc. Protein complexes were precipitated using Protein A-Sepharose and eluted by heating in SDS-PAGE sample buffer with or without β-mercaptoethanol at 95 °C for 5 min. Samples were separated using SDS-PAGE and analyzed using immunoblotting with an anti-VEGF-D monoclonal antibody as above.
Cell Growth and Survival Assays
Ba/F3-VEGFR-2 (9) and Ba/F3-VEGFR-3 (30) cells (received as generous gifts from Dr. Kari Alitalo) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 ng/ml rmIL-3 (Calbiochem), 1% penicillin-streptomycin solution (Sigma), and the required selection antibiotic: 500 μg/ml G418 (InvivoGen) for Ba/F3-VEGFR-2 and 200 μg/ml Zeocin (Invitrogen) for Ba/F3-VEGFR-3 cells. For the assays, cells were plated at 18,000 cells per well on 96-well plates in rmIL-3-free medium. Recombinant proteins or dilutions of the medium from the transfected 293T cells were added to the wells, and cell viability was quantified after 48 h of incubation at 37 °C and a humidified atmosphere of 5% CO2. Cell Titer Blue Reagent (20 μl; Promega) was added to each well, and the plates were incubated for two more hours. Fluorescence was read with Wallac Victor2 1420 Multilabel Counter (PerkinElmer Biosystems) using an excitation wavelength of 544 nm and an emission wavelength of 590 nm. The measured fluorescence values were normalized by defining the measured maximum response as 100 and the minimum response as 0. Results are expressed as the mean ± S.E.
Affinity Measurements
The relative VEGFR-2 and VEGFR-3 binding affinities were determined using solid phase competition assays. For the VEGFR-2 assay, 96-well plates were coated with 500 ng/ml VEGF-A121 recombinant protein and blocked with bovine serum albumin (Sigma). sVEGFR2-Fc protein, 1 μg/ml, was preincubated with a dilution series of the assayed VEGF ligands at 37 °C for 1 h. The preincubated samples were transferred onto the VEGF-A121-coated plate and incubated at 37 °C for 1 h. The amount of bound sVEGFR2-Fc was quantified using an anti-human Fc-AP (Sigma) and p-nitrophenyl phosphate substrate (Sigma) by measuring the absorbance at 405 nm. The VEGFR-3 binding affinities were determined similarly, but the 96-well plates were coated with 200 ng/ml VEGF-C, and preincubations were done with 500 ng/ml sVEGFR3-Fc. To determine the IC50 values of the proteins, sigmoidal dose response curves were fitted to measured data using non-linear regression in Prism 4 (GraphPad Prism Software).
Phosphorylation Assays
Porcine Aortic Endothelial (PAE)-KDR (31) and PAE-Flt4 (32) cells (received as generous gifts from Dr. Kari Alitalo) were cultured in F12 nutrient mixture (Invitrogen) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin solution (Sigma), and the required selection antibiotic (400 μg/ml G418 (InvivoGen) for PAE-KDR cells and 0.5 μg/ml puromycin (InvivoGen)) for PAE-Flt4 cells, at 37 °C and a humidified atmosphere of 5% CO2. Subconfluent cells were serum-starved overnight and preincubated for 5 min with 1 mm Na3VO4 in phosphate-buffered saline (Lonza). Phosphorylations were induced by adding the recombinant proteins to the cells in 5 ml of serum-free F12 nutrient mixture. Cells were washed with ice-cold phosphate-buffered saline containing 1 mm Na3VO4 and immediately lysed using ice-cold lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 10% glycerol, 1 mm Na3VO4, and protease inhibitor tablet (Roche Applied Science)). The lysates were clarified by centrifugation (10,000 × g at 4 °C for 15 min), and the total protein concentrations were determined with BCA Protein Assay kit (Thermo Scientific). The lysates were adjusted to the same total protein concentration, supplemented with SDS-PAGE sample buffer, and heated at 95 °C for 5 min. Proteins were separated using SDS-PAGE followed by transfer onto a nitrocellulose membrane (Pure Nitrocellulose Membrane, Bio-Rad).
Phosphorylated VEGFR-2 was immunoblotted with a phospho-VEGF receptor 2 (Tyr-1175) rabbit mAb (Cell Signaling), and total VEGFR-2 was immunoblotted with a VEGF receptor 2 rabbit mAb (Cell Signaling). Phosphorylated Akt was immunoblotted with a phospho-Akt (Ser-473) (Cell Signaling) antibody, and total Akt was immunoblotted with an Akt Antibody (Cell Signaling). Horseradish peroxidase-conjugated secondary antibodies (Pierce), a chemiluminescent-based detection system (SuperSignal West Dura Extended Duration Substrate, Pierce), and CL-XPosure films (Thermo Scientific) were used. After the detection of phosphorylated forms, the membranes were stripped for 15 min at room temperature with Restore Western blot Stripping Buffer (Pierce), and the total amount of protein was detected. Total VEGFR-3 was immunoprecipitated with a VEGFR-3 antibody (Abcam). The samples were separated using SDS-PAGE and immunoblotted with anti-phosphotyrosine (Upstate) and VEGFR-3 antibodies and detected using a chemiluminescent-based detection system as above.
VEGF-D Stability Assay
The samples of 2 μg/ml VEGF-DΔNΔC or C25L mutant were incubated in cell culture medium (Dulbecco's modified Eagle's medium, 10% fetal bovine serum, 1% penicillin-streptomycin solution) or Tris buffer (100 mm Tris, 150 mm NaCl, 1% bovine serum albumin, 0.1% Tween 20, 0.1 μg/ml heparin) from 0 to 48 h. Microtiter plates (96-well; Microtiter Assembly Strip, EB, Thermo Scientific) were coated overnight with 5 μg/ml anti-FLAG M2 (Sigma) antibody and blocked with bovine serum albumin (Sigma). The samples and standards generated by serial dilutions of both proteins were incubated in the coated plate and detected using sVEGFR2-Fc and an anti-human Fc-AP (Sigma) similar to the affinity measurements. The percentage of remaining activity was calculated in comparison to the 0-h samples.
Miles Assay
A Miles assay (33) for analysis of vascular permeability in the skin was performed as described earlier (34). Doses of 50, 100, 250, 500, and 1000 ng of recombinant proteins (VEGF-DΔNΔC, C25L mutant, or VEGF-A121) in 0.1 ml of phosphate-buffered saline were used.
RESULTS
Homology Models of VEGF-DΔNΔC
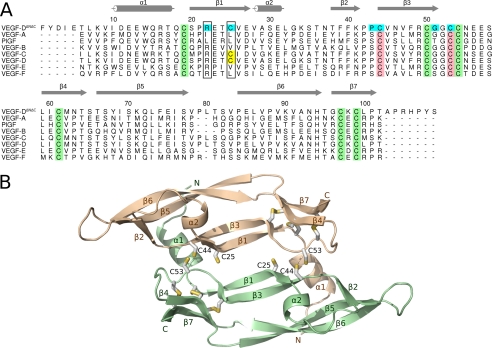
Homology models of human VEGF-DΔNΔC dimer were built based on the known x-ray structures of VEGF-A and the VEGF-F VR-1 to gain a better understanding of the unique architecture of the subunit interface of VEGF-D. According to the structure-based alignment of VEGF sequences (Fig. 1A), which was used for modeling, the sequence identity was 33% between VEGF-D and VEGF-A and 30% between VEGF-D and VR-1. The sequence identity was clearly lower (∼9%) at residues 75–85, where VEGF-D had two amino acids more than VEGF-A and one amino acid more than VR-1. This is likely an important region for receptor specificity (22, 35).
FIGURE 1.
The structure of VEGF-DΔNΔC. A, structure-based sequence alignment of the VEGF homology domain regions of the VEGF family members. The crystal structures of VEGF-A (PDB code 1FLT), PlGF (1RV6), VEGF-B (2C7W), VEGF-E (2GNN), and VEGF-F VR-1 (1WQ9) were superimposed as described under “Experimental Procedures.” The sequences of VEGF-D (UniProt accession code O43915) and VEGF-C (UniProt accession code P49767) were aligned to the structure-based alignment. Cysteines of the cystine knot are in green. Cysteines forming the (putative) intermolecular disulfide bond are in pink. The extra cysteine, found in VEGF-D and VEGF-C, is in yellow. Mutated amino acids of the dimer interface are in cyan. Amino acids equivalent to Arg-22 and Cys-25 in VEGF-D are framed. Secondary structure elements (according to VEGF-A structure (22)) are shown in gray. The figure was prepared using Alscript 2.04 (54), Inkscape 0.46, and Gimp 2.6. B, homology model of the VEGF-DΔNΔC dimer. Each monomer (pale green and wheat) consists of a central four-stranded β-sheet (β1, β3, β5, and β6), two additional short β-strands (β4 and β7), and two short α-helices (α1 and α2). Cysteines are shown as sticks. The figure was prepared using Pymol 1.1 (55) and Inkscape 0.46.
The monomers of the dimeric VEGF-DΔNΔC were built up of a central four-stranded β-sheet (β1, β3, β5, and β6) and two additional short β-strands (β4 and β7) which were extensions of β3 and β6 (Fig. 1B). The segment between β1 and β3 contained a short α-helix followed by a loop and the second β-strand, β2. The VEGF-DΔNΔC homodimer was antiparallel and had an elongated shape but was flat at the central part. Two intermolecular disulfide bonds formed by the conserved Cys-44 from one monomer and Cys-53 from the other one covalently linked the model structure. In the VEGF-DΔNΔC dimer, the partially helical N terminus of one VEGF-D monomer folded on top of the other monomer. The growth factor cysteine knot (14), consisting of three intramolecular disulfide bridges formed by six cysteine residues (Cys-19, -50, -54, -61, -97, and -99), conserved in all VEGFs (Fig. 1A), shaped the VEGF-D fold at one end of both monomers.
Apart from Cys-44 and Cys-53, VEGF-DΔNΔC had an additional cysteine residue, Cys-25, at the dimer interface (Fig. 1). Among the known members of the VEGF protein family, this cysteine was found only in VEGF-C, VEGF-D, and the newly discovered VEGF homolog PVF-1 (36). We wanted to examine whether an intramolecular disulfide bond could form between the residues Cys-25 and Cys-44 in the VEGF-D monomer and, accordingly, constructed a model of monomeric VEGF-DΔNΔC with a disulfide bond between these two cysteines. Based on the model, the interatomic distance between the sulfur atoms of these cysteines was 2.0 Å, and thus, an intramolecular disulfide bond might form between Cys-25 and Cys-44.
Alanine Scanning of the Cysteines at the Dimer Interface of VEGF-DΔNΔC
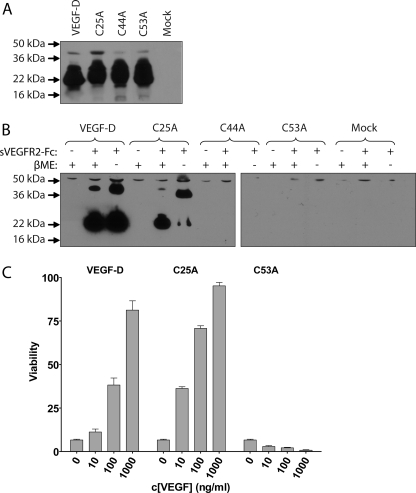
To test the biological significance of the three subunit interface cysteines (Cys-25, Cys-44, and Cys-53) of VEGF-DΔNΔC, we replaced these residues, one at a time, by alanine residues. The resulting expression constructs were named C25A, C44A, and C53A. The constructs were transiently transfected into 293T cells, and the protein expression was verified using immunoblotting with an anti-VEGF-D mAb (Fig. 2A). To evaluate the receptor binding activity of the VEGF-DΔNΔC mutants, the conditioned mediums were precipitated with a sVEGFR2-Fc (35) (Fig. 2B). Whereas the native VEGF-DΔNΔC and C25A proteins both efficiently bound to the soluble receptor, the C44A or C53A proteins did not bind to sVEGFR2-Fc. In immunoblot analysis, both after reducing and non-reducing SDS-PAGE, the receptor-bound native VEGF-DΔNΔC appeared mainly in a monomeric state even though some protein was seen as a dimer in both conditions. The C25A mutant behaved mainly as a monomeric protein on SDS-PAGE under reducing conditions but as a dimeric protein under non-reducing conditions. These results suggest that most of the C25A protein exists as a covalently bound dimer, and that the extra, putatively unpaired Cys-25 in the native VEGF-DΔNΔC may hinder the formation of disulfide bond-linked VEGF-DΔNΔC dimers.
FIGURE 2.
Expression, sVEGFR2-Fc binding and dimerization of VEGF-DΔNΔC variants. A, an immunoblot of transiently transfected 293T cell media separated with SDS-PAGE under reducing conditions using an anti-VEGF-D mAb. B, precipitation of receptor binding VEGF-D forms from transiently transfected 293T cell media. The media and sVEGFR2-Fc protein were incubated, and protein complexes were collected using protein A-Sepharose. Samples were separated with SDS-PAGE either with or without β-mercaptoethanol (βME) in the sample buffer and immunoblotted using an anti-VEGF-D mAb to analyze receptor binding and dimerization of the VEGF-D variants. C, Ba/F3-VEGFR-2 cell growth and survival assay of recombinant proteins produced in insect cells. Cells were supplemented with the indicated concentrations of the proteins, and viability was determined after 48 h of incubation.
The native VEGF-DΔNΔC and the three cysteine mutants were also produced in a larger scale in insect cells using BVboostFG baculovirus expression system (37), and the proteins were purified from the culture medium using metal affinity chromatography. All proteins were successfully expressed, but the C44A protein was repeatedly lost during dialysis after the purification. This may be due to structural changes in the protein caused by the mutation, which either promoted protein aggregation or instability. The biological activity of the purified recombinant proteins was evaluated on Ba/F3-VEGFR-2 (9) cells for their ability to induce cell growth and survival (Fig. 2C). Ba/F3-VEGFR-2 and Ba/F3-VEGFR-3 (30) cells, respectively, express chimeric receptors with the extracellular domain of VEGFR-2 or VEGFR-3 fused to the cytoplasmic and transmembrane domains of EpoR. The cells are dependent on IL-3, but in its absence ligand binding to VEGFR can rescue the cells. The C25A mutant gave an enhanced cell survival response when compared with the native form of VEGF-DΔNΔC. The C53A mutant, which also failed to bind to the recombinant sVEGFR2-Fc protein (see above; Fig. 2B), was inactive in this assay.
Molecular Modeling of the VEGF-DΔNΔC Dimer Interface
We found that the Cys-25 of VEGF-DΔNΔC was not essential for protein activity, but above all its replacement with alanine increased the ability of VEGF-DΔNΔC to promote Ba/F3-VEGFR-2 cell survival. The C25A protein also had an increased dimer to monomer ratio compared with the native form. This inspired us to test if we could create a mutant of VEGF-DΔNΔC that would solely be expressed as a dimer and whether it would have improved biological activity.
To design a totally dimeric VEGF-DΔNΔC mutant, we analyzed the dimer interface in the structurally known VEGFs. The homology model of VEGF-D and the crystal structures of VEGF-A, VEGF-B, VEGF-E, VEGF-F (VR-1), and PlGF were superimposed to compare their dimer interfaces. VEGF-A is known to be expressed entirely as a dimer, and therefore, we aimed at designing a VEGF-DΔNΔC mutant that would have a more VEGF-A-like dimer interface. In VEGF-A, Leu-32 and Ile-29 from different monomers form hydrophobic interactions. The corresponding residues in VEGF-DΔNΔC are Cys-25 and Arg-22 (Figs. 1A and 3A), which showed that VEGF-DΔNΔC has a considerably more hydrophilic dimer interface. Like VEGF-A, PlGF has a hydrophobic pair of amino acids (Leu-40 and Leu-37) at the dimer interface, whereas the other structurally known VEGFs (VEGF-F, VEGF-B, and VEGF-E) have a hydrophobic amino acid (Leu or Val) paired with an arginine at this position. In other words, they have characteristics of both the VEGF-A and the VEGF-D dimer interfaces. Based on these findings, the residues Arg-22 and Cys-25 were chosen as targets for site-directed mutagenesis.
FIGURE 3.
Amino acids of the dimer interface in homology models of the different VEGF-D variants. A, native VEGF-D. B–E, VEGF-D variants C53A (B), C25L (C), R22I/C25L (D), and G51C (E). Amino acids at the interface are shown as sticks. Mutated amino acids are in pink. Monomers are colored as in Fig. 1B. The figure was prepared using Pymol 1.1 (55) and Inkscape 0.46.
VEGF-DΔNΔC variants, R22I, R22L, C25L, R22I/ C25L, and R22L/C25L, were constructed based on the corresponding amino acids in the dimer interfaces of VEGF-A, PlGF, and VEGF-F (Fig. 1A). Additionally, the mutants G51C, P43S, and C25A/P43S were constructed (Fig. 3). By constructing the G51C variant (Fig. 3E), we wanted to introduce a cysteine residue opposite to Cys-25 in the dimer interface. The idea was that an intermolecular disulfide bridge could form between these two cysteines and stabilize the VEGF-D dimer. The P43S mutant was constructed on the basis that VEGF-A, PlGF, VEGF-B, and VEGF-F have a serine instead of proline (Pro-43) as in VEGF-D in this position (Fig. 1A). The double mutant C25A/P43S was constructed with the aim of further strengthening the dimer-forming ability of the C25A single mutant.
Screening the Biological Activity of the Mutants
The mutant proteins were all expressed in transiently transfected 293T cells, and their concentrations were measured by VEGF-D enzyme-linked immunosorbent assay (R&D Systems). The biological activities of the mutant forms were analyzed in Ba/F3-VEGFR-2 and Ba/F3-VEGFR-3 cell growth and survival assays by providing dilutions of the conditioned media from transfected 293T cells (Fig. 4, A and B). In both cell lines the C25A and C25L mutants had increased activity compared with the native VEGF-DΔNΔC, the C25L mutant being the most active one. The double mutants R22I/C25L and C25A/P43S had biological activity comparable with that of the native protein in Ba/F3-VEGFR-2 cells. However, in these cases the activity can be explained by the C25L and C25A mutations, respectively, as the single G51C, R22I, R22L, or P43S mutants could not induce cell survival. In other words, of the mutations at the VEGF-D dimer interface, only the single mutations of Cys-25 increased the biological activity.
FIGURE 4.
Screening of the biological activity of the VEGF-D mutants on Ba/F3-VEGFR-2 and Ba/F3-VEGFR-3 cell lines. VEGF-DΔNΔC and the mutants were produced in transiently transfected 293T cells. The concentrations of VEGF-DΔNΔC proteins in the media were measured using VEGF-D enzyme-linked immunosorbent assay (R&D Systems), and Ba/F3 cells were supplemented with the indicated concentrations of the proteins. Mock A, B, and C represent similar serial dilutions of media from mock-transfected cells as used for the VEGF-D-containing samples. A and C, assays on the Ba/F3-VEGFR-2 cells. B and D, assays on the Ba/F3-VEGFR-3 cells.
To study in detail which amino acid would be the most appropriate to replace Cys-25, we generated several additional mutants of VEGF-DΔNΔC by substituting Cys-25 with amino acids with different physicochemical properties. These mutants were tested as above (Fig. 4, C and D). The mutant forms with an aliphatic hydrophobic amino acid (Ile, Leu, and Val) in position Cys-25 yielded the highest activity on both Ba/F3-VEGFR-2 and Ba/F3-VEGFR-3 cells (Fig. 4, C and D). Interestingly, the substitution of Cys-25 with glycine led to inactivation of the growth factor. The mutants with Cys-25 replaced by Ala, Phe, Asn, Ser, or Trp also enhanced cell survival when compared with the native protein even though not as strongly as the mutants with Ile, Leu, or Val substituting Cys-25.
Dimerization of the Mutant Proteins
To determine how the different mutations affected the dimerization of VEGF-DΔNΔC, the proteins were analyzed from the conditioned media of transfected 293T cells using SDS-PAGE and immunoblotting (Fig. 5A). Similar to VEGF-DΔNΔC, the mutants formed both monomers and dimers. However, the C25I, C25L and C25V mutants were mainly in a presumably covalently bound dimeric form. This form migrated slightly faster on SDS-PAGE compared with the VEGF-DΔNΔC dimer. A similar, but much weaker, band was also detected from the mutants where Cys-25 was replaced by Ala, Phe, Asn, Ser, and Trp. The ability of the mutants to form covalent dimer correlated with their increased ability to induce Ba/F3-VEGFR-2 and Ba/F3-VEGFR-3 cell growth and survival as seen in Fig. 4. The mutants G51C, R22I/C25L, and R22L/C25L clearly had increased dimer to monomer ratios as well. The dimers of these mutants migrated similarly to the VEGF-DΔNΔC dimer; however, no increased activity was detected in the Ba/F3 cell assays for these mutants.
FIGURE 5.
The dimerization of VEGF proteins. A, VEGF-DΔNΔC and the mutants were produced in transiently transfected 293T cells. Samples of the media were separated using SDS-PAGE without reducing agents, and VEGF-D proteins were detected by immunoblotting using an anti-VEGF-D mAb. B and C, purified recombinant VEGF proteins were separated using SDS-PAGE under reducing conditions (B) and non-reducing conditions (C) and stained using Coomassie staining.
Dimerization, Activity, and Receptor Binding Affinity of the Purified Recombinant Proteins
Based on the studies above, selected mutants of VEGF-DΔNΔC together with native VEGF-DΔNΔC and VEGF-A121 (38) were expressed on a large scale in insect cells and purified using metal affinity chromatography. VEGF-A121 isoform was chosen because, as VEGF-DΔNΔC, it does not bind to heparan sulfates or neuropilins (39). The purified proteins were first characterized on SDS-PAGE (Fig. 5, B and C). The recombinant proteins with Cys-25 substitutions were found to have an increased dimer-to-monomer ratio compared with VEGF-DΔNΔC under non-reducing conditions. The C25L mutant was nearly completely dimeric, quite similarly to VEGF-A121. The activity of the proteins was compared in the Ba/F3-VEGFR-2 and Ba/F3-VEGFR-3 cell survival and growth assays over a broad range of concentrations (Fig. 6, A and B). As expected, the recombinant VEGF-A121 protein had the highest activity on Ba/F3-VEGFR-2 cells. VEGF-DΔNΔC protein derived from insect cells had only a modest activity on Ba/F3-VEGFR-2 cells, whereas the C25A and C25L mutants had increased activity, with C25L being more than 2 orders of magnitude more active than VEGF-DΔNΔC. In the Ba/F3-VEGFR-3 cell assay, the activity of both the native and mutant VEGF-DΔNΔC proteins was low. However, the Cys-25 mutants were more active than the native VEGF-DΔNΔC, which was practically inactive over the concentration range that was used, similarly to the negative control sample (the C53A mutant). The most active mutant in the Ba/F3-VEGFR-3 cell assays was C25L, as in the case of the Ba/F3-VEGFR-2 cells.
FIGURE 6.
Determination of biological activity and receptor binding affinity of the purified recombinant VEGFs. Ba/F3-VEGFR-2 (A) and Ba/F3-VEGFR-3 (B) cell growth and survival assays were performed with purified recombinant VEGF proteins. C, the relative VEGFR-2 binding affinities of recombinant VEGF proteins were measured using a solid phase competition assay. Dilution series of the recombinant VEGF proteins were incubated with sVEGFR2-Fc recombinant protein on VEGF-A121-coated 96-well plates. The amount of bound sVEGFR2-Fc was quantified using an anti-human Fc-AP antibody, and the values are expressed as mean ± S.E. D, the relative VEGFR-3 binding affinities of recombinant VEGF proteins were measured using a competition assay. Experiments were performed as above but using VEGF-C-coated plates and sVEGFR3-Fc recombinant protein. E, the determined IC50 values of each protein in the receptor affinity assays. Sigmoidal dose response curves were fitted to measured data using a non-linear regression in Prism to determine the IC50 values of each protein.
The relative binding affinities of VEGF-A121, VEGF-DΔNΔC, C25A, C25L, and C53A to sVEGFR2-Fc and to sVEGFR3-Fc were characterized using competition binding assays (Fig. 6, C and D). As expected, C53A did not bind either receptor, and VEGF-A121 showed the highest affinity to sVEGFR2-Fc (IC50 = 6.32 × 10−10 m). Of the VEGF-DΔNΔC proteins, C25L had the highest affinity for sVEGFR2-Fc (IC50 = 2.84 × 10−09 m). In contrast to the results from the Ba/F3-VEGFR-2 assay (see above; Fig. 6A), the affinity of VEGF-DΔNΔC (IC50 = 1.37 × 10−08 m) to sVEGFR2-Fc was higher than that of the C25A mutant (IC50 = 3.95 × 10−08 m). Similar changes in sVEGFR3-Fc binding affinity were observed with VEGF-DΔNΔC (IC50 = 4.21 × 10−08 m), C25A (IC50 = 1.88 × 10−07 m), and C25L (IC50 = 1.22 × 10−08 m). This suggests that the observed increase in protein activity in cell growth and survival assays (Fig. 6, A and B) are not solely due to changes in receptor affinity.
Effect of the VEGF-DΔNΔC Mutants on Receptor Phosphorylation
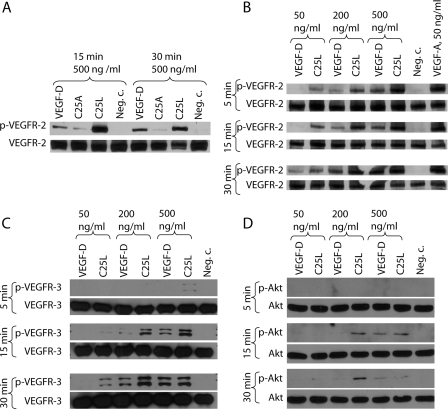
To further characterize the functions of the VEGF-DΔNΔC variants, we performed receptor phosphorylation assays on PAE cells stably expressing either VEGFR-2 (PAE-KDR) (31) or VEGFR-3 (PAE-Flt-4) (32). VEGF-DΔNΔC, C25A, and C25L all induced VEGFR-2 Tyr-1175 phosphorylation. In agreement with the affinity measurements, C25L was more effective but C25A was less effective compared with native VEGF-DΔNΔC (Fig. 7A). The C25L mutant was selected for a detailed comparison with VEGF-DΔNΔC and VEGF-A121 was used as a positive control (Fig. 7B). VEGF-A121 was found to induce VEGFR-2 Tyr-1175 phosphorylation efficiently at the used concentration of 50 ng/ml. Compared with VEGF-A, VEGF-DΔNΔC has previously been reported to induce VEGFR-2 phosphorylation only at higher concentrations and with slower receptor activation kinetics (40). In agreement with this, VEGF-A121 induced a strong receptor phosphorylation already at the 5-min time point, whereas VEGF-DΔNΔC-induced phosphorylation increased over time and was strongest at the 30-min time point. The C25L mutant induced VEGFR-2 phosphorylation already at lower concentrations than the native VEGF-DΔNΔC, and the concentration of 500 ng/ml gave an equal phosphorylation as 50 ng/ml VEGF-A121 at all time points.
FIGURE 7.
Phosphorylation of VEGFR-2, VEGFR-3, and Akt. Serum-starved cells were incubated with recombinant VEGF proteins, and the phosphorylation status of VEGFRs or Akt was analyzed from cell lysates. The results are representatives of replicate experiments yielding similar results. A, the effect of 500 ng/ml VEGF-DΔNΔC, C25A, and C25L on the phosphorylation of VEGFR-2 in PAE-KDR cells at 15- and 30-min time points. The phosphorylation of Tyr-1175 of VEGFR-2 was quantified from the cell lysates using immunoblotting with a specific antibody for VEGFR-2 phosphorylated at Tyr-1175 (Cell Signaling). Equal loading was confirmed using an antibody against total VEGFR-2 (Cell Signaling). B, the effect of VEGF-DΔNΔC, C25L, and VEGF-A121 on the phosphorylation of VEGFR-2 in PAE-KDR cells at 5-, 15-, and 30-min time points. C, the effect of VEGF-DΔNΔC and C25L on the phosphorylation of VEGFR-3 in PAE-Flt4 cells at 5-, 15-, and 30-min time points. Cell lysates were immunoprecipitated using an anti-VEGFR-3 (Abcam) antibody and analyzed by immunoblotting with an anti phosphotyrosine antibody (Upstate). Equal loading was confirmed using an anti-VEGFR-3 antibody (Abcam). D, the effect of VEGF-DΔNΔC and C25L on the phosphorylation of Akt in PAE-Flt4 cells at 5-, 15-, and 30-min time points. The phosphorylation of Akt was quantified from the cell lysates using immunoblotting with a specific antibody for Akt phosphorylated at Ser-473 (Cell Signaling). Equal loading was confirmed using an antibody against total Akt (Cell Signaling).
VEGFR-3 phosphorylation induced by VEGF-DΔNΔC or the C25L mutant was compared in PAE-Flt4 cells. As in the VEGFR-2 phosphorylation assay, the C25L mutant induced VEGFR-3 phosphorylation at slightly lower concentrations than the native protein (Fig. 7C). To further characterize the activation of the signaling pathway, we studied the activation of Akt which is a downstream signaling target of both VEGF-D receptors (41). Akt phosphorylation was detected in PAE-Flt-4 cells after stimulation using either VEGF-DΔNΔC or the C25L mutant (Fig. 7D).
Protein Stability Measurements
The stability of VEGF-DΔNΔC and the C25L mutant was compared in an assay measuring the amount of protein capable of binding to sVEGFR2-Fc after 0–48 h of incubation at 37 °C both in cell culture medium and Tris-buffered saline (Fig. 8A). The native VEGF-DΔNΔC rapidly lost its ability to bind sVEGFR2-Fc in both conditions, but this was clearly slower in Tris-buffered saline, whereas no change of activity was detected with the C25L mutant even at the 48-h time point in either condition. Immunoblot analysis of the samples confirmed that the actual polypeptide chains remained intact, and there was no unspecific binding to the test tubes. Furthermore, the dimer to monomer ratio of the protein remained unchanged, as VEGF-DΔNΔC was both monomeric and dimeric, and C25L was mainly dimeric (Fig. 8B).
FIGURE 8.
Stability of recombinant VEGF-DΔNΔC and its C25L mutant. A, insect cell -derived purified recombinant proteins were incubated both in cell culture medium or Tris-buffered saline from 0 to 48 h. VEGF-D proteins were captured onto anti-FLAG M2 mAb-coated 96-well plates, and the active proteins were detected using sVEGFR2-Fc recombinant protein and an anti-human Fc-AP antibody. A standard curve was generated for both proteins from serial dilutions of the 0-h sample. The amount of remaining active proteins is presented as the percentage of the active protein relative to the concentration in the 0-h sample. The values are expressed as the mean ± S.E. B, immunoblot of the samples from different time points separated under non-reducing conditions and using an anti-VEGF-D mAb. Dimeric (di) and monomer (mo) fractions are indicated. TBS, Tris-buffered saline.
In Vivo Studies of Vascular Permeability
The ability of the VEGF-A121, VEGF-DΔNΔC, and C25L recombinant proteins to induce vascular permeability in rabbit skin was evaluated using the Miles assay (33). Whereas 50 ng of VEGF-A121 was enough to induce detectable vascular permeability in this assay, the recombinant VEGF-DΔNΔC or C25L did not induce permeability even at a dose of 1000 ng (Fig. 9).
FIGURE 9.
Vascular permeability effects of the recombinant proteins in the skin of New Zealand White rabbits. The recombinant proteins were injected into the rabbit skin, and Evans Blue dye was administered intravenously. The rabbits were sacrificed after 30 min and perfused. The blue color indicates extravasation of proteins from the vasculature.
DISCUSSION
VEGF receptors are protein-tyrosine kinases that are activated by ligand-induced dimerization followed by phosphorylation of the intracellular kinase domains and subsequent activation of specific signaling pathways (42). The crystal structures of VEGF-A (22) and PlGF (23) in complex with VEGFR-1 have shown that the dimerization of these growth factors forms two identical receptor binding surfaces composed of both subunits, thus making dimerization essential for receptor binding. An alanine scanning study of VEGF-A has shown that the binding of VEGF-A to VEGFR-2 likely happens similarly (35). It is reasonable to assume that a corresponding mechanism where only a dimeric ligand can induce receptor dimerization is also responsible for the function of VEGF-D and other VEGFs.
Based on our model, the overall structure of the VEGF-D dimer is similar to the structurally known VEGFs. A unique feature in the VEGF-C and VEGF-D dimer interfaces is an additional cysteine residue (Cys-25), presumably located close to the conserved cysteines Cys-44 and Cys-53 (Fig. 3A). Recently, the pvf-1 gene from Caenorhabditis elegans has been shown to code for a VEGF/platelet-derived growth factor homolog, PVF-1, which binds to human VEGFR-1 and -2 and exists predominantly as a non-covalent dimer (36), similar to VEGF-CΔNΔC (8) and VEGF-DΔNΔC (20). The PVF-1 protein also has a homologous additional cysteine located at the dimer interface. Therefore, this cysteine residue may be as important a factor in maintaining these growth factors as non-covalently bound dimers. In agreement with this, the replacement of Cys-25 with several different amino acids increased the fraction of dimeric VEGF-D protein (Fig. 5A) and also the potency in cell survival and growth assays (Fig. 4). The aliphatic hydrophobic residues Ile, Leu, and Val replacing Cys-25 improved the activity of the proteins the most. The other mammalian VEGF family members, except VEGF-C, have either Leu or Val at the site equivalent to Cys-25 of VEGF-D (Fig. 1A), and therefore, the result is well in line with natural VEGFs. Furthermore, the VEGF-F (VR-1) has Arg and Leu at the positions corresponding to Arg-22 and Cys-25 in VEGF-D (Fig. 1A), and thus, it is logical for the VEGF-D C25L mutant to have a stable dimer interface.
The inactive C53A mutant was expressed as a stable protein. As it has no cysteine that could form the intersubunit disulfide bridge, it migrates as a monomer in SDS-PAGE. However, an intrasubunit disulfide bridge may have formed between the closely located Cys-25 and Cys-44 in the C53A mutant. It remains unclear whether this disulfide bridge could also occur in the native protein (Figs. 1B and 3A). The substitution of Cys-25 with Gly also led to inactivation of the VEGF-D (Fig. 4C). It may be that substitution of Cys-25 with substantially smaller Gly leads to a conformational change which disturbs receptor binding. The mutants that were expressed but had reduced VEGFR activation ability (C53A and C25G) (Figs. 4 and 6) may be potential tools for studying the effects of VEGF-D co-receptors, such as neuropilins (43) and integrins (44), on VEGF-D signaling, as it is possible that these point mutations do not affect co-receptor binding. For example, the neuropilin binding site of VEGF-D is reported to reside in the propeptides (43), and therefore, neuropilin binding is not likely affected.
The measured receptor binding affinities correlate well with their ability to induce phosphorylation of VEGFRs. However, these properties did not directly correlate with the potencies in the Ba/F3 bioassays. As a major difference, the affinity and phosphorylation assays measure the activity of the ligands in short time scale (minutes) compared with the Ba/F3 bioassays (48 h). The most likely explanation for the high activity of C25L is the increased stability (Fig. 8A) due to the elimination of the free thiol group, which facilitates formation of the intermolecular Cys-44–Cys-53 disulfide bond. There are examples of stabilization of proteins through introduction of a disulfide bond into their structures (45, 46). Similarly, mutation of free cysteine residues in human acidic fibroblast growth factor leads to increased half-life (47). However, to our knowledge, a similar mechanism where a third cysteine residue modulates the formation of a closely located disulfide bridge has not been previously described. It is probable that also the other Cys-25 mutant forms of VEGF-DΔNΔC inducing more sustained receptor activation in cell culture are more stable than the native form. However, some mutations lead to decreased receptor binding affinity as seen with the C25A mutant. This could be caused by conformational changes that are due to the substitution of the cysteine with amino acids that are unsuitable at this position. As free cysteines are quite hydrophobic (48), it is logical that the hydrophobic amino acids Ile, Leu, and Val, which have roughly similar size as C, are the most suitable replacements of Cys-25 at the VEGF-D dimer interface.
As the additional cysteine is evolutionarily conserved in VEGF-C and VEGF-D proteins of different species and in the PVF-1 protein of C. elegans, it is likely to be important for the function of these proteins. Both VEGF-C and VEGF-D are synthesized as dimeric proproteins (8, 20), which are cleaved by proteases to release the mature forms with highly increased affinity toward VEGFR-2 (49). In humans, VEGF-D is constitutively expressed in arteries (50), and therefore, it could be possible that the additional cysteine residue forms a control system that limits and modulates the activity of the highly angiogenic mature protein in vivo. Thus, the rate of exchange of the intersubunit disulfide bridges for intrasubunit disulfides, possibly in response to changes in extracellular redox environment, could be a regulatory mechanism, inherent to the function of VEGF-D and other members of the family that have the additional cysteine at the dimer interface, guiding the angiogenic activity to areas of need.
This stabilization of the VEGF-DΔNΔC protein by the Cys-25 mutations may also increase the half-life and activity of the protein in vivo and, consequently, lead to stronger angiogenic and lymphangiogenic responses. VEGF-DΔNΔC has been found to be a potent angiogenic growth factor when expressed in skeletal muscle (12), periadventitial space (51), or myocardium (13). Therefore, these improved VEGF-DΔNΔC variants are potential therapeutic agents for restoring compromised blood flow in conditions like myocardial ischemia and peripheral arterial disease by inducing angiogenesis in ischemic tissues. Therapy could be performed by introducing these agents into tissue either as recombinant proteins or using gene therapy vectors.
Increased vascular permeability and subsequent edema in tissues are side effects of VEGFs when used to induce therapeutic vascular formation (52). VEGF-DΔNΔC has previously been reported not to induce acute vascular permeability in skin (53) as opposed to VEGF-A and VEGF-C (8). Promisingly, also our most potent VEGF-DΔNΔC mutant, C25L, did not induce acute vascular permeability in rabbit skin. This is likely because the increased activity is, rather, due to the stabilization of the protein than major changes in its ability to trigger receptor activation.
Conclusions
We have studied the structural and functional properties of VEGF-D using homology modeling and site-directed mutagenesis. VEGF-D contains an additional cysteine residue, Cys-25, on its dimer interface when compared with the consensus sequence of VEGF family members. The mutation of Cys-25 to several other residues, preferably Ile, Leu, or Val, enhances the formation of disulfide-linked dimers. The formation of the interchain disulfides improves the activity of the VEGF-DΔNΔC mainly due to stabilization of the protein.
Acknowledgments
We thank Riikka Eisto, Joonas Malinen, Anneli Miettinen, and Tarja Taskinen for excellent technical assistance.
This work was supported by Ark Therapeutics Group Plc., the Academy of Finland, the ISB (National Graduate School in Informational and Structural Biology), the Foundation of Åbo Akademi (Centre of Excellence in Cell Stress), the Sigrid Jusélius Foundation, the Leducq Foundation, the Joe, Pentti, and Tor Borg Memorial Fund, the Finnish Cultural Foundation, and the Magnus Ehrnrooth Foundation.
- VEGF
- vascular endothelial growth factor
- PlGF
- placenta growth factor
- VEGFR
- VEGF receptor
- PAE cells
- porcine aortic endothelial cells
- sVEGFR2-Fc
- soluble VEGFR-2-human IgG Fc fragment fusion protein
- mAb
- monoclonal antibody
- AP
- alkaline phosphatase
- KDR
- kinase insert domain receptor.
REFERENCES
- 1.Byrne A. M., Bouchier-Hayes D. J., Harmey J. H. ( 2005) J. Cell Mol. Med. 9, 777– 794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tammela T., Enholm B., Alitalo K., Paavonen K. ( 2005) Cardiovasc. Res. 65, 550– 563 [DOI] [PubMed] [Google Scholar]
- 3.Lyttle D. J., Fraser K. M., Fleming S. B., Mercer A. A., Robinson A. J. ( 1994) J. Virol. 68, 84– 92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamazaki Y., Takani K., Atoda H., Morita T. ( 2003) J. Biol. Chem. 278, 51985– 51988 [DOI] [PubMed] [Google Scholar]
- 5.Holmes D. I., Zachary I. ( 2005) Genome Biol. 6, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibuya M., Claesson-Welsh L. ( 2006) Exp. Cell Res. 312, 549– 560 [DOI] [PubMed] [Google Scholar]
- 7.Terman B. I., Dougher-Vermazen M., Carrion M. E., Dimitrov D., Armellino D. C., Gospodarowicz D., Böhlen P. ( 1992) Biochem. Biophys. Res. Commun. 187, 1579– 1586 [DOI] [PubMed] [Google Scholar]
- 8.Joukov V., Sorsa T., Kumar V., Jeltsch M., Claesson-Welsh L., Cao Y., Saksela O., Kalkkinen N., Alitalo K. ( 1997) EMBO J. 16, 3898– 3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achen M. G., Jeltsch M., Kukk E., Mäkinen T., Vitali A., Wilks A. F., Alitalo K., Stacker S. A. ( 1998) Proc. Natl. Acad. Sci. U. S. A. 95, 548– 553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annex B. H., Simons M. ( 2005) Cardiovasc. Res. 65, 649– 655 [DOI] [PubMed] [Google Scholar]
- 11.Ylä-Herttuala S., Alitalo K. ( 2003) Nat. Med. 9, 694– 701 [DOI] [PubMed] [Google Scholar]
- 12.Rissanen T. T., Markkanen J. E., Gruchala M., Heikura T., Puranen A., Kettunen M. I., Kholová I., Kauppinen R. A., Achen M. G., Stacker S. A., Alitalo K., Ylä-Herttuala S. ( 2003) Circ. Res. 92, 1098– 1106 [DOI] [PubMed] [Google Scholar]
- 13.Rutanen J., Rissanen T. T., Markkanen J. E., Gruchala M., Silvennoinen P., Kivelä A., Hedman A., Hedman M., Heikura T., Ordén M. R., Stacker S. A., Achen M. G., Hartikainen J., Ylä-Herttuala S. ( 2004) Circulation 109, 1029– 1035 [DOI] [PubMed] [Google Scholar]
- 14.Murray-Rust J., McDonald N. Q., Blundell T. L., Hosang M., Oefner C., Winkler F., Bradshaw R. A. ( 1993) Structure 1, 153– 159 [DOI] [PubMed] [Google Scholar]
- 15.Muller Y. A., Christinger H. W., Keyt B. A., de Vos A. M. ( 1997) Structure 5, 1325– 1338 [DOI] [PubMed] [Google Scholar]
- 16.Iyer S., Leonidas D. D., Swaminathan G. J., Maglione D., Battisti M., Tucci M., Persico M. G., Acharya K. R. ( 2001) J. Biol. Chem. 276, 12153– 12161 [DOI] [PubMed] [Google Scholar]
- 17.Iyer S., Scotney P. D., Nash A. D., Ravi A. K. ( 2006) J. Mol. Biol. 359, 76– 85 [DOI] [PubMed] [Google Scholar]
- 18.Pieren M., Prota A. E., Ruch C., Kostrewa D., Wagner A., Biedermann K., Winkler F. K., Ballmer-Hofer K. ( 2006) J. Biol. Chem. 281, 19578– 19587 [DOI] [PubMed] [Google Scholar]
- 19.Suto K., Yamazaki Y., Morita T., Mizuno H. ( 2005) J. Biol. Chem. 280, 2126– 2131 [DOI] [PubMed] [Google Scholar]
- 20.Stacker S. A., Stenvers K., Caesar C., Vitali A., Domagala T., Nice E., Roufail S., Simpson R. J., Moritz R., Karpanen T., Alitalo K., Achen M. G. ( 1999) J. Biol. Chem. 274, 32127– 32136 [DOI] [PubMed] [Google Scholar]
- 21.Joukov V., Kumar V., Sorsa T., Arighi E., Weich H., Saksela O., Alitalo K. ( 1998) J. Biol. Chem. 273, 6599– 6602 [DOI] [PubMed] [Google Scholar]
- 22.Wiesmann C., Fuh G., Christinger H. W., Eigenbrot C., Wells J. A., de Vos A. M. ( 1997) Cell 91, 695– 704 [DOI] [PubMed] [Google Scholar]
- 23.Christinger H. W., Fuh G., de Vos A. M., Wiesmann C. ( 2004) J. Biol. Chem. 279, 10382– 10388 [DOI] [PubMed] [Google Scholar]
- 24.Lehtonen J. V., Still D. J., Rantanen V. V., Ekholm J., Björklund D., Iftikhar Z., Huhtala M., Repo S., Jussila A., Jaakkola J., Pentikäinen O., Nyrönen T., Salminen T., Gyllenberg M., Johnson M. S. ( 2004) J. Comput. Aided Mol. Des. 18, 401– 419 [DOI] [PubMed] [Google Scholar]
- 25.Eswar N., Webb B., Marti-Renom M. A., Madhusudhan M. S., Eramian D., Shen M. Y., Pieper U., Sali A. ( 2007) Curr. Protoc. Protein Sci., Chapter 2, Unit 2.9 [DOI] [PubMed] [Google Scholar]
- 26.Majumder K. ( 1992) Gene 110, 89– 94 [DOI] [PubMed] [Google Scholar]
- 27.Suzuki Y., Kagawa N., Fujino T., Sumiya T., Andoh T., Ishikawa K., Kimura R., Kemmochi K., Ohta T., Tanaka S. ( 2005) Nucleic Acids Res. 33, e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laitinen O. H., Airenne K. J., Hytönen V. P., Peltomaa E., Mähönen A. J., Wirth T., Lind M. M., Mäkelä K. A., Toivanen P. I., Schenkwein D., Heikura T., Nordlund H. R., Kulomaa M. S., Ylä-Herttuala S. ( 2005) Nucleic Acids Res. 33, e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Airenne K. J., Hiltunen M. O., Turunen M. P., Turunen A. M., Laitinen O. H., Kulomaa M. S., Ylä-Herttuala S. ( 2000) Gene Ther. 7, 1499– 1504 [DOI] [PubMed] [Google Scholar]
- 30.Achen M. G., Roufail S., Domagala T., Catimel B., Nice E. C., Geleick D. M., Murphy R., Scott A. M., Caesar C., Makinen T., Alitalo K., Stacker S. A. ( 2000) Eur. J. Biochem. 267, 2505– 2515 [DOI] [PubMed] [Google Scholar]
- 31.Waltenberger J., Claesson-Welsh L., Siegbahn A., Shibuya M., Heldin C. H. ( 1994) J. Biol. Chem. 269, 26988– 26995 [PubMed] [Google Scholar]
- 32.Pajusola K., Aprelikova O., Pelicci G., Weich H., Claesson-Welsh L., Alitalo K. ( 1994) Oncogene 9, 3545– 3555 [PubMed] [Google Scholar]
- 33.Miles A. A., Miles E. M. ( 1952) J. Physiol. (Lond.) 118, 228– 257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rissanen T. T., Markkanen J. E., Arve K., Rutanen J., Kettunen M. I., Vajanto I., Jauhiainen S., Cashion L., Gruchala M., Närvänen O., Taipale P., Kauppinen R. A., Rubanyi G. M., Ylä-Herttuala S. ( 2003) FASEB J. 17, 100– 102 [DOI] [PubMed] [Google Scholar]
- 35.Fuh G., Li B., Crowley C., Cunningham B., Wells J. A. ( 1998) J. Biol. Chem. 273, 11197– 11204 [DOI] [PubMed] [Google Scholar]
- 36.Tarsitano M., De Falco S., Colonna V., McGhee J. D., Persico M. G. ( 2006) FASEB J. 20, 227– 233 [DOI] [PubMed] [Google Scholar]
- 37.Airenne K. J., Peltomaa E., Hytönen V. P., Laitinen O. H., Ylä-Herttuala S. ( 2003) Nucleic Acids Res. 31, e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tischer E., Mitchell R., Hartman T., Silva M., Gospodarowicz D., Fiddes J. C., Abraham J. A. ( 1991) J. Biol. Chem. 266, 11947– 11954 [PubMed] [Google Scholar]
- 39.Soker S., Takashima S., Miao H. Q., Neufeld G., Klagsbrun M. ( 1998) Cell 92, 735– 745 [DOI] [PubMed] [Google Scholar]
- 40.Jia H., Bagherzadeh A., Bicknell R., Duchen M. R., Liu D., Zachary I. ( 2004) J. Biol. Chem. 279, 36148– 36157 [DOI] [PubMed] [Google Scholar]
- 41.Olsson A. K., Dimberg A., Kreuger J., Claesson-Welsh L. ( 2006) Nat. Rev. Mol. Cell Biol. 7, 359– 371 [DOI] [PubMed] [Google Scholar]
- 42.Schlessinger J. ( 2000) Cell 103, 211– 225 [DOI] [PubMed] [Google Scholar]
- 43.Kärpänen T., Heckman C. A., Keskitalo S., Jeltsch M., Ollila H., Neufeld G., Tamagnone L., Alitalo K. ( 2006) FASEB J. 20, 1462– 1472 [DOI] [PubMed] [Google Scholar]
- 44.Vlahakis N. E., Young B. A., Atakilit A., Sheppard D. ( 2005) J. Biol. Chem. 280, 4544– 4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagihara Y., Mine S., Uegaki K. ( 2007) J. Biol. Chem. 282, 36489– 36495 [DOI] [PubMed] [Google Scholar]
- 46.Bjørk A., Dalhus B., Mantzilas D., Eijsink V. G., Sirevåg R. ( 2003) J. Mol. Biol. 334, 811– 821 [DOI] [PubMed] [Google Scholar]
- 47.Culajay J. F., Blaber S. I., Khurana A., Blaber M. ( 2000) Biochemistry 39, 7153– 7158 [DOI] [PubMed] [Google Scholar]
- 48.Nagano N., Ota M., Nishikawa K. ( 1999) FEBS Lett. 458, 69– 71 [DOI] [PubMed] [Google Scholar]
- 49.McColl B. K., Paavonen K., Karnezis T., Harris N. C., Davydova N., Rothacker J., Nice E. C., Harder K. W., Roufail S., Hibbs M. L., Rogers P. A., Alitalo K., Stacker S. A., Achen M. G. ( 2007) FASEB J. 21, 1088– 1098 [DOI] [PubMed] [Google Scholar]
- 50.Rutanen J., Leppänen P., Tuomisto T. T., Rissanen T. T., Hiltunen M. O., Vajanto I., Niemi M., Häkkinen T., Karkola K., Stacker S. A., Achen M. G., Alitalo K., Ylä-Herttuala S. ( 2003) Cardiovasc. Res. 59, 971– 979 [DOI] [PubMed] [Google Scholar]
- 51.Bhardwaj S., Roy H., Gruchala M., Viita H., Kholova I., Kokina I., Achen M. G., Stacker S. A., Hedman M., Alitalo K., Ylä-Herttuala S. ( 2003) Hum. Gene Ther. 14, 1451– 1462 [DOI] [PubMed] [Google Scholar]
- 52.Vajanto I., Rissanen T. T., Rutanen J., Hiltunen M. O., Tuomisto T. T., Arve K., Närvänen O., Manninen H., Räsänen H., Hippeläinen M., Alhava E., Ylä-Herttuala S. ( 2002) J. Gene Med. 4, 371– 380 [DOI] [PubMed] [Google Scholar]
- 53.Stacker S. A., Vitali A., Caesar C., Domagala T., Groenen L. C., Nice E., Achen M. G., Wilks A. F. ( 1999) J. Biol. Chem. 274, 34884– 34892 [DOI] [PubMed] [Google Scholar]
- 54.Barton G. J. ( 1993) Protein Eng. 6, 37– 40 [DOI] [PubMed] [Google Scholar]
- 55.DeLano W. L. ( 2002) The PyMOL Molecular Graphics System, DeLano Scientific, Palo Alto, CA [Google Scholar]
- 56.Anisimov A., Alitalo A., Korpisalo P., Soronen J., Kaijalainen S., Leppänen V. M., Jeltsch M., Ylä-Herttuala S., Alitalo K. ( 2009) Circ. Res., in press [DOI] [PMC free article] [PubMed] [Google Scholar]