Abstract
mTOR (mammalian target of rapamycin) stimulates cell growth by phosphorylating and promoting activation of AGC (protein kinase A/protein kinase G/protein kinase C) family kinases such as Akt (protein kinase B), S6K (p70 ribosomal S6 kinase) and SGK (serum and glucocorticoid protein kinase). mTORC1 (mTOR complex-1) phosphorylates the hydrophobic motif of S6K, whereas mTORC2 phosphorylates the hydrophobic motif of Akt and SGK. In the present paper we describe the small molecule Ku-0063794, which inhibits both mTORC1 and mTORC2 with an IC50 of ∼10 nM, but does not suppress the activity of 76 other protein kinases or seven lipid kinases, including Class 1 PI3Ks (phosphoinositide 3-kinases) at 1000-fold higher concentrations. Ku-0063794 is cell permeant, suppresses activation and hydrophobic motif phosphorylation of Akt, S6K and SGK, but not RSK (ribosomal S6 kinase), an AGC kinase not regulated by mTOR. Ku-0063794 also inhibited phosphorylation of the T-loop Thr308 residue of Akt phosphorylated by PDK1 (3-phosphoinositide-dependent protein kinase-1). We interpret this as implying phosphorylation of Ser473 promotes phosphorylation of Thr308 and/or induces a conformational change that protects Thr308 from dephosphorylation. In contrast, Ku-0063794 does not affect Thr308 phosphorylation in fibroblasts lacking essential mTORC2 subunits, suggesting that signalling processes have adapted to enable Thr308 phosphorylation to occur in the absence of Ser473 phosphorylation. We found that Ku-0063794 induced a much greater dephosphorylation of the mTORC1 substrate 4E-BP1 (eukaryotic initiation factor 4E-binding protein 1) than rapamycin, even in mTORC2-deficient cells, suggesting a form of mTOR distinct from mTORC1, or mTORC2 phosphorylates 4E-BP1. Ku-0063794 also suppressed cell growth and induced a G1-cell-cycle arrest. Our results indicate that Ku-0063794 will be useful in delineating the physiological roles of mTOR and may have utility in treatment of cancers in which this pathway is inappropriately activated.
Keywords: Akt/protein kinase B (PKB), cancer, kinase inhibitor, phosphoinositide 3-kinase (PI3K), p70 ribosomal S6 kinase (S6K), serum and glucocorticoid protein kinase (SGK)
Abbreviations: AGC family, protein kinase A/protein kinase G/protein kinase C family; AMPK, AMP-activated protein kinase; DTT, dithiothreitol; eIF4E, eukaryotic initiation factor 4E; 4E-BP1, eIF4E-binding protein 1; ERK, extracellular-signal-regulated kinase; GST, glutathione transferase; HEK-293 cell, human embryonic kidney 293 cell; IGF, insulin-like growth factor; MAPK, mitogen-activated protein kinase; MEF, mouse embryonic fibroblast; mTOR, mammalian target of rapamycin; mTORC, mTOR complex; NDRG1, N-Myc downstream-regulated gene-1; PDK, 3-phosphoinositide-dependent protein kinase; PH domain, pleckstrin homology domain; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; PRAS40, proline-rich Akt substrate of 40 kDa; RSK, ribosomal S6 kinase; S6K, p70 ribosomal S6 kinase; SGK, serum and glucocorticoid protein kinase; SPHK, sphingosine kinase
INTRODUCTION
The mTOR (mammalian target of rapamycin) protein kinase lies at the nexus of signalling pathways that regulate cell growth and proliferation. Many cancer-driving mutations in genes such as receptor tyrosine kinases, Ras, PI3K (phosphoinositide 3-kinase) and PTEN (phosphatase and tensin homologue deleted on chromosome 10) stimulate proliferation, growth and survival by activating mTOR kinase. Two mTOR complexes have been characterized, termed mTORC1 (mTOR complex-1) and mTORC2 (reviewed in [1,2]). mTORC1 consists of mTOR, Raptor and mLST8 and is acutely inhibited by rapamycin [3–5]. mTORC1 is activated by insulin and growth factors via a PI3K-controlled pathway, involving Akt-mediated phosphorylation of PRAS40 (proline-rich Akt substrate of 40 kDa) and tuberous sclerosis complex-2 protein, and activation of the Rheb GTPase [6,7]. mTORC1 is also stimulated by amino acids through a pathway involving bidirectional transport of amino acids [8] and the Rag GTPase [9,10]. Low-energy conditions suppress cell growth by inhibiting mTORC1 via the LKB1–AMPK (AMP-activated protein kinase) pathway [11]. mTORC2 consists of mTOR, Rictor, Sin1, mLST8 as well as Protor and is insensitive to acute rapamycin treatment [12–18]. The activity of mTORC2 activity is controlled by PI3K, but is insensitive to amino acids or energy stress.
A key role of mTOR complexes is to phosphorylate and control the activity of a subgroup of related AGC (protein kinase A/protein kinase G/protein kinase C) family kinases that comprise isoforms of Akt, S6K (p70 ribosomal S6 kinase), PKC (protein kinase C) and SGK (serum and glucocorticoid protein kinase). mTOR complexes phosphorylate these enzymes at a conserved C-terminal non-catalytic residue, termed the ‘hydrophobic motif’. Activation of AGC kinases also requires phosphorylation of an additional site within their kinase catalytic domain termed the ‘T-loop motif’. This phosphorylation is executed by the PDK1 (3-phosphoinositide-dependent protein kinase-1) [19]. mTORC1 phosphorylates the hydrophobic motif of S6K [4,5], whereas mTORC2 phosphorylates the hydrophobic motifs of Akt [20], SGK [21] and certain isoforms of PKC [17,22]. In the case of S6K, SGK and PKC isoforms, hydrophobic motif phosphorylation creates a high-affinity docking site for PDK1, thereby promoting phosphorylation and activation of these enzymes by PDK1 [19,23]. In the case Akt, interaction of the PH (pleckstrin homology) domains in PDK1 and Akt with PtdIns(3,4,5)P3 (a product of PI3K activation), at the plasma membrane of cells, brings these enzymes together and induces a conformational change in Akt that enables it to be phosphorylated at Thr308 by PDK1 [24,25]. Recent data also indicates that mTORC2 controls the phosphorylation of Akt and PKC isoforms at a conserved phosphorylation site termed the ‘turn motif’ that stabilizes these enzymes in an active conformation [26,27]. mTORC1 also phosphorylates other substrates such as the eukaryotic translation initiation factor 4E-BP1 [eIF4E (eukaryotic initiation factor 4E)-binding protein 1]. Phosphorylation of 4E-BP1 by mTOR induces dissociation from eIF4E, promoting initiation of protein translation that is required to power cell growth and proliferation ([4,5],[28], but see [28a]). In the present study, we describe the characterization of Ku-0063794, a novel potent and highly specific small-molecule inhibitor of the mTOR protein kinase. We demonstrate that Ku-0063794 inhibits both mTORC1 and mTORC2 in vitro and in vivo and can be employed to dissect cellular functions of the mTOR pathway.
MATERIALS AND METHODS
Materials
Protein G–Sepharose and glutathione–Sepharose were purchased from Amersham Bioscience. [γ-32P]ATP was from PerkinElmer; IGF1 (insulin-like growth factor) was from Invitrogen. Tween 20, DMSO, PMA and dimethyl pimelimidate were from Sigma, and CHAPS and rapamycin were from Calbiochem. Akti-1/2, PI-103 and PD0325901-CL were synthesized by Dr Natalia Shpiro at the MRC Protein Phosphorylation Unit, University of Dundee. Ku-0063794 was synthesized at AstraZeneca. The wild-type control mLST8+/+ and mLST8−/− knockout MEFs (mouse embryonic fibroblasts) were described previously [17] and provided by David Sabatini (Whitehead Institute for Biomedical Research, Cambridge, MA, U.S.A.). The wild-type control Rictor+/+ and Rictor−/− knockout MEFs were described previously [29] and provided by Mark Magnuson (Vanderbilt University School of Medicine, Nashville, TN, U.S.A.). The wild-type control Sin1+/+ and Sin1−/− knockout MEFs were described previously [16] and provided by Bing Su (Yale University School of Medicine, New Haven, CT, U.S.A.).
Antibodies
The following antibodies were raised in sheep and affinity-purified on the appropriate antigen: anti-mLST8 (S837B, 3rd bleed) was raised against the human full-length mLST8 protein expressed in Escherichia coli (used for immunoblotting), anti-Raptor (S682B, 3rd bleed; residues 1–20 of human Raptor, MESEMLQSPLLGLGEEDEAD, used for immunoblotting and immunoprecipitation), anti-Rictor (S654B, 3rd bleed; residues 6–20 of human Rictor, RGRSLKNLRVRGRND, used for immunoblotting in HEK-293 (human embryonic kidney 293) cells and immunoprecipitation), anti-Rictor (S274C, 1st bleed; residues 6–20 of mouse Rictor, RGRSLKNLRIRGRND, used for immunoblotting), anti-Sin1 (S8C, 1st bleed) was raised against the human full-length Sin1 protein expressed in E. coli (used for immunoblotting). Anti-NDRG1 (N-Myc downstream-regulated gene-1) (S276B, 2nd bleed) was made in sheep using recombinant GST (glutathione transferase) fusion of full-length NDRG1 (used for immunoblotting). An antibody that recognizes NDRG1 phosphorylated at Thr346, Thr356 and Thr366 (S911B, 2nd bleed; termed pNDRG1 3×Thr-P) was raised against the nonapeptide RSRSHpTSEG, whose sequence is common to all three SGK1 phosphorylation sites on NDGR1 (used for immunoblotting). Anti-Akt1 (S695B, 3rd bleed; residues 466 – 480 of human Akt1 RPHFPQFSYSASGTA, used for immunoblotting and immunoprecipitation), anti-S6K (S417B, 2nd bleed; residues 25–44 of human S6K, AGVFDIDLDQPEDAGSEDEL, used for immunoblotting and immunoprecipitation). Anti-GST (S902A, 1st bleed) was raised against the GST tag expressed from pGex4T (used for immunoblotting). Anti-PRAS40 (S115B, 1st bleed; residues 238–256 of human PRAS40, DLPRPRLNTSDFQKLKRKY, used for immunoblotting). An antibody that recognizes PRAS40 phosphorylated at Thr246 (S114B, 2nd bleed) was raised against the residues 240–251 of human PRAS40, CRPRLNTpSDFQK (used for immunoblotting). Anti-RSK2 (ribosomal S6 kinase 2) (S382B, 1st bleed; residues 712–734 of human RNQSPVLEPVGRSTLAQRRGIKK, used for immunoblotting and immunoprecipitation); Anti-FoxO1 (S504A, 1st bleed) was made in sheep using recombinant GST-fusion FoxO1 (2–655, used for immunoblotting). Anti-β-tubulin (H-235, #sc-9104), phospho-RSK Thr227 (#sc-12445-R) and the total mTOR antibody (#sc-1549) were purchased from Santa Cruz Biotechnology. For phospho-immunoblotting of the hydrophobic motif of SGK1(Ser422), we employed the Thr389 S6K antibody (# 9205) from Cell Signaling Technology, which we previously demonstrated cross-reacted with the phosphorylated Ser422 of SGK1 [30]. The phospho-Akt Ser473 (#9271), Thr308 (#4056), Thr450 (#9267), phospho-S6K Thr389 (#9234), phospho S6 ribosomal protein Ser235 (#4856), total S6 ribosomal protein (#2217), 4E-BP1 total (#9452), phospho 4E-BP1 Thr37/Thr46 (#9459), phospho 4E-BP1 Ser65 (#9451), phospho-ERK (extracellular-signal-regulated kinase) Thr202/Tyr204 (#9101), total ERK (#9102), phospho-RSK Thr573 (#9346), phospho-RSK Thr380 (#9341), phospho-FoxO1/O3 Thr24/32 (#9464), phospho-GSK3α/β Ser21/Ser9 (#9331), the phospho-mTOR Ser2481 (#2974) and the phospho-mTOR Ser2448 (#2971) were purchased from Cell Signaling Technology. For phospho-immunoblotting of the phosphorylated T-loop of S6K, we employed the pan-PDK1 site antibody from Cell Signaling Technology (#9379) as previously described [31].The GSK3α/β antibody (44–610) was purchased from Biosource. The secondary antibodies coupled to horseradish peroxidase used for immunoblotting were obtained from Thermo Scientific.
General methods
Tissue culture, immunoblotting, restriction enzyme digests, DNA ligations and other recombinant DNA procedures were performed using standard protocols. DNA constructs used for transfection were purified from E. coli DH5α using Qiagen plasmid Mega or Maxi kits according to the manufacturer's protocol. All DNA constructs were verified by DNA sequencing, which was performed by The Sequencing Service, School of Life Sciences, University of Dundee, Dundee, U.K., using DYEnamic ET terminator chemistry (Amersham Biosciences) on Applied Biosystems automated DNA sequencers. For transfection studies, typically ten 10-cm-diameter dishes of HEK-293 cells were cultured, and each dish was transfected with 5–10 μg of the indicated plasmids using the polyethylenimine method [32]. Cellular levels of PtdIns(3,4,5)P3 were measured as described previously [33]. MEFs were cultured with additional non-essential amino acids and 1% sodium pyruvate solution.
Buffers
The following buffers were used: Tris lysis buffer [50 mM Tris/HCl, pH 7.5, 1 mM EGTA, 1 mM EDTA, 0.3% (w/v) CHAPS, 1 mM sodium orthovanadate, 10 mM sodium β-glycerophosphate, 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 0.27 M sucrose, 0.15 M NaCl, 0.1% (v/v) 2-mercaptoethanol, 1 mM benzamidine and 0.1 mM PMSF], Buffer A [50 mM Tris/HCl, pH 7.5, 0.1 mM EGTA and 0.1% (v/v) 2-mercaptoethanol], Hepes lysis buffer [40 mM Hepes, pH 7.5, 120 mM NaCl, 1 mM EDTA, 0.3% (w/v) CHAPS, 10 mM sodium pyrophosphate, 10 mM sodium β-glycerophosphate, 50 mM sodium fluoride, 0.5 mM sodium orthovanadate, 1 mM benzamidine and 0.1 mM PMSF], Hepes kinase buffer (25 mM Hepes, pH 7.5, 50 mM KCl and TBS-Tween Buffer [50 mM Tris/HCl, pH 7.5, 0.15 M NaCl and 0.1% (v/v) Tween 20] and Sample Buffer [50 mM Tris/HCl, pH 6.8, 6.5% (v/v) glycerol, 1% (w/v) SDS and 1% (v/v) 2-mercaptoethanol].
Cell lysis
HEK-293, HeLa (human cervical carcinoma) or MEF cells were cultured and treated as described in the Figure legends. Following treatment, cells were rapidly rinsed twice with ice-cold PBS and then lysed using Tris lysis buffer. Whole-cell lysates were centrifuged (18000 g at 4 °C for 20 min), supernatants were removed and stored in aliquots at −80 °C until required.
Specificity kinase panel
All assays were performed at The National Centre for Protein Kinase Profiling (http://www.kinase-screen.mrc.ac.uk/) as previously described [34]. Briefly, all assays were carried out robotically at room temperature (21 °C) and were linear with respect to time and enzyme concentration under the conditions used. Assays were performed for 30 min using Multidrop Micro reagent dispensers (Thermo Electron Corporation, Waltham, MA, U.S.A.) in a 96-well format. The abbreviations for each kinase are defined in legend to Table 1. The concentration of magnesium acetate in the assays was 10 mM and [γ-33P]ATP (∼800 c.p.m./pmol) was used at 5 μM for CK2α, DYRK3, EF2K, ERK1, ERK8, GSK3β, HIPK2, IGF1R, IRR, MARK3, MKK1, p38γ MAPK (mitogen-activated protein kinase), p38δ MAPK, PAK4, PIM2, Akt1, PLK1, PKCζ and PRK2; 20 μM for CaMKKβ, CDK2/cyclin A, CHK1, CHK2, CK1δ, CSK, EPH-B3, FGF-R1, IR, JNK1α1, JNK2α2, MAPKAP-K2, MSK1, MST2, MST4, p38β MAPK, PKA, PAK5, PAK6, PDK1, PIM1, PIM3, PKCα, ROCKII, PRAK, S6K1, SGK1, SYK, VEGFR and YES1; or 50 μM for AMPK, BRSK2, BTK, CaMK1, DYRK1a, DYRK2, EPH-A2, ERK2, IKKε, LCK, MELK, NEK2A, NEK6, p38α, PhKγ1, Akt2, PKD1, RSK1, RSK2, SRPK1 and TBK1, in order to be at or below the Km for ATP for each enzyme [34].
Table 1. Effect of Ku-0063794 on 76 protein kinases.
Results are presented as percentage of kinase activity compared with control incubations, in which Ku-0063794 was omitted. Protein kinases were assayed as described in the Materials and methods section. The results are an average of a triplicate determination±S.D. Asterisks indicate inhibition of >2-fold. Abbreviations not defined in main text: BRSK, brain-specific kinase; BTK, Bruton's tyrosine kinase; CaMK1, calmodulin-dependent kinase; CaMKK, CaMK kinase; CDK, cyclin-dependent kinase; CHK, checkpoint kinase; CK1, casein kinase 1; CSK, C-terminal Src kinase; DYRK, dual-specificity tyrosine-phosphorylated and regulated kinase; EF2K, elongation-factor-2 kinase; EPH, ephrin; FGF-R, fibroblast growth factor receptor; GSK3, glycogen synthase kinase 3; HIPK, homeodomain-interacting protein kinase; IGF1R, IGF1 receptor; IKK, inhibitory κB kinase; IR, insulin receptor; IRR, insulin-related receptor; JNK, c-Jun N-terminal kinase; Lck, lymphocyte cell-specific protein tyrosine kinase; MAPKAP-K, MAPK-activated protein kinase; MARK, microtubule-affinity-regulating kinase; MELK, maternal embryonic leucine-zipper kinase; MKK1, MAPK kinase-1; MLCK, smooth muscle myosin light-chain kinase; MNK, MAPK-integrating protein kinase; MSK, mitogen- and stress-activated protein kinase; MST, mammalian homologue Ste20-like kinase; NEK, NIMA (never in mitosis in Aspergillus nidulans)-related kinase; PAK, p21-activated protein kinase; PHK, phosphorylase kinase; PIM, provirus integration site for Moloney murine leukaemia virus; PKA, cAMP-dependent protein kinase; PKD, protein kinase D; PLK, polo-like kinase; PRAK, p38-regulated activated kinase; PRK, protein kinase C-related kinase; ROCK, Rho-dependent protein kinase; SRPK, serine-arginine protein kinase; SYK, spleen tyrosine kinase; TBK1, TANK-binding kinase 1; VEGFR, vascular endothelial growth factor receptor; YES1, Yamaguchi sarcoma viral oncogene homologue 1. n.d., not determined.
| Percentage of activity remaining | Percentage of activity remaining | ||||
|---|---|---|---|---|---|
| Kinase | Ku-0063794 (1 μM) | Ku-0063794 (10 μM) | Kinase | Ku-0063794 (1 μM) | Ku-0063794 (10 μM) |
| Akt1 | 98±8 | 90±1 | MLCK | 82±4 | 104±3 |
| Akt2 | 97±8 | 107±9 | MNK1 | 111±0 | 75±12 |
| AMPK | 97±1 | 106±3 | MNK2α | 87±5 | 92±1 |
| Aurora B | n.d. | 86±6 | MSK1 | 101±6 | 117±7 |
| BRSK2 | 90±1 | 72±7 | MST2 | 81±10 | 102±3 |
| BTK | 85±7 | 81±21 | MST4 | 87±5 | 86±8 |
| CAMK1 | 96±1 | 101±6 | NEK2A | 92±10 | 86±2 |
| CAMKKβ | 108±5 | 94±0 | NEK6 | 74±4 | 83±10 |
| CDK2/Cyclin A | 89±5 | 90±6 | p38α MAPK | 101±6 | 96±5 |
| CHK1 | 87±8 | 86±7 | p38β MAPK | 106±7 | 83±5 |
| CHK2 | 97±2 | 96±9 | p38γ MAPK | 89±8 | 91±1 |
| CK1δ | 112±15 | 109±12 | p38δ MAPK | 72±1 | 87±4 |
| CK2α | 104±0 | 85±12 | PAK4 | 110±1 | 75±4 |
| CSK | 90±1 | 99±3 | PAK5 | 101±1 | 81±3 |
| DYRK1a | 94±10 | 80±7 | PAK6 | 75±3 | 100±24 |
| DYRK2 | 92±1 | 96±12 | PDK1 | 89±5 | 91±2 |
| DYRK3 | 88±9 | 84±1 | PhKγ1 | 89±2 | 116±3 |
| EFK2 | 88±7 | 108±0 | PIM1 | 89±4 | 98±15 |
| EPH-A2 | 108±8 | 96±2 | PIM2 | 86±11 | 78±0 |
| EPH-B3 | 100±8 | 78±4 | PIM3 | 114±12 | 75±7 |
| ERK1 | 102±0 | 87±1 | PKA | 115±7 | 85±12 |
| ERK2 | 102±8 | 92±9 | PKCα | 106±13 | 86±18 |
| ERK8 | 88±1 | 92±5 | PKCζ | 87±9 | 84±0 |
| FGF-R1 | 102±8 | 87±10 | PKD1 | 80±4 | 101±3 |
| GSK3β | 95±11 | 91±5 | PLK1 | 76±2 | 94±2 |
| HIPK2 | 89±5 | 89±1 | PRAK | 67±4 | 87±8 |
| IGF1R | 117±0 | 89±1 | PRK2 | 95±5 | 105±12 |
| IKKβ | 104±3 | 89±3 | ROCKII | 75±4 | 86±5 |
| IKKε | 99±1 | 100±5 | RSK1 | 100±4 | 86±2 |
| IR | 81±1 | 67±5 | RSK2 | 103±7 | 104±1 |
| IRR | 105±7 | 71±4 | S6K1 | 104±11 | 98±4 |
| JNK1α1 | 80±5 | 93±4 | SGK1 | 76±6 | 64±6 |
| JNK2α2 | 101±4 | 103±13 | Src | n.d. | 86±5 |
| LCK | 69±5 | 104±9 | SRPK1 | 101±1 | 94±2 |
| MAPKAP-K2 | 98±3 | 81±10 | SYK | 88±3 | 83±5 |
| MARK3 | 93±6 | 93±15 | TBK1 | 97±8 | 89±4 |
| MELK | 91±6 | 72±4 | VEGFR | 92±8 | 92±8 |
| MKK1 | 96±4 | 43±7* | YES1 | 94±10 | 90±1 |
Lipid kinase panel
SPHK1 (sphingosine kinase 1) was assayed as follows: SPHK1 [diluted in 50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM EGTA and 1 mM DTT (dithiothreitol)] was assayed against sphingosine in a final volume of 50 μl containing 50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM EGTA, 10 μM sphingosine, 10 μM ATP and 1 mM DTT and incubated for 30 min at room temperature.
SPHK2 was assayed as follows: SPHK2 (diluted in 50 mM Tris/HCl, pH 7.5, 200 mM KCl, 5 mM MgCl2, 1 mM EGTA and 1 mM DTT) was assayed against sphingosine in a final volume of 50 μl containing 50 mM Tris/HCl, pH 7.5, 200 mM KCl, 5 mM MgCl2, 1 mM EGTA, 10 μM sphingosine, 1 μM ATP and 1 mM DTT and incubated for 30 min at room temperature.
Choline kinase was assayed as follows: choline kinase (diluted in 25 mM glycine/NaOH, pH 8.5, 67 mM KCl and 5 mM MgCl2) was assayed against choline in a final volume of 50 μl containing 25 mM glycine/NaOH, pH 8.5, 67 mM KCl, 5 mM MgCl2, 1 mM choline, 1 μM ATP and 1 mM DTT and incubated for 30 min at room temperature. These three assays were stopped by addition of 50 μl Kinase Glo Plus Reagent, incubated for 10 min at room temperature and read for 1 s/well.
Class 1 PI3Kα was assayed as follows: PI3Kα (diluted in 50 mM Hepes, pH 7.5, 150 mM NaCl, 0.02% sodium cholate and 1 mM DTT) was assayed against PtdIns(4,5)P2 diC8 in a final volume of 50 μl containing 37 mM Hepes, pH 7.5, 111 mM NaCl, 0.02% sodium cholate, 5 mM DTT, 5 mM MgCl2, 1 mM ATP and 2 μM PtdIns(4,5)P2 and incubated for 70 min at room temperature. Assays were stopped by addition of a 5.5 μl solution of 50 mM EDTA and 0.02% (w/v) sodium cholate. An aliquot (25 μl) of the resultant mixture was transferred to a Lumitrac 200 plate. Detection mix 1 [41 mM Hepes, pH 7.5, 123 mM NaCl, 1.7 μg GST–GRP1 (guanine-nucleotide-releasing protein 1), 0.16 μM PtdIns(3,4,5)P3–biotin, 1.6 μg streptavidin–allophycocyanin and 0.96 μg/ml europium-chelatelabelled antibody] was added to give a final volume of 50 μl and incubated for 20 min at room temperature before reading.
Class 1 PI3Kβ was assayed as follows: PI3Kβ (diluted in 50 mM Hepes, pH 7.5, 150 mM NaCl, 0.02% sodium cholate and 1 mM DTT) was assayed against PtdIns(4,5)P2–diC8 in a final volume of 50 μl containing 37 mM Hepes, pH 7.5, 111 mM NaCl, 0.02% sodium cholate, 5 mM DTT, 5 mM MgCl2, 1 mM ATP and 2 μM PtdIns(4,5)P2 and incubated for 70 min at room temperature. Assays were stopped by addition of 5.5 μl 50 mM EDTA and 25 μl assay mixture was transferred to Lumitrac 200 plate. Detection mix 1 was added to give a final volume of 50 μl and the mixture was incubated for 20 min at room temperature before reading.
Class 2 PI3KC2β was assayed as follows: PI3KC2β (diluted in 20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.5 mM EGTA and 0.02% CHAPS) was assayed against phosphoinositide substrate in a final volume of 50 μl containing 19 mM Tris/HCl, pH 7.5, 143 mM NaCl, 0.96 mM EDTA, 0.96 mM DTT, 0.48 mM EGTA, 0.02% CHAPS, 20 μM phosphatidylinositol, 0.2 mM ATP and 2 mM MgCl2 and incubated for 30 min at room temperature. Assays were stopped by addition of 5.5 μl 50 mM EDTA and 2% CHAPS, and 25 μl was transferred to Lumitrac 200 plate. Detection mix 2 (18.6 mM Tris/HCl, pH 7.5, 140 mM NaCl, 0.9 mM DTT, 0.02% CHAPS, 1.4 μg SGK PX, 0.06 μM PtdIns3P–biotin, 1.6 μg streptavidin–allophycocyanin and 0.64 μg/ml Eu chelate-labelled antibody) was added to give a final volume of 50 ml and incubated for 30 min at room temperature before reading.
Class 3 VPS34 was assayed as follows: VPS34 (diluted in 20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.5 mM EGTA and 0.02% CHAPS) was assayed against phosphoinositide substrate in a final volume of 50 μl containing 19 mM Tris/HCl, pH 7.5, 143 mM NaCl, 0.96 mM EDTA, 0.96 mM DTT, 0.48 mM EGTA, 0.02% CHAPS, 20 μM phosphatidylinositol, 0.2 mM ATP and 2 mM MnCl2 and incubated for 60 min at room temperature. Assays were stopped by addition of 5.5 μl 50 mM EDTA and 2% CHAPS and 25 μl was transferred to a Lumitrac 200 plate. Detection mix 2 was added to give a final volume of 50 ml and incubated for 30 min at room temperature before reading.
mTOR complexes kinase assays
HEK-293 cells were freshly lysed in Hepes lysis buffer. Lysate (1–4 mg) was pre-cleared by incubating with 5–20 μl of Protein G–Sepharose conjugated to pre-immune IgG. The lysate extracts were then incubated with 5–20 μl of Protein G–Sepharose conjugated to 5–20 μg of either anti-Rictor or anti-Raptor antibody, or pre-immune IgG. All antibodies were covalently conjugated to Protein G–Sepharose. Immunoprecipitations were carried out for 1 h at 4 °C on a vibrating platform. The immunoprecipitates were washed four times with Hepes lysis buffer, followed by two washes with Hepes kinase buffer. For Raptor immunoprecipitates used for phosphorylating S6K1, for the initial two wash steps the buffer included 0.5 M NaCl to ensure optimal kinase activity [7]. GST–Akt1 was isolated from serum-deprived HEK-293 cells incubated with PI-103 (1 μM for 1 h) [24]. GST–S6K1 was purified from serum-deprived HEK-293 cells incubated with rapamycin (0.1 μM for 1 h) [25]. mTOR reactions were initiated by adding 0.1 mM ATP and 10 mM MgCl2 in the presence or absence of Ku-0063794 and GST–Akt1 (0.5 μg) or GST–S6K1 (0.5 μg). Reaction were carried out for 30 min at 30 °C on a vibrating platform and stopped by addition of SDS sample buffer. Reaction mixtures were then filtered through a 0.22-μm-pore-size Spin-X filter and samples were subjected to electrophoresis and immunoblot analysis.
Kinase assays
HEK-293 were lysed in Tris lysis buffer. In order to perform Akt and S6K assays, 500 μg of lysate was incubated with 5 μg of the corresponding antibody conjugated to Protein G–Sepharose. To perform SGK1 activity assays, 50 μg of transfected lysate was incubated with 5 μg of glutathione–Sepharose. All the incubations were performed for 1 h at 4 °C on a vibrating platform. Kinase activity was assayed exactly as described previously [35] using the Crosstide peptide (GRPRTSSFAEG) at 30 μM. Incorporation of [32P]phosphate into the peptide substrate was determined by applying the reaction mixture to P81 phosphocellulose paper and liquid-scintillation counting of radioactivity after washing the papers in phosphoric acid. One unit of activity was defined as that which catalysed the incorporation of 1 nmol of [32P]phosphate into the substrate.
GST-pull down of transfected SGK1 for immunoblotting analysis
At 36 h post-transfection, HEK-293 cells were lysed in Tris lysis buffer. Lysate (0.5 mg) was affinity-purified on glutathione–Sepharose. Incubations were carried out for 1 h at 4 °C on a vibrating platform. The resultant precipitates were then washed twice with Tris lysis buffer containing 0.5 M NaCl, followed by two washes with Buffer A. The immunoprecipitates were resuspended in 20 μl of sample buffer, filtered through a 0.22-μmpore-size Spin-X filter, and samples were subjected to electrophoresis and immunoblot analysis as described below.
Immunoblotting
Total cell lysate (5–20 μg) or immunoprecipitated samples were heated at 95 °C for 5 min in sample buffer, and subjected to PAGE and electrotransfer to nitrocellulose membrane. Membranes were blocked for 1 h in TBS-Tween buffer containing 10% (w/v) non-fat dried skimmed milk powder. The membranes were probed with the indicated antibodies in TBS-Tween containing 5% (w/v) skimmed milk or 5% (w/v) BSA for 16 h at 4 °C. Detection was performed using horseradish peroxidase-conjugated secondary antibodies and the enhanced chemiluminescence reagent.
Cell growth
MEFs were seeded in 24-well plates (20000 cells per 1.91 cm2 well) and grown overnight in the presence of 10% foetal bovine serum. Cells were then treated in the presence or absence or rapamycin or Ku-0063794 and the medium was changed every 24 h with freshly dissolved inhibitors. Every 24 h of treatment, cells were washed once with PBS, and fixed in 4% (v/v) paraformaldehyde in PBS for 15 min. After washing once with water, the cells were stained with 0.1% Crystal Violet in 10% ethanol for 20 min and washed three times with water. Crystal Violet was extracted from cells with 0.5 ml of 10% (v/v) ethanoic (acetic) acid for 20 min. The eluate was then diluted 1:10 in water, and absorbance at 590 nm was quantified.
Flow cytometric analysis of cell cycle distribution
Adherent MEFs were harvested by trypsinization, washed once in PBS, and re-suspended in ice-cold aq. 70% (v/v) ethanol. Cells were washed twice in PBS plus 1% (w/v) BSA and stained for 20 min in PBS plus 0.1% (v/v) Triton X-100 containing 50 g/ml propidium iodide and 50 g/ml RNase A. The DNA content of cells was determined using a FACSCalibur flow cytometer (BD Biosciences) and CellQuest software. Red fluorescence (585±42 nm) was acquired on a linear scale, and pulse width analysis was used to exclude doublets. Cell-cycle distribution was determined using FlowJo software (Tree Star).
RESULTS
Ku-0063794 is a specific mTORC1 and mTORC2 inhibitor
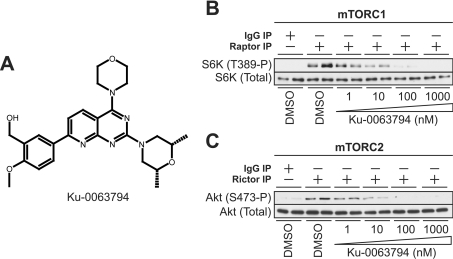
The structure of Ku-0063794 is shown in Figure 1(A). It inhibited the activity of endogenous immunoprecipitated mTORC1, assayed employing S6K1 as substrate (Figure 1B) and mTORC2, assayed using Akt as substrate (Figure 1C) with an IC50 of ∼10 nM. To evaluate the specificity of Ku-0063794, we studied the effect of Ku-0063794 at ATP concentrations, which approximate to the Km constant for ATP, towards a panel of 76 protein kinases. At 1 μM Ku-0063794, which completely suppressed mTORC1 and mTORC2 activity (Figures 1B and 1C), none of the kinases on the specificity panel were significantly inhibited (Table 1). Even at 10 μM, the only enzyme on the panel that was inhibited more than 2-fold was MAPK kinase-1, which was decreased ∼55% (Table 1). Ku-0063794, even at concentrations of 10 μM, did not significantly inhibit seven lipid kinases tested (Class I PI3Kα and PI3Kβ, Class II PI3K-B, Class III VPS34 as well as SPHK-1, SPHK-2 and choline kinase) (Table 2).
Figure 1. Ku-0063794 inhibits both mTORC1 and mTORC2 complexes in vitro.
(A) Structure of Ku-0063794. (B and C) HEK-293 cell extracts were subjected to immunoprecipitation (IP) with pre-immune (IgG), anti-Raptor (B) or anti-Rictor antibody (C). The immunoprecipitates were incubated with dephosphorylated GST–S6K1 (B) or GST–Akt1 (C) in the presence of the indicated concentrations of Ku-0063794 or vehicle (DMSO). Kinases assays were performed for 30 min in the presence of MgATP and then subjected to immunoblot analysis with the indicated antibodies. Similar results were obtained in three independent experiments. T389-P, phosphorylated Thr389; S473-P, phosphorylated Ser473.
Table 2. Effect of Ku-0063794 on seven lipid kinases.
Results are presented as percentage of lipid kinase activity compared with control incubations in which Ku-0063794 was omitted. Lipid kinases were assayed as described in the Materials and methods section, in the absence or presence of the indicated concentration of Ku-0063794. The results are an average of a triplicate determinations±S.D.
| Percentage of activity remaining | ||
|---|---|---|
| Kinase | Ku-0063794 (1 μM) | Ku-0063794 (10 μM) |
| PI3Ks | ||
| Class I | ||
| PI3Kα | 97±2 | 95±3 |
| PI3Kβ | 95±9 | 84±22 |
| Class II | ||
| PI3KC2β | 126±13 | 82±12 |
| Class III | ||
| VPS34 | 101±4 | 93±1 |
| Other lipid kinases | ||
| Choline kinase | 102±1 | 102±1 |
| SPHK1 | 108±3 | 99±7 |
| SPHK2 | 119±2 | 116±3 |
Ku-0063794 inhibits mTORC1 activity in HEK-293 cells
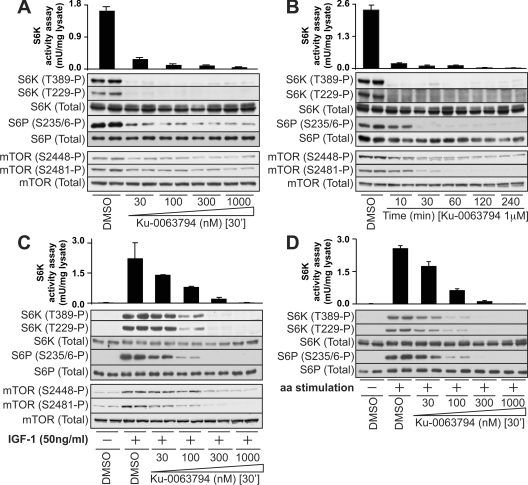
We next investigated the effect of adding increasing amounts of Ku-0063794 on the activity and phosphorylation of S6K1 in HEK-293 cells cultured in the presence of serum (Figure 2A). Under these conditions, with a concentration of Ku-0063794 as low as 30 nM, Ku-0063794 almost ablated S6K1 activity and phosphorylation of the hydrophobic motif (Thr389). Consistent with phosphorylation of the T-loop of S6K1 being dependent upon prior phosphorylation of the hydrophobic motif, Ku-0063794 inhibited phosphorylation of the T-loop residue (Thr229). Ku-0063794 also inhibited phosphorylation of the ribosomal S6 protein, an S6K substrate (Figure 2A). The ability of Ku-0063794 to suppress S6K1 activity and phosphorylation was rapid, with maximal inhibition seen within 10 min and sustained for at least 4 h, the longest time examined (Figure 2B). Ku-0063794 also suppressed S6K1 activity and phosphorylation induced by IGF1 stimulation of serum-starved HEK-293 cells, although higher concentrations were required to fully inhibit S6K1, compared with cells cultured in serum (compare Figure 2A with Figure 2C). For example, in the presence of serum, 30 nM Ku-0063794 suppressed S6K1 activity by ∼90%, whereas following IGF1 stimulation, 30 nM Ku-0063794 suppressed S6K1 activity by ∼50%. In the presence of IGF1, a concentration of 300 nM Ku-0063794 was necessary to reduce S6K1 activity ∼90%. mTORC1, and hence S6K1, is also activated by nutrients such as amino acids. We observed that Ku-0063794 induced a dose-dependent inhibition of the phosphorylation of S6K1 and S6 protein induced following the replenishment of amino acids to HEK-293 cells incubated in medium lacking these nutrients (Figure 2D). Concentrations of 100–300 nM Ku-0063794 were required to fully suppress amino-acid-induced phosphorylation of S6K1 and S6 protein (Figure 2D).
Figure 2. Ku-0063794 inhibits mTORC1 activity in vivo.
(A and B) HEK-293 cells cultured in the presence of 10% foetal bovine serum, were treated for 30 min in the absence or presence of the indicated concentrations of Ku-0063794 (A) or with 1 μM Ku-0063794 for the indicated time (B). Cells were lysed and S6K1 immunoprecipitated and catalytic activity assessed employing the Crosstide substrate. Each bar represents the mean specific activity±S.E.M. from three different samples, with each sample assayed in duplicate. Cell lysates were also analysed by immunoblotting with the indicated antibodies. (C) As in (A), except that cells were deprived of serum for 16 h prior to stimulation with 50 ng/ml of IGF-1 for 20 min. (D) As in (C), except that, after 16 h of serum deprivation, cells were incubated for 1 h in amino-acid-free EBSS (Earle's Balanced Salt Solution) medium containing 10% dialysed serum. Cells were then incubated in the absence or presence or the indicated amount of Ku-0063794 for 30 min prior to re-addition of physiological levels of amino acids for an additional 30 min. Immunoblots are representative of three different experiments.
Recent work has shown that mTORC1 is phosphorylated at Ser2448 [36], a reaction probably mediated by S6K1 [37,38]. In contrast, mTORC2 autophosphorylates at Ser2481 [36]. We observed that Ku-0063795 suppressed phosphorylation of both Ser2448 and Ser2481 in a dose-dependent and time-dependent manner, similar to S6K1 (lower panels of Figures 2A–2C).
Ku-0063794 inhibits mTORC2 activity in HEK-293 cells
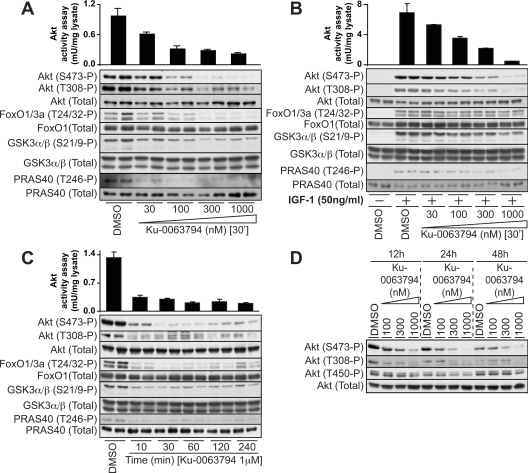
To investigate whether Ku-0063794 inhibited mTORC2, we studied how it affected Akt activity and hydrophobic motif (Ser473) phosphorylation in HEK-293 cells. In the presence of serum (Figure 3A) or following IGF1 stimulation (Figure 3B), Ku-0063794 caused a dose-dependent suppression of Akt activity, accompanied by inhibition of Ser473 phosphorylation. As observed with S6K1, the activity and phosphorylation of Akt was inhibited by lower concentrations of Ku-0063794 when cells were cultured in serum compared with stimulation with IGF1 (compare Figure 3A with Figure 3B). IGF1 induces a ∼6-fold greater activation of Akt than serum (compare Figure 3A with Figure 3B), which is likely to account for the higher concentrations of Ku-0063794 required to inhibit Akt and S6K in IGF1-stimulated cells compared with serum. The effects of Ku-0063794 on Akt activity and phosphorylation were observed within 10 min and were sustained for up to 48 h (Figures 3C and 3D). Phosphorylation of the Akt substrates PRAS40 at Thr246 [39], GSK3α/GSK3β at Ser21/Ser9 [40] and Foxo-1/3a at Thr24/Thr32 [41] was also inhibited to a similar extent by Ku-0063794 as Akt activity and phosphorylation in cells cultured in serum (Figure 3A). In cells stimulated with IGF1, which induced a greater activation of Akt than observed with serum, even concentrations of 1 μM Ku-0063794 did not inhibit Akt phosphorylation and activation completely (Figure 3B). At 1 μM Ku-0063794 in IGF-1 stimulated cells, Akt1 activity was reduced to ∼0.5 units/mg, which is ∼50% of the Akt activity observed with cells cultured in serum (Figure 3A). This may explain why phosphorylation of GSK3 and Foxo are not completely suppressed with 1 μM Ku-00637954 in IGF1-stimulated compared with serum-treated cells (compare Figure 3A with Figure 3B).
Figure 3. Ku-0063794 ablates mTORC2 in vivo.
HEK-293 cells were cultured in serum, and stimulated with IGF-1 in the absence or presence of Ku-0063794 as described in the legend to Figure 2. Akt1 was immunoprecipitated and catalytic activity assessed employing the Crosstide substrate. Each bar represents the mean specific activity±S.E.M. from three different samples, with each sample assayed in duplicate. Cell lysates were analysed by immunoblotting with the indicated antibodies. Immunoblots are representative of three different experiments. In (D), medium containing freshly dissolved Ku-0063794 was replaced every 12 h. P, phosphorylated.
We also studied the effect that Ku-0063794 had on the turn-motif Thr450 residue of Akt1, whose phosphorylation is regulated by mTORC2 [26,27]. Even after 48 h treatment with 1 μM Ku-0063794, phosphorylation of Akt at Thr450 was only moderately reduced (Figure 3D). This is consistent with phosphorylation of Thr450 being introduced in Akt shortly after its synthesis and turning over very slowly [26,27].
Ku-0063794 inhibits phosphorylation of Akt at Thr308 in HEK-293 cells
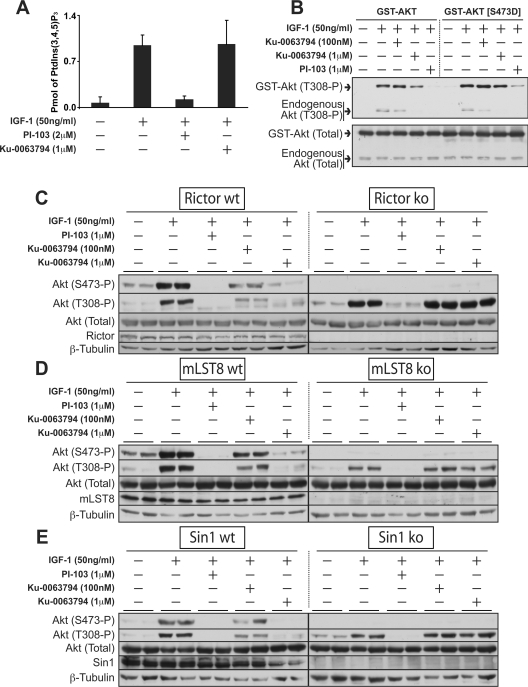
We observed that, in addition to inhibiting Akt Ser473 phosphorylation, Ku-0063794 suppressed phosphorylation of Akt at Thr308 (Figures 3A–3D). This was unexpected, as some previous work suggested that the phosphorylation of Thr308 by PDK1 is not directly influenced by mTORC2. Ku-0063794 was not suppressing Thr308 phosphorylation by inhibiting PI3K or PtdIns(3,4,5)P3 production, as IGF1 induced a similar enhancement of PtdIns(3,4,5)P3 levels in the absence or presence of 1 μM Ku-0063794, under conditions which the PI3K inhibitor PI-103 ablated IGF1-induced PtdIns(3,4,5)P3 production (Figure 4A). To further evaluate the effect that Ku-0063794 had on Thr308 phosphorylation, we overexpressed in HEK-293 cells a mutant form of GST–Akt1 in which Ser473 was changed to aspartate in an attempt to mimic the Akt-phosphorylated form of mTORC2 and tested how Ku-0063794 affected phosphorylation at Thr308. We found that IGF1-induced phosphorylation of GST–Akt1(S473D) at Thr308 was not suppressed by Ku-0063794 to the same extent as endogenous Akt in these cells, suggesting that Ku-0063794 is not directly inhibiting PDK1-mediated phosphorylation of Thr308 (Figure 4B). In parallel studies, Ku-0063794 also suppressed Thr308 phosphorylation of wild-type GST–Akt1 to a greater extent than GST–Akt1(S473D) (Figure 4B). The dual PI3K and mTOR inhibitor PI-103 inhibited phosphorylation of Thr308 on both GST–Akt1(S473D) and wild-type Akt1 (Figure 4B). We also examined the effect that Ku-0063794 had on the phosphorylation of Akt at Thr308 in MEFs lacking the critical mTORC2 subunits Rictor [29] (Figure 4C), mLST8 [17] (Figure 4D) and Sin1 [16] (Figure 4E). Consistent with previous work, IGF1 induced phosphorylation of Thr308, but not Ser473, in mTORC2-deficient cells (Figures 4C–4E). However, Ku-0063794 did not inhibit phosphorylation of Thr308 in any of the mTORC2-deficient cells under conditions where it suppressed Thr308 phosphorylation in control wild-type cells (Figures 4C–4E). Treatment of mTORC2-deficient cells with PI-103 abolished phosphorylation of Thr308.
Figure 4. Further investigation of the effect of Ku-0063794 on Akt Thr308 phosphorylation.
(A) HEK-293 cells were deprived of serum for 16 h, treated for 30 min in the absence or presence of the indicated concentrations of Ku-0063794 or PI-103, then stimulated with 50 ng/ml of IGF-1 for 10 min. Cell were lysed with 0.5 M trichloroacetic acid and amounts of PtdIns(3,4,5)P3 were determined. Each bar represents the means±S.E.M. from three independent samples. (B) HEK-293 cells were transfected with a DNA construct encoding wild-type or mutant GST–Akt1. At 24 h post-transfection, cells were deprived of serum for 16 h and stimulated with IGF-1 for 20 min, in the presence or absence of inhibitors as described in (A). Cell lysates were analysed by immunoblotting with the indicated antibodies and immunoblots shown are representative of three separate experiments. (C–E) The indicated wild-type (wt) or knockout (ko) MEFs were deprived and stimulated with IGF-1 for 20 min, in the presence or absence of inhibitors as described in (A). Cell lysates were analysed by immunoblotting with the indicated antibodies. Similar results were obtained in three independent experiments. P, phosphorylated.
Ku-0063794 inhibits SGK1 but not RSK
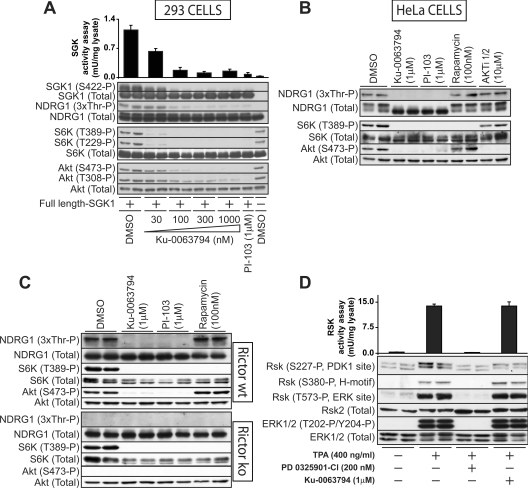
We have shown that SGK1 activity and phosphorylation of its hydrophobic motif (Ser422) is regulated by mTORC2 but not mTORC1 [21]. This finding has recently been supported by elegant genetic studies in Caenorhabditis elegans [42,43]. SGK1 activity and hydrophobic motif phosphorylation should therefore be inhibited by Ku-0063794, but not rapamycin. As it is not possible to assay phosphorylation and activity of endogenous SGK1 [21], we investigated how Ku-0063794 affected activity and phosphorylation of overexpressed full-length SGK1 in HEK-293 cells cultured in the presence of serum (Figure 5A). We observed that Ku-0063794 inhibited SGK1 activity and Ser422 phosphorylation in a dose-dependent manner, to the same extent as Akt and S6K1 phosphorylation (Figure 5A). We also examined the phosphorylation of NDRG1, a well-characterized substrate for SGK1, which is not phosphorylated in SGK1 knockout mouse tissues [44]. Employing an antibody recognizing the SGK1-phosphorylated form of NDRG1, we found that Ku-0063794 inhibited NDRG1 phosphorylation to the same extent as it suppressed SGK1 activity. Phosphorylation of endogenous NDRG1 was also inhibited by Ku-0063794 in HeLa (Figure 5B) cells as well as wild-type MEFs (Figure 5C). The dual PI3K/mTOR inhibitor PI-103, but not rapamycin or the Akt inhibitor Akti-1/2 [45], reduced phosphorylation of NDRG1 in both HeLa cells and wild-type MEFs.
Figure 5. Ku-0063794 suppresses hydrophobic motif phosphorylation and activation of SGK1 but not RSK.
(A) HEK-293 cells were transfected with a DNA construct encoding GST–SGK1 (full-length enzyme). Cells were cultured in the presence of 10% foetal bovine serum in order to maintain PI3K pathway activity. At 36 h post-transfection, cells were treated for 30 min in the absence or presence of the indicated concentrations of Ku-0063794 or PI-103. Cells were lysed, SGK1 was affinity-purified on glutathione–Sepharose and catalytic activity was assessed employing the Crosstide substrate. Each bar represents the mean specific activity±S.E.M. from three different samples, with each sample assayed in duplicate. Affinity purified SGK1 was also subjected to immunoblotting with an anti-GST antibody (SGK1-Total) as well as an anti-Ser422 phosphospecific antibody (S422-P). Cell lysates were also analysed by immunoblotting with the indicated non-SGK antibodies. (B and C) HeLa cells or the indicated wild-type (wt) or knockout (ko) MEFs were cultured in the presence of 10% serum, then treated for 30 min in the absence or presence of the indicated concentrations of inhibitors. Cells were lysed and extracts were analysed by immunoblotting with the indicated antibodies. Immunoblots are representative of three different experiments. (D) HEK-293 cells were deprived of serum for 16 h, treated for 30 min in the absence or presence of 1 μM Ku-0063794 or 0.2 μM PD 0325901 then stimulated with 400 ng/ml of PMA for 15 min. RSK was immunoprecipitated with an antibody recognizing all isoforms and catalytic activity assessed employing the Crosstide substrate. Each bar represents the mean specific activity±S.E.M. from two different samples, with each sample assayed in duplicate. Cell lysates were also analysed by immunoblotting with the indicated antibodies. P, phosphorylated.
To ensure Ku-0063794 was not inhibiting the phosphorylation and activity of all AGC kinases, we studied the effect that Ku-0063794 had on the activation of the RSK, which is activated by ERK1/ERK2 pathway and not regulated by mTOR. HEK-293 cells were stimulated with phorbol ester, which markedly enhanced ERK as well as RSK phosphorylation and activity (Figure 5D). Ku-0063794 did not inhibit phorbol ester-induced ERK or RSK phosphorylation and RSK activation under conditions in which the MAPK kinase inhibitor PD 0325901-CL [46] ablated ERK phosphorylation and RSK activation (Figure 5D).
Ku-0063794 induces complete dephosphorylation of 4E-BP1
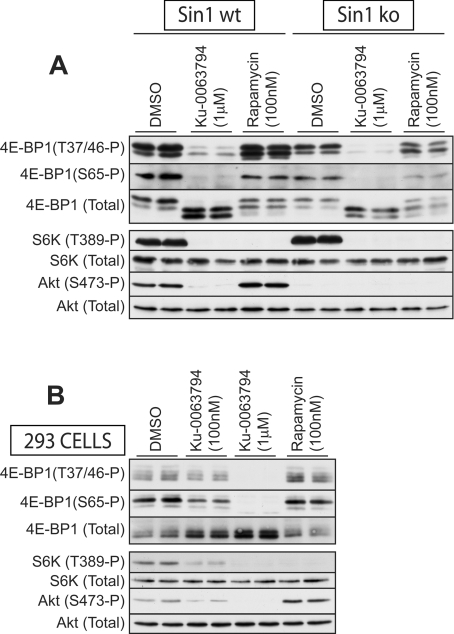
Consistent with mTOR regulating 4E-BP1 phosphorylation, we found that treatment of wild-type MEFs (Figure 6A) or HEK-293 cells (Figure 6B) with Ku-0063794 induced a marked increase in the electrophoretic mobility of 4E-BP1, which was accompanied by complete dephosphorylation of Thr37, Thr46 and Ser65. We observed that rapamycin reduced phosphorylation of 4E-BP1 and enhanced electrophoretic mobility to a significantly lower extent than Ku-0063794, even in mTORC2-deficient Sin1 knockout MEFs (Figures 6A and 6B).
Figure 6. Ku-0063794 induces a marked dephosphorylation of 4E-BP1.
(A and B) The indicated wild-type (wt) or knockout (ko) MEFs (A) or HEK-293 cells (B) were cultured in the presence of 10% serum, and treated for 30 min in the absence or presence of the indicated concentrations of inhibitors. Cells were lysed and extracts analysed by immunoblotting with the indicated antibodies. Immunoblots are representative of three different experiments. P, phosphorylated.
Ku-0063794 suppresses cell growth and induces G1-cell cycle arrest
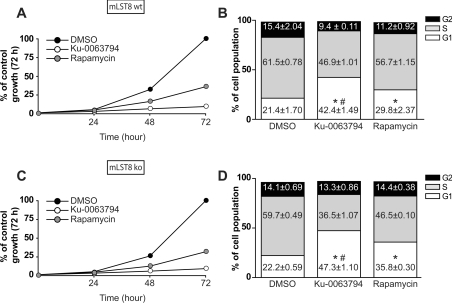
We compared the effect that Ku-0063794 and rapamycin had on growth of wild-type and mLST8 deficient MEFs. We selected these cells, as despite lacking mTORC2, they proliferate at similar rates in serum as wild-type control fibroblasts. Moreover, mLST8-fibroblast cell lines proliferate more rapidly than Rictor- or Sin1-deficient MEFs (results not shown). We observed that Ku-0063794 suppressed growth of both wild-type (Figure 7A) and mLST8-deficient (Figure 7C) MEFs more markedly than rapamycin. Flow-cytometric analysis indicated that Ku-0063794 increased the proportion of wild-type (Figure 7B) and mLST8 knockout MEFs (Figure 7D) in the G1 cell cycle state approx. 2-fold, compared with rapamycin, which increased the proportion of cells in G1 by 1.5-fold.
Figure 7. Ku-0063794 inhibits cell growth and induces a G1 cell cycle arrest.
The indicated wild-type (wt) or knockout (ko) MEFs were passaged at low density (20000 cells per 1.91 cm2 dish) and cells were cultured for 1, 2 or 3 days in the absence (DMSO) or presence of 3 μM Ku-0063794 or 100 nM rapamycin. (A and C) Cell numbers were measured in quadruplicate using a Crystal Violet staining as described in the Materials and methods section. The results are presented as means±S.E.M. of the percentage of cells relative to the DMSO control. Flow cytometric analysis was also undertaken as described in the Materials and methods section. (B and D) DNA was stained with propidium iodide, and cellular content was analysed. The percentage of cells in G1, S, or G2 phases were determined from triplicate dishes for each condition using CellQuest software. Two-way ANOVA and Bonferroni posttests were performed, *P<0.001 compared with DMSO control. #P<0.001 compared with rapamycin.
DISCUSSION
Our results from the present study establish that Ku-0063794 is a potent and highly specific inhibitor of mTOR as it does not significantly inhibit 76 other protein kinases tested as well as seven lipid kinases, including class 1a PI3Kα and PI3Kβ. mTOR belongs to the PI3K-related kinases (PIKK) subgroup, as it possesses significant similarity to PI3Ks [47]. Many widely used PI3K inhibitors, including wortmannin [48], LY294002 [48], PI-103 [49] and NVP-BEZ235 [50], inhibit mTOR with a potency similar to that towards Class 1 PI3Ks. Recently, specific PI3K inhibitors have been described that do not inhibit mTOR, such as GDC-0941 [51]. Our results from the present study demonstrate that Ku-0063794 does not inhibit PI3K in vivo, as Ku-0063794 does not suppress increases in cellular levels of PtdIns(3,4,5)P3 following IGF-1 stimulation of HEK-293 cells (Figure 4A). Moreover, Ku-0063794 does not inhibit Akt Thr308 phosphorylation in three different mTORC2-deficient MEF cell lines (Figures 4C–4E) under conditions which PI-103 inhibited PtdIns(3,4,5)P3 production and Thr308 phosphorylation.
Two other mTOR specific inhibitors that do not inhibit PI3K have recently been described, namely PP242 [52] and Torin [53]. Although the chemical structure of Torin has thus far not been disclosed, the chemical structures of PP242 and Ku-0063794 are quite distinct. We have synthesized PP242 and compared its specificity with Ku-0063794 and observed that it is less selective. At 1 μM PP242, 22 of the 76 kinases tested were inhibited by >50%, whereas at 10 μM PP242, 46 out of the 76 kinases were inhibited (results available upon request from D.R.A.). In contrast, 10 μM Ku-0063794 only suppressed 1 out of 76 kinases over 2-fold (Table 1). Torin was reported to be highly selective [53]. The availability of chemically unrelated mTOR inhibitors will be useful in confirming specificity of physiological responses observed when using these reagents. Our findings using Ku-0063794 are similar to those reported for the PP242 [52] and Torin [53], as all three inhibitors reduced hydrophobic motif as well as T-loop phosphorylation of endogenous S6K as well as Akt. We also demonstrated Ku-0063794, but not rapamycin or Akti-1/2, inhibited phosphorylation and activation of SGK1 as well as its physiological substrate NDGR1. This is consistent with our findings that SGK1 is regulated by mTORC2 and not the mTORC1 inhibitor rapamycin (reviewed in [54]). We would expect Torin and PP242 to also inhibit SGK1 activation and phosphorylation.
The finding that Ku-0063794 as well as Torin [53] and PP242 [52] inhibited Akt Thr308 phosphorylation is arguably unexpected, as previous work suggested that Thr308 and Ser473 phosphorylation can occur independently from each other. The initial evidence for this was that an Akt-T308A mutant overexpressed in IGF1-stimulated HEK-293 cells was still phosphorylated at Ser473 and vice versa [55]. Moreover, in cells lacking mTORC2 activity, Akt is still phosphorylated at Thr308 despite not being phosphorylated at Ser473 [16,17,29]. In PDK1-deficient cells [56] and in mouse tissues lacking PDK1 [57,58], Akt is still phosphorylated at Ser473, but not at Thr308. One explanation for the effect that mTOR inhibitors such as Ku-0063794 have on Akt Thr308 phosphorylation would be if Akt phosphorylation of Ser473 generated a docking site for PDK1 to interact and phosphorylate Thr308, in the same way in which phosphorylation of the hydrophobic motifs of S6K/SGK by mTOR promotes T-loop phosphorylation of these enzymes by PDK1. The evidence against this stems from previous studies undertaken in knockin cells [59,60] and in mouse tissues [61] that express a PIF-pocket mutant form of PDK1 that is unable to interact with the hydrophobic motifs of S6K and SGK1. In these knockin studies, it was demonstrated that Akt was normally phosphorylated at Thr308, under conditions which S6K and SGK are not phosphorylated at their T-loop residue, suggesting that Akt Thr308 phosphorylation was not dependent on PDK1 interacting with Akt via its PIF-pocket. How can the effects on Thr308 phosphorylation in mTOR inhibitor and knockin/knockout studies be reconciled? One possibility is that in wild-type cells, phosphorylation of Akt at Ser473 does indeed promote PDK1 interaction and contributes towards maximal phosphorylation of Thr308 by PDK1. However, in knockout cells permanently lacking mTORC2 activity or in PDK1 PIF-pocket mutant knockin cells, Akt activation may be so essential for survival that signalling networks adapt so that Akt can be phosphorylated by PDK1 at Thr308 independently of Ser473 phosphorylation. Evidence to support the idea that PDK1 might interact with Ser473 after its phosphorylation by mTORC2 might stem from recent observations made in PDK1-PH domain knockin mice that express a form of PDK1 that can not interact with PtdIns(3,4,5)P3. In tissues or cells derived from these animals, Akt is still phosphorylated at Thr308, albeit to ∼3-fold lower level than in wild-type tissues or cells [62]. This observation was unexpected, as previous evidence indicated that PDK1 was needed to interact with phosphoinositides at the plasma membrane in order to phosphorylate Akt at Thr308 [19]. However, if phosphorylation at Ser473 by mTORC2 does stimulate interaction of PDK1 with Akt, this may bypass the need for PDK1 to interact with PtdIns(3,4,5)P3 and enhance phosphorylation of Thr308 without the need of PDK1 binding to phosphoinositides. In future work, it would important to investigate the effect on Akt Thr308 phosphorylation of allosteric PIF-pocket PDK1 inhibitors being elaborated [63,64] or mTORC2-specific inhibitors (if such compounds could be developed).
Another possibility to account for the effect of mTOR inhibitors on Akt Thr308 phosphorylation is if the lack of Akt Ser473 phosphorylation leads to destabilization of Akt, promoting dephosphorylation of Thr308 by protein phosphatase(s). Indeed, this idea is supported by structural analysis of Akt2, where it has been demonstrated that hydrophobic motif phosphorylation induces a marked disorder-to-order transition of the kinase domain, resulting in stabilization of the alphaC helix with concomitant restructuring of the T-loop and reconfiguration of the structure of the catalytic domain [65]. Treatment of cells with Ku-0063794 will inhibit Akt Ser473 phosphorylation and may lead to less stable Akt conformation, with Thr308 being more exposed and accessible to becoming dephosphorylated by protein phosphatase(s). It would be interesting to investigate whether in mTORC2-deficient knockout cells, in which Thr308 phosphorylation is unaffected, the activity of the protein phosphatase(s) that act on Thr308 is reduced, or if Akt is stabilized in some way that enables it to function without Ser473 phosphorylation.
Ku-0063794 (Figure 6), as well as Torin [53] and PP242 [52], induce a much greater dephosphorylation of 4E-BP1 than rapamycin, suggesting that these drugs will more effectively suppress protein synthesis resulting from overactivation of the PI3K/Akt pathways. Both mTORC1 and mTORC2 activity would be inhibited in mTORC2-deficient cells treated with rapamycin. However, we still observed significant phosphorylation of 4E-BP1 at Thr37/Thr46 and Ser65 in Sin-1 knockout MEFs treated with rapamycin, under conditions in which the hydrophobic motif of both S6K and Akt are not phosphorylated (in agreement with lack of mTORC1 and mTORC2 activity). In contrast, treatment of Sin-1 knockout MEFs with Ku-0063794 led to a much greater dephosphorylation of 4E-BP1 than observed with rapamycin. Similar results were also found with the Torin [53] and PP242 [52] inhibitors. Taken together, these results indicate that rapamycin may not inhibit the phosphorylation of 4E-BP1 by mTORC1 and/or there may be a rapamycin-resistant form of mTOR, distinct from mTORC2. The existence of such a complex might also explain why Ku-0063794 inhibits proliferation of mTORC2-deficient mLST8 knockout cells more effectively than rapamycin (Figures 7C–7D). It would be interesting to see whether it might be possible to isolate a form of mTOR capable of phosphorylating 4E-BP1, which was inhibited by Ku-0063794, but not by rapamycin.
There is great expectation that inhibitors of the PI3K-PDK1-Akt-mTOR signalling axis will have utility as anti-cancer drugs, as this signalling network is inappropriately activated in the majority of human cancers. Rapamycin and its analogues are being extensively deployed for the treatment of various cancers [66]. It will be interesting to evaluate whether an inhibitor such as Ku-0063794, which targets both mTOR complexes, is a more effective anti-cancer agent than rapamycin. This might be expected, as mTOR inhibitors such as Ku-0063794, unlike rapamycin derivatives, will inhibit Akt and SGK isoforms that are likely to play vital roles in driving the proliferation of many cancers. The finding that mTOR inhibitors such as Ku-0063794 induce more marked dephosphorylation of 4E-BP1 than rapamycin also holds promise that these drugs will be more effective at suppressing protein synthesis required for growth and proliferation of cancer cells than rapamycin derivatives. Ku-0063794 and other mTOR inhibitors will also be very useful reagents to dissect and validate the molecular roles that mTOR plays and hence extend our repertoire of knowledge of how this master regulator of cell growth operates.
AUTHOR CONTRIBUTION
Juan M. García-Martínez, Sabina C. Cosulich, Christine M. Chresta and Dario R. Alessi planned and analysed experimental data. Juan M. García-Martínez undertook most of the experimentation. Jennifer Moran undertook kinase profiling, Rosemary G. Clarke undertook FACS analysis and Alex Gray measured PtdIns(3,4,5)P3 levels. Sabina C. Cosulich and Christine M. Chresta were involved in the development of Ku-0063794. Juan M. García-Martínez and Dario R. Alessi wrote the manuscript.
ACKNOWLEDGEMENTS
We thank Mark Magnuson, David Sabatini, and Bing Su for provision of reagents. We are also grateful to the Sequencing Service for DNA sequencing, the Post Genomics and Molecular Interactions Centre for Mass Spectrometry facilities (School of Life Sciences, University of Dundee, Dundee, Scotland, U.K.) and the protein production and antibody purification teams [Division of Signal Transduction Therapy (DSTT), University of Dundee, Dundee, Scotland, U.K.] co-ordinated by Hilary McLauchlan and James Hastie for expression and purification of antibodies.
FUNDING
J. M. G. M. is funded by a grant from AstraZeneca. We also thank the Medical Research Council for financial support.
References
- 1.Wullschleger S., Loewith R., Hall M. N. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Sarbassov D. D., Ali S. M., Sabatini D. M. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J. L., Bonenfant D., Oppliger W., Jenoe P., Hall M. N. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 4.Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., Tokunaga C., Avruch J., Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 5.Kim D. H., Sarbassov D. D., Ali S. M., King J. E., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 6.Hay N., Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 7.Sancak Y., Thoreen C. C., Peterson T. R., Lindquist R. A., Kang S. A., Spooner E., Carr S. A., Sabatini D. M. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Nicklin P., Bergman P., Zhang B., Triantafellow E., Wang H., Nyfeler B., Yang H., Hild M., Kung C., Wilson C., et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sancak Y., Peterson T. R., Shaul Y. D., Lindquist R. A., Thoreen C. C., Bar-Peled L., Sabatini D. M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim E., Goraksha-Hicks P., Li L., Neufeld T. P., Guan K. L. Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alessi D. R., Sakamoto K., Bayascas J. R. LKB1-dependent signaling pathways. Annu. Rev. Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 12.Jacinto E., Loewith R., Schmidt A., Lin S., Ruegg M. A., Hall A., Hall M. N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 13.Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q., Inoki K., Ikenoue T., Guan K. L. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frias M. A., Thoreen C. C., Jaffe J. D., Schroder W., Sculley T., Carr S. A., Sabatini D. M. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S. Y., Huang Q., Qin J., Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 17.Guertin D. A., Stevens D. M., Thoreen C. C., Burds A. A., Kalaany N. Y., Moffat J., Brown M., Fitzgerald K. J., Sabatini D. M. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev. Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Pearce L. R., Huang X., Boudeau J., Pawlowski R., Wullschleger S., Deak M., Ibrahim A. F., Gourlay R., Magnuson M. A., Alessi D. R. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem. J. 2007;405:513–522. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mora A., Komander D., Van Aalten D. M., Alessi D. R. PDK1, the master regulator of AGC kinase signal transduction. Semin. Cell Dev. Biol. 2004;15:161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Martinez J. M., Alessi D. R. mTOR complex-2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum and glucocorticoid induced protein kinase-1 (SGK1) Biochem. J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 22.Parekh D., Ziegler W., Yonezawa K., Hara K., Parker P. J. Mammalian TOR controls one of two kinase pathways acting upon nPKCδ and nPKCε. J. Biol. Chem. 1999;274:34758–34764. doi: 10.1074/jbc.274.49.34758. [DOI] [PubMed] [Google Scholar]
- 23.Biondi R. M. Phosphoinositide-dependent protein kinase 1, a sensor of protein conformation. Trends Biochem. Sci. 2004;29:136–142. doi: 10.1016/j.tibs.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Alessi D. R., Deak M., Casamayor A., Caudwell F. B., Morrice N., Norman D. G., Gaffney P., Reese C. B., MacDougall C. N., Harbison D., et al. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 25.Alessi D. R., Kozlowski M. T., Weng Q. P., Morrice N., Avruch J. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr. Biol. 1998;8:69–81. doi: 10.1016/s0960-9822(98)70037-5. [DOI] [PubMed] [Google Scholar]
- 26.Facchinetti V., Ouyang W., Wei H., Soto N., Lazorchak A., Gould C., Lowry C., Newton A. C., Mao Y., Miao R. Q., et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–1943. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikenoue T., Inoki K., Yang Q., Zhou X., Guan K. L. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hara K., Yonezawa K., Weng Q. P., Kozlowski M. T., Belham C., Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 28a.Erratum J. Biol. Chem. 1998;273:22160. [Google Scholar]
- 29.Shiota C., Woo J. T., Lindner J., Shelton K. D., Magnuson M. A. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev. Cell. 2006;11:583–589. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Lizcano J. M., Deak M., Morrice N., Kieloch A., Hastie C. J., Dong L., Schutkowski M., Reimer U., Alessi D. R. Molecular basis for the substrate specificity of NIMA-related kinase-6 (NEK6). Evidence that NEK6 does not phosphorylate the hydrophobic motif of ribosomal S6 protein kinase and serum- and glucocorticoid-induced protein kinase in vivo. J. Biol. Chem. 2002;277:27839–27849. doi: 10.1074/jbc.M202042200. [DOI] [PubMed] [Google Scholar]
- 31.Collins B. J., Deak M., Murray-Tait V., Alessi D. R. in vivo role of the phosphate-binding groove of PDK1 defined by knockin mutation. J. Cell Sci. 2005;118:5023–5034. doi: 10.1242/jcs.02617. [DOI] [PubMed] [Google Scholar]
- 32.Durocher Y., Perret S., Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30:E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray A., Olsson H., Batty I. H., Priganica L., Peter Downes C. Nonradioactive methods for the assay of phosphoinositide 3-kinases and phosphoinositide phosphatases and selective detection of signaling lipids in cell and tissue extracts. Anal. Biochem. 2003;313:234–245. doi: 10.1016/s0003-2697(02)00607-3. [DOI] [PubMed] [Google Scholar]
- 34.Bain J., Plater L., Elliott M., Shpiro N., Hastie C. J., McLauchlan H., Klevernic I., Arthur J. S., Alessi D. R., Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi T., Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3- phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem. J. 1999;339:319–328. [PMC free article] [PubMed] [Google Scholar]
- 36.Copp J., Manning G., Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–1827. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiang G. G., Abraham R. T. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J. Biol. Chem. 2005;280:25485–25490. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- 38.Holz M. K., Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J. Biol. Chem. 2005;280:26089–26093. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]
- 39.Kovacina K. S., Park G. Y., Bae S. S., Guzzetta A. W., Schaefer E., Birnbaum M. J., Roth R. A. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J. Biol. Chem. 2003;278:10189–10194. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- 40.Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 41.Brunet A., Bonni A., Zigmond M. J., Lin M. Z., Juo P., Hu L. S., Anderson M. J., Arden K. C., Blenis J., Greenberg M. E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 42.Jones K. T., Greer E. R., Pearce D., Ashrafi K. Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1. PLoS Biol. 2009;7:e60. doi: 10.1371/journal.pbio.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soukas A. A., Kane E. A., Carr C. E., Melo J. A., Ruvkun G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009;23:496–511. doi: 10.1101/gad.1775409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray J. T., Campbell D. G., Morrice N., Auld G. C., Shpiro N., Marquez R., Peggie M., Bain J., Bloomberg G. B., Grahammer F., et al. Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem. J. 2004;384:477–488. doi: 10.1042/BJ20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green C. J., Goransson O., Kular G. S., Leslie N. R., Gray A., Alessi D. R., Sakamoto K., Hundal H. S. Use of Akt inhibitor and a drug-resistant mutant validates a critical role for protein kinase B/Akt in the insulin-dependent regulation of glucose and system A amino acid uptake. J. Biol. Chem. 2008;283:27653–27667. doi: 10.1074/jbc.M802623200. [DOI] [PubMed] [Google Scholar]
- 46.Barrett S. D., Bridges A. J., Dudley D. T., Saltiel A. R., Fergus J. H., Flamme C. M., Delaney A. M., Kaufman M., LePage S., Leopold W. R., et al. The discovery of the benzhydroxamate MEK inhibitors CI-1040 and PD 0325901. Bioorg. Med. Chem. Lett. 2008;18:6501–6504. doi: 10.1016/j.bmcl.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 47.Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 48.Brunn G. J., Williams J., Sabers C., Wiederrecht G., Lawrence J. C., Jr, Abraham R. T. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 49.Fan Q. W., Knight Z. A., Goldenberg D. D., Yu W., Mostov K. E., Stokoe D., Shokat K. M., Weiss W. A. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maira S. M., Stauffer F., Brueggen J., Furet P., Schnell C., Fritsch C., Brachmann S., Chene P., De Pover A., Schoemaker K., et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 51.Folkes A. J., Ahmadi K., Alderton W. K., Alix S., Baker S. J., Box G., Chuckowree I. S., Clarke P. A., Depledge P., Eccles S. A., et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin -4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J. Med. Chem. 2008;51:5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 52.Feldman M. E., Apsel B., Uotila A., Loewith R., Knight Z. A., Ruggero D., Shokat K. M. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thoreen C. C., Kang S. A., Chang J. W., Liu Q., Zhang J., Gao Y., Reichling L. J., Sim T., Sabatini D. M., Gray N. S. An ATP-competitive mTOR inhibitor reveals rapamycin-insensitive functions of mTORC1. J. Biol. Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alessi D. R., Pearce L. R., Garcia-Martinez J. M. New insights into mTOR Signaling: mTORC2 and beyond. Science Signaling. 2009;2:pe27. doi: 10.1126/scisignal.267pe27. [DOI] [PubMed] [Google Scholar]
- 55.Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 56.Williams M. R., Arthur J. S., Balendran A., van der Kaay J., Poli V., Cohen P., Alessi D. R. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr. Biol. 2000;10:439–448. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 57.Mora A., Davies A. M., Bertrand L., Sharif I., Budas G. R., Jovanovic S., Mouton V., Kahn C. R., Lucocq J. M., Gray G. A., et al. Deficiency of PDK1 in cardiac muscle results in heart failure and increased sensitivity to hypoxia. EMBO J. 2003;22:4666–4676. doi: 10.1093/emboj/cdg469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mora A., Lipina C., Tronche F., Sutherland C., Alessi D. R. Deficiency of PDK1 in liver results in glucose intolerance, impairment of insulin-regulated gene expression and liver failure. Biochem. J. 2005;385:639–648. doi: 10.1042/BJ20041782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Collins B. J., Deak M., Arthur J. S., Armit L. J., Alessi D. R. in vivo role of the PIF-binding docking site of PDK1 defined by knock-in mutation. EMBO J. 2003;22:4202–4211. doi: 10.1093/emboj/cdg407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collins B. J., Deak M., Murray-Tait V., Storey K. G., Alessi D. R. in vivo role of the phosphate groove of PDK1 defined by knockin mutation. J. Cell Sci. 2005;118:5023–5034. doi: 10.1242/jcs.02617. [DOI] [PubMed] [Google Scholar]
- 61.Bayascas J. R., Sakamoto K., Armit L., Arthur J. S., Alessi D. R. Evaluation of approaches to generate PDK1 tissue specific knock-in mice. J. Biol. Chem. 2006;281:28772–28781. doi: 10.1074/jbc.M606789200. [DOI] [PubMed] [Google Scholar]
- 62.Bayascas J. R., Wullschleger S., Sakamoto K., Garcia-Martinez J. M., Clacher C., Komander D., van Aalten D. M., Boini K. M., Lang F., Lipina C., et al. Mutation of the PDK1 PH domain inhibits protein kinase B/Akt, leading to small size and insulin resistance. Mol. Cell Biol. 2008;28:3258–3272. doi: 10.1128/MCB.02032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stockman B. J., Kothe M., Kohls D., Weibley L., Connolly B. J., Sheils A. L., Cao Q., Cheng A. C., Yang L., Kamath A. V., et al. Identification of allosteric PIF-pocket ligands for PDK1 using NMR-based fragment screening and 1H-15N TROSY experiments. Chem. Biol. Drug Des. 2009;73:179–188. doi: 10.1111/j.1747-0285.2008.00768.x. [DOI] [PubMed] [Google Scholar]
- 64.Engel M., Hindie V., Lopez-Garcia L. A., Stroba A., Schaeffer F., Adrian I., Imig J., Idrissova L., Nastainczyk W., Zeuzem S., et al. Allosteric activation of the protein kinase PDK1 with low molecular weight compounds. EMBO J. 2006;25:5469–5480. doi: 10.1038/sj.emboj.7601416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang J., Cron P., Thompson V., Good V. M., Hess D., Hemmings B. A., Barford D. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol. Cell. 2002;9:1227–1240. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
- 66.Baldo P., Cecco S., Giacomin E., Lazzarini R., Ros B., Marastoni S. mTOR pathway and mTOR inhibitors as agents for cancer therapy. Curr. Cancer Drug Targets. 2008;8:647–665. doi: 10.2174/156800908786733513. [DOI] [PubMed] [Google Scholar]