Abstract
As an important metabolic pathway, phosphatidylinositol metabolism generates both constitutive and signalling molecules that are crucial for plant growth and development. Recent studies using genetic and molecular approaches reveal the important roles of phospholipid molecules and signalling in multiple processes of higher plants, including root growth, pollen and vascular development, hormone effects and cell responses to environmental stimuli plants. The present review summarizes the current progress in our understanding of the functional mechanism of phospholipid signalling, with an emphasis on the regulation of Ins(1,4,5)P3–Ca2+ oscillation, the second messenger molecule phosphatidic acid and the cytoskeleton.
Keywords: phospholipid, plant, signalling
Abbreviations: ABA, abscisic acid; AtCP, actin-capping protein; AtIPK2, Arabidopsis thaliana inositol polyphosphate kinase 2; AVR4, avirulence 4; CTR1, constitutive triple response 1; DAG, diacylglycerol; DGK, DAG kinase; F-actin, filamentous actin; IAA, indoleacetic acid; IP3R, Ins(1,4,5)P3 receptor; LOX2, lipoxygenase 2; LPP, lipid phosphate phosphatase; MAPK, mitogen-activated protein kinase; OsPIPK, Oryza sativa phosphoinositide phosphate kinase; PA, phosphatidic acid; PDK1, phosphoinositide-dependent kinase 1; PI3K, phosphoinositide 3-kinase; PI4K, phosphoinositide 4-kinase; PIPK, phosphoinositide phosphate kinase; PIP5K, phosphoinositide phosphate 5-kinase; PLC, phospholipase C; PLD, phospholipase D; PP2A, protein phosphatase 2A; 5PTase, inositol polyphosphate 5-phosphatase; RACK1, receptor for activated C-kinase 1; SNX1, sorting nexin 1; ZmPLC1, Zea mays PLC1
BACKGROUND
Membranes act as barriers to hydrophilic molecules and ions because of the hydrophobic core of the phospholipid bilayer [1]. Phospholipid molecules are one of the main structural components of membranes, and they have emerged as important second messengers [2] to regulate plant growth and development and cellular responses to environmental change or stress [3].
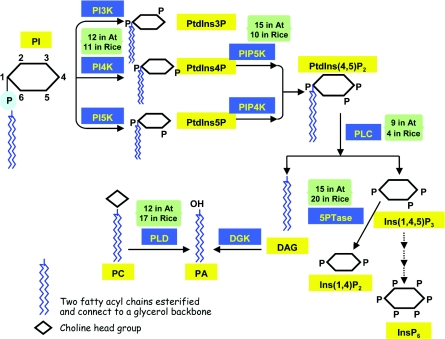
Phosphorylations of the inositol ring of phosphatidylinositol are carried out by specific phosphoinositide kinases, including PI3Ks (phosphoinositide 3-kinases) [4], PI4Ks (phosphoinositide 4-kinases) [5] and PI5Ks (phosphoinositide 5-kinases) [6] at the D-3, D-4 or D-5 positions to generate PtdIns3P, PtdIns4P or PtdIns5P respectively. Sequential phosphorylation by phosphoinositide 4-phosphate 5-kinase [7] or phosphoinositide 5-phosphate 4-kinase [8] will generate PtdIns(4,5)P2, which is then hydrolysed by PLC (phospholipase C), resulting in the production of two important second messenger molecules: Ins(1,4,5)P3 and DAG (diacylglycerol) [2]. The inositol polyphosphates can be phosphorylated to generate InsP6 or dephosphorylated by inositol polyphosphate phosphatases, which are classified into four groups on the basis of the position of the phosphates [9]. In addition, PLD (phospholipase D) hydrolyses phospholipids at the terminal phosphodiester bond and generates PA (phosphatidic acid) [10] (Figure 1).
Figure 1. The phosphoinositide metabolic pathway in higher plants.
The isoform numbers of the key enzymes in Arabidopsis (At) and rice are indicated. Abbreviations: PI, phosphoinositide; PI5K, phosphoinositide 5-kinase.
The presence of many isoforms of the key enzymes in phosphoinositide metabolism has been demonstrated. Some 12 PI3/4K-domain-containing proteins are predicted to be PI4K genes [11], and there are 15 isoforms of PIP5Ks (phosphoinositide phosphate 5-kinases) [11], 15 isoforms of 5PTases (inositol polyphosphate 5-phosphatases) [12], 12 PLD isoforms [13], 12 PI4K isoforms [10] and nine PLC isoforms [10,14] in Arabidopsis. In rice, a monocotyledon, the distribution and functions of relevant isoforms have been less studied. An analysis showed that there are 17 PLD members [15] and four PLC members [14] in rice. Our preliminary analysis using BLAST and CLUSTW shows that there are ten PIP5Ks, 11 PI4Ks and 20 5PTase members in rice (Figure 1).
In the present review, we focus on the functions and mechanisms of PIP5K, PLC, 5PTase, PLD and the phospholipid molecules PtdIns4P, PtdIns(4,5)P2, Ins(1,4,5)P3 and PA, as well as Ca2+, in plant growth and development.
CRITICAL EFFECTS OF PHOSPHOLIPIDS IN PLANT GROWTH AND STRESS RESPONSES
Root growth and root hair patterning
Mutation in XIPOTL1, which encodes a PEAMT (S-adenosyl-L-methionine:phosphoethanolamine N-methyltransferase) and is critical in phosphatidylcholine biosynthesis, resulted in a short primary root and short epidermal cells due to the cell death induced by decreased phosphatidylcholine content [16]. Arabidopsis lines with a deficiency of INT1 (INOSITOL TRANSPORTER 1) show increased intracellular myo-inositol concentrations and reduced root growth, which is possibly due to sequestration of inositol in vacuoles [17]. Arabidopsis PIP5K9 negatively regulates sugar-mediated root cell elongation through interaction with a cytosolic invertase, CINV1 [18]. Conversely, suppressed AtIPK2α (Arabidopsis thaliana inositol polyphosphate kinase 2α) expression and application of exogenous Ins(1,4,5)P3 lead to enhanced root growth [19].
Seedlings treated with the PLD inhibitor butan-1-ol or deficient in PLDζ2 display suppressed primary root elongation and inhibited lateral root formation [20], and PLD-derived PA production is an early signalling event during adventitious root formation induced by auxin and NO in cucumber explants [21]. In addition, accumulated PtdIns(4,5)P2 and Ins(1,4,5)P3 under SAC9 deficiency result in shorter primary roots and fewer lateral roots [22]. PA takes part in root growth, root hair and pollen tube patterning by regulating cytoskeleton and vesicle trafficking [20,21,23] (Figure 2).
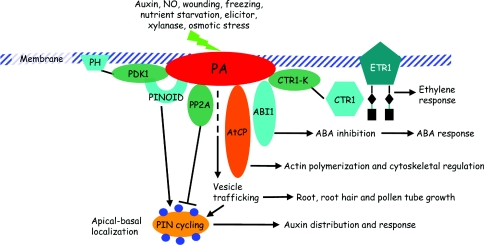
Figure 2. PA functions in hormone responses and growth of roots, root hairs and pollen tubes.
PA accumulates in response to treatment with auxin and NO, wounding, freezing, nutrient starvation, elicitor, xylanase, and osmotic stress. PA could bind CTR1 and block the interaction with ethylene receptor ETR1 to regulate the ethylene response. PA binds and decreases the activity of ABI1, resulting in the insensitive response to ABA treatment. PA promotes actin polymerization by binding to and inhibiting the activity of AtCP. PA regulates cycling of PIN proteins through directly mediating vesicle trafficking, or binding and mediating the activity of auxin transport upstream proteins PDK1, PINOID and PP2A. Abbreviations: CTR1-K, CTR1 kinase domain; PH, pleckstrin homology domain.
Regarding the root hair pattern, a deficiency of AtSfh1, which encodes a PITP (phosphatidylinositol transfer protein), compromises polarized root hair expansion in a manner that coincides with a loss of tip-directed PtdIns(4,5)P2 [24]. Decreased AtIPK2α and PLDζ1 expression confers insensitivity of root hair growth to calcium depletion [19] and leads to random initiation of root hairs [25] respectively, whereas increased PLDζ1 expression induces branched and swollen root hairs [25]. Treatment with a PI3K-specific inhibitor inhibits root hair growth by regulating PtdIns3P-mediated vesicle trafficking and ROS (reactive oxygen species) production [26]. The double mutant PI4Kβ1/PI4Kβ2 displays aberrant root hair morphologies due to altered calcium signalling [27], whereas mutation in a phosphoinositide 4-phosphate phosphatase gene (rhd4) results in shorter and morphologically aberrant root hairs due to the altered accumulation of PtdIns4P together with PI4Kβ1 in membrane compartments [28]. Studies by two independent groups have shown that Arabidopsis PIP5K3 is essential for root hair formation through its production of PtdIns(4,5)P2 at the root hair apex. Furthermore, reduced expression of PIP5K3 caused significantly shortened root hairs, whereas overexpression resulted in increasingly deformed root hairs [29,30]. In Medicago truncatula, inhibition of PI3K and PI-PLC (phosphatidylinositol-specific PLC) prevented root hair curling and the formation of infection threads [31].
Pollen development
Phospholipid signalling is involved in pollen development, including pollen viability, germination and pollen tube elongation, through regulation of the production of PtdIns3P, PtdIns4P, PtdIns(3,5)P2, PtdIns(4,5)P2, PA, Ins(1,4,5)P3 and downstream [Ca2+]cyt levels. Studies reveal that PI3K and PI4K are involved in regulation of pollen viability through different mechanisms. PI3K regulates vacuole reorganization and nuclear division in pollen grains, possibly by regulating the products PtdIns3P and PtdIns(3,5)P2, which are essential for protein targeting to vacuoles and normal vacuole function and morphology respectively [32], whereas PI4Kγ1 performs crucial intracellular trafficking regulation for tapetum and microspore development [33]. In addition, PtdIns(4,5)P2 leads to increased [Ca2+]cyt, transient growth perturbation and inhibition of apical secretion, whereas Ins(1,4,5)P3 treatment causes a transient [Ca2+]cyt increase of similar magnitude and stimulates apical secretion and severe growth perturbation, indicating the different targets of these two molecules [23]. Recent reports have shown that deficiency of two Arabidopsis pollen-expressed PIP5Ks, PIP5K4 and PIP5K5, reduced pollen germination and pollen tube elongation, which is consistent with the effect of their product, PtdIns(4,5)P2 [34,35]. However, overexpression of either PIP5K4 or PIP5K5 results in multiple pollen tip branching, which is thought to occur by regulating membrane trafficking and apical pectin secretion [34,35].
PA plays an important role in pollen tube polarity by regulating microfilament polymerization and membrane transport. A reduced level of PA leads to inhibited apical plasma membrane recycling and formation of thick, but non-directional, microfilaments [23]. PLD-derived PA stimulates pollen germination and tube elongation [36] and expands the pollen tube apical region [37] (Figure 2).
In addition, because both PtdIns(4,5)P2 and DAG are accumulated in an apical domain of the plasma membrane at the pollen tube tips, disruption of this domain causes defective pollen tube growth, which highlights the importance of maintaining an apical domain enriched in PtdIns(4,5)P2 and DAG for polar cell growth [38].
Vascular development
Although several studies have demonstrated the role of phospholipid signalling in vascular growth and development, the mechanism is still unclear. CVP2, encoding a type I inositol polyphosphate 5-phosphatase 6 (At5PTase6), promotes vascular cell proliferation and prevents the termination of premature veins (the cvp2-knockout mutant shows an open cotyledon vein [39]). The type II At5PTase13 regulates cotyledon vein formation by regulating auxin homoeostasis [40]. FRA3, a type II 5PTase gene, plays an essential role in secondary wall synthesis in fibre cells and xylem vessels, and the fra3-knockout mutant contains higher amounts of PtdIns(4,5)P2 and Ins(1,4,5)P3 and shows reduced thickness of the secondary wall and decreased stem strength owing to the disruption in actin organization [41]. AtSAC1 exhibits phosphatase activity toward PtdIns(3,5)P2, and mutation of AtSAC1 causes a dramatic decrease in the cell wall thickness of fibre cells and vessel elements, resulting in a weak stem [42], suggesting the important effect of the PtdIns(3,5)P2 pool in the formation of aberrant cell shape.
Hormone effects
Altered phospholipid signalling shows changed responses to various hormones. Seedlings deficient in 5TPase1, 5PTase2 [43] and cvp2 [39] are hypersensitive to exogenous ABA (abscisic acid), whereas a deficiency in 5PTase13 causes insensitivity to ABA in seed germination [40]. PA, generated by PLDα1, binds to ABI1 and decreases the phosphatase activity of ABI1, resulting in the insensitivity of stomatal closure of PLDα1-null mutant leaves to ABA treatment [44] (Figure 2). In addition, PLDα1 directly interacts with and inactivates the Gα subunit of heterotrimeric G-proteins to mediate ABA inhibition of stomatal opening [45], and suppression of rice PLDβ1 results in reduced sensitivity to ABA in seed germination [15].
Ins(1,4,5)P3 oscillation mediates auxin transport and is involved in gravistimulation responses [46]. Arabidopsis 5PTase13 and PLDζ2 regulate the cotyledon vein [40] and root development [20] respectively by modulating auxin synthesis and transport. PLA activity is rapidly induced by auxin within 2 min [47], and Arabidopsis sPLA2 regulates shoot gravitropism by regulating auxin-induced cell elongation [48].
Besides auxin and ABA, involvement of phospholipid signalling in other hormone cascades is seldom reported. Testerink et al. [49] demonstrated that PA regulates CTR1 activity to influence ethylene signalling. Lin et al. [50] pointed out that phospholipid signalling could be regulated by multiple hormones, hinting at the cross-talk between them.
Stress responses
The involvement of phospholipid signalling in the cell responses to stress stimuli, including salt, osmotic, temperature and pathogen stressors, has been well demonstrated, and multiple members of the phosphoinositide pathway are supposed to mediate the stress responses through different mechanisms.
Salt stress
Salinity is a major stress that threatens plant growth and crop production. Arabidopsis plants show rapidly increased PtdIns(4,5)P2 synthesis in response to treatments with NaCl, KCl and sorbitol [51], suggesting an important role of PtdIns(4,5)P2 in plant salt tolerance. PtdIns(4,5)P2 and its derivative Ins(1,4,5)P3 are accumulated in plants under salt, cold and osmotic stresses, and sac (suppressor of actin) mutants harbour accumulated PtdIns(4,5)P2 and Ins(1,4,5)P3 and show a constitutive stress response [22]. Consistent with this, pharmacological studies using U73122 (a specific inhibitor of PLC) have shown that, in salt-treated seedlings, PLC activity, as well as Ins(1,4,5)P3 and calcium, are necessary for the expression of pyrroline-5-carboxylate synthetase and resultant pyrroline levels [52].
Recent studies revealed that salt-stress-induced association of PtdIns(4,5)P2 with clathrin-coated vesicles [53] and PI3K-mediated endocytosis are necessary for plant salt tolerance [54], highlighting the importance of vesicle trafficking in the salt response. Multiple PLDs, including PLDα1, α3, δ and ε, are required for salt and water deficit tolerance in Arabidopsis, possibly by regulating PA production and membrane lipid compositions [55,56].
Drought stress
Drought directly leads to water deficiency and inhibits plant growth. Studies have shown that members of both PLC and PLD families are involved in drought tolerance. Expression of maize ZmPLC1 (Zea mays PLC1) is up-regulated under dehydration and this enhanced expression improves the drought tolerance of maize [57]. Expression pattern analysis revealed that six of nine Arabidopsis PLC genes are stimulated by dehydration [14], suggesting a similar function of Arabidopsis PLCs to that of ZmPLC1. PLDs and the derived PA are also widely involved in plants' drought tolerance. Both PLDα1 and PLDα3 are necessary for drought tolerance in Arabidopsis [58]; however, the regulatory mechanisms are different. PLDα1 mediates early drought response and ABA signalling [59], whereas PLDα3 is involved in promoting root growth and stress avoidance under hyperosmotic conditions [55]. PLDδ is accumulated under dehydration and contributes to the dehydration-induced PA accumulation [60], whose knockout mutants display hypersensitivity to water deficit [56]. Recently, Perera et al. [61] showed that Arabidopsis transgenic plants overexpressing mammalian type I 5PTase present significantly enhanced drought tolerance.
Cold stress
A transcriptomic analysis of cold-treated Arabidopsis suspension cells in the presence of U73122 or ethanol, the inhibitors for PLC and PLD respectively, revealed that both PLC- and PLD-mediated signal pathways are activated simultaneously, which leads to the activation of different clusters of cold-response genes [62]. Genetic analysis reveals that different PLD isoforms have opposite effects in cold response. Arabidopsis plants that are deficient for PLDα1 show improved tolerance to freezing through activating the cold-responsive genes and increasing osmolyte accumulation [63,64], whereas deficiency of the membrane-associated PLDδ rendered hypersensitivity to freezing (PLDδ overexpression resulted in increased freezing tolerance [65]). Suppression of PLDα1 decreases phospholipid hydrolysis and PA production in both freezing and post-freezing phases, whereas ablation of PLDδ increases lipid hydrolysis and PA production in post-freezing recovery [66]. Chilling and freezing result in the increased Ins(1,4,5)P3–Ca2+ influx [67] and transgenic plants overexpressing mammalian 5PTase display a ∼30% decrease in the rapid transient Ca2+ peak in response to cold or salt stimuli [61].
Oxidative stress
H2O2 treatment activates the PLD activity, and deficiency of PLDδ displays hypersensitivity to H2O2-induced cell death, suggesting a crucial role of PA in H2O2-induced cell death [68]. This is consistent with the fact that PA could trigger an oxidative burst through activating a MAPK (mitogen-activated protein kinase) cascade [69] and indicates PA as a possible important regulator of Rop-regulated ROS generation to mediate the process of cell death [70]. In addition, PI3K can stimulate plasma membrane endocytosis and produce ROS, subsequently inducing root hair curling [31].
Other stress
Wounding triggers a rapid activation of PLD-mediated phospholipid hydrolysis. The Ca2+ increase upon wounding mediates the translocation of PLD protein from cytosol to membrane [71]. Multiple members of PLD are activated by wounding, and suppression of PLDα by an antisense approach decreases the wound-induced expression of LOX2 (lipoxygenase 2), suggesting that LOX2 is probably a downstream target through PLD-mediated production of jasmonic acid [72]. MAPK signalling is activated by wounding in soya bean seedlings, and wound-induced activation of MAPK signalling is suppressed when PA production is inhibited with n-butanol, which indicates that PA acts as a second messenger in wound-induced MAPK signalling [73]. In addition, PLDζ2 plays a key role in phosphate cycling, and pldζ2 mutants exhibit hypersensitivity to phosphate starvation [74,75]. PLDε regulates the root surface area to improve the nitrogen uptake and utilization [76].
Besides abiotic stress, phospholipid signalling is also regulated by biotic stress. The AVR4 (avirulence 4) gene encodes an elicitor protein and displays resistance in tomato plants carrying the Cf-4 resistance gene to the fungus Cladosporium fulvum. In response to the infection, PA is rapidly produced in a few minutes when AVR4 interacts with Cf-4, indicating that the PA accumulation is an early response in the Cf-4–AVR4 interaction [77].
Others
In addition to the above-mentioned developmental processes and stress responses, phospholipid signalling is involved in embryo development, stomata closure, and light and sugar signal transduction. Deficiency of PECT (phosphorylethanolamine cytidylyltransferase), a rate-limiting enzyme in phosphatidylethanolamine biosynthesis, results in embryo abortion before the octant stage, delays embryo maturation and reduces seed fertility [78]. Deficiency of LPAT (lysophosphatidyl acyltransferase), which is responsible for PA biosynthesis, caused embryo lethality (arrested embryo at the globular stage [79]).
The Arabidopsis 5PTase13 deficiency mutant (5pt13) displays shorter hypocotyls under blue light [80]. Mutants 5pt1 and 5pt2 grow faster and have elongated hypocotyls in the dark [43]. The ITPK-1 (inositol 1,3,4-trisphosphate 5/6-kinase) knockout mutant is hypersensitive to red light, similar to the csn (COP9 signalosome) mutant [81]. In addition, PtdIns(4,5)P2 and Ins(1,4,5)P3-mediated Ca2+ oscillation are important for stomatal opening [82,83]. Arabidopsis 5PTase13 regulates SnRK1 (sucrose non-fermenting-1-related kinase) activity, which differs according to nutrient availability, and deficiency of 5PTase13 results in a shortened root under no-nutrient or low-nutrient conditions and is less sensitive to sugar and insensitive to ABA [84].
The altered growth patterns of the loss-of-function knockout mutant and transgenic lines with overexpression or decreased expression of phospholipid signalling-related genes are summarized in Table 1.
Table 1. Altered growth of loss-of-function knockout mutants (KO) and transgenic lines with overexpression (OE) or decreased expression (DE) of phospholipid signalling-related genes.
Unless indicated otherwise, results are from Arabidopsis.
| Gene | Protein | Type | Phenotype | Reference(s) |
|---|---|---|---|---|
| VSP34 | PI3K | KO | Short root hairs, and decreased pollen viability, germination and pollen tube growth | [26,32] |
| PI4Kβ1/PI4Kβ2 | PI4Kβ1, PI4Kβ2 | KO | (Double mutant) Aberrant root hair morphology | [27] |
| PI4Kγ1 | PI4Kγ1 | KO | Decreased pollen viability, abnormal tapetum and microspore development | [33] |
| RHD4 | PI4P phosphatase | KO | Short and morphologically aberrant root hairs | [28] |
| PIP5K3 | PIP5K3 | DE | Shorter root hair | [29,30] |
| OE | Deformed root hair | |||
| PIP5K4 | PIP5K4 | KO | Reduced stomatal opening, and impaired pollen germination, tube growth and polarity | [34,35,82] |
| OE | Perturbed pollen tube growth, multiple pollen tip branching | |||
| PIP5K5 | PIP5K5 | OE | Multiple pollen tip branching | [34] |
| PIP5K9 | PIP5K9 | OE | Shortened primary root | [18] |
| SAC1 (fra7) | SAC domain phosphoinositide phosphatase 1 | KO | Weak stem, decreased cell wall thickness | [42] |
| SAC9 | SAC domain phosphoinositide phosphatase 9 | KO | Shorter primary root and fewer lateral roots | [22] |
| DAD1 | PLA1 | KO | Defects in anther dehiscence, pollen maturation and flower opening | [85] |
| AtsPLA2β | AtsPLA2β | DE | Shortened leaf petioles and stems, delayed light-induced stomatal opening | [48,86] |
| OE | Prolonged leaf petioles and inflorescence stems, faster stomatal opening | |||
| AtPLC1 | AtPLC1 | DE | Insensitive to ABA in seed germination and growth | [87] |
| ZmPLC1 | ZmPLC1 | OE | Improved drought tolerance | [57] |
| NtPLC3 | NtPLC3 | OE | Inhibited pollen tube growth | [38] |
| OsPLDβ1 | OsPLDβ1 | KO | Reduced sensitivity to ABA during seed germination | [15] |
| PLDα1 | PLDα1 | KO | Decreased wound-induced synthesis of jasmonic acid, decreased drought tolerance, enhanced seed quality after storage, insensitive response of stomatal closure to ABA, enhanced sensitivity to high salinity, increased freezing tolerance | [56,58,64,65,72,88] |
| PLDα3 | PLDα3 | KO | Increased sensitivities to salinity and water deficiency, later flowering in drought conditions | [55] |
| OE | Decreased sensitivities to salinity and water deficiency, earlier flowering in drought conditions | |||
| PLDε | PLDε | KO | Decreased root growth and biomass accumulation, decreased lateral root elongation | [76] |
| OE | Increased root growth and biomass accumulation, increased lateral root and root hair elongation | |||
| PLDδ | PLDδ | KO | Increased sensitivity to H2O2-induced cell death, sensitive to freezing | [68,89] |
| OE | Increased freezing tolerance | |||
| PLDζ1 | PLDζ1 | OE | Branched and swollen root hairs | [25] |
| DE | Random initiation of root hairs | |||
| PLDζ1/PLDζ2 | PLDζ1, PLDζ2 | KO | (Double mutant) Hypersensitive to phosphate deficiency in root growth | [74,75] |
| PLDζ2 | PLDζ2 | KO | Suppressed primary root elongation and inhibited lateral root formation, less sensitive to auxin, reduced root gravitropism | [20] |
| OE | Enhanced primary root growth, root gravitropism, hypersensitive to auxin. | |||
| 5PTase1/5PTase2 | 5PTase1, 5PTase2 | KO | (Double mutant) Faster germination and longer hypocotyl in the dark, hypersensitive to ABA | [43] |
| 5PTase5 | 5PTase5 | KO | Disrupted root-hair tip growth | [90] |
| CVP2 | 5PTase6 | KO | Open reticulum and increased free vein endings | [39] |
| 5PTase11 | 5PTase11 | KO | Slower germination and decreased hypocotyl growth when grown in the dark | [91] |
| FRA3 | 5PTase12 | KO | Dramatic reduction in secondary wall thickness and a concomitant decrease in stem strength | [41] |
| 5PTase13 | 5PTase13 | KO | Defect in development of the cotyledon vein, shortened hypocotyls and expanded cotyledons under blue light, hypersensitive to sugar and ABA in seed germination | [40,80,84] |
| Itpk-1 | ITPK-1 | KO | Decreased hypocotyl length under red light | [81] |
| OsITL1 | OsITPK1 | OE | (In tobacco) Decreased tolerance to NaCl during germination and seedling development. | [92] |
| ATS2 | LPAAT (lysophosphatidic acid acyltransferase β) | KO | Embryo lethality | [79] |
| XIPOTL1 | PEAMT | KO | Short primary root and induced cell death | [16] |
| PECT | PECT | KO | Embryo abortion before the octant stage, delayed embryo maturation and reduced seed fertility | [78] |
| INT1 | INT1 | KO | Reduced root length | [17] |
| AtSfh1 | PITP | KO | Short root hairs | [24] |
REGULATION OF PHOSPHOLIPID SIGNALLING
Regulation of key enzymes and molecules in phosphoinositide signalling by hormones and environmental stimuli
Studies of expression patterns of genes encoding key enzymes in phosphoinositide signalling employing DNA chip technology and RT (reverse transcription)–PCR reveal that the expression of multiple enzymes is widely and differentially regulated by various hormones and abiotic stressors, especially in the families of PIPK (phosphoinositide phosphate kinase), PLD and 5PTase [50]. Of nine Arabidopsis PLC genes, four are induced by salt, three are induced by ABA, four respond to cold, and six are stimulated by dehydration [14]. Arabidopsis PLD is activated by various hyperosmotic stresses, including high salinity [93], dehydration [60] and freezing [63,65], as well as ABA [44]. Similarly, many rice PLD genes are induced by salt and drought stress [15]. PIP5K family genes are reported to be stimulated by water stress and ABA [50,59,94] and repressed by cold [18]. In addition, the plant hormone auxin is broadly related to phosphoinositide signalling, inducing the expression of AtIPK2β [95] and PLDζ2 [20], and suppressing the expression of 5PTase13 [40].
Some phospholipid molecules, such as PA, PtdInsP and PtdIns(4,5)P2, are accumulated after treatment with auxin, NO [21], wounding [72,96], freezing [63], nutrient starvation [74,97], elicitor [98], xylanase [99] or under osmotic stress [100].
Ins(1,4,5)P3-mediated Ca2+ oscillation
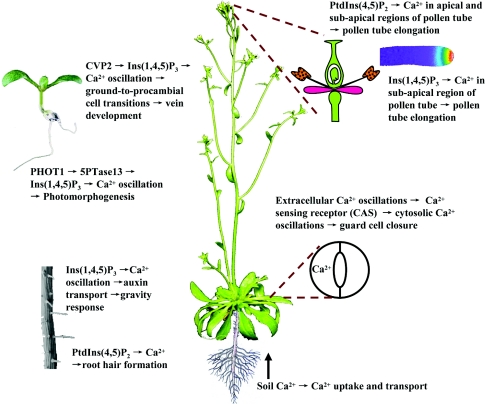
PtdIns(4,5)P2 throughout the membrane establishes the basis for rapid and transient increases of Ins(1,4,5)P3 in response to environmental stimuli, which leads to the rapid oscillation of Ca2+. Many studies have shown that Ins(1,4,5)P3-mediated Ca2+ influx is important for plant cell growth (Figure 3). In cvp2 mutants, the Ins(1,4,5)P3 levels increase approx. 3-fold compared with wild-type, and Ins(1,4,5)P3 releases Ca2+ from internal stores to tightly control the number of ground-to-procambial cell transitions and to regulate the vein formation [39]. The 5pt13 mutant has a higher Ins(1,4,5)P3 level compared with wild-type and mediates the effects of blue light through Ins(1,4,5)P3-mediated Ca2+ oscillations [80].
Figure 3. The Ca2+ oscillation, mediated by PtdIns(4,5)P2 and Ins(1,4,5)P3, is crucial for multiple processes of plant growth including root tropism, root hair formation, cotyledon vein development, photomorphogenesis, guard cell closure, pollen tube elongation and hormone effects.
Gravistimulation induced differential lateral IAA (indoleacetic acid) transport, which is consistent with the changed Ins(1,4,5)P3 levels in maize and oat pulvini [46]. Previous studies have shown that the level of Ins(1,4,5)P3 increases approx. 3-fold in the first 5 min of gravistimulation, and the second peak of Ins(1,4,5)P3 appears by 15–20 min [101]. After 30 min, the Ins(1,4,5)P3 level returns to baseline [101], indicating that the oscillation of Ins(1,4,5)P3 is a key component of the response to gravity stimuli.
Environmental stimuli also evoke a transient increase of Ins(1,4,5)P3 levels, leading to the following question: is the long-term increase or decrease of the Ins(1,4,5)P3 levels associated with cell growth? The shorter hypocotyls of the 5pt13 mutant under blue light provide evidence that a higher Ins(1,4,5)P3 level, and hence increased [Ca2+]cyt under blue light irradiation, regulates cell growth [80]. Similarly, in maize pulvini, the inositol lipids' rate of turnover is associated with an increase in membrane biogenesis and Ins(1,4,5)P3–Ca2+ release from internal stores to initiate cell growth upon gravistimulation [102].
The PtdIns(4,5)P2–Ca2+ gradient plays a key role in pollen tube growth [103] and root hair formation [24]. Because Ins(1,4,5)P3–Ca2+ influx across the plasma membrane is required to maintain the Ca2+ gradient in the pollen tube cell and Ins(1,4,5)P3-sensitive Ca2+ channels have not been identified [10], it was speculated that the function of Ca2+ influx was a direct result of Ins(1,4,5)P3 in pollen tube growth [38]. When PtdIns(4,5)P2 and Ins(1,4,5)P3 were loaded into an Agapanthus pollen tube, Ins(1,4,5)P3 was more effective than PtdIns(4,5)P2 [23], indicating that Ins(1,4,5)P3–Ca2+ and PtdIns(4,5)P2–Ca2+ are spatially different. PtdIns(4,5)P2–Ca2+ is increased in both apical and sub-apical regions, whereas Ins(1,4,5)P3–Ca2+ is increased mainly in sub-apical regions [23], indicating that both PtdIns(4,5)P2 and Ins(1,4,5)P3 cause transient increases in Ca2+ levels; however, the effects are markedly different.
Transient and rapid increase in intracellular Ca2+ have been demonstrated in response to abiotic stresses, including cold, salt and osmotic stress [104,105], and Ins(1,4,5)P3-mediated Ca2+ oscillation is also involved in the response to these stimuli.
In addition, Ca2+ is important for the effects of phospholipids and activity of relevant enzymes. The interaction of the PLD C2 domain with Ca2+ is stimulated by phospholipids [106], and Ca2+ sensitivities of different PLD isoforms vary from the micromolar to the millimolar range [10,107,108]. Increased [Ca2+]cyt evokes conformational changes in PLDs to assist with the recruitment of substrate PtdIns(4,5)P2 [109]. Additionally, enzymatic activity of PLC is regulated by Ca2+ through binding with the EF-hand motif of PLC [110], and PLA2s are classified as Ca2+-dependent or -independent members [2].
Cross-talk with hormone signalling
Auxin transporters is regulated by phosphorylation, and PINOID plays a fundamental role in the asymmetrical localization of membrane proteins during polar auxin transport by regulating the polarity of PIN's apical-basal localization [111]. Interestingly, PDK1 (phosphoinositide-dependent kinase 1) enhances PINOID activity and is involved in polar auxin transport regulation [111]. Both PDK1 and PINOID can bind to PA, PtdIns3P, PtdIns(3,4)P2 and PtdIns(4,5)P2 through the PH (pleckstrin homology) domain [111,112]. PP2A (protein phosphatase 2A) and PINOID act antagonistically on mediating PIN apical-basal polar localization [113] and PP2A can bind to PA [114]. In addition, PLDζ2 and product PA are required for the normal cycling of PIN2-containing vesicles to maintain auxin transport, and the deficiency of PLDζ2 results in suppressed sensitivity to auxin and reduced root gravitropism [20]. These findings suggest that membrane phospholipid turnover is important for regulating polar auxin transport (Figure 2).
Recently several studies revealed the crucial functions of phospholipids in vesicular trafficking. The trafficking of auxin efflux carrier PIN2 protein is dependent on SNX1 (sorting nexin 1)-containing endosomes which are sensitive to the PI3K inhibitor wortmannin [115]. SNX1 co-localizes with PtdIns3P-enriched membrane subdomains, and is a candidate downstream effector of PI3K because of the presence of phosphatidylinositol-binding PX (phox homology) domain of SNX1 [116]. During the regulation of turnover of auxin transporters, PIN2 is targeted to the vacuole for degradation, and the trafficking of PIN2-containing vacuolar depends on the phosphatidylinositol pathway [116]. Wortmannin affects the recycling of vacuolar sorting receptors between the PVC (prevacuolar compartment) and the TGN (trans-Golgi network) [116–118] and whose treatment inhibits the trafficking and degradation of PIN2, and increases the total PIN2 protein level in membrane [116]. Such cross-talk prompts us to ask questions. Do the membrane compositions affected by phospholipids influence the localization of membrane protein PINs? Do phospholipids serve as mediators to recruit the proteins to membrane sites and to promote signal transduction? Although the current studies suggest that the latter speculation may be the case, the relevant mechanisms are still unclear.
At5PTase13 regulates the expression of CYP83B1, which is important for auxin homoeostasis [40], and overexpression of AtIPK2β leads to more axillary shoot branching and an IAA-related phenotype by mediating the transcription of the IAA-related genes CYP83B1, PIN4, MAX4 and SPS [95].
Only PA was reported to bind CTR1 (constitutive triple response 1), which negatively regulates ethylene responses in Arabidopsis [119] to disrupt the intramolecular interaction between the CTR1 kinase domain and the CTR1 N-terminal regulatory domain to inhibit its kinase activity [49] (Figure 2).
Regulation through PA
PA, the simplest membrane phospholipid, has emerged as a new class of lipid mediator involved in various cellular processes, such as signal transduction, membrane trafficking, secretion and cytoskeletal rearrangement [10]. Cellular PA is generated mainly via two routes: DGK (DAG kinase) phosphorylating DAG or PLD hydrolysing structural phospholipids. In plants, PA serves as a second messenger and is triggered in response to various biotic and abiotic stresses, including pathogen infection [77,120], drought, salinity, wounding, cold, cell death [68,70,98] and oxylipin production [71–73]. The PA signal production is fast (minutes) and transient.
PA plays a role in the ABA response during stomatal movement and seed germination. PLDα1-derived PA binds to ABI1, a negative regulator of the ABA response, and inhibits its activity to promote stomata closure [44]. T-DNA (transferred DNA) insertional mutants of PA catabolic enzyme LPP (lipid phosphate phosphatase), lpp2-1 and lpp2-2, are hypersensitive to ABA during germination due to the increase in PA levels [121].
Regarding the roles of PA in the abiotic stress response, it is suggested that PA produced by high PLDα1 activity destabilizes the membrane and increases membrane leakage, whereas a regulated increase of plasma membrane PA by PLDδ may produce signalling PA species that mitigate stress damage [63,65]. The PA level increases during chilling and cold acclimatization, as well as in response to freezing [63,122], and the results of microarray analysis imply that PA produced from the PLD and PLC/DGK pathways are involved in two different pathways in cold responses [62]. Osmotic stress also induces the rapid accumulation of PA [60,123], which may be involved in regulation of proline biosynthesis [124]. Studies using soya bean cells show that PA can bind MAPK6-related protein, an important mediator in stress and ethylene signalling, suggesting a different mechanism of PA involvement in the stress response [125] (Figure 2). Furthermore, the PA-interacting/binding proteins should be studied in more detail to illustrate the downstream mechanisms.
Regulation through cytoskeleton
Both the actin and microtubular cytoskeletons can be regulated by phospholipids and participate in regulating the tip growth of the root hair and pollen tube. PtdIns(4,5)P2, Ins(1,4,5)P3, PA and the related PI3K, PI4K and PIP5K are involved in the organization of actin filaments; the microtubular cytoskeleton is mainly modulated by PLD.
Phospholipids play an integral role in regulating the structure and dynamics of the cytoskeleton through interaction between PtdIns(4,5)P2 and many actin-binding proteins, including profilin, gelsolin, α-actin, cofilin, filamin and vinculin [126–130]. It has been shown that PtdIns(4,5)P2 controls the dynamic organization of F-actin (filamentous actin) through regulating profilin, a G-actin (globular actin)-binding protein [131], and actin remodelling in root hairs and pollen tubes, sites of rapid growth in plants, is sensitive to alterations of PtdIns(4,5)P2 biosynthesis. Expression of mutant Arabidopsis Rac2 in tobacco pollen tubes decreases the plasma membrane PtdIns(4,5)P2 and disrupts the normal actin filament orientation [132]. Arabidopsis PIP5K1, which synthesizes PtdIns(4,5)P2 in vivo, interacts directly with actin and recruits PI4Kβ1 to the actin cytoskeleton [133]. Although it has not been confirmed, Stenzel et al. [30] speculated that the regulation of membrane-associated PIP5K3 in root hair elongation might relate to the control of F-actin (Figure 4).
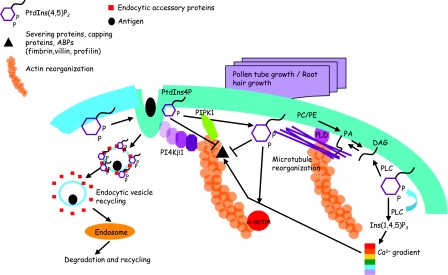
Figure 4. Phospholipids participate in the tip growth of the root hair and pollen tube through regulating actin and microtubule cytoskeletons.
PtdIns(4,5)P2 is synthesized on the plasma membrane and assembles a number of endocytic accessory proteins to induce the antigen-stimulated endocytosis. PIP5K1 and PtdIns(4,5)P2 interact directly with actin and recruit PI4Kβ1 to actin. PtdIns(4,5)P2 interacts and suppresses the activity of actin-binding proteins, including severing proteins, capping proteins, ABPs (actin-binding proteins: fimbrin, villin, profilin), and induces the activity of α-actin to regulate actin remodelling. PA turnover from DAG is the substrate for PLD which is involved in microtubule reorganization. Ins(1,4,5)P3–Ca2+ oscillation regulates the activity of actin-binding proteins to participate in actin remodelling. Abbreviations: PC, phosphatidylcholine; PE, phosphatidylethanolamine.
The PLC-mediated PtdIns(4,5)P2 turnover also affects the actin structure and normal pollen growth [38,134]. Elevation of intracellular PtdIns(4,5)P2 [through photolysis of caged PtdIns(4,5)P2] leads to the perturbation of apical morphology and the appearance in this region of a fine cortical mesh of microfilaments [23].
PA promotes actin polymerization by binding to and inhibiting the activity of AtCP, an actin-capping protein [135]. Arabidopsis PLDβ1 can bind directly to actin, and the activity of PLD is, in turn, regulated by the actin cytoskeleton [136]. AtSAC1 and FRA3, which encode PtdIns(3,5)P2 phosphatase and 5PTase respectively, are both required for actin organization and cell wall synthesis [41,42]. Deficiency of AtSfh1, a PITP-encoding gene, results in arrested root hair pattern formation by altering both actin and the microtubular cytoskeleton [24]. A recent study shows that both PtdIns3P and PtdIns4P modulate actin filament organization in guard cells of day flowers [137].
The involvement of the microtubular cytoskeleton in phospholipid signalling is mediated by PLD. Treatment with the PLD inhibitor butan-1-ol disrupts cortical microtubule organization in soya bean cells and fucoid alga [138,139] and promotes microtubule depolymerization and release from the plasma membrane in Arabidopsis [140]. Unlike in mammalian cells, cortical microtubules lie beneath the plasma membrane in plant cells, and PLD is postulated to be the linker [141].
Others
Other kinds of regulation of phospholipids and relevant key proteins also exist. PLD activity, with the exception of ζ-class PLDs, is dependent on calcium, whereas β-, γ- and ζ-class PLDs require PtdIns(4,5)P2 for in vitro activity [142]. A recent study shows that aluminium ions inhibit PLD in a microtubule-dependent manner [143]. The activity of AtPIP5K1 is inhibited by phosphorylation through protein kinase A [144]. In addition, the MORN motifs in OsPIPK1 (Oryza sativa PIPK1) and the highly charged region of OsPI4K2 are involved in the regulation of subcellular localization and substrate binding [145,146]. The WD40 domain of 5PTase13 interacts with SnRK1 and acts as a positive regulator of SnRK1 activity to stabilize the protein and suppress its degradation [84].
CONCLUSIONS AND PERSPECTIVES
Substantial evidence demonstrates that phospholipid signalling plays an important role in various aspects of plant growth and development, stress response and hormone signalling, and extensive progress has been made toward understanding the regulation and function of phospholipid molecules and related enzymes.
It has been noted that several gene families involved in phospholipid signalling contain more than ten isoforms in Arabidopsis, including PIP5K, PLD and 5PTase, and isoforms in the same family have distinct expression patterns and may have specific functions. The precise analysis of the expression patterns in specific tissues, at different developmental stages and under different stimulations of individual isoforms will facilitate the dissection of their specific functions.
Although post-transcriptional regulation is an important process in functional modulation, studies of the regulation of phospholipid-related enzymes and protein level are limited. PIP5K activity can be inhibited by phosphorylation and modulated by changes in subcellular localization and substrate binding [145,146], shedding light on the cellular regulation of phospholipid signalling.
The IP3Rs [Ins(1,4,5)P3 receptors] have been identified from animal and yeast cells; however, none are known in plants. Ins(1,4,5)P3 activates IP3R in the endoplasmic reticulum, resulting in the Ca2+ release and leading to the [Ca2+]i oscillations. In plants, an external Ca2+-sensing receptor (CAS) serves as a receptor to regulate Ins(1,4,5)P3 levels and then in turn trigger the Ca2+ release. Although cell-surface receptors and IP3Rs are unknown [2,83], it is reasonable to speculate that CAS may also act as a candidate receptor for Ins(1,4,5)P3 after studies using biochemical and single-cell imaging analyses [83]. RACK1 (receptor for activated C-kinase 1), which has highly conserved WD40 repeats, acts as a scaffold protein through interacting with IP3R and other proteins in animal nervous systems [147]. In Arabidopsis, the first crystal structure of RACK1 isoform A has been characterized [148]. Does the WD40 domain of 5PTase family also play a role as a scaffold protein for IP3R in plants? In addition, the functions of Ins(1,4,5)P3-mediated Ca2+ oscillation seems to be more important than Ins(1,4,5)P3 itself in Arabidopsis; do Ins(1,4,5)P3 and Ca2+ share the same receptor?
Until now, most studies have been performed using the dicotyledonous model plant Arabidopsis; few studies have been carried out in rice [15,149]. Whether the function is conserved in monocotyledons (such as rice) is not well understood. Preliminary analysis reveals a difference in the domain structure and isoform numbers of key enzymes in the PLD family [15], suggesting a functional divergence between dicotyledons and monocotyledons. Understanding these differences may provide further information for agricultural improvement.
FUNDING
The study was supported by the National Science Foundation of China [grant numbers 30740006 and 30721061] and Science and Technology Commission of Shanghai Municipality [grant number 08XD14049].
References
- 1.Kuypers F. A. Red cell membrane lipids in hemoglobinopathies. Curr. Mol. Med. 2008;8:633–638. doi: 10.2174/156652408786241429. [DOI] [PubMed] [Google Scholar]
- 2.Munnik T., Irvine R. F., Musgrave A. Phospholipid signalling in plants. Biochim. Biophys. Acta. 1998;1389:222–272. doi: 10.1016/s0005-2760(97)00158-6. [DOI] [PubMed] [Google Scholar]
- 3.Xue H., Chen X., Li G. Involvement of phospholipid signaling in plant growth and hormone effects. Curr. Opin. Plant Biol. 2007;10:483–489. doi: 10.1016/j.pbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Lingaraj T., Donovan J., Li Z., Li P., Doucette A., Harrison S., Ecsedy J. A., Dang L., Zhang W. A high-throughput liposome substrate assay with automated lipid extraction process for PI 3-kinase. J. Biomol. Screen. 2008;13:906–911. doi: 10.1177/1087057108324498. [DOI] [PubMed] [Google Scholar]
- 5.Carricaburu V., Lamia K. A., Lo E., Favereaux L., Payrastre B., Cantley L. C., Rameh L. E. The phosphatidylinositol (PI)-5-phosphate 4-kinase type II enzyme controls insulin signaling by regulating PI-3,4,5-trisphosphate degradation. Proc. Natl. Acad. Sci. U.S.A. 2003;100:9867–9872. doi: 10.1073/pnas.1734038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coburn R. F., Labelle E. F., Baron C. B. Polyamines, PI(4,5)P2, and actin polymerization. J. Cell. Physiol. 2006;209:405–412. doi: 10.1002/jcp.20729. [DOI] [PubMed] [Google Scholar]
- 7.D'Angelo G., Vicinanza M., Di Campli A., De Matteis M. A. The multiple roles of PtdIns(4)P, not just the precursor of PtdIns(4,5)P2. J. Cell Sci. 2008;121:1955–1963. doi: 10.1242/jcs.023630. [DOI] [PubMed] [Google Scholar]
- 8.Coronas S., Ramel D., Pendaries C., Gaits-Iacovoni F., Tronchere H., Payrastre B. PtdIns5P: a little phosphoinositide with big functions? Biochem. Soc. Symp. 2007:117–128. doi: 10.1042/BSS0740117. [DOI] [PubMed] [Google Scholar]
- 9.Liu L., Damen J. E., Ware M., Hughes M., Krystal G. SHIP, a new player in cytokine-induced signalling. Leukemia. 1997;11:181–184. doi: 10.1038/sj.leu.2400559. [DOI] [PubMed] [Google Scholar]
- 10.Meijer H. J., Munnik T. Phospholipid-based signaling in plants. Annu. Rev. Plant Biol. 2003;54:265–306. doi: 10.1146/annurev.arplant.54.031902.134748. [DOI] [PubMed] [Google Scholar]
- 11.Mueller-Roeber B., Pical C. Inositol phospholipid metabolism in Arabidopsis: characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol. 2002;130:22–46. doi: 10.1104/pp.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berdy S. E., Kudla J., Gruissem W., Gillaspy G. E. Molecular characterization of At5PTase1, an inositol phosphatase capable of terminating inositol trisphosphate signaling. Plant Physiol. 2001;126:801–810. doi: 10.1104/pp.126.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin C., Wang X. The Arabidopsis phospholipase D family: characterization of a calcium-independent and phosphatidylcholine-selective PLDζ1 with distinct regulatory domains. Plant Physiol. 2002;128:1057–1068. doi: 10.1104/pp.010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tasma I. M., Brendel V., Whitham S. A., Bhattacharyya M. K. Expression and evolution of the phosphoinositide-specific phospholipase C gene family in Arabidopsis thaliana. Plant Physiol. Biochem. 2008;46:627–637. doi: 10.1016/j.plaphy.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Li G., Lin F., Xue H.-W. Genome-wide analysis of the phospholipase D family in Oryza sativa and functional characterization of PLDβ1 in seed germination. Cell Res. 2007;17:881–894. doi: 10.1038/cr.2007.77. [DOI] [PubMed] [Google Scholar]
- 16.Cruz-Ramírez A., López-Bucio J., Ramírez-Pimentel G., Zurita-Silva A., Sánchez-Calderon L., Ramírez-Chávez E., González-Ortega E., Herrera-Estrella L. The xipotl mutant of Arabidopsis reveals a critical role for phospholipid metabolism in root system development and epidermal cell integrity. Plant Cell. 2004;16:2020–2034. doi: 10.1105/tpc.103.018648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider S., Beyhl D., Hedrich R., Sauer N. Functional and physiological characterization of Arabidopsis INOSITOL TRANSPORTER1, a novel tonoplast-localized transporter for myo-inositol. Plant Cell. 2008;20:1073–1087. doi: 10.1105/tpc.107.055632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lou Y., Gou J. Y., Xue H.-W. PIP5K9, an Arabidopsis phosphatidylinositol monophosphate kinase, interacts with a cytosolic invertase to negatively regulate sugar-mediated root growth. Plant Cell. 2007;19:163–181. doi: 10.1105/tpc.106.045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J., Brearley C. A., Lin W. H., Wang Y., Ye R., Mueller-Roeber B., Xu Z. H., Xue H.-W. A role of Arabidopsis inositol polyphosphate kinase, AtIPK2α, in pollen germination and root growth. Plant Physiol. 2005;137:94–103. doi: 10.1104/pp.104.045427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li G., Xue H.-W. Arabidopsis PLDζ2 regulates vesicle trafficking and is required for auxin response. Plant Cell. 2007;19:281–295. doi: 10.1105/tpc.106.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanteri M. L., Laxalt A. M., Lamattina L. Nitric oxide triggers phosphatidic acid accumulation via phospholipase D during auxin-induced adventitious root formation in cucumber. Plant Physiol. 2008;147:188–198. doi: 10.1104/pp.107.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams M. E., Torabinejad J., Cohick E., Parker K., Drake E. J., Thompson J. E., Hortter M., Dewald D. B. Mutations in the Arabidopsis phosphoinositide phosphatase gene SAC9 lead to overaccumulation of PtdIns(4,5)P2 and constitutive expression of the stress-response pathway. Plant Physiol. 2005;138:686–700. doi: 10.1104/pp.105.061317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monteiro D., Liu Q., Lisboa S., Scherer G. E., Quader H., Malho R. Phosphoinositides and phosphatidic acid regulate pollen tube growth and reorientation through modulation of [Ca2+]c and membrane secretion. J. Exp. Bot. 2005;56:1665–1674. doi: 10.1093/jxb/eri163. [DOI] [PubMed] [Google Scholar]
- 24.Vincent P., Chua M., Nogue F., Fairbrother A., Mekeel H., Xu Y., Allen N., Bibikova T. N., Gilroy S., Bankaitis V. A. A Sec14p-nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. J. Cell Biol. 2005;168:801–812. doi: 10.1083/jcb.200412074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohashi Y., Oka A., Rodrigues-Pousada R., Possenti M., Ruberti I., Morelli G., Aoyama T. Modulation of phospholipid signaling by GLABRA2 in root-hair pattern formation. Science. 2003;300:1427–1430. doi: 10.1126/science.1083695. [DOI] [PubMed] [Google Scholar]
- 26.Lee Y., Bak G., Choi Y., Chuang W. I., Cho H. T. Roles of phosphatidylinositol 3-kinase in root hair growth. Plant Physiol. 2008;147:624–635. doi: 10.1104/pp.108.117341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preuss M. L., Schmitz A. J., Thole J. M., Bonner H. K., Otegui M. S., Nielsen E. A role for the RabA4b effector protein PI-4Kβ1 in polarized expansion of root hair cells in Arabidopsis thaliana. J. Cell Biol. 2006;172:991–998. doi: 10.1083/jcb.200508116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thole J. M., Vermeer J. E., Zhang Y., Gadella T. W., Jr, Nielsen E. ROOT HAIR DEFECTIVE4 encodes a phosphatidylinositol-4-phosphate phosphatase required for proper root hair development in Arabidopsis thaliana. Plant Cell. 2008;20:381–395. doi: 10.1105/tpc.107.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kusano H., Testerink C., Vermeer J. E., Tsuge T., Shimada H., Oka A., Munnik T., Aoyama T. The Arabidopsis phosphatidylinositol phosphate 5-kinase PIP5K3 is a key regulator of root hair tip growth. Plant Cell. 2008;20:367–380. doi: 10.1105/tpc.107.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stenzel I., Ischebeck T., Konig S., Holubowska A., Sporysz M., Hause B., Heilmann I. The type B phosphatidylinositol-4-phosphate 5-kinase 3 is essential for root hair formation in Arabidopsis thaliana. Plant Cell. 2008;20:124–141. doi: 10.1105/tpc.107.052852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peleg-Grossman S., Volpin H., Levine A. Root hair curling and Rhizobium infection in Medicago truncatula are mediated by phosphatidylinositide-regulated endocytosis and reactive oxygen species. J. Exp. Bot. 2007;58:1637–1649. doi: 10.1093/jxb/erm013. [DOI] [PubMed] [Google Scholar]
- 32.Lee Y., Kim E. S., Choi Y., Hwang I., Staiger C. J., Chung Y. Y. The Arabidopsis phosphatidylinositol 3-kinase is important for pollen development. Plant Physiol. 2008;147:1886–1897. doi: 10.1104/pp.108.121590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alves-Ferreira M., Wellmer F., Banhara A., Kumar V., Riechmann J. L., Meyerowitz E. M. Global expression profiling applied to the analysis of Arabidopsis stamen development. Plant Physiol. 2007;145:747–762. doi: 10.1104/pp.107.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ischebeck T., Stenzel I., Heilmann I. Type B phosphatidylinositol-4-phosphate 5-kinases mediate Arabidopsis and Nicotiana tabacum pollen tube growth by regulating apical pectin secretion. Plant Cell. 2008;20:3312–3330. doi: 10.1105/tpc.108.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sousa E., Kost B., Malho R. Arabidopsis phosphatidylinositol-4-monophosphate 5-kinase 4 regulates pollen tube growth and polarity by modulating membrane recycling. Plant Cell. 2008;20:3050–3064. doi: 10.1105/tpc.108.058826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potocky M., Elias M., Profotova B., Novotna Z., Valentova O., Zarsky V. Phosphatidic acid produced by phospholipase D is required for tobacco pollen tube growth. Planta. 2003;217:122–130. doi: 10.1007/s00425-002-0965-4. [DOI] [PubMed] [Google Scholar]
- 37.Zonia L., Munnik T. Osmotically induced cell swelling versus cell shrinking elicits specific changes in phospholipid signals in tobacco pollen tubes. Plant Physiol. 2004;134:813–823. doi: 10.1104/pp.103.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helling D., Possart A., Cottier S., Klahre U., Kost B. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell. 2006;18:3519–3534. doi: 10.1105/tpc.106.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carland F. M., Nelson T. COTYLEDON VASCULAR PATTERN2-mediated inositol (1,4,5) triphosphate signal transduction is essential for closed venation patterns of Arabidopsis foliar organs. Plant Cell. 2004;16:1263–1275. doi: 10.1105/tpc.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin W. H., Wang Y., Mueller-Roeber B., Brearley C. A., Xu Z. H., Xue H.-W. At5PTase13 modulates cotyledon vein development through regulating auxin homeostasis. Plant Physiol. 2005;139:1677–1691. doi: 10.1104/pp.105.067140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong R., Burk D. H., Morrison W. H., 3rd, Ye Z. H. FRAGILE FIBER3, an Arabidopsis gene encoding a type II inositol polyphosphate 5-phosphatase, is required for secondary wall synthesis and actin organization in fiber cells. Plant Cell. 2004;16:3242–3259. doi: 10.1105/tpc.104.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong R., Burk D. H., Nairn C. J., Wood-Jones A., Morrison W. H., Ye Z. H. Mutation of SAC1, an Arabidopsis SAC domain phosphoinositide phosphatase, causes alterations in cell morphogenesis, cell wall synthesis, and actin organization. Plant Cell. 2005;17:1449–1466. doi: 10.1105/tpc.105.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunesekera B., Torabinejad J., Robinson J., Gillaspy G. E. Inositol polyphosphate 5-phosphatases 1 and 2 are required for regulating seedling growth. Plant Physiol. 2007;143:1408–1417. doi: 10.1104/pp.106.089474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W., Qin C., Zhao J., Wang X. Phospholipase Dα1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9508–9513. doi: 10.1073/pnas.0402112101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishra G., Zhang W., Deng F., Zhao J., Wang X. A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science. 2006;312:264–266. doi: 10.1126/science.1123769. [DOI] [PubMed] [Google Scholar]
- 46.Yun H. S., Joo S. H., Kaufman P. B., Kim T. W., Kirakosyan A., Philosoph-Hadas S., Kim S. K., Chang S. C. Changes in starch and inositol 1,4,5-trisphosphate levels and auxin transport are interrelated in graviresponding oat (Avena sativa) shoots. Plant Cell Environ. 2006;29:2100–2111. doi: 10.1111/j.1365-3040.2006.01584.x. [DOI] [PubMed] [Google Scholar]
- 47.Scherer G. F., Zahn M., Callis J., Jones A. M. A role for phospholipase A in auxin-regulated gene expression. FEBS Lett. 2007;581:4205–4211. doi: 10.1016/j.febslet.2007.07.059. [DOI] [PubMed] [Google Scholar]
- 48.Lee H. Y., Bahn S. C., Kang Y. M., Lee K. H., Kim H. J., Noh E. K., Palta J. P., Shin J. S., Ryu S. B. Secretory low molecular weight phospholipase A2 plays important roles in cell elongation and shoot gravitropism in Arabidopsis. Plant Cell. 2003;15:1990–2002. doi: 10.1105/tpc.014423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Testerink C., Larsen P. B., van der Does D., van Himbergen J. A., Munnik T. Phosphatidic acid binds to and inhibits the activity of Arabidopsis CTR1. J. Exp. Bot. 2007;58:3905–3914. doi: 10.1093/jxb/erm243. [DOI] [PubMed] [Google Scholar]
- 50.Lin W. H., Ye R., Ma H., Xu Z. H., Xue H.-W. DNA chip-based expression profile analysis indicates involvement of the phosphatidylinositol signaling pathway in multiple plant responses to hormone and abiotic treatments. Cell Res. 2004;14:34–45. doi: 10.1038/sj.cr.7290200. [DOI] [PubMed] [Google Scholar]
- 51.DeWald D. B., Torabinejad J., Jones C. A., Shope J. C., Cangelosi A. R., Thompson J. E., Prestwich G. D., Hama H. Rapid accumulation of phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate correlates with calcium mobilization in salt-stressed Arabidopsis. Plant Physiol. 2001;126:759–769. doi: 10.1104/pp.126.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parre E., Ghars M. A., Leprince A. S., Thiery L., Lefebvre D., Bordenave M., Richard L., Mazars C., Abdelly C., Savoure A. Calcium signaling via phospholipase C is essential for proline accumulation upon ionic but not nonionic hyperosmotic stresses in Arabidopsis. Plant Physiol. 2007;144:503–512. doi: 10.1104/pp.106.095281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konig S., Hoffmann M., Mosblech A., Heilmann I. Determination of content and fatty acid composition of unlabeled phosphoinositide species by thin-layer chromatography and gas chromatography. Anal. Biochem. 2008;378:197–201. doi: 10.1016/j.ab.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 54.Leshem Y., Seri L., Levine A. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J. 2007;51:185–197. doi: 10.1111/j.1365-313X.2007.03134.x. [DOI] [PubMed] [Google Scholar]
- 55.Hong Y., Pan X., Welti R., Wang X. Phospholipase Dα3 is involved in the hyperosmotic response in Arabidopsis. Plant Cell. 2008;20:803–816. doi: 10.1105/tpc.107.056390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bargmann B. O., Laxalt A. M., ter Riet B., van Schooten B., Merquiol E., Testerink C., Haring M. A., Bartels D., Munnik T. Multiple PLDs required for high salinity and water deficit tolerance in plants. Plant Cell Physiol. 2009;50:78–89. doi: 10.1093/pcp/pcn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang C. R., Yang A. F., Yue G. D., Gao Q., Yin H. Y., Zhang J. R. Enhanced expression of phospholipase C 1 (ZmPLC1) improves drought tolerance in transgenic maize. Planta. 2008;227:1127–1140. doi: 10.1007/s00425-007-0686-9. [DOI] [PubMed] [Google Scholar]
- 58.Sang Y., Zheng S., Li W., Huang B., Wang X. Regulation of plant water loss by manipulating the expression of phospholipase Dα. Plant J. 2001;28:135–144. doi: 10.1046/j.1365-313x.2001.01138.x. [DOI] [PubMed] [Google Scholar]
- 59.Mane S. P., Vasquez-Robinet C., Sioson A. A., Heath L. S., Grene R. Early PLDα-mediated events in response to progressive drought stress in Arabidopsis: a transcriptome analysis. J. Exp. Bot. 2007;58:241–252. doi: 10.1093/jxb/erl262. [DOI] [PubMed] [Google Scholar]
- 60.Katagiri T., Takahashi S., Shinozaki K. Involvement of a novel Arabidopsis phospholipase D, AtPLDδ, in dehydration-inducible accumulation of phosphatidic acid in stress signalling. Plant J. 2001;26:595–605. doi: 10.1046/j.1365-313x.2001.01060.x. [DOI] [PubMed] [Google Scholar]
- 61.Perera I. Y., Hung C. Y., Moore C. D., Stevenson-Paulik J., Boss W. F. Transgenic Arabidopsis plants expressing the type 1 inositol 5-phosphatase exhibit increased drought tolerance and altered abscisic acid signaling. Plant Cell. 2008;20:2876–2893. doi: 10.1105/tpc.108.061374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vergnolle C., Vaultier M. N., Taconnat L., Renou J. P., Kader J. C., Zachowski A., Ruelland E. The cold-induced early activation of phospholipase C and D pathways determines the response of two distinct clusters of genes in Arabidopsis cell suspensions. Plant Physiol. 2005;139:1217–1233. doi: 10.1104/pp.105.068171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Welti R., Li W., Li M., Sang Y., Biesiada H., Zhou H. E., Rajashekar C. B., Williams T. D., Wang X. Profiling membrane lipids in plant stress responses: role of phospholipase Dα in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- 64.Rajashekar C. B., Zhou H. E., Zhang Y., Li W., Wang X. Suppression of phospholipase Dα1 induces freezing tolerance in Arabidopsis: response of cold-responsive genes and osmolyte accumulation. J. Plant Physiol. 2006;163:916–926. doi: 10.1016/j.jplph.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Li W., Li M., Zhang W., Welti R., Wang X. The plasma membrane-bound phospholipase Dδ enhances freezing tolerance in Arabidopsis thaliana. Nat. Biotechnol. 2004;22:427–433. doi: 10.1038/nbt949. [DOI] [PubMed] [Google Scholar]
- 66.Li W., Wang R., Li M., Li L., Wang C., Welti R., Wang X. Differential degradation of extraplastidic and plastidic lipids during freezing and post-freezing recovery in Arabidopsis thaliana. J. Biol. Chem. 2008;283:461–468. doi: 10.1074/jbc.M706692200. [DOI] [PubMed] [Google Scholar]
- 67.Viswanathan C., Zhu J. K. Molecular genetic analysis of cold-regulated gene transcription. Philos. Trans. R. Soc. London Ser. B. 2002;357:877–886. doi: 10.1098/rstb.2002.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang W., Wang C., Qin C., Wood T., Olafsdottir G., Welti R., Wang X. The oleate-stimulated phospholipase D, PLDδ, and phosphatidic acid decrease H2O2-induced cell death in Arabidopsis. Plant Cell. 2003;15:2285–2295. doi: 10.1105/tpc.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laxalt A. M., Munnik T. Phospholipid signalling in plant defence. Curr. Opin. Plant Biol. 2002;5:332–338. doi: 10.1016/s1369-5266(02)00268-6. [DOI] [PubMed] [Google Scholar]
- 70.Park J., Gu Y., Lee Y., Yang Z., Lee Y. Phosphatidic acid induces leaf cell death in Arabidopsis by activating the Rho-related small G protein GTPase-mediated pathway of reactive oxygen species generation. Plant Physiol. 2004;134:129–136. doi: 10.1104/pp.103.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryu S. B., Wang X. Activation of phospholipase D and the possible mechanism of activation in wound-induced lipid hydrolysis in castor bean leaves. Biochim. Biophys. Acta. 1996;1303:243–250. doi: 10.1016/0005-2760(96)00096-3. [DOI] [PubMed] [Google Scholar]
- 72.Wang C., Zien C. A., Afitlhile M., Welti R., Hildebrand D. F., Wang X. Involvement of phospholipase D in wound-induced accumulation of jasmonic acid in Arabidopsis. Plant Cell. 2000;12:2237–2246. doi: 10.1105/tpc.12.11.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee S., Hirt H., Lee Y. Phosphatidic acid activates a wound-activated MAPK in Glycine max. Plant J. 2001;26:479–486. doi: 10.1046/j.1365-313x.2001.01037.x. [DOI] [PubMed] [Google Scholar]
- 74.Cruz-Ramírez A., Oropeza-Aburto A., Razo-Hernandez F., Ramírez-Chávez E., Herrera-Estrella L. Phospholipase Dζ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Arabidopsis roots. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6765–6770. doi: 10.1073/pnas.0600863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li M., Qin C., Welti R., Wang X. Double knockouts of phospholipases Dζ1 and Dζ2 in Arabidopsis affect root elongation during phosphate-limited growth but do not affect root hair patterning. Plant Physiol. 2006;140:761–770. doi: 10.1104/pp.105.070995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hong Y., Devaiah S. P., Bahn S. C., Thamasandra B. N., Li M., Welti R., Wang X. Phospholipase Dε and phosphatidic acid enhance Arabidopsis nitrogen signaling and growth. Plant J. 2009;58:376–387. doi: 10.1111/j.1365-313X.2009.03788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Jong C. F., Laxalt A. M., Bargmann B. O., de Wit P. J., Joosten M. H., Munnik T. Phosphatidic acid accumulation is an early response in the Cf-4/Avr4 interaction. Plant J. 2004;39:1–12. doi: 10.1111/j.1365-313X.2004.02110.x. [DOI] [PubMed] [Google Scholar]
- 78.Mizoi J., Nakamura M., Nishida I. Defects in CTP:PHOSPHORYLETHANOLAMINE CYTIDYLYLTRANSFERASE affect embryonic and postembryonic development in Arabidopsis. Plant Cell. 2006;18:3370–3385. doi: 10.1105/tpc.106.040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu B., Wakao S., Fan J., Benning C. Loss of plastidic lysophosphatidic acid acyltransferase causes embryo-lethality in Arabidopsis. Plant Cell Physiol. 2004;45:503–510. doi: 10.1093/pcp/pch064. [DOI] [PubMed] [Google Scholar]
- 80.Chen X., Lin W. H., Wang Y., Luan S., Xue H.-W. An inositol polyphosphate 5-phosphatase functions in PHOTOTROPIN1 signaling in Arabidopsis by altering cytosolic Ca2+ Plant Cell. 2008;20:353–366. doi: 10.1105/tpc.107.052670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qin Z. X., Chen Q. J., Tong Z., Wang X. C. The Arabidopsis inositol 1,3,4-trisphosphate 5/6 kinase, AtItpk-1, is involved in plant photomorphogenesis under red light conditions, possibly via interaction with COP9 signalosome. Plant Physiol. Biochem. 2005;43:947–954. doi: 10.1016/j.plaphy.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 82.Lee Y., Kim Y. W., Jeon B. W., Park K. Y., Suh S. J., Seo J., Kwak J. M., Martinoia E., Hwang I., Lee Y. Phosphatidylinositol 4,5-bisphosphate is important for stomatal opening. Plant J. 2007;52:803–816. doi: 10.1111/j.1365-313X.2007.03277.x. [DOI] [PubMed] [Google Scholar]
- 83.Tang R. H., Han S., Zheng H., Cook C. W., Choi C. S., Woerner T. E., Jackson R. B., Pei Z. M. Coupling diurnal cytosolic Ca2+ oscillations to the CAS-IP3 pathway in Arabidopsis. Science. 2007;315:1423–1426. doi: 10.1126/science.1134457. [DOI] [PubMed] [Google Scholar]
- 84.Ananieva E. A., Gillaspy G. E., Ely A., Burnette R. N., Erickson F. L. Interaction of the WD40 domain of a myoinositol polyphosphate 5-phosphatase with SnRK1 links inositol, sugar, and stress signaling. Plant Physiol. 2008;148:1868–1882. doi: 10.1104/pp.108.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishiguro S., Kawai-Oda A., Ueda J., Nishida I., Okada K. The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell. 2001;13:2191–2209. doi: 10.1105/tpc.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seo J., Lee H. Y., Choi H., Choi Y., Lee Y., Kim Y. W., Ryu S. B., Lee Y. Phospholipase A2β mediates light-induced stomatal opening in Arabidopsis. J. Exp. Bot. 2008;59:3587–3594. doi: 10.1093/jxb/ern208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanchez J. P., Chua N. H. Arabidopsis PLC1 is required for secondary responses to abscisic acid signals. Plant Cell. 2001;13:1143–1154. doi: 10.1105/tpc.13.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Devaiah S. P., Roth M. R., Baughman E., Li M., Tamura P., Jeannotte R., Welti R., Wang X. Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a phospholipase Dα1 knockout mutant. Phytochemistry. 2006;67:1907–1924.. doi: 10.1016/j.phytochem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 89.Li W., Li M., Zhang W., Wang X. The plasma membrane bound phospholipase Dδ enhances freezing tolerance in Arabidopsis thaliana. Nat. Biotechnol. 2004;22:427–433. doi: 10.1038/nbt949. [DOI] [PubMed] [Google Scholar]
- 90.Jones M. A., Raymond M. J., Smirnoff N. Analysis of the root-hair morphogenesis transcriptome reveals the molecular identity of six genes with roles in root-hair development in Arabidopsis. Plant J. 2006;45:83–100. doi: 10.1111/j.1365-313X.2005.02609.x. [DOI] [PubMed] [Google Scholar]
- 91.Ercetin M. E., Ananieva E. A., Safaee N. M., Torabinejad J., Robinson J. Y., Gillaspy G. E. A phosphatidylinositol phosphate-specific myo-inositol polyphosphate 5-phosphatase required for seedling growth. Plant Mol. Biol. 2008;67:375–388. doi: 10.1007/s11103-008-9327-3. [DOI] [PubMed] [Google Scholar]
- 92.Niu X., Chen Q., Wang X. OsITL1 gene encoding an inositol 1,3,4-trisphosphate 5/6-kinase is a negative regulator of osmotic stress signaling. Biotechnol. Lett. 2008;30:1687–1692. doi: 10.1007/s10529-008-9730-5. [DOI] [PubMed] [Google Scholar]
- 93.Testerink C., Munnik T. Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci. 2005;10:368–375. doi: 10.1016/j.tplants.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Mikami K., Katagiri T., Iuchi S., Yamaguchi-Shinozaki K., Shinozaki K. A gene encoding phosphatidylinositol-4-phosphate 5-kinase is induced by water stress and abscisic acid in Arabidopsis thaliana. Plant J. 1998;15:563–568. doi: 10.1046/j.1365-313x.1998.00227.x. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Z. B., Yang G., Arana F., Chen Z., Li Y., Xia H. J. Arabidopsis inositol polyphosphate 6-/3-kinase (AtIpk2β) is involved in axillary shoot branching via auxin signaling. Plant Physiol. 2007;144:942–951. doi: 10.1104/pp.106.092163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zien C. A., Wang C., Wang X., Welti R. in vivo substrates and the contribution of the common phospholipase D, PLDα, to wound-induced metabolism of lipids in Arabidopsis. Biochim. Biophys. Acta. 2001;1530:236–248. doi: 10.1016/s1388-1981(01)00091-9. [DOI] [PubMed] [Google Scholar]
- 97.Lakin-Thomas P. L. Effects of inositol starvation on the levels of inositol phosphates and inositol lipids in Neurospora crassa. Biochem. J. 1993;292:805–811. doi: 10.1042/bj2920805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamaguchi T., Minami E., Ueki J., Shibuya N. Elicitor-induced activation of phospholipases plays an important role for the induction of defense responses in suspension-cultured rice cells. Plant Cell Physiol. 2005;46:579–587. doi: 10.1093/pcp/pci065. [DOI] [PubMed] [Google Scholar]
- 99.Gonorazky G., Laxalt A. M., Testerink C., Munnik T., de la Canal L. Phosphatidylinositol 4-phosphate accumulates extracellularly upon xylanase treatment in tomato cell suspensions. Plant Cell Environ. 2008;31:1051–1062. doi: 10.1111/j.1365-3040.2008.01818.x. [DOI] [PubMed] [Google Scholar]
- 100.Meijer H. J., Berrie C. P., Iurisci C., Divecha N., Musgrave A., Munnik T. Identification of a new polyphosphoinositide in plants, phosphatidylinositol 5-monophosphate (PtdIns5P), and its accumulation upon osmotic stress. Biochem. J. 2001;360:491–498. doi: 10.1042/0264-6021:3600491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perera I. Y., Hung C. Y., Brady S., Muday G. K., Boss W. F. A universal role for inositol 1,4,5-trisphosphate-mediated signaling in plant gravitropism. Plant Physiol. 2006;140:746–760. doi: 10.1104/pp.105.075119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perera I. Y., Heilmann I., Boss W. F. Transient and sustained increases in inositol 1,4,5-trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5838–5843. doi: 10.1073/pnas.96.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holdaway-Clarke T. L., Weddle N. M., Kim S., Robi A., Parris C., Kunkel J. G., Hepler P. K. Effect of extracellular calcium, pH and borate on growth oscillations in Lilium formosanum pollen tubes. J. Exp. Bot. 2003;54:65–72. doi: 10.1093/jxb/erg004. [DOI] [PubMed] [Google Scholar]
- 104.Knight H., Trewavas A. J., Knight M. R. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Knight H., Knight M. R. Abiotic stress signalling pathways: specificity and cross-talk. Trends Plant Sci. 2001;6:262–267. doi: 10.1016/s1360-1385(01)01946-x. [DOI] [PubMed] [Google Scholar]
- 106.Pappan K., Zheng L., Krishnamoorthi R., Wang X. Evidence for and characterization of Ca2+ binding to the catalytic region of Arabidopsis thaliana phospholipase Dβ. J. Biol. Chem. 2004;279:47833–47839. doi: 10.1074/jbc.M402789200. [DOI] [PubMed] [Google Scholar]
- 107.Pappan K., Zheng S., Wang X. Identification and characterization of a novel plant phospholipase D that requires polyphosphoinositides and submicromolar calcium for activity in Arabidopsis. J. Biol. Chem. 1997;272:7048–7054. doi: 10.1074/jbc.272.11.7048. [DOI] [PubMed] [Google Scholar]
- 108.Zheng L., Krishnamoorthi R., Zolkiewski M., Wang X. Distinct Ca2+ binding properties of novel C2 domains of plant phospholipase Dα and β. J. Biol. Chem. 2000;275:19700–19706. doi: 10.1074/jbc.M001945200. [DOI] [PubMed] [Google Scholar]
- 109.Zheng L., Shan J., Krishnamoorthi R., Wang X. Activation of plant phospholipase Dβ by phosphatidylinositol 4,5-bisphosphate: characterization of binding site and mode of action. Biochemistry. 2002;41:4546–4553. doi: 10.1021/bi0158775. [DOI] [PubMed] [Google Scholar]
- 110.Rebecchi M. J., Pentyala S. N. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- 111.Zegzouti H., Anthony R. G., Jahchan N., Bogre L., Christensen S. K. Phosphorylation and activation of PINOID by the phospholipid signaling kinase 3-phosphoinositide-dependent protein kinase 1 (PDK1) in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6404–6409. doi: 10.1073/pnas.0510283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Anthony R. G., Henriques R., Helfer A., Meszaros T., Rios G., Testerink C., Munnik T., Deak M., Koncz C., Bogre L. A protein kinase target of a PDK1 signalling pathway is involved in root hair growth in Arabidopsis. EMBO J. 2004;23:572–581. doi: 10.1038/sj.emboj.7600068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Michniewicz M., Zago M. K., Abas L., Weijers D., Schweighofer A., Meskiene I., Heisler M. G., Ohno C., Zhang J., Huang F., et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell. 2007;130:1044–1056. doi: 10.1016/j.cell.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 114.Testerink C., Dekker H. L., Lim Z. Y., Johns M. K., Holmes A. B., Koster C. G., Ktistakis N. T., Munnik T. Isolation and identification of phosphatidic acid targets from plants. Plant J. 2004;39:527–536. doi: 10.1111/j.1365-313X.2004.02152.x. [DOI] [PubMed] [Google Scholar]
- 115.Jaillais Y., Fobis-Loisy I., Miege C., Rollin C., Gaude T. AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature. 2006;443:106–109. doi: 10.1038/nature05046. [DOI] [PubMed] [Google Scholar]
- 116.Kleine-Vehn J., Leitner J., Zwiewka M., Sauer M., Abas L., Luschnig C., Friml J. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc. Natl. Acad. Sci. U.S.A. 2008;105:17812–17817. doi: 10.1073/pnas.0808073105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matsuoka K., Bassham D. C., Raikhel N. V., Nakamura K. Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J. Cell Biol. 1995;130:1307–1318. doi: 10.1083/jcb.130.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.daSilva L. L., Taylor J. P., Hadlington J. L., Hanton S. L., Snowden C. J., Fox S. J., Foresti O., Brandizzi F., Denecke J. Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell. 2005;17:132–148. doi: 10.1105/tpc.104.026351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kieber J. J., Rothenberg M., Roman G., Feldmann K. A., Ecker J. R. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- 120.den Hartog M., Musgrave A., Munnik T. Nod factor-induced phosphatidic acid and diacylglycerol pyrophosphate formation: a role for phospholipase C and D in root hair deformation. Plant J. 2001;25:55–65. doi: 10.1046/j.1365-313x.2001.00931.x. [DOI] [PubMed] [Google Scholar]
- 121.Katagiri T., Ishiyama K., Kato T., Tabata S., Kobayashi M., Shinozaki K. An important role of phosphatidic acid in ABA signaling during germination in Arabidopsis thaliana. Plant J. 2005;43:107–117. doi: 10.1111/j.1365-313X.2005.02431.x. [DOI] [PubMed] [Google Scholar]
- 122.Ruelland E., Cantrel C., Gawer M., Kader J. C., Zachowski A. Activation of phospholipases C and D is an early response to a cold exposure in Arabidopsis suspension cells. Plant Physiol. 2002;130:999–1007. doi: 10.1104/pp.006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Munnik T., Meijer H. J., Ter Riet B., Hirt H., Frank W., Bartels D., Musgrave A. Hyperosmotic stress stimulates phospholipase D activity and elevates the levels of phosphatidic acid and diacylglycerol pyrophosphate. Plant J. 2000;22:147–154. doi: 10.1046/j.1365-313x.2000.00725.x. [DOI] [PubMed] [Google Scholar]
- 124.Thiery L., Leprince A. S., Lefebvre D., Ghars M. A., Debarbieux E., Savoure A. Phospholipase D is a negative regulator of proline biosynthesis in Arabidopsis thaliana. J. Biol. Chem. 2004;279:14812–14818. doi: 10.1074/jbc.M308456200. [DOI] [PubMed] [Google Scholar]
- 125.Liu Y., Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell. 2004;16:3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Janmey P. A. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu. Rev. Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- 127.Simonsen A., Wurmser A. E., Emr S. D., Stenmark H. The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol. 2001;13:485–492. doi: 10.1016/s0955-0674(00)00240-4. [DOI] [PubMed] [Google Scholar]
- 128.Takenawa T., Itoh T. Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim. Biophys. Acta. 2001;1533:190–206. doi: 10.1016/s1388-1981(01)00165-2. [DOI] [PubMed] [Google Scholar]
- 129.Yin H. L., Janmey P. A. Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- 130.Downes C. P., Gray A., Lucocq J. M. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 2005;15:259–268. doi: 10.1016/j.tcb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 131.Meagher R. B., McKinney E. C., Kandasamy M. K. Isovariant dynamics expand and buffer the responses of complex systems: the diverse plant actin gene family. Plant Cell. 1999;11:995–1006. doi: 10.1105/tpc.11.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kost B., Lemichez E., Spielhofer P., Hong Y., Tolias K., Carpenter C., Chua N. H. Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol. 1999;145:317–330. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Davis A. J., Im Y. J., Dubin J. S., Tomer K. B., Boss W. F. Arabidopsis phosphatidylinositol phosphate kinase 1 binds F-actin and recruits phosphatidylinositol 4-kinase β1 to the actin cytoskeleton. J. Biol. Chem. 2007;282:14121–14131. doi: 10.1074/jbc.M611728200. [DOI] [PubMed] [Google Scholar]
- 134.Dowd P. E., Coursol S., Skirpan A. L., Kao T. H., Gilroy S. Petunia phospholipase C1 is involved in pollen tube growth. Plant Cell. 2006;18:1438–1453. doi: 10.1105/tpc.106.041582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee S., Park J., Lee Y. Phosphatidic acid induces actin polymerization by activating protein kinases in soybean cells. Mol. Cells. 2003;15:313–319. [PubMed] [Google Scholar]
- 136.Kusner D. J., Barton J. A., Qin C., Wang X., Iyer S. S. Evolutionary conservation of physical and functional interactions between phospholipase D and actin. Arch. Biochem. Biophys. 2003;412:231–241. doi: 10.1016/s0003-9861(03)00052-3. [DOI] [PubMed] [Google Scholar]
- 137.Choi Y., Lee Y., Jeon B., Staiger C. J., Lee Y. Phosphatidylinositol 3- and 4-phosphate modulate actin filament reorganization in guard cells of day flower. Plant Cell Environ. 2008;31:366–377. doi: 10.1111/j.1365-3040.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- 138.Gardiner J. C., Collings D. A., Harper J. D. I., Marc J. The effects of the phospholipase D-antagonist 1-butanol on seedling development and microtubule organisation in Arabidopsis. Plant Cell Physiol. 2003;44:687–696. doi: 10.1093/pcp/pcg095. [DOI] [PubMed] [Google Scholar]
- 139.Peters N. T., Logan K. O., Miller A. C., Kropf D. L. Phospholipase D signaling regulates microtubule organization in the fucoid alga Silvetia compressa. Plant Cell Physiol. 2007;48:1764–1774. doi: 10.1093/pcp/pcm149. [DOI] [PubMed] [Google Scholar]
- 140.Dhonukshe P., Laxalt A. M., Goedhart J., Gadella T. W. J., Munnik T. Phospholipase D activation correlates with microtubule reorganization in living plant cells. Plant Cell. 2003;15:2666–2679. doi: 10.1105/tpc.014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gardiner J. C., Harper J. D. I., Weerakoon N. D., Collings D. A., Ritchie S., Gilroy S., Marc J. A 90-kD phospholipase D from tobacco binds to microtubules and the plasma membrane. Plant Cell. 2001;13:2143–2158. doi: 10.1105/TPC.010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bargmann B. O., Munnik T. The role of phospholipase D in plant stress responses. Curr. Opin. Plant Biol. 2006;9:515–522. doi: 10.1016/j.pbi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 143.Pejchar P., Pleskot R., Schwarzerová K., Martinec J., Valentová O., Novotna Z. Aluminum ions inhibit phospholipase D in a microtubule-dependent manner. Cell Biol. Int. 2008;32:554–556. doi: 10.1016/j.cellbi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 144.Westergren T., Dove S. T., Sommarin M., Pical C. AtPIP5K1, an Arabidopsis thaliana phosphatidylinositol phosphate kinase, synthesizes PtdIns(3,4)P2 and PtdIns(4,5)P2 in vitro and is inhibited by phosphorylation. Biochem. J. 2001;359:583–589. doi: 10.1042/0264-6021:3590583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lou Y., Ma H., Lin W. H., Chu Z. Q., Mueller-Roeber B., Xu Z. H., Xue H.-W. The highly charged region of plant β-type phosphatidylinositol 4-kinase is involved in membrane targeting and phospholipid binding. Plant Mol. Biol. 2006;61:215–226. doi: 10.1007/s11103-005-5548-x. [DOI] [PubMed] [Google Scholar]
- 146.Ma H., Lou Y., Lin W. H., Xue H.-W. MORN motifs in plant PIPKs are involved in the regulation of subcellular localization and phospholipid binding. Cell Res. 2006;16:466–478. doi: 10.1038/sj.cr.7310058. [DOI] [PubMed] [Google Scholar]
- 147.Sklan E. H., Podoly E., Soreq H. RACK1 has the nerve to act: structure meets function in the nervous system. Prog. Neurobiol. 2006;78:117–134. doi: 10.1016/j.pneurobio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 148.Ullah H., Scappini E. L., Moon A. F., Williams L. V., Armstrong D. L., Pedersen L. C. Structure of a signal transduction regulator, RACK1, from Arabidopsis thaliana. Protein Sci. 2008;17:1771–1780. doi: 10.1110/ps.035121.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ma H., Xu S. P., Luo D., Xu Z. H., Xue H.-W. OsPIPK1, a rice phosphatidylinositol monophosphate kinase, regulates rice heading by modifying the expression of floral induction genes. Plant Mol. Biol. 2004;54:295–310. doi: 10.1023/B:PLAN.0000028796.14336.24. [DOI] [PubMed] [Google Scholar]