Abstract
Context: Type 2 diabetes is associated with higher fracture risk at a given bone mineral density. Advanced glycation endproducts (AGEs) accumulate in bone collagen with age and diabetes and may weaken bone.
Objective: The aim was to determine whether urine pentosidine, an AGE, was associated with fractures in older adults with and without diabetes.
Design: We performed an observational cohort study.
Setting: We used data from the Health, Aging and Body Composition prospective study of white and black, well-functioning men and women ages 70–79 yr.
Participants: Participants with (n = 501) and without (n = 427) diabetes were matched on gender, race, and study site.
Predictor: Urine pentosidine was assayed from frozen stored baseline specimens.
Main Outcome Measures: Incident clinical fractures and baseline vertebral fractures were measured.
Results: Despite higher bone mineral density, clinical fracture incidence (14.8 vs. 12.6%) and vertebral fracture prevalence (2.3 vs. 2.9%) were not lower in those with diabetes (P > 0.05). In multivariable models, pentosidine was associated with increased clinical fracture incidence in those with diabetes [relative hazard, 1.42; 95% confidence interval (CI), 1.10, 1.83, for 1 sd increase in log pentosidine] but not in those without diabetes (relative hazard, 1.08; 95% CI, 0.79, 1.49; P value for interaction = 0.030). In those with diabetes, pentosidine was associated with increased vertebral fracture prevalence (adjusted odds ratio, 5.93; 95% CI, 2.08, 16.94, for 1 sd increase in log pentosidine) but not in those without diabetes (adjusted odds ratio, 0.74; 95% CI, 0.30, 1.83; P value for interaction = 0.005).
Conclusions: Higher pentosidine levels are a risk factor for fracture in older adults with diabetes and may account in part for reduced bone strength in type 2 diabetes.
In older adults with type 2 diabetes, higher urine pentosidine is a risk factor for incident clinical fractures and prevalent vertebral fractures, independent of bone density.
Older age is associated with bone that is weaker for a given bone density. Similarly, in those with type 2 diabetes, fracture risk appears to be higher for a given bone density (1,2). Accumulation of advanced glycation endproducts (AGEs) in bone collagen has been proposed as a factor reducing bone strength with aging and diabetes. AGEs are formed through a series of nonenzymatic reactions between glucose and proteins resulting in a highly stable cross-linked product. Contributing factors include hyperglycemia, oxidative stress, and reduced renal function (3). Although all proteins are prone to AGE formation, deleterious AGE accumulation occurs in tissues with low turnover, including collagen. This accumulation subtly alters collagen structure and function, increasing stiffness in arteries, skin, cartilage, and bone.
Two studies have reported an association between higher levels of pentosidine, an AGE, and risk of vertebral fractures. In older diabetic women, but not men, increased serum pentosidine was associated with prevalent vertebral fractures (4). In older nondiabetic women, urine pentosidine was associated with prevalent and incident vertebral fractures (5). However, prospective data on AGEs and incident clinical fracture or bone loss are not available. To investigate the role of AGEs in bone health, we used data from the Health, Aging, and Body Composition (Health ABC) Study to determine whether urine pentosidine was associated with incident clinical fracture or prevalent vertebral fracture, as well as bone loss at the hip and bone turnover markers, in older adults with and without diabetes.
Subjects and Methods
Health ABC participants
The Health ABC Study is a prospective study investigating whether changes in body composition act as a common pathway by which multiple diseases affect morbidity, disability, and risk of mortality. The cohort consists of 3075 men and women aged 70–79 yr recruited at the University of Pittsburgh and the University of Tennessee, Memphis. Participants were excluded if they reported any difficulty with activities of daily living, walking up 10 steps without resting, or walking a quarter of a mile. Study procedures were approved by the institutional review boards, and written informed consent was provided by all participants. The baseline examination took place during 1997–1998.
Diabetes
For this report, diabetes was defined as use of hypoglycemic medication or elevated fasting glucose (≥126 mg/dl). Using these criteria, 527 participants had diabetes at baseline. Assays of urine pentosidine were available on 501 participants. Of these, 395 (79%) had been previously diagnosed with diabetes, and 106 (21%) participants were found to have diabetes at the baseline visit, based on an elevated fasting glucose.
A sample of 427 participants without diabetes at baseline was taken from those who did not self-report a diagnosis of diabetes or use of hypoglycemic medication and had a fasting glucose less than 110 mg/dl and an oral glucose tolerance test less than 200 mg/dl. These cutpoints were based on the American Diabetes Association (ADA) definition of normal glucose homeostasis in effect from 1997 to 2002 (6). The selected participants were matched to diabetic participants on race, gender, and clinic site. Of the nondiabetic participants, 60 had a fasting glucose between 100 and 110 mg/dl, defined as impaired fasting glucose in current ADA guidelines (7).
Biological specimens
Serum and urine were obtained at baseline after an overnight fast. Specimens were frozen at −70 C and stored at McKesson Bioservices (Rockville, MD). Specimens were retrieved and assayed for bone turnover markers and pentosidine after approximately 5 yr of storage.
Urine pentosidine
Urinary pentosidine was measured on hydrolysated samples by an HPLC technique using purified bone pentosidine as a standard (8). The pentosidine recovery rate was 93 ± 4%, and the assay was linear over the validated amount range of 0–0.5 nmol with a detection limit below 0.02 pmol. Intra- and interassay coefficients of variation (CV) are less than 8% and less than 15%, respectively. Pentosidine measurements were performed by Dr. Evelyne Gineyts (Institut National de la Santé et de la Recherche Médicale Research Unit 831, Lyon, France). Results are reported normalized to urine creatinine (Cr).
Bone turnover markers
Serum N-propeptide of type I procollagen (P1NP) was measured with a two-site immunoassay based on monoclonal antibodies raised against purified intact human P1NP and detecting both intact monomeric and trimeric forms, but not fragments using an automated analyzer (Elecsys; Roche Diagnostics, Indianapolis, IN). Intraassay CV is less than 2%, and interassay CV is less than 4%. Urinary N-terminal crosslinking telopeptide of type I collagen (U-NTX) was measured by ELISA using the Osteomark assay (Ostex International Inc., Seattle, OR) on an automated analyzer (Vitros Eci; Ortho Clinical Diagnostics, Rochester, NY). The intra- and interassay CV are both less than 7%. Results are reported normalized to urine Cr. Serum bone alkaline phosphatase (bone ALP) was measured by an immunochemiluminescence assay using the Ostase reagent on an automatic analyzer (Ostase, Access; Beckman Coulter, Fullerton, CA). The intra- and interassay CV are less than 5% and less than 8%, respectively, and the cross-reactivity with the liver isoenzyme is 13%. Serum C-terminal cross-linking telopeptide of type I collagen (S-CTX) was measured by a two-site assay using monoclonal antibodies raised against an 8-amino acid sequence from the C-telopeptide of human type I collagen by an automatic analyzer (Elecsys; Roche Diagnostics). Intraassay CV is less than 3%, and interassay CV is less than 8%.
Incident clinical fracture
Every 6 months, at clinic visits or by telephone, participants were asked about the occurrence of fractures. Reported fractures were included in analyses if verified by a radiology report. Fractures of the ribs, chest/sternum, skull/face, fingers, toes, and cervical vertebra as well as fractures due to a pathological process, such as cancer, were excluded. Fractures were not excluded based on reported degree of trauma because fractures at all trauma levels are associated with lower bone density (9).
Prevalent vertebral fracture
Lateral scout scans were obtained at the baseline visit to determine placement for computed tomography abdominal scans. The scout scans covered T4 through the upper sacrum. Images were obtained in Pittsburgh using a 9800 Advantage (General Electric, Milwaukee, WI) and in Memphis using a Somatom Plus 4 (Siemens, Erlangen, Germany) or a Picker PQ 2000S (Marconi Medical Systems, Cleveland, OH).
A study comparing CT scout scans with conventional spinal radiographs for the identification of vertebral deformities reported a 23% reduction in vertebral fractures detected from the scout scans, mainly due to reduced sensitivity in the thoracic spine (10). The CT lateral scout scans were assessed and graded by a radiologist for prevalent vertebral deformities (10,11). A semi-quantitative grade of 1 indicates a mild vertebral deformity with 20–25% reduction in anterior, middle, and/or posterior height; grade 2 indicates a moderate deformity with a 25–40% height reduction; and grade 3 indicates a severe deformity with greater than 40% height reduction. The radiologist was blinded to the diabetes status of the participants. Because moderate/severe deformities are more strongly associated with subsequent vertebral fractures than the mild deformities, we defined vertebral fracture as a deformity grading of 2 or greater (12).
Bone mineral density (BMD)
BMD was measured at the proximal femur using dual energy x-ray absorptiometry (DXA) (QDR 4500A, software version 9.03; Hologic, Inc., Bedford, MA). DXA quality assurance procedures, including use of daily and cross-calibration phantoms, were performed at both study sites to ensure scanner reliability. The precision of DXA scans of the hip is 1–2% (13). Measurements were obtained at the baseline exam and at the fourth follow-up exam, with an average time between scans of 4.0 ± 0.1 yr.
Covariates
At the baseline visit, weight was measured with a calibrated balance beam scale. Participants were asked whether they had lost more than 5 pounds in the year before the baseline visit. Height was measured with a Harpenden stadiometer. Body mass index was calculated as weight (kilograms) divided by height (meters) squared. Participants were asked if they had fallen in the previous year. To assess balance, participants were asked to maintain a semitandem, full tandem, and then one-leg balance stand for as long as possible up to 30 sec each. The three times were summed into a standing balance test time (range, 0–90 sec).
Participants were asked to bring prescription and over-the-counter medications used in the previous week to the visit. Medications were coded according to the Iowa Drug Information System (14). In these analyses, bisphosphonates, calcitonin, and raloxifene were grouped together as “osteoporosis medications.”
Laboratory measurements were performed on baseline specimens at the Laboratory of Clinical Biochemistry at the University of Vermont. For measurements of plasma glucose, blood was drawn after an overnight fast (≥8 h). Immediately afterward, participants ingested 75 g glucose in solution (glucola), and a second blood sample was drawn 2 h later. Cr and hemoglobin A1c (A1C) were measured in serum specimens using standard laboratory procedures. Estimated glomerular filtration rate (eGFR) was calculated using the abbreviated Modification of Diet in Renal Disease study equation that includes serum Cr, age, gender, and race (15). Cystatin-C, a marker of renal function, was measured on baseline serum stored at −70 C for an average of 6.5 yr using a BNII nephelometer (Dade Behring Inc., Deerfield, IL) using a particle-enhanced immunonepholometric assay (N Latex Cystatin C; Dade Behring Inc., Deerfield, IL). Intra- and interassay CV for cystatin-C are 2.0–2.8 and 2.3–3.1%, respectively.
Statistical analyses
Baseline characteristics are presented separately for those with and without diabetes. χ2 tests were calculated for categorical variables, and ANOVA was used for continuous variables to test for statistical differences between the two groups. Levels of pentosidine and bone turnover markers were log-transformed to normalize their distributions.
Generalized linear regression was used to analyze the associations between log-transformed pentosidine levels, bone loss at the hip over 4 yr, and baseline bone turnover markers. Proportional hazards models with time to first fracture as the outcome were used to analyze the association between log-transformed pentosidine and risk of clinical fractures. Logistic regression was used to analyze the association between log-transformed pentosidine levels at baseline and odds of prevalent vertebral fractures. We tested for interaction between pentosidine levels and diabetes status in the multivariate models, and reported results separately where statistically significant (P < 0.05) interaction was found.
Results for pentosidine and bone turnover markers, initially expressed as adjusted mean values of the log bone markers for those with and without diabetes, were back-transformed to the original units for ease of interpretation. Geometric means are reported. All statistical analyses were performed in SAS version 9.1 (SAS Institute Inc., Cary, NC).
Results
As reported in previous studies, urine pentosidine levels increased with age and were higher in women (data not shown). Higher pentosidine was associated with poorer renal function (eGFR and cystatin-C), weight loss (>5 pounds) in the year before baseline, a history of falls, and poorer standing balance.
Pentosidine levels were similar in those with and without diabetes in unadjusted models (Table 1) and after adjustment for age, gender, eGFR, and previous weight loss. When participants were stratified into previously diagnosed diabetes, newly diagnosed diabetes, impaired fasting glucose, and normoglycemic participants, pentosidine levels did not differ across these four groups. Pentosidine was not correlated with A1C levels in the diabetic participants (r = 0.021; P = 0.64) or in all participants (r = −0.008; P = 0.81).
Table 1.
Characteristics of older adults by diabetes status (Health Aging and Body Composition Study)
| Diabetes
|
||
|---|---|---|
| No | Yes | |
| n | 427 | 501 |
| Age at baseline (yr) | 73.4 ± 2.9 | 73.6 ± 2.9 |
| Gender (% men) | 57.4 | 56.9 |
| Race (% white) | 43.8 | 43.3 |
| Clinic site (% Memphis) | 49.6 | 49.5 |
| BMI (kg/m2) | 26.9 ± 4.7 | 29.6 ± 4.9a |
| Weight (kg) | 75.8 ± 15.4 | 82.9 ± 14.8a |
| Lost 5+ pounds in previous 12 months (% yes) | 28.4 | 48.9a |
| Cystatin-C (mg/liter) | 1.02 ± 0.29 | 1.10 ± 0.35a |
| Renal insufficiencyb | 16.9 | 24.6a |
| Fell in year before baseline (% yes) | 20.7 | 22.1 |
| Standing balance time (sec) | 70.7 ± 22.2 | 62.0 ± 25.4a |
| Current smoker (% yes) | 12.0 | 9.2 |
| Calcium supplement use (% yes) | 13.3 | 9.4 |
| Vitamin D supplement use (% yes) | 7.3 | 3.8a |
| Oral estrogen use (women only) (% yes) | 20.9 | 14.4 |
| Thiazide diuretic use (% yes) | 18.0 | 21.4 |
| Statin use (% yes) | 9.1 | 13.6a |
| Osteoporosis drug use (% yes)c | 2.6 | 1.6 |
| A1C (%) | 6.0 ± 0.5 | 8.1 ± 1.5a |
| Pentosidine (pmol/mmol Cr)d | 11.8 ± 6.9 | 11.6 ± 6.9 |
| Baseline BMD (g/cm2) | ||
| Hip total | 0.90 ± 0.17 | 0.96 ± 0.17a |
| Femoral neck | 0.75 ± 0.14 | 0.81 ± 0.14a |
| Trochanter | 0.70 ± 0.14 | 0.74 ± 0.15a |
| Change in BMD (%/yr) | ||
| Hip total | −0.5 ± 1.1 | −0.5 ± 1.1 |
| Femoral neck | −0.4 ± 1.4 | −0.6 ± 1.4 |
| Trochanter | −0.5 ± 1.4 | −0.5 ± 1.3 |
| Prevalent vertebral fracture | ||
| Moderate/severe (SQ grade ≥2) (% with at least one deformity) | 2.9 | 2.3 |
| Clinical fracture (% with at least one fracture) | 12.6 | 14.8 |
| In diabetic participants only | ||
| Insulin use (% yes) | 22.0 | |
| Oral hypoglycemic use (% yes) | 52.5 | |
| Diabetes duration (yr) | 9.9 ± 11.5 | |
Data are expressed as mean ± sd or percentage.
P < 0.05, comparing those with and without diabetes.
Defined as eGFR <60 ml/min per 1.73 m2. eGFR estimated with the abbreviated Modification of Diet in Renal Disease (MDRD) study equation that includes serum Cr, age, gender, and race.
Includes bisphosphonates, calcitonin, and raloxifene.
Geometric means based on log-transformed pentosidine.
Incident clinical fracture
During a mean follow-up of 7.5 (sd 2.7) yr, 128 participants experienced at least one confirmed fracture, including 35 hip, 25 clinical spine, and 24 lower forearm fractures. In models adjusted for age, race, and gender, pentosidine was associated with higher risk of clinical fracture in those with diabetes, but not those without diabetes (Table 2). After adjustment for additional potential confounders, pentosidine remained associated with fracture risk among those with diabetes [multivariate-adjusted relative hazard (RH), 1.42; 95% confidence interval (CI), 1.10, 1.83; 1 sd increase in log pentosidine] but was not associated with fracture among those without diabetes (multivariate-adjusted RH, 1.08; 95% CI, 0.79, 1.49; P value for interaction = 0.030). Results were similar after further adjustment for a history of falls and standing balance performance (data not shown). We also considered the possibility of interaction between renal function and pentosidine for the outcome of clinical fractures because renal function is a risk factor for fracture that was correlated with both diabetes and pentosidine, but found no evidence of interaction (P = 0.545).
Table 2.
Relative riska and 95% CI for clinical fractures and prevalent vertebral deformities associated with 1 sd increase in pentosidineb
| ne | Minimally adjustedc
|
Multivariable modeld
|
|||||
|---|---|---|---|---|---|---|---|
| RRa | 95% CI | P | RRa | 95% CI | P | ||
| Clinical fracturef | |||||||
| Diabetes | 74 | 1.50 | 1.22, 1.85 | <0.001 | 1.42 | 1.10, 1.83 | 0.007 |
| No diabetes | 54 | 0.97 | 0.72, 1.30 | 0.817 | 1.08 | 0.79, 1.49 | 0.630 |
| Moderate or severe vertebral deformityg | |||||||
| Diabetes | 11 | 1.67 | 1.05, 2.65 | 0.029 | 5.93 | 2.08, 16.94 | 0.001 |
| No diabetes | 12 | 0.52 | 0.23, 1.17 | 0.112 | 0.74 | 0.30, 1.83 | 0.519 |
Relative hazard for clinical fracture models; odds ratio for vertebral deformity models.
Log-transformed pentosidine.
Adjusted for age, race, and gender.
Adjusted for age, race, gender, current smoker, baseline BMD, baseline weight, weight loss of 5+ pounds in year before baseline, cystatin-C, A1C, and use of vitamin D supplements, calcium supplements, oral steroids, osteoporosis drugs (bisphosphonates, calcitonin, raloxifene), thiazide diuretics, statins, oral estrogen and, in models with diabetic participants, use of insulin, metformin, sulfonylureas, thiazolidinediones, and other oral hypoglycemic medications.
Number of participants with at least one fracture.
P value for interaction between log pentosidine and diabetes status = 0.030.
P value for interaction between log pentosidine and diabetes status = 0.005.
Prevalent vertebral fracture
Prevalent vertebral fractures, defined as moderate/severe vertebral deformities, were found in 23 (2.6%) participants. The expected prevalence in this population, based on previous studies using spine radiographs, is about 6% (16,17,18). The relatively low prevalence of vertebral deformities may result from the use of CT Scout scans, rather than spine radiographs, for detection of vertebral deformities (10). The prevalence was similar in those with and without diabetes (Table 1). In adjusted models (Table 2), pentosidine was associated with prevalent vertebral fracture among those with diabetes (multivariate-adjusted odds ratio, 5.93; 95% CI, 2.08, 16.94; 1 sd increase in log pentosidine), but not in those without diabetes (multivariate-adjusted odds ratio, 0.74; 95% CI, 0.30, 1.83; P value for interaction = 0.005). Results were similar for those with and without diabetes after further adjustment for a history of falls and standing balance performance (data not shown). We did not find evidence of an interaction between renal function and pentosidine for the outcome of prevalent vertebral fractures (P = 0.276).
Bone turnover markers
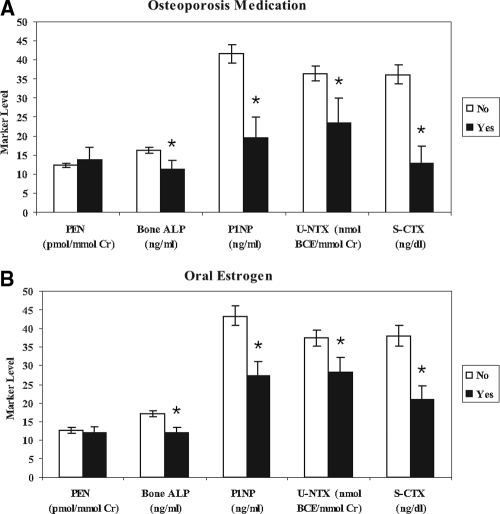
Markers of bone formation and resorption had small but statistically significant positive associations with pentosidine levels. The correlations between log pentosidine and log-transformed bone turnover markers were 0.087 (P = 0.009) for bone ALP, 0.151 (P < 0.001) for P1NP, 0.122 (P < 0.001) for S-CTX, and 0.199 (P < 0.001) for U-NTX. The correlations between bone turnover markers and pentosidine did not differ significantly for those with and without diabetes. To determine whether increased pentosidine was simply a marker of bone turnover, we compared pentosidine levels in women who were and were not using bone-active medications. As expected, bone turnover markers were lower in the women using oral estrogen or an osteoporosis drug, but pentosidine did not differ significantly (Fig. 1). Mean pentosidine was 12.0 and 12.6 pmol/mmol Cr for women who reported estrogen use or no use, respectively. For osteoporosis drugs, mean pentosidine was 13.8 and 12.4 pmol/mmol Cr in users and nonusers, respectively.
Figure 1.
In women, mean (95% CI) levels of pentosidine (PEN) and bone turnover markers were stratified by baseline use of osteoporosis medications (bisphosphonates, raloxifene, or calcitonin) (A) and hormone replacement therapy (B). Bone turnover markers (bone ALP, P1NP, U-NTX, S-CTX) were lower (*, P < 0.01) in those reporting use of an osteoporosis medication or hormone replacement therapy, but pentosidine levels did not differ.
BMD
Higher pentosidine was associated with lower baseline BMD at the total hip and trochanter, but not the femoral neck, in age-adjusted analyses (data not shown). Higher pentosidine was associated with increased bone loss at the total hip, femoral neck, and trochanter over 4 yr of follow-up in minimally adjusted models (Table 3). After adjustment for additional potential confounders, an increase of 1 sd in pentosidine continued to be associated with additional bone loss of −0.13% per year at the femoral neck and −0.16% per year at the trochanter. Association with total hip bone loss was only marginally significant in adjusted models (−0.072% per year; P = 0.11) (Table 3). There was no evidence of interaction with diabetes status.
Table 3.
Annualized percentage change in BMD over 4 yr of follow-up for 1 sd increase in baseline pentosidine (log-transformed)
| Minimally adjusteda
|
Multivariable modelb
|
|||||
|---|---|---|---|---|---|---|
| Regression coefficient | 95% CI | P | Regression coefficient | 95% CI | P | |
| Total hip | −0.146 | (−0.243, −0.050) | 0.003 | −0.072 | (−0.161, 0.017) | 0.112 |
| Femoral neck | −0.185 | (−0.307, −0.062) | 0.003 | −0.125 | (−0.248, −0.002) | 0.047 |
| Trochanter | −0.218 | (−0.334, −0.101) | <0.001 | −0.156 | (−0.271, −0.041) | 0.008 |
Adjusted for age, race, and gender.
Adjusted for age, race, gender, current smoker, baseline BMD, baseline weight, weight change during 4-yr follow-up, cystatin- C, A1C, diabetes, and use of the following: insulin, metformin, sulfonylureas, thiazolidinediones, other oral hypoglycemic medications, vitamin D supplements, calcium supplements, oral steroids, osteoporosis drugs (bisphosphonates, calcitonin, raloxifene), thiazide diuretics, statins, and oral estrogen.
Discussion
We found that higher urine pentosidine levels are associated with incident clinical fractures and prevalent vertebral fractures in older adults with diabetes, independent of BMD and other risk factors for fracture. This is the first study to report an association between the level of an AGE and incident clinical fracture risk. Surprisingly, we did not find an association between pentosidine and fracture risk in those without diabetes.
Two previous studies have reported an association between pentosidine and vertebral fracture. Yamamoto et al. (4) found an increased risk of prevalent vertebral fracture with higher urine pentosidine among women, but not men, with type 2 diabetes. In our cohort of older adults, we did not find evidence of gender differences in the association between pentosidine and prevalent vertebral fracture. Shiraki et al. (5) reported an increased risk of prevalent and incident vertebral fracture with higher serum pentosidine in older women without diabetes, in contrast to our findings of no increased risk in those without diabetes.
The association that we found between urine pentosidine and fracture is consistent with the hypothesis that increased AGE levels in bone collagen result in weaker bone for a given BMD. In this study, those with diabetes have higher bone density but do not have a concomitant reduction in fracture risk. As reported in this cohort as a whole (2) and in other studies (19), the higher BMD associated with type 2 diabetes fails to provide the expected protective effect for fractures. Similarly, older age is associated with increased fracture risk independent of bone density. Higher AGE levels in bone collagen may contribute to bone fragility. Cadaver studies have found higher levels of pentosidine associated with reduced strength in vertebral bone (20,21,22). A study using surgical specimens reported higher levels of pentosidine in bone from patients with a femoral neck fracture, compared with controls who did not have a fracture (23). In spontaneously diabetic WBN/Kob rats, impaired bone mechanical properties were associated with an increase in pentosidine levels without a reduction in BMD (24).
Direct studies of AGE levels in bone collagen require invasive measures, and these are not feasible in most studies. In this study, we measured pentosidine levels in urine and did not have direct measurements of AGE levels in bone. However, peripheral AGE levels in serum and urine may provide a marker for levels in bone collagen. Odetti et al. (25) reported that pentosidine levels in plasma correlated with levels in cortical bone from the femur (r = 0.25) in specimens removed during surgery.
In addition to possible direct effects on the material properties of bone collagen, higher AGE levels may influence bone cells. The effect on osteoclasts is controversial, with reports of increased (26) and decreased (27) osteoclast activity in the presence of higher AGE levels. AGEs may also impair osteoblast attachment to collagen matrix (28). Higher AGE levels have been reported in patients with osteoporosis (29).
These mechanisms for the effects of AGEs on bone are not mutually exclusive, and both may be operating. However, in this study, we found very modest correlations between bone turnover and pentosidine levels. Similarly, pentosidine was associated with increased bone loss, but the degree of additional bone loss for a 1 sd difference in baseline pentosidine was small (∼0.1% per year). Average bone loss at these hip sites in the Health ABC cohort was about 0.5% per year. Thus, although we found evidence for a modest association between pentosidine and bone loss, this degree of bone loss seems unlikely to account for the increased fracture risk with higher pentosidine levels.
It is possible that increased bone turnover releases greater quantities of pentosidine and other AGEs from the bone collagen into the circulation. However, our finding that pentosidine levels were not reduced in women using bone-active medications suggests that this is not a substantial influence on urine pentosidine levels.
Previous studies have reported that reduced renal function is associated with the accumulation of AGEs in serum, urine, and tissues (30,31). In our study as well, pentosidine levels correlated with renal function. Because poor renal function has been shown to predict fracture (32,33), it is possible that poor renal function is a confounder of the association between pentosidine and fracture in our study. However, our models showing an association between pentosidine and fracture were adjusted for renal function. Similarly, poor balance and history of falls are associated with higher pentosidine levels in this cohort and could serve as confounders of the relationship between pentosidine levels and fracture risk. However, when we included these factors in our models they did not account for the observed associations between pentosidine and fracture.
The reasons for the effect modification of the association between pentosidine and fracture risk by diabetes status are not clear. If not a chance finding, the results may reflect structural differences in diabetic bone. There is emerging evidence that those with type 2 diabetes may have bone volume at the spine and cross-sectional area at the spine, hip, and radius that are reduced relative to their body size (34,35). This structural difference may make diabetic bone more vulnerable to weakening from accumulated AGEs.
In this study, we did not find higher levels of pentosidine among those with type 2 diabetes, in contrast to previous reports (36,37). Because AGEs increase with aging, the differences in pentosidine between those with and without diabetes may be reduced in this older cohort.
This study has several important strengths. The pentosidine levels were measured in urine obtained at baseline in a defined cohort before clinical fractures were ascertained. Diabetes was well characterized, and the presence or absence of diabetes was confirmed through fasting glucose and 2-h oral glucose tolerance test. BMD and bone turnover measurements were available on participants. A limitation of the study is the lack of direct measurement of AGEs in bone collagen. In addition, prevalent vertebral fractures were assessed from CT scout scans rather than traditional spine radiographs, possibly underestimating the presence of vertebral deformities. Such underassessment is likely to be nondifferential with respect to the levels of pentosidine and would thus have attenuated any association. Finally, the study is limited by its observational nature, making causal inferences speculative.
In conclusion, these results indicate that higher levels of advanced glycation endproducts are a risk factor for fracture in older adults with type 2 diabetes, a finding that might account at least in part for the higher fracture risks for a given bone density observed with type 2 diabetes. Further research is needed to identify the underlying mechanisms for this association.
Acknowledgments
We thank Lisa Palermo, M.S., M.A., for statistical analyses and Dr. Evelyne Gineyts for expert technical assistance in pentosidine measurements.
Footnotes
This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging (NIA) (Grants NIA N01-AG-6-2106, N01-AG-6-2101, N01-AG-6-2103, and NIA R01 AG17482); an American Diabetes Association Junior Faculty Award (to A.V.S.), and a grant from the Alliance for Better Bone Health (to A.V.S.).
Disclosure Summary: T.A.H., D.E.S., E.S.S., K.R.F., H.E.R., F.A.T., D.M.B., S.R.C., T.B.H. and D.C.B. have nothing to declare. A.V.S. and P.G. received research grant support from the Alliance for Better Bone Health (Procter & Gamble and Aventis).
First Published Online April 21, 2009
Abbreviations: A1C, Hemoglobin A1c; AGE, advanced glycation endproduct; ALP, alkaline phosphatase; BMD, bone mineral density; CI, confidence interval; Cr, creatinine; CV, coefficient(s) of variation; DXA, dual energy x-ray absorptiometry; eGFR, estimated glomerular filtration rate; P1NP, N-propeptide of type I procollagen; RH, relative hazard; S-CTX, serum C-terminal cross-linking telopeptide of type I collagen; U-NTX, urinary N-terminal crosslinking telopeptide of type I collagen.
References
- Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR 2001 Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab 86:32–38 [DOI] [PubMed] [Google Scholar]
- Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB 2005 Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med 165:1612–1617 [DOI] [PubMed] [Google Scholar]
- Goh SY, Cooper ME 2008 Clinical review: the role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab 93:1143–1152 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T 2008 Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 93:1013–1019 [DOI] [PubMed] [Google Scholar]
- Shiraki M, Kuroda T, Tanaka S, Saito M, Fukunaga M, Nakamura T 2008 Nonenzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures. J Bone Miner Metab 26:93–100 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association 2002 Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 25:S5–S20 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association 2006 Standards of Medical Care in Diabetes-2006. Diabetes Care 29:S4–S42 [PubMed] [Google Scholar]
- Tsukahara H, Shibata R, Ohta N, Sato S, Hiraoka M, Ito S, Noiri E, Mayumi M 2003 High levels of urinary pentosidine, an advanced glycation end product, in children with acute exacerbation of atopic dermatitis: relationship with oxidative stress. Metabolism 52:1601–1605 [DOI] [PubMed] [Google Scholar]
- Mackey DC, Lui LY, Cawthon PM, Bauer DC, Nevitt MC, Cauley JA, Hillier TA, Lewis CE, Barrett-Connor E, Cummings SR 2007 High-trauma fractures and low bone mineral density in older women and men. JAMA 298:2381–2388 [DOI] [PubMed] [Google Scholar]
- Takada M, Wu CY, Lang TF, Genant HK 1998 Vertebral fracture assessment using the lateral scoutview of computed tomography in comparison with radiographs. Osteoporos Int 8:197–203 [DOI] [PubMed] [Google Scholar]
- Genant HK, Wu CY, van Kuijk C, Nevitt MC 1993 Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148 [DOI] [PubMed] [Google Scholar]
- Black DM, Palermo L, Nevitt MC, Genant HK, Epstein R, San Valentin R, Cummings SR 1995 Comparison of methods for defining prevalent vertebral deformities: the Study of Osteoporotic Fractures. J Bone Miner Res 10:890–902 [DOI] [PubMed] [Google Scholar]
- Bates DW, Black DM, Cummings SR 2002 Clinical use of bone densitometry: clinical applications. JAMA 288:1898–1900 [DOI] [PubMed] [Google Scholar]
- Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P 1994 Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol 10:405–411 [DOI] [PubMed] [Google Scholar]
- Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G 2003 National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 139:137–147 [DOI] [PubMed] [Google Scholar]
- Black D, Palermo L 1995 Use of vertebral reference values to define prevalent vertebral deformities. In: Genant H, Jergas M, van Kuijk C, eds. Vertebral fracture in osteoporosis. San Francisco: Radiology Research and Education Foundation; 189–204 [Google Scholar]
- Cauley JA, Palermo L, Vogt M, Ensrud KE, Ewing S, Hochberg M, Nevitt MC, Black DM 2008 Prevalent vertebral fractures in black women and white women. J Bone Miner Res 23:1458–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy JK, Meyer WA, Grigoryan M, Fan B, Flores RH, Genant HK, Resnik C, Hochberg MC 2006 Racial differences in the prevalence of vertebral fractures in older men: the Baltimore Men’s Osteoporosis Study. Osteoporos Int 17:99–104 [DOI] [PubMed] [Google Scholar]
- Vestergaard P 2007 Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int 18:427–444 [DOI] [PubMed] [Google Scholar]
- Wang X, Shen X, Li X, Agrawal CM 2002 Age-related changes in the collagen network and toughness of bone. Bone 31:1–7 [DOI] [PubMed] [Google Scholar]
- Viguet-Carrin S, Roux JP, Arlot ME, Merabet Z, Leeming DJ, Byrjalsen I, Delmas PD, Bouxsein ML 2006 Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone 39:1073–1079 [DOI] [PubMed] [Google Scholar]
- Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, DeGroot J, Bank RA, Keaveny TM 2005 Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone 37:825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Fujii K, Marumo K 2006 Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcif Tissue Int 79:160–168 [DOI] [PubMed] [Google Scholar]
- Saito M, Fujii K, Mori Y, Marumo K 2006 Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int 17:1514–1523 [DOI] [PubMed] [Google Scholar]
- Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, Federici A 2005 Advanced glycation end products and bone loss during aging. Ann NY Acad Sci 1043:710–717 [DOI] [PubMed] [Google Scholar]
- Miyata T, Notoya K, Yoshida K, Horie K, Maeda K, Kurokawa K, Taketomi S 1997 Advanced glycation end products enhance osteoclast-induced bone resorption in cultured mouse unfractionated bone cells and in rats implanted subcutaneously with devitalized bone particles. J Am Soc Nephrol 8:260–270 [DOI] [PubMed] [Google Scholar]
- Valcourt U, Merle B, Gineyts E, Viguet-Carrin S, Delmas PD, Garnero P 2007 Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J Biol Chem 282:5691–5703 [DOI] [PubMed] [Google Scholar]
- McCarthy AD, Uemura T, Etcheverry SB, Cortizo AM 2004 Advanced glycation endproducts interfere with integrin-mediated osteoblastic attachment to a type-I collagen matrix. Int J Biochem Cell Biol 36:840–848 [DOI] [PubMed] [Google Scholar]
- Hein G, Wiegand R, Lehmann G, Stein G, Franke S 2003 Advanced glycation end-products pentosidine and Nε-carboxymethyllysine are elevated in serum of patients with osteoporosis. Rheumatology 42:1242–1246 [DOI] [PubMed] [Google Scholar]
- Monnier VM, Bautista O, Kenny D, Sell DR, Fogarty J, Dahms W, Cleary PA, Lachin J, Genuth S 1999 Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes 48:870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita Z, Radoff S, Rayfield EJ, Yang Z, Skolnik E, Delaney V, Friedman EA, Cerami A, Vlassara H 1991 Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med 325:836–842 [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, Cauley JA, Jamal SA, Antoniucci DM, Cummings SR 2007 Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med 167:133–139 [DOI] [PubMed] [Google Scholar]
- Dukas L, Schacht E, Stähelin HB 2005 In elderly men and women treated for osteoporosis a low creatinine clearance of <65 ml/min is a risk factor for falls and fractures. Osteoporos Int 16:1683–1690 [DOI] [PubMed] [Google Scholar]
- Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Zmuda JM, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB 2004 Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: The Health, Aging, and Body Composition Study. J Bone Miner Res 19:1084–1091 [DOI] [PubMed] [Google Scholar]
- Melton 3rd LJ, Riggs BL, Leibson CL, Achenbach SJ, Camp JJ, Bouxsein ML, Atkinson EJ, Robb RA, Khosla S 2008 A bone structural basis for fracture risk in diabetes. J Clin Endocrinol Metab 93:4804–4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Hoshino H, Kushida K, Kawana K, Inoue T 1996 Direct quantification of pentosidine in urine and serum by HPLC with column switching. Clin Chem 42:1439–1444 [PubMed] [Google Scholar]
- Krapfenbauer K, Birnbacher R, Vierhapper H, Herkner K, Kampel D, Lubec G 1998 Glycoxidation, and protein and DNA oxidation in patients with diabetes mellitus. Clin Sci 95:331–337 [PubMed] [Google Scholar]