Abstract
Context: Stress-mediated hypothalamic-pituitary-adrenal axis activation, regulated by arginine vasopressin (AVP), may have a role in the pathophysiology of metabolic syndrome (MetSyn).
Objective: The objective of the study was to investigate whether plasma C-terminal provasopressin fragment (copeptin), a surrogate for circulating AVP, was associated with measures of insulin resistance and presence of MetSyn.
Design, Setting, and Participants: This was a multicenter, community-based study, investigating novel biomarkers for vascular disease. Participants included 1293 African-Americans (AA) (64 ± 9 yr) and 1197 non-Hispanic whites (NHW) (59 ± 10 yr) belonging to hypertensive sibships.
Main Outcome Measures: Plasma copeptin levels were measured by an immunoluminometric assay. MetSyn was defined per Adult Treatment Panel III criteria. Generalized estimating equations were used to assess whether plasma copeptin was associated with measures of insulin resistance and MetSyn.
Results: The prevalence of MetSyn was 50% in AA and 49% in NHW. In each group, after adjustment for age and sex, plasma copeptin levels significantly correlated with body mass index, fasting plasma glucose and insulin, homeostasis model assessment of insulin resistance, triglycerides, and (inversely) high-density lipoprotein cholesterol (P < 0.05 for each variable). In multivariable logistic regression models that adjusted for age, sex, smoking, statin use, serum creatinine, education, physical activity, and diuretic use, plasma copeptin levels in the highest quartile were associated with an increased odds ratio of having MetSyn compared with bottom quartile: odds ratio (95% confidence interval) in AA, 2.07 (1.45–2.95); in NHW, 1.74 (1.21–2.5).
Conclusions: Our findings indicate a novel cross-sectional association between plasma copeptin and measures of insulin resistance and MetSyn.
Plasma carboxy-terminal pro-vasopressin (copeptin) is associated with measures of insulin resistance and metabolic syndrome in hypertensive adults.
Metabolic syndrome is a major public health burden with nearly a quarter of the world’s adult population affected (1). The syndrome is associated with a 5-fold higher risk of developing type 2 diabetes (2) and 2- to 3-fold higher risk of cardiovascular disease (2). The pathophysiology of metabolic syndrome is not well defined, and several investigators have sought to identify a unifying factor that could explain all the components of the syndrome. In addition to insulin resistance/hyperinsulinemia (3), investigators have found several biomarkers to be associated with metabolic syndrome including leptin (2), epinephrine and norepinephrine (3), brain natriuretic peptide (4), oxidized low-density lipoprotein cholesterol (5), uric acid (6), C-reactive protein (2), plasminogen activator inhibitor-1 (2), and aldosterone (2), highlighting diverse pathophysiological perturbations in metabolic syndrome.
Chronic psychosocial stress, defined as feelings of fatigue, lack of energy, irritability, demoralization, and hostility (7), has been linked to metabolic syndrome (7,8). Chronic psychosocial stress is frequently accompanied by increased plasma levels of arginine vasopressin (AVP), which is an amplifier of the hypothalamic-pituitary-adrenal (HPA) axis along with CRH (9,10,11). Several reports suggest that stress-mediated activation of the HPA axis may have a role in the pathogenesis of insulin resistance and metabolic syndrome (8).
We therefore hypothesized that plasma AVP would be associated with measures of insulin resistance and metabolic syndrome. We tested this hypothesis in a cohort of adults belonging to sibships ascertained on the basis of hypertension and well characterized for presence of metabolic syndrome and its components. We measured plasma levels of the C-terminal pro-AVP fragment also known as copeptin, as a surrogate for circulating AVP levels. Copeptin is secreted in equimolar amounts to AVP and therefore directly reflects the release of AVP into the circulation. Unlike AVP, it remains stable ex vivo for several days at room temperature in serum or plasma (12).
Subjects and Methods
The study methods have been described previously (13). Briefly, the study was part of the Proteomic Markers of Arteriosclerosis Study, which is investigating the association of multiple markers in various etiologic pathway of vascular disease with several phenotypes of arteriosclerosis. The non-Hispanic white participants were recruited from the Olmsted County, Minnesota, whereas the African-American participants were recruited from Jackson, MS. If the eligible proband, at either center, had at least one sibling with hypertension, all available full biological siblings of the index hypertensive including normotensive siblings were invited to participate in interviews, physical examinations, and phlebotomy at their respective centers. The only exclusionary criterion at enrollment at either center was the presence of a secondary cause of hypertension (such as documented renal artery stenosis or advanced renal insufficiency) in the index siblings (13). The study was approved by the Institutional Review Boards of the University of Mississippi Medical Center (Jackson MS) and Mayo Clinic (Rochester MN). Written informed consent was obtained from each participant. The present study included 2490 participants (1293 African-Americans and 1197 non-Hispanic whites). We excluded two participants with copeptin levels greater than 100 μl/liter.
Height was measured by stadiometer, weight by electronic balance, and body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Ever smoking was defined as having smoked more than 100 cigarettes. Resting systolic blood pressure and diastolic blood pressure were measured by random zero sphygmomanometer (Hawskley and Sons, London, UK). The diagnosis of hypertension was established based on blood pressure (BP) levels measured at the study visit (≥140/90 mm Hg) or a prior diagnosis of hypertension and current treatment with antihypertensive medications. Diabetes was considered present if the subject had history of diabetes, was being treated with insulin or oral agents, or had a fasting glucose level of 126 mg/dl or greater. Information about the use of BP medications and statins was obtained from the participants at the time of the study visit. We constructed a physical activity score by considering the responses to questions on how many hours per day of heavy activity, moderate activity, slight activity, and sedentary activity the participant engaged in. Specifically, the physical activity score was derived as follows: 3heavy + 2moderate + 1light (hours).
Blood was collected by venipuncture after an overnight fast, and the plasma/serum samples were stored at −80 C until analyzed. Serum total cholesterol and high-density lipoprotein (HDL) cholesterol were measured by standard enzymatic methods. Insulin was determined by a RIA method using the human insulin specific RIA kit (Linco Research, St. Charles, MO). The sensitivity of the assay was 0.03 μU/ml with an interassay coefficient of variation (CV) of less than 10%. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as insulin (microinternational units per milliliter) × [glucose (milligrams per deciliter) × 0.055/22.5] (14). Metabolic syndrome was defined as the presence of three or more of the following components: 1) waist circumference of 35 in. or greater (≥88.9 cm) in women or 40 in. or greater (≥101.6 cm) in men; 2) BP 130/85 mm Hg or greater or treatment for hypertension; 3) fasting triglycerides 150 mg/dl or greater (≥1.70 mmol/liter); 4) HDL cholesterol 40 mg/dl or less (≤1.04 mmol/liter) in men or 50 mg/dl or less (≤1.30 mmol/liter) in women; and 5) fasting blood glucose 110 mg/dl or greater (≥6.11 mmol/liter) or treatment for diabetes (15,16). Copeptin was measured in a blinded fashion in a single batch with a commercial sandwich immunoluminometric assay (LUMItest C-terminal pro-AVP; BRAMHS AG, Hennigsdorf/Berlin, Germany) as described previously (12,17). The assay yielded results within 3 h. The analytical detection limit was 1.84 pg/ml (1.7 pmol/liter) and the interassay CV was less than 20% for values greater than 2.44 pg/ml (>2.25 pmol/liter) (12).
Statistical methods
Because of the presence of siblingships in the sample, regression analyses were performed using generalized estimating equations to correct for intrafamilial correlations (18). Continuous data were summarized as either mean ± sd or median and quartiles, and categorical data were expressed as percentages. Due to significant differences in age and the proportion of women in the two ethnic groups, participant characteristics were assessed after adjustment for age and sex. All analyses were stratified by ethnicity. Serum creatinine and alcohol intake were log transformed to reduce skewness. We calculated Spearman correlation coefficients (adjusted for age and sex) between plasma copeptin and the following variables: 1) conventional risk factors for cardiovascular disease (total cholesterol, HDL cholesterol, triglycerides, systolic BP, smoking, and diabetes); 2) measures of adiposity and insulin resistance (BMI, waist circumference, fasting blood sugar, insulin level, and HOMA-IR); and 3) lifestyle variables (physical activity score, alcohol intake, and education).
To evaluate the association of copeptin with metabolic syndrome, we constructed multivariable logistic regression models in each ethnic group to assess whether plasma copeptin was independently associated with metabolic syndrome. We calculated the odds ratio for the presence of metabolic syndrome in quartiles of copeptin with participants in the lowest quartile of copeptin considered the referent group. Adjustments were performed for age and sex; and age, sex, serum creatinine, smoking, statin use, history of myocardial infarction/stroke, lifestyle variables (physical activity score and educational status), and diuretic use. Alcohol intake was not associated with copeptin levels in the present study; therefore, this was not used in the final model (see Table 2). The above analyses were repeated in nondiabetic individuals in both ethnic groups. A two-sided P< 0.05 was used for statistical significance. Statistical analyses were carried out using SAS version 9.1 software package (SAS Institute, Cary, NC).
Table 2.
Age- and sex-adjusted (Spearman) correlations between copeptin and select variables
| African-Americans (n = 1286)
|
Non-Hispanic whites (n = 1197)
|
|||
|---|---|---|---|---|
| r | P | r | P | |
| Waist | 0.16 | <0.001 | 0.20 | <0.001 |
| BMI | 0.14 | <0.001 | 0.19 | <0.001 |
| Plasma glucose | 0.12 | <0.001 | 0.17 | <0.001 |
| Plasma insulina | 0.14 | <0.001 | 0.22 | <0.001 |
| HOMA-IRa | 0.17 | <0.001 | 0.23 | <0.001 |
| Diabetes | 0.13 | <0.001 | 0.07 | 0.014 |
| HDL cholesterol | −0.07 | 0.014 | −0.08 | 0.009 |
| Triglycerides | 0.09 | 0.002 | 0.09 | 0.002 |
| Systolic BP | 0.06 | 0.022 | −0.02 | 0.59 |
| Heart rate | 0.07 | 0.015 | 0.07 | 0.025 |
| Metabolic score | 0.16 | <0.001 | 0.14 | <0.001 |
| Physical activity score | −0.10 | <0.001 | −0.07 | 0.018 |
| Education | −0.06 | 0.002 | −0.004 | 0.89 |
| Alcohol intake | 0.02 | 0.45 | −0.005 | 0.074 |
n = 1070 in the African-Americans and 1142 in the non-Hispanic whites, respectively, for both HOMA-IR and insulin correlations.
Results
There was a greater proportion of women than men in both African-American and non-Hispanic white cohorts and African-Americans were older. After adjustment for age and sex, African-Americans had a higher prevalence of diabetes than their non-Hispanic white counterparts and lower use of statins. However the prevalence of metabolic syndrome was similar in the two ethnic groups (Table 1). In the African-Americans (n = 1293), 652 (50%) subjects met the criteria for metabolic syndrome, whereas 584 (49%) subjects met this criteria in the non-Hispanic whites (n = 1197).
Table 1.
Participant characteristics (n = 2490)
| African-Americans (n = 1293) | Non-Hispanic whites (n = 1197) | P value | |
|---|---|---|---|
| Age, yra | 63.6 ± 9.3 | 58.9 ± 10.2 | <0.001 |
| Men, n (%)a | 373 (28.9) | 510 (42.6) | <0.001 |
| BMI, kg/m² | 31.6 ± 6.6 | 30.8 ± 6.3 | 0.47 |
| Waist circumference, cm | 103.6 ± 14.5 | 100.6 ± 15.9 | 0.83 |
| Plasma glucose, mg/dl | 112.7 ± 47.7 | 104.9 ± 24.5 | <0.001 |
| Plasma insulin, IU/ml | 9.3 ± 8.2 | 8.0 ± 6.0 | 0.005 |
| HOMA-IR | 3.4 ± 10.4 | 2.3 ± 2.7 | 0.011 |
| Diabetes, n (%) | 385 (29.8) | 177 (14.8) | <0.001 |
| Total cholesterol, mg/dl | 201.7 ± 41 | 197.3 ± 34.8 | 0.004 |
| HDL cholesterol, mg/dl | 57.7 ± 18.3 | 51.9 ± 15.3 | <0.001 |
| Triglycerides, mg/dl | 101.5 (77.5–142) | 135 (97–192) | <0.001 |
| Hypertension | 1034 (80) | 875 (73) | <0.001 |
| Systolic BP, mm Hg | 138.6 ± 20.9 | 131.1 ± 17.1 | <0.001 |
| Diastolic BP, mm Hg | 79 ± 10.8 | 73.8 ± 9.3 | <0.001 |
| Heart rate | 67 ± 11.6 | 65.4 ± 10.9 | <0.001 |
| Serum creatinine, mg/dl | 0.9 ± 0.3 | 0.9 ± 0.2 | <0.001 |
| Physical activity score | 9.8 ± 3.4 | 13.3 ± 5.2 | 0.67 |
| Smoking, n (%) | 525 (40.6) | 584 (48.8) | 0.17 |
| Education, yr in school | 12.1 ± 3.6 | 13.4 ± 2.3 | <0.001 |
| Alcohol intake, oz/month | 1.5 ± 5.5 | 5.6 ± 10.5 | <0.001 |
| Previous history of MI or stroke | 152 (11.8) | 129 (10.8) | 0.24 |
| Statin use, n (%) | 242 (18.7) | 346 (28.9) | <0.001 |
| Aspirin, n (%) | 430 (33.3) | 490 (40.9) | <0.001 |
| Diuretic use, n (%) | 605 (47) | 452 (35) | <0.001 |
| β-Blockers, n (%) | 221 (17) | 393 (33) | <0.001 |
| RAAS blockers, n (%) | 517 (40) | 422 (35) | <0.001 |
| Ca-channel blockers, n (%) | 371 (29) | 178 (15) | <0.001 |
| Copeptin (pmol/liter) | 8.0 (5.0–12.7) | 5.0 (3.3–7.9) | <0.001 |
Continuous variables are presented as means ± sd or median and interquartile range, whereas categorical variables are presented as counts and percentages. MI, Myocardial infarction; RAAS, renin-angiotensin-aldosterone system; Ca-channel, calcium channel.
P values for variables age and men are unadjusted. All other variables are adjusted for age and sex.
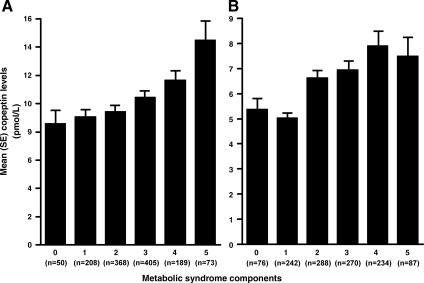
In African-Americans, mean copeptin levels in participants without and with metabolic syndrome were 9.48 and 11.51 pmol/liter, respectively P < 0.001. After adjustment for age and sex, plasma copeptin positively correlated (P < 0.05) with BMI, waist circumference, fasting plasma glucose and insulin level, HOMA-IR, presence of diabetes, and serum triglycerides and inversely correlated with HDL cholesterol and physical activity score (Table 2). After additional adjustment for BMI, plasma copeptin remained positively correlated (P < 0.05) with waist circumference, fasting plasma glucose and insulin level, HOMA-IR, presence of diabetes, systolic BP, and serum triglycerides (analyses not shown). Plasma copeptin levels increased with increasing number of metabolic syndrome components (P < 0.001; Fig. 1A). In multivariable logistic regression analyses that adjusted for age and sex, plasma copeptin levels in the third and fourth quartile were significantly associated with higher odds ratios (OR) of having metabolic syndrome, OR 1.49 (P = 0.010) and 2.18 (P < 0.001), respectively. This association remained significant after additional adjustment for serum creatinine, statin use, past history of myocardial infarction/stroke, lifestyle variables (history of smoking, educational status, and physical activity score), and diuretic use, with ORs of 1.49 (P = 0.017) and 2.07 (P < 0.001) for the third and fourth copeptin quartile, respectively (Table 3). In the subset of participants without diabetes (n = 908), having a plasma copeptin level in the fourth quartile was significantly associated with the presence of metabolic syndrome (OR 1.54; P = 0.036) after adjustment for age and sex. This association was weakened after adjustment for additional covariates (OR 1.42; P = 0.10).
Figure 1.
Association of plasma copeptin levels with the number of metabolic syndrome components in African-Americans (A) and non-Hispanic whites (B), respectively.
Table 3.
Association of plasma copeptin with the presence of metabolic syndrome: logistic regression analyses
| OR (95% CI)
|
||||
|---|---|---|---|---|
|
P value
|
||||
| First quartile | Second quartile | Third quartile | Fourth quartile | |
| African-Americans | ||||
| Copeptin, pmol/liter | <5.0 | 5.0–8.0 | 8.0–12.7 | >12.7 |
| Unadjusted | 1 | 1.35 (1.01–1.81) | 1.36 (1.01–1.83) | 2.04 (1.48–2.81) |
| 0.045 | 0.042 | <0.001 | ||
| Age and sex adjusted | 1 | 1.40 (1.04–1.89) | 1.49 (1.10–2.02) | 2.18 (1.56–3.06) |
| 0.025 | <0.001 | <0.001 | ||
| Fully adjusted | 1 | 1.42 (1.05–1.93) | 1.49 (1.07–2.06) | 2.07 (1.45–2.95) |
| 0.024 | 0.017 | <0.001 | ||
| Non-Hispanic whites | ||||
| Copeptin, pmol/liter | <3.32 | 3.32–5.0 | 5.0–7.91 | >7.91 |
| Unadjusted | 1 | 1.17 (0.84–1.62) | 1.82 (1.33–2.48) | 2.0 (1.41–2.71) |
| 0.36 | <0.001 | <0.001 | ||
| Age and sex adjusted | 1 | 1.16 (0.84–1.62) | 1.81 (1.32–2.49) | 1.91 (1.36–2.66) |
| 0.38 | <0.001 | <0.001 | ||
| Fully adjusted | 1 | 1.12 (0.79–1.59) | 1.79 (1.27–2.51) | 1.74 (1.21–2.5) |
| 0.52 | <0.001 | 0.003 | ||
The fully adjusted model includes age, sex, serum creatinine, history of ever smoking, statin use, past history of myocardial infarction/stroke, physical activity, educational status, and diuretic use. CI, Confidence interval.
In non-Hispanic whites, mean copeptin levels in participants without and with metabolic syndrome were 6.13 and 8.27 pmol/liter, respectively P < 0.001. After adjustment for age and sex, plasma copeptin positively correlated (P < 0.05) with BMI, waist circumference, fasting serum glucose and insulin levels, HOMA-IR, presence of diabetes, and serum triglycerides and inversely correlated with HDL cholesterol and physical activity score (Table 2). After additional adjustment for BMI, plasma copeptin remained positively correlated (P < 0.05) with waist circumference, fasting plasma glucose and insulin levels, and HOMA-IR (analyses not shown). Plasma copeptin levels increased with increasing number of metabolic syndrome components (P < 0.001, Fig. 1B). In multivariable logistic regression analyses that adjusted for age and sex, plasma copeptin levels in the third and fourth quartile were significantly associated with a higher odds of having metabolic syndrome: OR of 1.81 (P < 0.001) and 1.91 (P < 0.001), respectively. This association remained significant after additional adjustment for age, sex, serum creatinine, statin and aspirin use, past history of myocardial infarction/stroke, lifestyle variables (history of smoking, educational status, and physical activity score), and diuretic use, with an OR of 1.79 (P < 0.001) and 1.74 (P = 0.003) for the third and fourth copeptin quartile, respectively (Table 3). In the subset of participants without diabetes (n = 1021), having a plasma copeptin level in the third or fourth quartile was significantly associated with the presence of metabolic syndrome (OR 1.83, P < 0.001; OR 1.82, P = 0.001, respectively) after adjustment for age and sex. This association remained significant after adjustment for additional covariates (OR 1.84, P = 0.001; OR 1.70, P = 0.007, for the third and fourth copeptin quartiles, respectively).
Discussion
The present study, to the best of our knowledge, is the first to report that plasma copeptin, a surrogate for plasma AVP release, is associated with measures of insulin resistance (fasting plasma glucose and insulin, and HOMA-IR) and the presence of metabolic syndrome. Participants with metabolic syndrome had higher mean copeptin levels than those without metabolic syndrome and participants in the third or fourth quartiles for plasma copeptin had 70–100% higher odds of metabolic syndrome compared with those in the bottom quartile (Table 3). In addition, plasma copeptin levels were higher in participants who had greater clustering of metabolic syndrome components (Fig. 1, A and B). These associations were independent of adiposity, highlighting AVP as a novel marker of insulin resistance and metabolic syndrome. The relatively high prevalence of metabolic syndrome in both groups (∼50%) in this study is consistent with the high prevalence of hypertension and the older age of study participants (1,2).
AVP is a vasoactive neurohypophysial hormone that plays a central role in the regulation of water and electrolyte balance, its release stimulated by a change in plasma tonicity, hypovolemia, or low blood pressure (19). Several experimental studies have shown that under chronic psychosocial stress states, AVP becomes a key amplifier of ACTH release from the anterior pituitary in concert with CRH (9,10,20). Also, AVP levels more closely correlate with ACTH levels than do CRH levels in these chronic stress studies, suggesting a more dynamic role for AVP in activity of the stress axis and a primarily permissive function for CRH (9,10).
We speculate that the activation of HPA axis by AVP (copeptin) in chronic psychosocial stress may be one of the mediators of its association with insulin resistance/metabolic syndrome. Multiple endocrine perturbations that favor insulin resistance ensue (summarized in Fig. 2) including a decrease in thyroid and GH, reduction in testosterone and estrogen level (21), and an increase in cortisol levels (9,10,20). AVP is also known to directly stimulate cortisol release in both cows and humans by activating the V1a receptors on the adrenal cortex cells (22). Cortisol impedes the action of insulin to promote glucose uptake in cells and stimulates glucagon secretion and glycogenolysis (23), which results in higher plasma glucose levels. It also induces stress-related excessive feeding behavior (24). Furthermore, plasma cortisol deficiency leads to elevated plasma AVP, suggesting that glucocorticoids may be feedback inhibitors of AVP secretion (25). AVP activates the V1b receptors on the chromaffin cells in the adrenal medulla to increase epinephrine levels, which may subsequently contribute to hyperglycemia by stimulating glycogenolysis in liver (26,27).
Figure 2.
Mechanisms by which AVP may lead to insulin resistance and metabolic syndrome.
AVP directly induces hepatic glycogenolysis (26,28), glucogenolysis (28), and gluconeogenesis in perfused rat liver via activation of V1a receptors in hepatocytes (28). AVP activates V1b receptors on the α-cells of the pancreatic islets to increases the secretion of glucagon (23,26,29) and potentiates insulin release from the β-cells of the pancreatic islets, in the presence of glucose, by activation of the phosphoinositide receptor pathway (23,29,30). In diabetic rats, AVP had a greater impact on glucagon secretion than insulin secretion (23). Infusion of AVP in both mice and humans results in higher plasma glucose levels (31). The combined effect of these perturbations may lead to insulin resistance as manifested by higher fasting glucose and insulin levels and greater HOMA-IR. Diabetes is known to be associated with higher AVP levels (32); therefore, we repeated the analyses in the subset of participants without diabetics. Inferences remained similar, although in African-Americans the association was of borderline significance.
In addition to measures of insulin resistance, we found plasma copeptin to be independently associated with several components of metabolic syndrome including adiposity (higher waist circumference and BMI) and dyslipidemia (lower HDL cholesterol and higher triglyceride levels) (Table 2). In multivariable logistic regression analyses that adjusted for age, sex, serum creatinine, history of myocardial infarction and stroke, and conventional cardiovascular risk factors (including diabetes and hypertension), higher plasma copeptin was independently associated with measures of adiposity including BMI and waist circumference (P < 0.001 for both BMI and waist circumference for both ethnic groups (analyses not shown). In a retrospective study of 52 patients with tumors involving the hypothalamic region, 52% patients were found to be obese after a median of 5 yr of follow-up. Use of Desmopressin (a vasopressin analog) was associated with the highest odds (OR 13) of weight gain and obesity after diagnosis (33). The association of plasma copeptin with obesity may be due to AVP’s role in increasing cortisol levels, which then may lead to weight gain by increasing appetite, altering body fat distribution, and increasing truncal adiposity (24).
Plasma copeptin was significantly associated with higher triglyceride levels and lower HDL cholesterol levels (Table 2). Intraperitoneal injection of AVP in goats resulted in an increase in plasma triglyceride levels (34). The association of copeptin with higher triglyceride levels may be secondary to increased hepatic synthesis of triglycerides under the influence of glucocorticoids, glucagon, and epinephrine released under stress, all of which are up-regulated by AVP (34) (Fig. 2). Plasma copeptin levels were found to be inversely associated with physical activity (Table 2). Aerobic fitness has been associated with a lower HPA axis response to psychological stress, suggesting that aerobic physical activity may blunt the activation of the HPA axis due to stress (35).
Increased levels of other vasoactive peptides have been associated with features of metabolic syndrome. For example, higher levels of epinephrine and norepinephrine, reflective of increased sympathetic nervous system activity, are associated with metabolic syndrome (3). In contrast, lower levels of brain natriuretic peptide, a vasodilator hormone, have been found to be associated with several traits of metabolic syndrome (4). Thus, an imbalance between vasoconstrictor, antidiuretic hormones, and vasodilator natriuretic peptides may contribute to the pathophysiology of metabolic syndrome.
Study strengths and limitations
Strengths of the present study include the use of uniform protocols in the two ethnic groups including questionnaires, anthropometric measurements, availability of conventional clinical covariates, and the novel copeptin assay. Hitherto, assessing the role of AVP in various pathophysiologic states has been difficult because of its instability in isolated plasma; binding to platelets, which leads to interference in its measurement; rapid clearance from plasma given short half-life of 24 min; and the need for 12–24 h of incubation in conventional assays; all of above factors lead to difficulty in precise measurement of AVP. In contrast, copeptin is a sandwich immunoassay with high sensitivity (analytical detection limit of 1.7 pmol/liter) and good precision (interlaboratory CV < 20%) (17). Copeptin levels show identical changes under disordered water states as previously shown for AVP, with higher values in fasting states or with infusion of hypertonic saline and a rapid decline in vivo during hypotonic saline infusion (36). The assay can detect plasma copeptin in 97% of the healthy population regardless of osmolality, whereas AVP is often not detectable in plasma samples with medium or low osmolality (17). Copeptin has emerged as a validated surrogate for AVP in numerous recently published studies (17,36,37).
Several limitations of our study need to be mentioned. The study is cross-sectional and therefore precludes direct inferences concerning causality or a temporal relationship between higher copeptin, measures of insulin resistance, and metabolic syndrome, even though studies of social stress in nonhuman primates (38) and mice (39) have established that increased activity of HPA axis precedes by several months to several years the development of metabolic syndrome (38,39). We did not measure psychosocial stress in the participants or plasma levels of cortisol, the final mediator of a perturbed HPA axis. We calculated HOMA-IR as a measure of insulin resistance, and although not the gold standard, it has been validated as a reliable and clinically useful index of insulin sensitivity in type 2 diabetic patients (40). Diuretic use may lead to volume depletion and a compensatory rise in copeptin levels. Participants on diuretics had higher plasma copeptin levels than those not on diuretics (analyses not shown). We repeated the multivariable analyses relating copeptin to metabolic syndrome, stratified by diuretic use. Inferences remained similar in these stratified analyses, although the use of diuretics was associated with higher mean copeptin levels (analyses not shown). Measures of plasma tonicity were not available, and we were therefore unable to adjust for any possible influence of plasma tonicity on AVP levels. However, our study included ambulatory participants who were not acutely ill and thus unlikely to be volume depleted or hypernatremic. Finally, the study was conducted in a predominantly hypertensive cohort and the generalizability of our findings to normotensive adults needs to be established.
Conclusions
In summary, higher copeptin levels, a surrogate for AVP release, are associated with measures of insulin resistance (higher fasting plasma glucose and insulin, and greater HOMA-IR), adiposity (BMI and waist circumference), and metabolic syndrome, independent of potential confounders. Higher plasma copeptin levels may lead to a perturbed HPA axis in individuals exposed to chronic psychosocial stress. Such neuroendocrine dysregulation may lead to higher cortisol, decreased energy expenditure, increased appetite and food consumption, increased peripheral vascular resistance, and increased insulin levels. In turn this could lead to visceral obesity, insulin resistance, dyslipidemia, and hypertension and the development of metabolic syndrome. Copeptin may be a novel marker of insulin resistance, and further work is needed to assess its utility as a predictor of incident diabetes mellitus and metabolic syndrome. Our findings suggest a novel pathophysiological mechanism underlying insulin resistance and metabolic syndrome.
Footnotes
This work was supported by Grant HL-81331 from the National Institutes of Health.
Disclosure Summary: A.B. holds ownership in BRAHMS AG, owns patent rights to the markers of the study, and is a member of the board of directors of BRAHMS AG. J.S. holds patent rights to the markers and is an employee of BRAHMS AG. N.G.M. is an employee of BRAHMS AG, a midsized company based in Hennigsdorf, Germany, that commercializes immunoassays and has developed the copeptin assay, for which it owns patent rights. The present study was not financed by BRAHMS AG. The remaining authors report no conflict of interest.
First Published Online April 14, 2009
Abbreviations: AVP, Arginine vasopressin; BMI, body mass index; BP, blood pressure; CV, coefficient of variation; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; HPA, hypothalamic-pituitary-adrenal; OR, odds ratio.
References
- Grundy SM 2008 Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 28:629–636 [DOI] [PubMed] [Google Scholar]
- Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH 2008 The metabolic syndrome. Endocr Rev 29:777–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven GM, Lithell H, Landsberg L 1996 Hypertension and associated metabolic abnormalities—the role of insulin resistance and the sympathoadrenal system. N Engl J Med 334:374–381 [DOI] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS 2007 Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation 115:1345–1353 [DOI] [PubMed] [Google Scholar]
- Austin MA, Edwards KL 1996 Small, dense low density lipoproteins, the insulin resistance syndrome and noninsulin-dependent diabetes. Curr Opin Lipidol 7:167–171 [DOI] [PubMed] [Google Scholar]
- Coutinho Tde A, Turner ST, Peyser PA, Bielak LF, Sheedy 2nd PF, Kullo IJ 2007 Associations of serum uric acid with markers of inflammation, metabolic syndrome, and subclinical coronary atherosclerosis. Am J Hypertens 20:83–89 [DOI] [PubMed] [Google Scholar]
- Raikkonen K, Keltikangas-Jarvinen L, Adlercreutz H, Hautanen A 1996 Psychosocial stress and the insulin resistance syndrome. Metabolism 45:1533–1538 [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R 1999 Hypothalamic origin of the metabolic syndrome X. Ann NY Acad Sci 892:297–307 [DOI] [PubMed] [Google Scholar]
- Antoni FA 1993 Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol 14:76–122 [DOI] [PubMed] [Google Scholar]
- Scott LV, Dinan TG 1998 Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: implications for the pathophysiology of depression. Life Sci 62:1985–1998 [DOI] [PubMed] [Google Scholar]
- Volpi S, Rabadan-Diehl C, Aguilera G 2004 Regulation of vasopressin V1b receptors and stress adaptation. Ann NY Acad Sci 1018:293–301 [DOI] [PubMed] [Google Scholar]
- Morgenthaler NG, Struck J, Alonso C, Bergmann A 2006 Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52:112–119 [DOI] [PubMed] [Google Scholar]
- Khaleghi M, Saleem U, Morgenthaler NG, Turner ST, Bergmann A, Struck J, Mosley TH, Kullo IJ 2009 Plasma midregional pro-atrial natriuretic peptide is associated with blood pressure indices and hypertension severity in adults with hypertension. Am J Hypertens 22:425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- 2001 Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285:2486–2497 [DOI] [PubMed] [Google Scholar]
- Grundy SM, Brewer Jr HB, Cleeman JI, Smith Jr SC, Lenfant C 2004 Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109:433–438 [DOI] [PubMed] [Google Scholar]
- Morgenthaler NG, Struck J, Jochberger S, Dunser MW 2008 Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab 19:43–49 [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY 1986 Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42:121–130 [PubMed] [Google Scholar]
- Penit J, Faure M, Jard S 1983 Vasopressin and angiotensin II receptors in rat aortic smooth muscle cells in culture. Am J Physiol 244:E72–E82 [DOI] [PubMed] [Google Scholar]
- Volpi S, Rabadan-Diehl C, Aguilera G 2004 Vasopressinergic regulation of the hypothalamic pituitary adrenal axis and stress adaptation. Stress 7:75–83 [DOI] [PubMed] [Google Scholar]
- Rosmond R, Dallman MF, Bjorntorp P 1998 Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab 83:1853–1859 [DOI] [PubMed] [Google Scholar]
- Gallo-Payet N, Guillon G 1998 Regulation of adrenocortical function by vasopressin. Horm Metab Res 30:360–367 [DOI] [PubMed] [Google Scholar]
- Yibchok-anun S, Abu-Basha EA, Yao CY, Panichkriangkrai W, Hsu WH 2004 The role of arginine vasopressin in diabetes-associated increase in glucagon secretion. Regul Pept 122:157–162 [DOI] [PubMed] [Google Scholar]
- Cavagnini F, Croci M, Putignano P, Petroni ML, Invitti C 2000 Glucocorticoids and neuroendocrine function. Int J Obes Relat Metab Disord 24(Suppl 2):S77–S79 [DOI] [PubMed] [Google Scholar]
- Papanek PE, Raff H 1994 Physiological increases in cortisol inhibit basal vasopressin release in conscious dogs. Am J Physiol 266:R1744–R1751 [DOI] [PubMed] [Google Scholar]
- Montero S, Mendoza H, Valles V, Lemus M, Alvarez-Buylla R, de Alvarez-Buylla ER 2006 Arginine-vasopressin mediates central and peripheral glucose regulation in response to carotid body receptor stimulation with Na-cyanide. J Appl Physiol 100:1902–1909 [DOI] [PubMed] [Google Scholar]
- Grazzini E, Breton C, Derick S, Andres M, Raufaste D, Rickwaert F, Boccara G, Colson P, Guerineau NC, Serradeil-le Gal C, Guillon G 1999 Vasopressin receptors in human adrenal medulla and pheochromocytoma. J Clin Endocrinol Metab 84:2195–2203 [DOI] [PubMed] [Google Scholar]
- Hems DA, Whitton PD 1973 Stimulation by vasopressin of glycogen breakdown and gluconeogenesis in the perfused rat liver. Biochem J 136:705–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning BE, Moltz JH, Fawcett CP 1984 Modulation of insulin and glucagon secretion from the perfused rat pancreas by the neurohypophysial hormones and by desamino-d-arginine vasopressin (DDAVP). Peptides 5:871–875 [DOI] [PubMed] [Google Scholar]
- Lu M, Soltoff SP, Yaney GC, Boyd 3rd AE 1993 The mechanisms underlying the glucose dependence of arginine vasopressin-induced insulin secretion in β-cells. Endocrinology 132:2141–2148 [DOI] [PubMed] [Google Scholar]
- Spruce BA, McCulloch AJ, Burd J, Orskov H, Heaton A, Baylis PH, Alberti KG 1985 The effect of vasopressin infusion on glucose metabolism in man. Clin Endocrinol (Oxf) 22:463–468 [DOI] [PubMed] [Google Scholar]
- Zerbe RL, Vinicor F, Robertson GL 1979 Plasma vasopressin in uncontrolled diabetes mellitus. Diabetes 28:503–508 [DOI] [PubMed] [Google Scholar]
- Daousi C, Dunn AJ, Foy PM, MacFarlane IA, Pinkney JH 2005 Endocrine and neuroanatomic features associated with weight gain and obesity in adult patients with hypothalamic damage. Am J Med 118:45–50 [DOI] [PubMed] [Google Scholar]
- Rossi R, Scharrer E 1993 Mechanisms of the effects of vasopressin on plasma levels of free fatty acids and triglycerides in pygmy goats. Comp Biochem Physiol Comp Physiol 104:287–290 [DOI] [PubMed] [Google Scholar]
- Traustadottir T, Bosch PR, Matt KS 2005 The HPA axis response to stress in women: effects of aging and fitness. Psychoneuroendocrinology 30:392–402 [DOI] [PubMed] [Google Scholar]
- Szinnai G, Morgenthaler NG, Berneis K, Struck J, Muller B, Keller U, Christ-Crain M 2007 Changes in plasma copeptin, the C-terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocrinol Metab 92:3973–3978 [DOI] [PubMed] [Google Scholar]
- Khan SQ, Dhillon OS, O'Brien RJ, Struck J, Quinn PA, Morgenthaler NG, Squire IB, Davies JE, Bergmann A, Ng LL 2007 C-terminal provasopressin (copeptin) as a novel and prognostic marker in acute myocardial infarction: Leicester Acute Myocardial Infarction Peptide (LAMP) study. Circulation 115:2103–2110 [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA, Williams JK 1996 Psychosocial factors, sex differences, and atherosclerosis: lessons from animal models. Psychosom Med 58:598–611 [DOI] [PubMed] [Google Scholar]
- Henry JP, Liu YY, Nadra WE, Qian CG, Mormede P, Lemaire V, Ely D, Hendley ED 1993 Psychosocial stress can induce chronic hypertension in normotensive strains of rats. Hypertension 21:714–723 [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Emoto M, Fujiwara S, Motoyama K, Morioka T, Komatsu M, Tahara H, Koyama H, Shoji T, Inaba M, Nishizawa Y 2004 Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment are useful indexes of insulin resistance in type 2 diabetic patients with wide range of fasting plasma glucose. J Clin Endocrinol Metab 89:1481–1484 [DOI] [PubMed] [Google Scholar]