Abstract
Context: Progesterone (P4) promotes its own secretion and the survival of human granulosa/luteal (GL) cells.
Objective: The objective of these studies was to determine whether progesterone receptor membrane component-1 (PGRMC1) mediates P4’s actions.
Design: In vitro studies were conducted on GL cells from women undergoing in vitro fertilization and GL5 cells, which are derived from GL cells.
Setting and Patients: GL cells were obtained from women undergoing fertility treatment at a university-based clinic and used for in vitro studies.
Main Outcome Measures: PCR, Western blot, and immunocytochemistry were used to detect various progestin binding proteins. 3H-P4 binding kinetics were assessed on partially purified PGRMC1. Apoptosis was determined after culture by either TUNEL or DAPI staining. P4 was measured by an ELISA assay. PGRMC1 was depleted using small interfering RNA.
Results: GL and GL5 cells expressed several P4 binding proteins including the nuclear progesterone receptor (PGR), progestin/adipoQ receptors (PAQR 7, 8, and 5) and PGRMC1. Ligand binding studies revealed that both P4 and the progestin, R5020, bound PGRMC1 with an EC50 of approximately 10 nm. Interestingly, P4 inhibited apoptosis at concentrations in the 10 nm range, whereas R5020 stimulated P4 secretion at concentrations of at lease 16 μm. Depleting PGRMC1 attenuated P4’s antiapoptotic action but failed to influence R5020-induced P4 secretion.
Conclusions: These studies conclusively demonstrate that in human GL cells PGRMC1 functions as a receptor through which P4 activates a signal cascade that prevents apoptosis. In contrast, PGRMC1 does not mediate P4’s ability to acutely promote its own secretion.
In human granulosa/luteal cells, PGRMC1 functions as a receptor through which P4 activates a signal cascade that prevents apoptosis but it does not mediate P4’s ability to acutely promote its own secretion.
Progesterone (P4) plays a key role in the reproductive process (1). Recently, the gonads have been shown not only to be the major site of P4 synthesis but also to be one of its target organs (2). Studies in rats have shown that P4, acting through its nuclear receptors [P4 receptors (PGRs)], regulates the expression of several genes that are required for ovulation (3). However, P4 also influences other ovarian functions such as steroidogenesis (4,5,6) and survival of granulosa (7) and luteal cells (7). The survival function of P4 has been observed in both rat (7,8,9,10,11) and human (9,11,12) luteal cells. Although rat luteal cells do not express PGR (13,14), human luteal cells do (12), thereby setting up a controversy as to whether PGR mediates P4’s antiapoptotic action in human luteal cells.
If not PGR, then there must be another progestin binding protein that mediates P4’s survival and steroidogenic actions in human luteal cells. Potential candidates include members of the progestin/adipoQ receptor family that have been described by the Thomas lab (15,16). These receptors are G protein-coupled seven-transmembrane receptors that ultimately regulate intracellular cAMP levels and MAPK activity (15,16). Unfortunately, their expression has not been characterized in human luteal cells. Another potential mediator of P4’s action is progesterone receptor membrane component-1 (PGRMC1) (7). PGRMC1 is expressed in human luteal cells, and blocking antibody studies suggest but do not prove that it is a mediator of P4’s antiapoptotic actions (12).
Because granulosa/luteal (GL) cells have to be harvested from individual patients, the identification of a human GL cell line that is responsive to P4 would help to resolve the controversies surrounding the mechanism through which P4 influences the survival and steroidogenic capacity of human luteal cells. Several years ago, a human GL cell line was developed by Dr. Bruce Carr (University of Texas Dallas) and his associates (17). These cells, referred to as GL5 cells, have a similar morphological appearance to that of human GL cells and maintain their capacity to secrete steroids (17). Therefore, in the first series of studies, the expression of several progestin binding proteins as well as P4’s actions was assessed in both GL and GL5 cells. The second series of studies used small interfering RNA (siRNA) to deplete PGRMC1 in GL5 cells, and then the changes in P4’s ability to prevent apoptosis and to promote P4 secretion were monitored.
Materials and Methods
GL cell and GL5 cell culture
Culture conditions
Human GL cells were harvested from women undergoing ovulation induction for the treatment of infertility as previously described (12). This protocol was approved by the Institutional Review Board of the University of Connecticut Health Center. GL cells were cultured as previously described (12,18). GL cells were cultured in DMEM/F12 medium supplemented with 5% fetal bovine serum and 2 IU/ml of human chorionic gonadotropin (Sigma Chemical Co., St. Louis, MO). On the day of each experiment, GL cells were collected from two to three patents, pooled, plated in the appropriate culture dish, and treated according to the specific experimental protocol. GL5 cells were provided from Dr. Bruce Carr and maintained in culture as described by Rainey et al. (17), with the exception that 5% horse serum and 2% Ultroser G were not included into the culture media.
Depending on the experimental design, GL and GL5 cells were cultured with the following reagents. P4 (1–1000 nm) was purchased from Sigma Chemical Co. The 8-bromo-cGMP (8-br-cGMP) (20 μm) and protein kinase G (PKG) inhibitor, DT-3 (0.25 μm), were obtained from EMD Chemicals (La Jolla, CA). R5020 (8–64 μm) was purchased from Perkin-Elmer (Waltham, MA). Note that R5020 was used as a surrogate for P4 in the steroidogenesis studies because it was not detected in the P4 ELISA assay.
Treatment with PGRMC1 siRNA
PGRMC1 was depleted from GL5 cells as follows. GL5 cells (2 × 105 cells) were plated in 35-mm culture dishes. After culture for 24 h, the medium was removed, and the cells were transfected with either PGRMC1 siRNA (cat. no. AM16708 siRNA, ID no. 18248; Ambion, Austin, TX) or scramble siRNA (cat. no. AM4611; Ambion) using the siPORT NeoFx transfection kit (Ambion) according to manufacturer’s instructions. GL5 cells were transfected to yield a final concentration of 30 or 60 nm siRNA. Reagents were gently mixed and incubated for 15 min at room temperature. After transfection, the cells were incubated for 72 h and then assigned to an experiment.
Detection of progestin binding proteins by PCR
PCR
Total mRNA was isolated from GL and GL5 cells, and cDNA was generated as previously described (19). The presence of PGR, PAQR 7, PAQR 8, PAQR 5, and PGRMC1 was then assessed by conducting 35 cycles of PCR (denaturation phase of 40 sec at 94 C, an annealing phase of 40 sec at 58 C, and an extension phase of 60 sec at 72 C). The specific primers used to detect each mRNA that encoded a progestin binding protein are shown in Supplemental Table 1 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). These primers were identified using Primer 3 software (http://frodo.wi.mit.edu/) except for the primers for PGR, which were taken from Misao et al. (20).
Real time PCR for the measurement of PGRMC1 mRNA
Quantitative measurements of PGRMC1 mRNA were done as previously described (19). Briefly, primers to human PGRMC1 were designed using ABI Prism Primer Express 2.0 software (Applied Biosystems, Foster City, CA). Primers were evaluated with NCBI blast to confirm product specificity, and products were designed to cross intron/exon borders. Primers used were: forward, 5′-CGGGCTGCTGCATGAGATTT-3′; and reverse, 5′-CCGCGCACGATCTTGTAGAG-3′. Quantitative real time PCR was performed on the iCycler Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Samples were resuspended in 25 μl of the SYBR Green RT-PCR Reaction Mix (Bio-Rad) containing appropriate primers. Relative gene expression was evaluated with Bio-Rad iCycler software using the ΔCT method (21). Values were expressed as a percentage of the scramble control treatment.
Immunodetection of PGRMC1
GL5 cells were lysed in RIPA buffer (50 mm Tris, 150 mm sodium chloride, 1.0 mm EDTA, 1% Nonidet P40, and 0.25% sodium-deoxycholate, pH 7.0) which was supplemented with complete protease inhibitor cocktail (50 μl/ml of RIPA buffer; Roche, Mannheim, Germany) and phosphatase inhibitor cocktail 1 (10 μl/ml of RIPA buffer; Sigma Chemical Co.) and then centrifuged at 1000 × g at 4 C for 5 min. The supernatant was run on a 10% acrylamide gel and transferred to nitrocellulose. The nitrocellulose was then incubated with 5% nonfat dry milk overnight at 4 C, washed, and subsequently incubated with the goat PGRMC1 antibody (1:150; Abcam Inc., Cambridge, MA) for either 1 h at room temperature or 24 h at 4 C. Western blots were processed as previously described, and an Enhanced Chemiluminescence detection system was used to reveal the presence of PGRMC1 (12). As a negative control, either the primary antibody was omitted or a goat IgG was used in place of the PGRMC1 antibody.
For PGRMC1 localization studies, GL5 cells were grown on glass cover slips. When the cells reached a confluence of 70–80%, they were fixed in 4% paraformaldehyde as previously described (12). The cover slips were incubated overnight at 4 C with the PGRMC1-NT antibody (1:50), which was provided by Drs. R. Losel and M. Wehling (University of Heidelberg, Heidelberg, Germany). After a washing with PBS to remove the primary antibody, the cover slips were incubated for 1 h at room temperature in the dark with Alexa Fluor 488-goat antirabbit IgG (1:100; Invitrogen, Carlsbad, CA). The cover slips were again washed with PBS, mounted in Anti-Fade, and observed under the fluorescent microscope. Negative controls were also processed as described for the Western blots.
Detection of apoptotic nuclei
Apoptotic nuclei were detected by either terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay or in situ DNA staining using 4′,6-diamidino-2-phenylindole (DAPI). For the TUNEL assay, GL5 cells were cultured for 24 h in the presence or absence of serum and then fixed in 1% paraformaldehyde for 10 min at room temperature. The cells were stained using the Apoptag Peroxidase in Situ kit staining according to manufacturer’s instructions (Chemicon, Temecula, CA).
In situ DNA staining was accomplished by adding DAPI directly to the culture medium at a final concentration of 2 μg/ml. The cultures were incubated for 10 min at 37 C in the dark. After staining, the cells were observed under epifluorescent optics. Under these conditions only cells with condensed or fragmented nuclei were stained intensely with DAPI. These cells were considered to be apoptotic (12). At least 100 cells/culture well were counted, and the percentage of apoptotic nuclei in each well was determined.
3H-P4 binding studies
3H-P4 binding to partially purified green fluorescent protein (GFP)-human PGRMC1 was determined per our previously published protocol (22). Briefly, SIGC cells (1 × 107) were transfected using lipofectamine with GFP-human PGRMC1 fusion protein and after 24 h were isolated using the protocol and reagents provided by Miltenyi Biotec (Auburn, CA) (22). Displacement studies were conducted to determine the effect of increasing concentrations of nonradioactive P4 or R5020 on 3H-P4 binding. The ability of unlabeled P4 or R5020 to displace 3H-P4 binding was assessed by fitting the data to a single binding site model using Prism software (GraphPad v. 5.0; GraphPad, La Jolla, CA). In addition to binding studies, the partially purified GFP-human PGRMC1 protein was also separated on a 10% acrylamide gel and processed for Western blot analysis using an antibody to either GFP (1:2000; Cell Signaling, Danvers, MA) or PGRMC1-NT (1:2000).
P4 assay
To monitor the effect of R5020 on P4 secretion, cells were washed with serum-free medium and then cultured in serum-free medium in the presence or absence of R5020. R5020 was used at final concentrations of 8, 16, 32, and 64 μm. Vehicle (absolute ethanol) was used to supplement the control cultures. Cells were incubated with these treatments for 3 h at 37 C. The medium was then removed, and P4 concentrations within the media were determined using a progesterone ELISA kit (Bio-Quant, San Diego, CA). All samples from each replicate were run on the same assay. P4 secretion was expressed as a fold-increase over control values.
Statistical analysis
All experiments were repeated at least three times, with each experiment yielding essentially identical results. When appropriate, the values from each experimental replicate were pooled to generate means ± se values and analyzed by either a Student t test or a one-way ANOVA followed by a Student-Newman-Keuls test, if more than two treatments groups were being compared. P values of less than 0.05 were considered to be significant.
Results
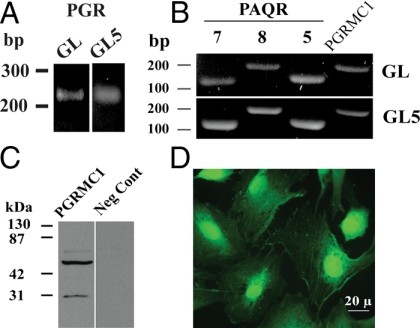
mRNAs that encode several progestin-binding proteins including PGR (Fig. 1A), PAQR 7, PAQR 8, PAQR 5, and PGRMC1 were detected in both GL and GL5 cells (Fig. 1B). Western blot analysis (Fig. 1C) revealed the presence of both the monomeric (≈28 kDa) and presumed dimeric (≈56 kDa) forms of PGRMC1 in GL5 cells. This finding is similar to the published PGRMC1 Western blots for GL cells (12). The immunocytochemical studies revealed the presence of PGRMC1 in the nucleus, cytoplasm, and at segments of the plasma membrane (Fig. 1C). A similar localization pattern was observed in GL cells (12).
Figure 1.
The mRNA levels of PGR (A), PAQR 7, PAQR 8, PAQR 5, and PGRMC1 (B) as revealed by PCR. Western blot and immunocytochemical detection of PGRMC1 in GL5 cells is shown in C and D, respectively. Negative control (i.e. omission of the PGRMC1 antibody) for the immunocytochemical staining of PGRMC1 failed to show any staining.
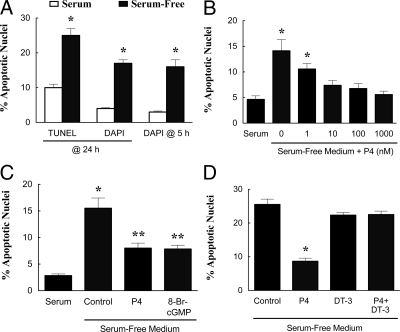
As illustrated by either TUNEL or DAPI staining, GL5 cells underwent apoptosis after being placed in serum-free medium for 24 h (Fig. 2A). DAPI staining also revealed a significant increase in GL5 cell apoptosis within 5 h in serum-free medium (Fig. 2A). Based on these findings, apoptosis was assessed in all subsequent studies after 5 h in serum-free culture using DAPI staining to detect apoptotic nuclei.
Figure 2.
The effect of serum withdrawal, P4, and PKG regulators on the rate of apoptosis of GL5 cells. A, The effect of serum withdrawal on GL5 apoptosis was assessed by TUNEL assay after 24 h (six observations per group collected from two experimental replicates) and by DAPI staining after 24 h (16 observations per group collected from three experimental replicates) or 5 h (12 observations per group collected from three experimental replicates). In this and subsequent panels, values represent means and se values. *, Means that are different from control values (P < 0.05). B, The dose-response characteristics of P4 on GL5 cell apoptosis are shown (eight observations per group collected from three experimental replicates). In panel B and all subsequent panels, apoptosis was assessed by DAPI staining after 5 h of culture. The effects of P4 (1 μm), 8-br-cGMP (20 μm), and the PKG inhibitor, DT-3 (0.25 μm), on the percentage of GL5 apoptotic nuclei are shown in C and D, respectively. *, A mean that is different from control; **, a mean that is greater than the serum control but less than the serum-free value.
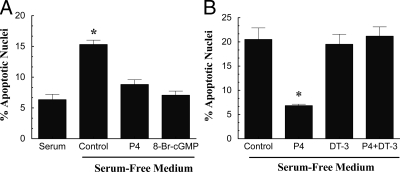
As previously observed for GL cells (12,23), P4 inhibited serum withdrawal-induced apoptosis of GL5 cells in a dose-dependent manner with an effective dose of 10 nm (Fig. 2B). Moreover, P4’s antiapoptotic action was mimicked by 8-br-cGMP (Fig. 2C) and attenuated by the PKG inhibitor, DT-3 (Fig. 2D). Similar responses to 8-br-cGMP and DT-3 were also observed in GL cells (Fig. 3, A and B).
Figure 3.
The effect of P4 (A and B), 8-br-cGMP (A), and the PKG inhibitor DT-3 (B) on the percentage of GL apoptotic nuclei. P4, 8-br-cGMP, and DT-3 were used at final concentrations of 1, 20, and 0.25 μm, respectively. In both panels, means are of six observations per group collected from three experimental replicates. *, A mean that is different from control.
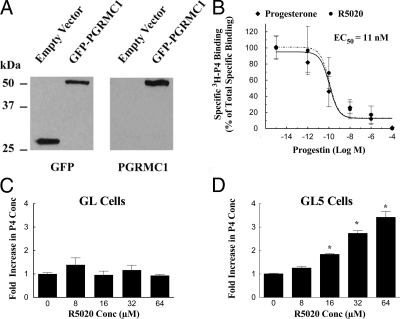
GFP-human PGRMC1 was effectively isolated from transfected cells as revealed by Western blots probed with antibodies to either GFP or PGRMC1 (Fig. 4A). Ligand binding studies conducted on partially purified GFP-human PGRMC1 demonstrated that this fusion protein specifically binds 3H-P4 at a single site with an EC50 of 11 nm (Fig. 4B). In addition, R5020 displaced 3H-P4 bound to GFP-human PGRMC1 with the same characteristics as nonlabeled P4 (Fig. 4B).
Figure 4.
P4 binding characteristic of partially purified human PGRMC1. SIGC cells were transfected with either an empty vector that encoded GFP or a vector that encoded a GFP-human PGRMC1 fusion protein. GFP proteins were isolated and assessed using Western blot. The Western blots that were probed with GFP antibody detected either the 28-kDa GFP protein (empty vector lane) or the 56-kDa GFP-PGRMC1 fusion protein (GFP-PGRMC1 lane) (A). Probing the Western blot with the PGRMC1 antibody confirmed that the 56-kDa protein was the GFP-PGRMC1 fusion protein (A). Ligand binding studies revealed that both P4 and R5020 displaced 3H-P4 with the same dose-dependent characteristics. Curve fitting the binding data revealed an EC50 of 11 nm for both P4 and R5020 (B) (six observations per group collected from three experimental replicates). The effect of increasing doses of the progestin, R5020, on P4 secretion from GL or GL5 is shown in panels C (12 observations per group collected from three experimental replicates) and D (12 observations per group collected from three experimental replicates), respectively. For these studies, both GL and GL5 cells were cultured for 3 d as described in the Materials and Methods section and then cultured for 3 h in serum-free medium. *, A mean that is different from control.
The basal P4 secretion rate for GL cells averaged 50 ± 26 pg/ml/3 h and was not altered by increasing doses of R5020 (Fig. 4C). The basal P4 secretion rate for GL5 cells was only about 2 ± 0.1 pg/ml/3 h, and this rate was increased in a dose-dependent manner by R5020 (Fig. 4D). The lowest stimulatory dose of R5020 was 16 μm, with the maximum dose of 64 μm inducing a 3- to 4-fold increase in P4 secretion (Fig. 4D).
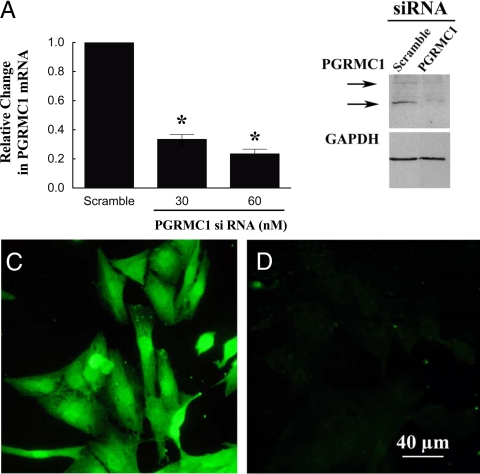
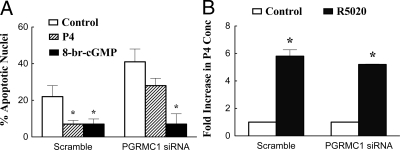
To determine whether PGRMC1 mediates either of P4’s actions, GL5 cells were treated with scramble or PGRMC1 siRNA. After 3 d of culture with PGRMC1 siRNA, PGRMC1 levels were reduced by approximately 80% as assessed by real-time PCR (Fig. 5A), Western blot analysis (Fig. 5B), and immunocytochemistry (compare Figs. 5C and 5D). PGRMC1 siRNA treatment did not alter glyceraldehyde-3-phosphate dehydrogenase levels (Fig. 5B). Importantly, treatment with PGRMC1 siRNA attenuated P4’s antiapoptotic action but did not affect the ability of 8-br-cGMP to prevent serum withdrawal-induced apoptosis (Fig. 6A). Conversely, treatment with PGRMC1 siRNA did not influence R5020-induced P4 secretion (Fig. 6B).
Figure 5.
The effect of PGRMC1 siRNA on the levels of PGRMC1 in GL5 cells. GL5 cells were cultured with either scramble (60 nm) or PGRMC1 siRNA (30 or 60 nm) for 3 d. PGRMC1 was assessed by real-time PCR (A) (n = 3 with each sample collected on a different day), Western blot (B), or immunocytochemistry (C and D). C, Cells cultured with scramble; D, cells treated with PGRMC1 siRNA. Images shown in B, C, and D were taken after treatment with 60 nm siRNA. *, A mean that is different from control.
Figure 6.
The effect of PGRMC1 siRNA on the percentage of apoptotic GL5 nuclei (A) (eight to nine observations per group collected from three experimental replicates) and P4 secretion (B) (22 observations collected over four experimental replicates). A, GL5 cells were treated with scramble or PGRMC1 siRNA and then control (serum-free) medium, P4 (1 μm), or 8-br-cGMP (20 μm). B, The effect of R5020 (60 μm) on P4 secretion after 3 d of treatment with either scramble or PGRMC1 siRNA. *, A mean that is different from control.
Discussion
The present study demonstrates that both GL and GL5 cells express several progesterone binding proteins, including PGR, PAQR 7, PAQR 8, PAQR 5, and PGRMC1. In addition, P4 regulates human GL cell function, but the receptor or receptors through which P4 acts is unknown. The elucidation of P4’s mechanism of action therefore would be facilitated by the identification of a P4-responsive human GL cell line. The present studies demonstrate that GL5 cells respond to P4, which inhibited apoptosis in a dose-dependent manner with an EC50 of approximately 10 nm. This is consistent with previous studies that demonstrate that P4 inhibits the rate of apoptosis in GL cells with similar dose-response characteristics (12,23). Furthermore, in both GL and GL5 cells, P4 stimulates a survival pathway that likely involves the activation of PKG because an activator of PKG (8-br-cGMP) mimics and an inhibitor of PKG (DT-3) attenuates P4’s antiapoptotic action. Taken together, these findings indicate that GL5 cells are suitable surrogates for assessing P4’s antiapoptotic mechanism of action in human GL cells.
Although blocking antibody studies suggest that PGRMC1 mediates P4’s antiapoptotic actions in human GL cells (12), additional biochemical and genetic studies are required to prove this concept. The first step in proving this concept is to characterize the ability of human PGRMC1 to bind P4. Previous studies have shown that partially purified rat PGRMC1 binds P4 with a high affinity (i.e. ≈35 nm) at a single site (22). Structurally, rat and human PGRMC1 are similar, with 94% amino acid homology (2). Moreover, the transmembrane and the heme binding domains are identical. One species-specific difference is that human PGRMC1 has a serine at amino acid 56 (2). This results in a putative casein kinase 2 site (http://scansite.mit.edu/motifscan) within the proposed P4 binding region (i.e. amino acids 20 to 70) (22). The present studies demonstrate that human PGRMC1 binds P4 at a single high-affinity binding site (EC50 ≈ 10 nm). This study indicates that the structural differences between rat and human PGRMC1 do not appreciably influence its capacity to bind P4 and that the EC50 is consistent with the dose-response characteristics of P4’s antiapoptotic action.
Having demonstrated that PGRMC1 binds P4, the next step in establishing a role for PGRMC1 in regulating human GL cell viability is to ablate PGRMC1 expression and then monitor P4’s ability to prevent apoptosis. To do this, siRNA was used to selectively deplete PGRMC1 from GL5. This treatment depletes PGRMC1 levels as shown by real-time PCR, Western blot, and immunocytochemistry. As a result of PGRMC1 siRNA treatment, P4’s ability to prevent apoptosis is attenuated. That this was due to the selective depletion of PGRMC1 is supported by the observation that an activator of PKG (i.e. 8-br-cGMP) is capable of inhibiting apoptosis of PGRMC1-depleted GL5 cells. This suggests that the survival pathway downstream of PKG activation was not affected by PGRMC1 siRNA treatment. Finally, this PGRMC1 siRNA study clearly demonstrates that P4 binding to any of the other P4 binding proteins does not activate a survival pathway that is sufficient to inhibit apoptosis.
That P4 activation of other P4 binding proteins (i.e. PGR and PAQRs) does not inhibit apoptosis is at odds with some previously published reports. For example, human GL cells as well as GL5 cells express PGR, and antagonists of PGR (RU486 and Org 31710) induce apoptosis in human GL cells (11). Importantly, RU486 was only effective in promoting apoptosis at 25 μm or higher doses (12). However, when RU486 was used at a 5-fold molar excess, it was unable to attenuate P4’s antiapoptotic action in human GL cells (12). Because the Kd for P4 binding to PGR is in the 1 to 5 nm range and RU486 binds to PGR at a slightly higher affinity than P4 (24), the 5-fold molar excess of RU486 should have been sufficient to block P4’s action. Because this was not observed, it suggests that apoptotic action of PGR antagonists is not due to a specific inhibition of PGR but rather a generalized toxic effect. The present studies provide genetic-based evidence that supports the concept that P4 activation of PGR is not sufficient to prevent apoptosis because P4 fails to prevent apoptosis in PGRMC1-deplete cells.
In addition to maintaining cell viability, the present studies demonstrate that P4 (R5020) stimulates its own secretion in GL5 cells but not GL cells. The inability of R5020 to stimulate P4 secretion from GL cells is likely due to their steroidogenic capacity being maximally stimulated by both in vivo and in vitro exposure to human chorionic gonadotropin. This is consistent with their higher basal P4 secretion rate (i.e. 50 pg/ml/3 h) compared with GL5 cells (i.e. 2 pg/ml/3 h). The ability of P4 (R5020) to influence steroidogenesis has also been observed in rat granulosa cells (25,26), luteal cells (6), and MA10 cells (27). To our knowledge, the present study is the first to reveal that R5020 stimulates P4 secretion from human GL cells (GL5 cells).
Interestingly, R5020 stimulates P4 in a dose-dependent manner with the lowest effective dose of 16 μm. Although this is an extremely high concentration, mean follicular fluid levels of P4 within human preovulatory follicles collected from women during either natural or gonadotropin-stimulated cycles range from 15 to 60 μm (28,29,30). This indicates that the P4-stimulating doses of R5020 are in a physiological range. Because R5020 binds human PGRMC1 with the same affinity as P4 (i.e. EC50 of ≈10 nm), maximal binding of R5020 to human PGRMC1 should be achieved in the nanomolar range. Therefore, if R5020 activation of PGRMC1 is involved in the mechanism through which it enhances P4 secretion, it should have been effective in the nanomolar, not the micromolar range. Moreover, R5020 was capable of promoting P4 secretion in PGRMC1-deplete GL5 cells. These observations provide compelling evidence that in GL5 cells, P4’s ability to acutely stimulate its own secretion is not mediated by PGRMC1. These studies, however, do not rule out the possibility that PGRMC1 could have genomic effects that might require a longer culture period to be detected. Alternatively, R5020’s steroidogenic action could be mediated by one of the other progestin binding proteins that are expressed by both GL and GL5 cells.
In summary, the present studies demonstrate that GL5 cells are a suitable cell line in which to investigate P4’s antiapoptotic mechanism of action in human GL cells. These studies also support the conclusion that P4 activation of PGRMC1 stimulates a PKG-dependent pathway that ultimately inhibits apoptosis, which is consistent with studies conducted with rodent granulosa cells (31,32,33). Finally, the role of PGRMC1 in promoting ovarian cell survival has clinical relevance because depletion and point mutations of PGRMC1 have been shown to be present in some women with premature ovarian failure (34). This underscores the importance of understanding the mechanism through which P4-PGRMC1 interaction mediates the survival of GL cells and thereby regulates the functional life span of the ovary.
Supplementary Material
Acknowledgments
The authors acknowledge Jonathan Romak for his help in conducting the P4 binding studies. We also thank Drs. R. Losel and M. Wehling of the University of Heidelberg (Heidelberg, Germany) for the gift of the PGRMC1-NT antibody.
Footnotes
This work was supported by National Institutes of Health Grant HD 052740.
Disclosure Summary: The authors have nothing to declare.
First Published Online May 5, 2009
Abbreviations: 8-br-cGMP, 8-Bromo-cGMP; DAPI, 4′,6-diamidino-2-phenylindole; GFP, green fluorescent protein; GL, granulosa/luteal; P4, progesterone; PGR, P4 receptor; PGRMC1, PGR membrane component-1; PKG, protein kinase G; siRNA, small interfering RNA; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
References
- Conneely OM, Lydon JP 2000 Progesterone receptors in reproduction: functional impact of the A and B isoforms. Steroids 65:571–577 [DOI] [PubMed] [Google Scholar]
- Peluso JJ 2007 Non-genomic actions of progesterone in the normal and neoplastic mammalian ovary. Semin Reprod Med 25:198–207 [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Yoshioka S, Sharma SC, Lydon JP, O'Malley BW, Espey LL, Richards JS 2000 Ovulation: a multi-gene, multi-step process. Steroids 65:559–570 [DOI] [PubMed] [Google Scholar]
- Schreiber JR, Nakamura K, Erickson GF 1981 Progestins inhibit FSH-stimulated granulosa estrogen production at a post-cAMP site. Mol Cell Endocrinol 21:161–170 [DOI] [PubMed] [Google Scholar]
- Fortune JE, Vincent SE 1983 Progesterone inhibits the induction of aromatase activity in rat granulosa cells in vitro. Biol Reprod 28:1078–1089 [DOI] [PubMed] [Google Scholar]
- Rothchild I 1996 The corpus luteum revisited: are the paradoxical effects of RU486 a clue to how progesterone stimulates its own secretion? Biol Reprod 55:1–4 [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A, Losel R, Wehling M 2006 Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone’s antiapoptotic action. Endocrinology 147:3133–3140 [DOI] [PubMed] [Google Scholar]
- Quirk SM, Cowan RG, Harman RM 2004 Progesterone receptor and the cell cycle modulate apoptosis in granulosa cells. Endocrinology 145:5033–5043 [DOI] [PubMed] [Google Scholar]
- Rung E, Friberg PA, Shao R, Larsson DG, Nielsen ECh, Svensson PA, Carlsson B, Carlsson LM, Billig H 2005 Progesterone-receptor antagonists and statins decrease de novo cholesterol synthesis and increase apoptosis in rat and human periovulatory granulosa cells in vitro. Biol Reprod 72:538–545 [DOI] [PubMed] [Google Scholar]
- Svensson EC, Markström E, Andersson M, Billig H 2000 Progesterone receptor-mediated inhibition of apoptosis in granulosa cells isolated from rats treated with human chorionic gonadotropin. Biol Reprod 63:1457–1464 [DOI] [PubMed] [Google Scholar]
- Svensson EC, Markström E, Shao R, Andersson M, Billig H 2001 Progesterone receptor antagonists Org 31710 and RU 486 increase apoptosis in human periovulatory granulosa cells. Fertil Steril 76:1225–1231 [DOI] [PubMed] [Google Scholar]
- Engmann L, Losel R, Wehling M, Peluso JJ 2006 Progesterone regulation of human granulosa/luteal cell viability by an RU486-independent mechanism. J Clin Endocrinol Metab 91:4962–4968 [DOI] [PubMed] [Google Scholar]
- Park OK, Mayo KE 1991 Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol 5:967–978 [DOI] [PubMed] [Google Scholar]
- Park-Sarge OK, Parmer TG, Gu Y, Gibori G 1995 Does the rat corpus luteum express the progesterone receptor gene? Endocrinology 136:1537–1543 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bond J, Thomas P 2003 Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA 100:2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P 2003 Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA 100:2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey WH, Sawetawan C, Shay JW, Michael MD, Mathis JM, Kutteh W, Byrd W, Carr BR 1994 Transformation of human granulosa cells with the E6 and E7 regions of human papillomavirus. J Clin Endocrinol Metab 78:705–710 [DOI] [PubMed] [Google Scholar]
- VandeVoort CA, Overstreet JW, Lasley BL, Stewart DR 2000 Effects of progesterone receptor blockers on human granulosa-luteal cell culture secretion of progesterone, estradiol, and relaxin. Biol Reprod 62:200–205 [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Liu X, Saunders MM, Claffey KP, Phoenix K 2008 Regulation of ovarian cancer cell viability and sensitivity to cisplatin by progesterone receptor membrane component-1. J Clin Endocrinol Metab 93:1592–1599 [DOI] [PubMed] [Google Scholar]
- Misao R, Nakanishi Y, Iwagaki S, Fujimoto J, Tamaya T 1998 Expression of progesterone receptor isoforms in corpora lutea of human subjects: correlation with serum oestrogen and progesterone concentrations. Mol Hum Reprod 4:1045–1052 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Romak J, Liu X 2008 Progesterone receptor membrane component-1 (PGRMC1) is the mediator of progesterone’s anti-apoptotic action in spontaneously immortalized granulosa cells as revealed by PGRMC1 siRNA treatment and functional analysis of PGRMC1 mutations. Endocrinology 149:534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrigiannakis A, Coukos G, Christofidou-Solomidou M, Montas S, Coutifaris C 2000 Progesterone is an autocrine/paracrine regulator of human granulosa cell survival in vitro. Ann NY Acad Sci 900:16–25 [DOI] [PubMed] [Google Scholar]
- Leonhardt SA, Boonyaratanakornkit V, Edwards DP 2003 Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids 68:761–770 [DOI] [PubMed] [Google Scholar]
- Fanjul LF, Ruiz de Galarreta CM, Hsueh AJ 1983 Progestin augmentation of gonadotropin-stimulated progesterone production by cultured rat granulosa cells. Endocrinology 112:405–407 [DOI] [PubMed] [Google Scholar]
- Ruiz de Galarreta CM, Fanjul LF, Hsueh AJ 1985 Progestin regulation of progesterone biosynthetic enzymes in cultured rat granulosa cells. Steroids 46:987–1002 [DOI] [PubMed] [Google Scholar]
- Schwarzenbach H, Manna PR, Stocco DM, Chakrabarti G, Mukhopadhyay AK 2003 Stimulatory effect of progesterone on the expression of steroidogenic acute regulatory protein in MA-10 Leydig cells. Biol Reprod 68:1054–1063 [DOI] [PubMed] [Google Scholar]
- Enien WM, el Sahwy S, Harris CP, Seif MW, Elstein M 1995 Human chorionic gonadotrophin and steroid concentrations in follicular fluid: the relationship to oocyte maturity and fertilization rates in stimulated and natural in-vitro fertilization cycles. Hum Reprod 10:2840–2844 [DOI] [PubMed] [Google Scholar]
- Kamel MA, Zabel G, Bernart W, Neulen J, Breckwoldt M 1994 Comparison between prolactin, gonadotrophins and steroid hormones in serum and follicular fluid after stimulation with gonadotrophin-releasing hormone agonists and human menopausal gonadotrophin for an in-vitro fertilization programme. Hum Reprod 9:1803–1806 [DOI] [PubMed] [Google Scholar]
- Tarlatzis BC, Pazaitou K, Bili H, Bontis J, Papadimas J, Lagos S, Spanos E, Mantalenakis S 1993 Growth hormone, oestradiol, progesterone and testosterone concentrations in follicular fluid after ovarian stimulation with various regimes for assisted reproduction. Hum Reprod 8:1612–1616 [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Liu X, Romak J 2007 Progesterone maintains basal intracellular adenosine triphosphate levels and viability of spontaneously immortalized granulosa cells by promoting an interaction between 14-3-3ς and ATP synthase β/precursor through a protein kinase G-dependent mechanism. Endocrinology 148:2037–2044 [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A 2004 Progesterone regulates granulosa cell viability through a protein kinase G-dependent mechanism that may involve 14–3-3ς. Biol Reprod 71:1870–1878 [DOI] [PubMed] [Google Scholar]
- Peluso JJ 2003 Progesterone as a regulator of granulosa cell viability. J Steroid Biochem Mol Biol 85:167–173 [DOI] [PubMed] [Google Scholar]
- Mansouri MR, Schuster J, Badhai J, Stattin EL, Lösel R, Wehling M, Carlsson B, Hovatta O, Karlström PO, Golovleva I, Toniolo D, Bione S, Peluso J, Dahl N 2008 Alterations in the expression, structure and function of progesterone receptor membrane component-1 (PGRMC1) in premature ovarian failure. Hum Mol Genet 17:3776–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.