Abstract
Context: PCSK9 is a secreted protein that influences plasma levels of low-density lipoprotein cholesterol (LDL-C) and susceptibility to coronary heart disease. PCSK9 is present in human plasma, but the factors that contribute to differences in plasma concentrations of PCSK9 and how they impact on the levels of lipoproteins have not been well-characterized.
Objective: The aim of the study was to measure PCSK9 levels in a large, ethnically diverse population (n = 3138) utilizing a sensitive and specific sandwich ELISA.
Design: We conducted an observational study in the Dallas Heart Study, a multiethnic, probability-based sample of Dallas County.
Results: Plasma levels of PCSK9 varied over approximately 100-fold range (33–2988 ng/ml; median, 487 ng/ml). Levels were significantly higher in women (517 ng/ml) than in men (450 ng/ml), and in postmenopausal women compared to premenopausal women (P < 0.0001), irrespective of estrogen status. Plasma levels of PCSK9 correlated with plasma levels of LDL-C (r = 0.24) but explained less than 8% of the variation in LDL-C levels (r2 = 0.073). Other factors that correlated with PCSK9 levels included plasma levels of triglycerides, insulin, and glucose. Individuals with loss-of-function mutations in PCSK9 and reduced plasma levels of LDL-C also had significantly lower plasma levels of PCSK9 after adjusting for age, gender, and LDL-C levels (P < 0.0001).
Conclusion: Multiple metabolic and genetic factors contribute to variation in plasma levels of PCSK9 in the general population. Although levels of PCSK9 correlate with plasma levels of LDL-C, they account for only a small proportion of the variation in the levels of this lipoprotein.
Plasma levels of PCSK9 correlate with levels of low density lipoprotein-cholesterol (LDL-C), but account for only a small proportion of the variation in the LDL-C concentration.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a member of the proprotein convertase family of zymogens (1). Sequence variation in PCSK9 is a major determinant of circulating levels of low-density lipoprotein cholesterol (LDL-C) in humans (2,3). Gain-of-function mutations in PCSK9 are rare and cause an autosomal dominant form of severe hypercholesterolemia and premature coronary heart disease (4), whereas loss-of-function mutations are more common and are associated with reduced plasma levels of LDL-C and protection from coronary heart disease (2,5).
PCSK9 modulates plasma levels of LDL-C by promoting the degradation of LDL receptors (LDLRs), the primary conduit for the removal of LDL from the circulation (6). LDL is formed in the circulation as a metabolic product of triglyceride-rich lipoproteins and binds to ligand-binding repeats in the extracellular domains of LDLRs on the sinusoidal surfaces of hepatocytes. The LDL-LDLR complexes are internalized via clathrin-coated pits and delivered to endosomes, where the LDLR dissociates from LDL and recycles to the cell surface (6). PCSK9 appears to interdict recycling of the LDLR and reroutes the receptor to the lysosome for degradation, leading to reduced clearance of LDL and accumulation of LDL-C in the circulation (7).
Several lines of evidence indicate that PCSK9 engages LDLRs on the cell surface. Addition of recombinant PCSK9 to the medium of cultured hepatocytes depletes the cells of LDLRs, and parabiosis experiments in mice demonstrated that circulating PCSK9 can mediate degradation of hepatic LDLRs (8,9). Although PCSK9 is expressed in several tissues, experiments in tissue-specific PCSK9 knockout mice suggest that almost all circulating PCSK9 is derived from the liver (8). Plasma levels of PCSK9 in humans have been measured using a variety of assays (10,11,12) in relatively small samples of individuals of European descent. To define further the factors that contribute to differences in plasma PCSK9 levels in the population and the relationship between circulating PCSK9 and LDL-C levels, we used a sensitive and specific sandwich ELISA to measure PCSK9 in the Dallas Heart Study, a large, ethnically diverse population (13).
Subjects and Methods
Subjects
Fasting blood samples were obtained from participants in the Dallas Heart Study, a multiethnic, probability-based sample of Dallas County that was collected between 2000 and 2002 (13). Ethnicity was self-assigned in accordance with U.S. census categories. “African-Americans” and “European-Americans” refer to individuals who identified themselves as non-Hispanic black and non-Hispanic white, respectively. The study protocol was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center, and written informed consent was obtained from each participant.
Blood sampling and PCSK9 ELISA
Venous blood samples were obtained after an overnight fast. The blood was maintained at 4 C until the plasma and serum were separated, aliquoted, and stored at −80 C. Genomic DNA was isolated from leukocytes using PureGene (Gentra Systems, Minneapolis, MN). Plasma concentrations of lipoproteins and lipids were measured using commercial enzymatic reagents (13).
PCSK9 levels in plasma were assayed using a sandwich ELISA assay similar to that previously reported for humans and monkeys (9,14). A rabbit antihuman PCSK9 polyclonal antibody (228B) was diluted in Buffer B [20 mm NaPO4 (pH 7.5), 100 mm NaCl) to a final concentration of 10 μg/ml and attached to Nunc Maxisorp white plates overnight at 4 C. The wells were washed three times with PBS containing 0.08% Tween 20 (pH 7.4) (PBST), blocked with 0.5% BSA for 1–2 h, and then washed again three times with PBST. Plasma samples were thawed at room temperature, mixed by inverting the tube several times, and centrifuged briefly; then, 1 μl of plasma was added to 99 μl of Buffer B containing 0.5% BSA and 0.08% Tween 20. The sample (100 μl) was added to the assay plate and incubated for 2.5 h at 37 C with shaking. At the end of the incubation, the wells were washed three times in PBST before the addition of the detection antibodies.
PCSK9 was detected using a mixture of two biotinylated mouse antihuman monoclonal antibodies: 13D3, which recognizes the catalytic domain, and 15A6, which recognizes the carboxyl terminus of PCSK9 (9). The antibodies were diluted to a concentration of 10 μg/ml each in Buffer B plus 0.5% BSA, and 100 μl detection solution was added to each well and incubated for 2 h at room temperature with shaking. The wells were then washed three times with PBST, and 100 μl Avidin-horseradish peroxidase (150 ng/ml in Buffer B plus 0.5% BSA) was added. Finally, plates were washed three times with PBST, followed by the addition of 100 μl Pierce SuperSignal ELISA Femto substrate (following the manufacturers’ instructions; Pierce, Rockford, IL), and luminescence was quantified using a Luminoskan Ascent (Thermo Electron Corporation, Waltham, MA). Standard curves were prepared by serial dilutions of purified recombinant human PCSK9 protein produced in human embryonic kidney (HEK) 293S cells (9) added to plasma from an individual who is a compound heterozygote for two inactivating mutations in PCSK9 and has no immunodetectable circulating PCSK9 (15). The intraassay coefficient of variation (CV) was 6.7%. The interassay CV was 10.1%.
To assess day-to-day variation in plasma levels of PCSK9, fasting blood samples were taken from 200 individuals on two separate occasions, 6 wk apart. The intraindividual CV for each subject was calculated using the formula: CV = sd/mean.
Sequencing and genotyping
Sequence variations were identified by PCR-mediated amplification and DNA sequencing as previously described (3). Apolipoprotein E (APOE) variants were assayed using the TaqMan system (Applied Biosystems, Foster City, CA). The assays were performed on an HT7900 Real-Time PCR system with probes and primers designed in house (see Supplemental Table S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Assay reagents were purchased from Applied Biosystems. Observed genotype frequencies were tested for Hardy-Weinberg equilibrium as described (3).
Statistical methods
Statistical analyses were performed using SAS software version 9.1 (SAS Institute, Cary, NC). Individuals using lipid-lowering medications (n = 214) were excluded from all analyses. Menopause was defined as age at least 55 yr, a history of bilateral oophorectomy, or a serum FSH level of over 30 IU/liter. When assessing differences in PCSK9 levels by menopausal status, women who were perimenopausal were excluded to avoid misclassification (n = 307); these individuals included women under the age of 55 yr who had not had a menstrual period for over 12 months and had an FSH above 10 IU/liter or whose last menstrual period was between 3 and 12 months earlier and had an FSH above 20 IU/liter (16). Because the distribution of PCSK9 levels in the general population was skewed, nonparametric tests were used to compare median levels of PCSK9 across groups. Correlations between PCSK9 and other variables were assessed using Spearman’s ranked correlation. Generalized linear models were used to determine the associations between sequence variants and plasma PCSK9 levels after adjusting for age and sex in ethnic-specific models. A separate model was assessed for association between sequence variants and plasma LDL-C concentration. A regression model was used to determine the percentage variance of PCSK9 explained by standard covariates; the model included age, sex, ethnicity, body mass index (BMI), systolic blood pressure, menopausal status, statin use, and fasting levels of glucose, LDL-C, high-density lipoprotein cholesterol (HDL-C), triglycerides, and C-reactive protein (CRP). Ethnic-specific models included these variables plus PCSK9 genotypes (encoding Y142X or C679X in African-Americans and R46L in European-Americans).
Results
Distribution of plasma PCSK9 levels in the general population
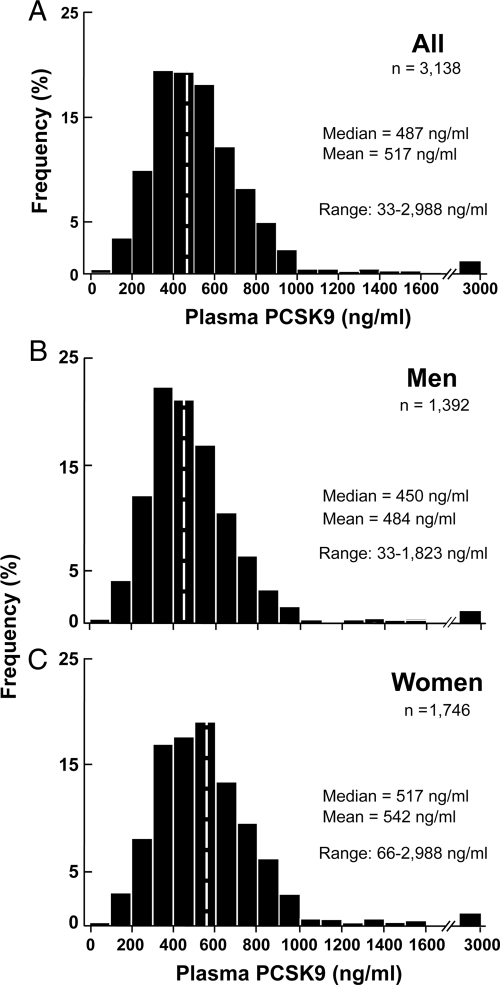
Plasma levels of PCSK9 varied over almost a 100-fold range, from 33 to 2988 ng/ml, among the participants in the Dallas Heart Study. Because statin treatment is associated with an increase in plasma PCSK9 levels (17), individuals taking statins were not included in the analysis (see below). The distribution of PCSK9 levels in the population was right-skewed with a median concentration of 487 ng/ml (Fig. 1A). Median levels were significantly higher in women (517 ng/ml) than in men (450 ng/ml) (P < 0.0001) (Fig. 1B). Significant differences in plasma levels of PCSK9 persisted between the sexes after adjusting for age, ethnicity, BMI, systolic blood pressure, menopausal status, and fasting levels of glucose, LDL-C, HDL-C, triglycerides, and CRP (P < 0.0001). Median plasma levels of PCSK9 were higher in Hispanics (508 ng/ml) and slightly lower in African-Americans (478 ng/ml) than in European-Americans (490 ng/dl) (Supplemental Fig. S1). After adjusting for age, gender, and plasma levels of triglycerides, the differences in median plasma PCSK9 concentrations between the groups were not statistically significant (P = 0.29).
Figure 1.
The distribution of fasting plasma concentrations of PCSK9 in the Dallas Heart Study (n = 3138) excluding individuals on statins (97 women and 117 men) in all subjects (A), in men only (B; n = 1392), and in women only (C; n = 1746). Levels of PCSK9 were measured using a sandwich ELISA in plasma samples obtained after an overnight fast, as described in Subjects and Methods. Significant differences were observed between men and women after adjustment for age, ethnicity, BMI, diabetes, and plasma levels of LDL-C, HDL-C, and triglycerides (P < 0.0001).
Effects of age and menopausal status on plasma levels of PCSK9
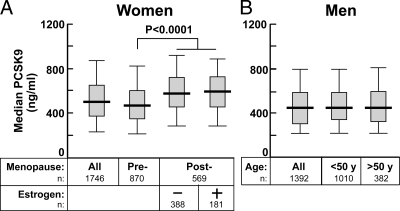
To examine further the gender differences in PCSK9 concentrations, we compared plasma levels of PCSK9 in pre- and postmenopausal women and in men as a function of age (Fig. 2). Median plasma concentrations of PCSK9 were slightly higher in premenopausal women than in men (479 vs. 450 ng/ml; P = 0.03), and this difference persisted when the comparison was made with men below age 50 yr.
Figure 2.
Effect of menopause status and estrogen usage in women (A) and effect of age in men (B) on median fasting plasma concentrations of PCSK9 in the Dallas Heart Study. The median level is denoted by a horizontal line. The gray boxes denote the 75th and 25th percentiles; the whiskers represent the 95th and 5th percentiles. A, Premenopausal women had significantly higher plasma levels of PCSK9 than did postmenopausal women (P = 0.001). Estrogen treatment did not significantly affect significant fasting PCSK9 levels in postmenopausal women. B, No difference in plasma levels of PCSK9 was apparent between men over age 50 yr and men younger than 50 yr. Subjects on statins were not included in the analysis.
Postmenopausal women had significantly higher median plasma levels of PCSK9 than did premenopausal women (P < 0.0001). Estrogen replacement therapy was not associated with a difference in median PCSK9 among the postmenopausal women (P = 0.6) (Fig. 2A). These data suggest that menopause has an effect on PCSK9 levels that is independent of estrogen status. Only a modest correlation was found between PCSK9 levels and age in women (r = 0.2), and there was no significant relationship between PCSK9 levels and age in men (Fig. 2B and Table 1).
Table 1.
Correlation between clinical parameters and plasma PCSK9 levels in the Dallas Heart Study (n = 3138)
| All | P value | Women | P value | Men | P value | |
|---|---|---|---|---|---|---|
| Age (yr) | 0.18 | <0.0001 | 0.2 | <0.0001 | 0.008 | 0.75 |
| BMI (k/m2) | 0.12 | <0.0001 | 0.13 | <0.0001 | 0.05 | 0.1 |
| Systolic BP (mm Hg) | 0.07 | 0.0001 | 0.15 | <0.0001 | 0.02 | 0.44 |
| Diastolic BP (mm Hg) | 0.08 | <0.0001 | 0.16 | <0.0001 | 0.04 | 0.1 |
| LDL-C (mg/dl) | 0.24 | <0.0001 | 0.31 | <0.0001 | 0.20 | <0.0001 |
| HDL-C (mg/dl) | 0.08 | 0.003 | 0.06 | 0.01 | 0.02 | 0.50 |
| Triglycerides (mg/dl) | 0.25 | <0.0001 | 0.29 | <0.0001 | 0.26 | <0.0001 |
| Glucose (mg/dl) | 0.17 | <0.0001 | 0.20 | <0.0001 | 0.17 | <0.0001 |
| Insulin (U) | 0.19 | <0.0001 | 0.13 | <0.0001 | 0.18 | <0.0001 |
| HOMA-IR | 0.21 | <0.0001 | 0.20 | <0.0001 | 0.20 | <0.0001 |
| CRP | 0.11 | <0.0001 | 0.14 | <0.0001 | 0.003 | 0.90 |
| CAC (Agatson U) | 0.07 | <0.0001 | 0.14 | <0.0001 | 0.05 | 0.11 |
| Lipoprotein(a) | 0.02 | 0.33 | 0.020 | 0.50 | −0.01 | 0.84 |
| Hepatic TG contenta | 0.13 | <0.0001 | 0.14 | <0.0001 | 0.12 | <0.0001 |
Values in are Spearman correlation coefficients between plasma concentrations of PCSK9 and clinical parameters and the associated P values. Statin users were excluded. CAC, Coronary artery calcium.
Hepatic triglyceride (TG) content was determined by proton magnetic spectroscopy in 2027 subjects (26).
Correlation of PCSK9 levels with demographic and metabolic variables
In univariate analysis, plasma concentrations of PCSK9 were significantly correlated with several variables (Table 1), although the correlation coefficients were low (<0.20) for many of them (age, BMI, total body fat, blood pressure, hepatic triglyceride content, and plasma levels of HDL-C and CRP). All of these correlations were higher in women than in men. The highest correlations found were between plasma levels of PCSK9 and the concentration of LDL-C and triglycerides in the blood (Table 1 and Supplemental Fig. S2). A 100 ng/ml increase in plasma PCSK9 level was associated with a 4.5-mg/dl increase in plasma LDL-C concentration in women and a 3.2-mg/dl increase in LDL-C in men. Levels of PCSK9 correlated less robustly with plasma concentrations of HDL-C. PCSK9 levels also correlated significantly (P < 0.001) with several indices of glucose metabolism, including fasting serum glucose, insulin, and homeostasis model assessment-insulin resistance (HOMA-IR), which is an indicator of insulin sensitivity; the strength of these correlations was similar in men and women. Obesity and diabetes were associated with significantly higher plasma levels of PCSK9 (P < 0.0001) (Supplemental Table S1). Hypertension, but not coronary artery calcium scores, was modestly associated with PCSK9 levels.
Levels of CRP, an acute phase protein, were positively correlated with PCSK9 levels in women but not in men, whereas CRP concentration correlated with levels of LDL-C (r = 0.1) in both sexes. The association between plasma levels of CRP and PCSK9 in women did not remain significant after adjustment for BMI and menopausal status (P = 0.37).
PCSK9 levels, LDL-C levels, and APOE genotypes
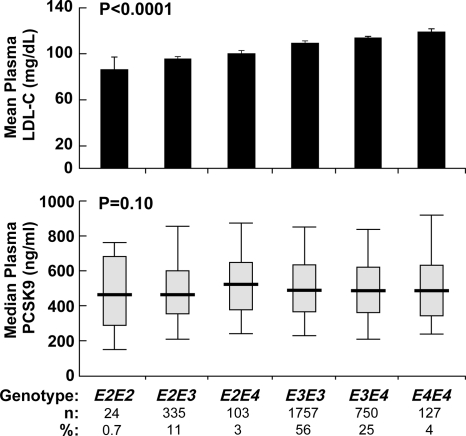
To assess the nature of the relationship between plasma levels of LDL-C and PCSK9, we tested for an association between polymorphisms in APOE and plasma concentrations of PCSK9. APOE mediates the binding of very low-density lipoprotein (VLDL) and chylomicron remnants to the LDLR, and polymorphisms of APOE are associated with differences in plasma levels of LDL-C (18). Generalized linear modeling indicated that APOE genotypes were significantly associated with plasma levels of LDL-C in the Dallas Heart Study (P < 0.0001; Fig. 3). No significant relationship was found between APOE genotypes and plasma PCSK9 concentrations (P = 0.10; Fig. 3). Thus, although plasma concentrations of PCSK9 and LDL-C correlate with each other, a change in LDL-C concentration does not necessarily result in a corresponding change in plasma level of PCSK9. Only 7% of the variance of LDL-C was explained by plasma PCSK9 concentration (5% in men and 10% in women). Exclusion of subjects with the R46L, Y142X, or C679X alleles did not appreciably alter the proportion of variation in LDL-C that could be attributed to PCSK9 levels (6 vs. 7%).
Figure 3.
Plasma concentrations of LDL-C (mean ± se; top) and PCSK9 (median; bottom) in relationship to APOE genotypes in the Dallas Heart Study. The APOE genotypes were determined using a TaqMan assay with custom-made probes. The APOE112 and APOE158 single nucleotide polymorphisms were combined to create the APOE genotypes. The mean plasma levels of LDL-C were significantly different between the APOE genotypes using one-way ANOVA (P < 0.0001). PCSK9 levels were not significantly different among APOE genotypes (P = 0.10) in the Dallas Heart Study. The P values reflect a model adjusted for age, ethnicity, gender, and BMI. Subjects on statins were not included in the analysis.
Effects of loss-of-function mutations in PCSK9 on plasma concentrations of PCSK9
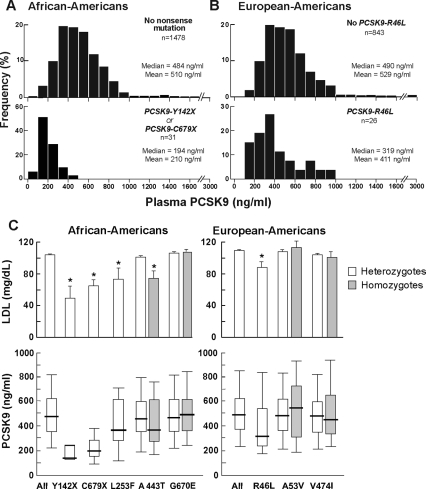
Next, we examined the effect of sequence variations in PCSK9 on plasma levels of PCSK9. Previously, we showed that African-Americans in the Dallas Heart Study who were heterozygous for nonsense mutations in PCSK9 (PCSK9:Y142X or PCSK9:C679X) (n = 31) had on average a 40% reduction in plasma level of LDL-C (2). Median PCSK9 concentrations were significantly lower in these individuals (194 ng/ml) than in those who did not have a null allele (484 ng/ml) (Fig. 4A). The reduction in plasma PCSK9 remained significant after adjusting for age, sex, and LDL-C (P < 0.0001).
Figure 4.
The distribution of plasma PCSK9 levels in African-Americans who were heterozygous for a nonsense mutation in PCSK9 (Y142X or C679X) (A); European-Americans heterozygous or homozygous for PCSK9:R46L (B); and median plasma levels of PCSK9 in African-Americans and European-Americans with various sequence variations in PCSK9 (C). A, African-American individuals heterozygous for a nonsense mutation had significantly lower median PCSK9 concentrations compared with those without the mutation after adjusting for age and sex (P < 0.0001) and after adjusting for LDL-C (P < 0.0001). B, In European-Americans, individuals heterozygous for R46L had significantly lower plasma PCSK9 in age and sex-adjusted models (P = 0.0004) and after adjusting for plasma LDL-C levels (P = 0.004). C, Relationship between nonsynonymous variants in PCSK9 and plasma levels of LDL-C in African-Americans (All, n = 1607) (left) and European-Americans (All, n = 909) (right) in the Dallas Heart Study. Individuals heterozygous for nonsynonymous variants not associated with changes in LDL-C were included in the analysis (PCSK9: G670E, A53V, V474I). Subjects taking statins were excluded.*, P < 0.05.
European-Americans heterozygous for PCSK9:R46L, a missense mutation in the prodomain of the PCSK9, have a mean reduction in LDL-C of 21% in the Dallas Heart Study (3). The reason this mutation is associated with lower plasma levels of LDL-C is not known. Unlike the two nonsense alleles, PCSK9:R46L is synthesized and secreted normally when the recombinant protein is expressed in cultured cells (15). This sequence variation was also associated with a significantly lower median plasma concentration of PCSK9 (319 vs. 490 ng/ml) in age- and sex-adjusted models (P = 0.0004) (Fig. 4B). Even after adjusting for plasma levels of LDL-C, the individuals with PCSK9:R46L had a reduced plasma level of PCSK9 (P = 0.004).
Multivariable linear regression modeling of PCSK9 levels
In multivariable linear regression modeling of the entire population, 20% of variance in lnPCSK9 was explained by the following covariates: age, sex, ethnicity, BMI, systolic blood pressure (BP), menopausal status, statin use, and fasting levels of glucose, LDL-C, HDL-C, triglycerides, and CRP. Of these variables, gender, plasma levels of LDL-C, triglycerides, and statin use were most strongly associated with PCSK9 levels (P < 0.0001 for all), while BMI (P = 0.005) and fasting glucose (P = 0.003) were also significant independent contributors in the model. Addition of the R46L mutation to the model marginally increased the variance explained (from 22 to 23% of PCSK9 levels in whites). In the African-Americans, inclusion of the nonsense mutations (PCSK9:Y142X or PCSK9:C679X) increased the proportion of variance explained from 19 to 24%. These results indicate that several key cardiovascular risk factors, as well as statin use, are independently associated with circulating levels of PCSK9 and that additional independent information is gained from assessing the presence of PCSK9 mutations.
Plasma levels of PCSK9 were also lower (P = 0.03) in seven individuals heterozygous for a missense mutation that substitutes a phenylalanine for a leucine at residue 253 (L253F), a rare sequence variation that severely impairs PCSK9 secretion in cultured cells and is associated with a 31% reduction in plasma levels of LDL-C (3). Conversely, PCSK9 levels were not significantly lower in individuals carrying PCSK9 alleles encoding PCSK9:A443T (n = 257), which is associated with a more modest (3.7%) reduction in LDL-C (3), or PCSK9:A53V and G670E, which are not associated with any significant differences in plasma LDL-C levels in the Dallas Heart Study (3).
Statin treatment and plasma concentrations of PCSK9
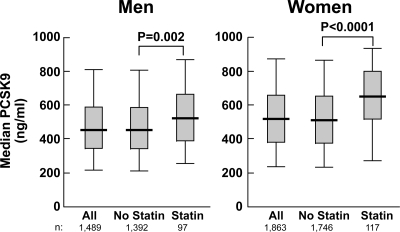
We examined the effect of 3-hydroxy-3-methylglutaryl coenzyme-A reductase inhibitors (“statins”) on plasma concentrations of PCSK9. Statins increase the expression of PCSK9 in cultured cells (19), in the livers of mice (20), and in the plasma of humans (12,17). As reported previously (12,17), statin use was associated with an increase in circulating levels of PCSK9 among both men (P = 0.002) and women (P < 0.0001) in the Dallas Heart Study (Fig. 5).
Figure 5.
Effect of statins on plasma concentrations of PCSK9 in men (left) and women (right) in the Dallas Heart Study. Statin use was determined by interview and medication reconciliation.
Discussion
The major finding of this study is that plasma concentrations of PCSK9 vary over a very wide range (∼100-fold) among normal, apparently healthy individuals. Some participants had plasma levels of PCSK9 that were barely detectable (33 ng/ml), whereas others had levels more than 5-fold greater than the median value for the population. The causes of the remarkable variation in plasma PCSK9 concentrations are largely unknown. Several demographic and metabolic parameters, including age, sex, menopausal status, body habitus, and circulating indices of lipid and glucose metabolism were correlated with PCSK9 levels, but together these factors accounted for only 23% of the variation observed. Loss-of-function mutations in PCSK9 were associated with large reductions in plasma PCSK9 concentrations, but these mutations are present in only a small fraction of the individuals within the population (3). Whereas genetic variation in PCSK9 activity has striking effects on the plasma levels of LDL-C (7), the association between plasma levels of PCSK9 and LDL-C was modest. Variation in plasma levels of PCSK9 accounted for only approximately 7% of the variation in plasma levels of LDL-C. Taken together, these findings indicate that circulating levels of PCSK9 provide only a limited indication of functional PCSK9 activity.
The present results confirmed in a much larger population the prior observation that plasma levels of PCSK9 are correlated with plasma levels of LDL-C (11,21). The factors responsible for this correlation have not been determined. The joint regulation of LDLR and PCSK9 by the cholesterol-responsive transcription factor, sterol regulatory element binding protein (SREBP)-2, likely contributes to the correlation between PCSK9 and LDL-C levels (22). Posttranscriptional factors also may contribute to the correlation between these two plasma proteins because PCSK9 promotes the degradation of LDLR (9), thus causing an increase in plasma levels of LDL-C. However, it is not clear that all circulating PCSK9 is capable of mediating degradation of the LDLR. The relatively modest correlation between circulating levels of PCSK9 and LDL-C (Supplemental Fig. S2) suggests that circulating PCSK9 levels provide at best a limited indication of PCSK9 activity.
It is also possible that the observed correlation between plasma levels of PCSK9 and LDL-C is due to both constituents being cleared from the circulation by the same pathway. Genetic deletion of LDLR in mice doubles the half-life of PCSK9 in the circulation (from ∼5 min to ∼10 min) (23), thus implicating LDLR as contributing significantly to the clearance of PCSK9. A recent study reported that a portion of the circulating PCSK9 is located in the same plasma fractions as LDL (24). It is possible that LDL binds to PCSK9 and delays the clearance of the protein. Our finding that sequence variations in APOE that were associated with significant differences in plasma LDL-C levels were not associated with changes in PCSK9 level is not consistent with the notion that plasma LDL-C levels directly affect circulating levels of PCSK9.
Differences in removal efficiency may also contribute to the wide differences between individuals in plasma levels of PCSK9. The processes that mediate LDLR-independent PCSK9 degradation and removal from plasma are not known and may influence steady-state plasma concentrations.
Plasma levels of PCSK9 were also significantly associated with plasma triglyceride concentrations both in men (r = 0.26) and in women (r = 0.29). There is currently no direct evidence that PCSK9 circulates in association with VLDL, but we cannot exclude the possibility that PCSK9 initially associates with nascent VLDL particles in the secretory pathway. Alternatively, the association between PCSK9 and plasma triglyceride levels may be indirect and caused by processes that drive the production of both particles by the liver, such as insulin resistance. PCSK9 levels correlated with many factors associated with insulin resistance (BMI, HOMA-IR, and fasting levels of glucose, insulin, and CRP). Insulin treatment results in increased expression of PCSK9 mRNA and protein in cultured hepatocytes and in the livers of mice (25).
PCSK9 sequence variants that interfered with PCSK9 synthesis or secretion in cultured cells (Y142X and C679X) (15) were associated with markedly lower plasma concentrations of PCSK9. Thus, levels of circulating PCSK9 do reflect the rate of synthesis of the protein. A third PCSK9 variant associated with lower plasma levels of LDL-C in the general population, R46L, was not associated with a reduction in the amount of PCSK9 synthesized or secreted from cultured cells (15). Here we found that carriers of PCSK9:R46L also had significantly lower plasma levels of PCSK9, even after adjusting for the lower levels of LDL-C (Fig. 4). The reason plasma levels of PCSK9 are lower in individuals with the R46L allele remains unknown. The allele may cause a reduction in the secretion of PCSK9 from hepatocytes that failed to be detected when the recombinant protein was expressed in human kidney cells (15). Alternatively, the substitution of leucine for arginine at position 46, which interrupts an α-helix in the prodomain, may cause the protein to be cleared more rapidly from the circulation.
Plasma levels of PCSK9 were higher in women than in men (517 vs. 450 ng/ml), and this gender difference persisted after accounting for potential confounding factors. Postmenopausal women had higher levels of PCSK9 than did premenopausal women or men, although these differences appeared not to be due to estrogen status because plasma levels of PCSK9 did not differ between the postmenopausal women taking estrogens and those not on estrogen replacement therapy. These results are similar to those reported by Mayne et al. (21) and may reflect an increase in the synthesis of PCSK9 or reduced clearance of the protein from the circulation.
Finally, we confirmed that statin therapy is associated with an increase in plasma levels of PCSK9, as previously reported (12,17). Statin treatment activates SREBP-2 and is associated with an increase in PCSK9 mRNA levels both in cultured human hepatocytes (19) and in mice (20). Thus, the increase in circulating PCSK9 levels associated with statin treatment presumably reflects transcriptional activation of PCSK9 by SREBP-2 (22). Conditions that increase SREBP-2 levels increase PCSK9 transcription and production, but they also increase the expression of the LDLR, which is important in the clearance of PCSK9 from plasma. The coregulation may explain why PCSK9 concentrations are elevated only with higher doses of statins, a state in which the increase in PCSK9 production might be greater than the increase in LDLR protein (17).
Supplementary Material
Acknowledgments
We thank Norma Anderson for excellent technical assistance and the Dallas Heart Study investigators and participants.
Footnotes
This work was supported by National Institute of Health Grants PO1 HL 20948 and UL1RR024923 and by the Donald W. Reynolds Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 7, 2009
Abbreviations: APOE, Apolipoprotein E; BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; CV, coefficient of variation; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment-insulin resistance; LDL, low-density lipoprotein; LDL-C, LDL-cholesterol; LDLR, LDL receptor; PBST, PBS containing 0.08% Tween 20; PCSK9, proprotein convertase subtilisin/kexin type 9; SREBP, sterol regulatory element binding protein; VLDL, very low-density lipoprotein.
References
- Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chretien M 2003 The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA 100:928–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH 2005 Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet 37:161–165 [DOI] [PubMed] [Google Scholar]
- Kotowski IK, Pertsemlidis A, Luke A, Cooper RS, Vega GL, Cohen JC, Hobbs HH 2006 A spectrum of PCSK9 alleles contributes to plasma levels of low-density lipoprotein cholesterol. Am J Hum Genet 78:410–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derré A, Villéger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C 2003 Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 34:154–156 [DOI] [PubMed] [Google Scholar]
- Cohen JC, Boerwinkle E, Mosley Jr TH, Hobbs HH 2006 Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 354:1264–1272 [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL 1986 A receptor-mediated pathway for cholesterol homeostasis. Science 232:34–47 [DOI] [PubMed] [Google Scholar]
- Horton JD, Cohen JC, Hobbs HH 2007 Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci 32:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid A, Roubtsova A, Essalmani R, Marcinkiewicz J, Chamberland A, Hamelin J, Tremblay M, Jacques H, Jin W, Davignon J, Seidah NG, Prat A 2008 Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology 48:646–654 [DOI] [PubMed] [Google Scholar]
- Lagace TA, Curtis DE, Garuti R, McNutt MC, Park SW, Prather HB, Anderson NN, Ho YK, Hammer RE, Horton JD 2006 Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest 116:2995–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alborn WE, Cao G, Careskey HE, Qian YW, Subramaniam DR, Davies J, Conner EM, Konrad RJ 2007 Serum proprotein convertase subtilisin kexin type 9 is correlated directly with serum LDL cholesterol. Clin Chem 53:1814–1819 [DOI] [PubMed] [Google Scholar]
- Lambert G, Ancellin N, Charlton F, Comas D, Pilot J, Keech A, Patel S, Sullivan DR, Cohn JS, Rye KA, Barter PJ 2008 Plasma PCSK9 concentrations correlate with LDL and total cholesterol in diabetic patients and are decreased by fenofibrate treatment. Clin Chem 54:1038–1045 [DOI] [PubMed] [Google Scholar]
- Mayne J, Dewpura T, Raymond A, Cousins M, Chaplin A, Lahey KA, Lahaye SA, Mbikay M, Ooi TC, Chrétien M 2008 Plasma PCSK9 levels are significantly modified by statins and fibrates in humans. Lipids Health Dis 7:22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH 2004 The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 93:1473–1480 [DOI] [PubMed] [Google Scholar]
- Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, Butler D, Charisse K, Dorkin R, Fan Y, Gamba-Vitalo C, Hadwiger P, Jayaraman M, John M, Jayaprakash KN, Maier M, Nechev L, Rajeev KG, Read T, Röhl I, Soutschek J, Tan P, Wong J, Wang G, Zimmermann T, de Fougerolles A, Vornlocher HP, Langer R, Anderson DG, Manoharan M, Koteliansky V, Horton JD, Fitzgerald K 2008 Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA 105:11915–11920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Tuakli-Wosornu Y, Lagace TA, Kinch L, Grishin NV, Horton JD, Cohen JC, Hobbs HH 2006 Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet 79:514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AY, Abdullah SM, Jain T, Stanek HG, Das SR, McGuire DK, Auchus RJ, de Lemos JA 2007 Associations among androgens, estrogens, and natriuretic peptides in young women: observations from the Dallas Heart Study. J Am Coll Cardiol 49:109–116 [DOI] [PubMed] [Google Scholar]
- Careskey HE, Davis RA, Alborn WE, Troutt JS, Cao G, Konrad RJ 2008 Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res 49:394–398 [DOI] [PubMed] [Google Scholar]
- Boerwinkle E, Utermann G 1988 Simultaneous effects of the apolipoprotein E polymorphism on apolipoprotein E, apolipoprotein B, and cholesterol metabolism. Am J Hum Genet 42:104–112 [PMC free article] [PubMed] [Google Scholar]
- Dubuc G, Chamberland A, Wassef H, Davignon J, Seidah NG, Bernier L, Prat A 2004 Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 24:1454–1459 [DOI] [PubMed] [Google Scholar]
- Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, Hammer RE, Moon YA, Horton JD 2005 Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci USA 102:5374–5379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne J, Raymond A, Chaplin A, Cousins M, Kaefer N, Gyamera-Acheampong C, Seidah NG, Mbikay M, Chrétien M, Ooi TC 2007 Plasma PCSK9 levels correlate with cholesterol in men but not in women. Biochem Biophys Res Commun 361:451–456 [DOI] [PubMed] [Google Scholar]
- Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL 2003 Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA 100:12027–12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefhorst A, McNutt MC, Lagace TA, Horton JD 2008 Plasma PCSK9 preferentially reduces liver LDL receptors in mice. J Lipid Res 49:1303–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Warren L, Xia D, Jensen H, Sand T, Petras S, Qin W, Miller KS, Hawkins J 6 December 2008 Function and distribution of circulating human PCSK9 expressed extrahepatically in transgenic mice. J Lipid Res, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costet P, Cariou B, Lambert G, Lalanne F, Lardeux B, Jarnoux AL, Grefhorst A, Staels B, Krempf M 2006 Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J Biol Chem 281:6211–6218 [DOI] [PubMed] [Google Scholar]
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH 2004 Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40:1387–1395 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.