Abstract
Context: The stimulatory effect of teriparatide on bone mineral density (BMD) and bone turnover is initially exuberant, but then diminishes.
Objective: Our objective was to determine whether retreating with teriparatide after a drug-free period can restore the initial exuberant response to teriparatide.
Design and Setting: This was a planned extension of a randomized controlled trial conducted in a single university hospital.
Patients and Intervention: Subjects previously participated in a 30-month randomized trial comparing the effects of alendronate (group 1), teriparatide (group 2), or both (group 3) on BMD and bone turnover in men and women with low BMD (phase 1). Subjects who completed phase 1 on their assigned therapy entered phase 2 (months 30–42), during which teriparatide was stopped in groups 2 and 3. Teriparatide was administered to all subjects during months 42 to 54 (phase 3).
Main Outcome Measures: We compared changes in BMD and markers of bone turnover (serum osteocalcin, N-terminal propeptide of type 1 collagen, and N-telopeptide) between phase 1 and 3 in subjects receiving teriparatide alone.
Results: Posterior-anterior and lateral spine BMD increased 12.5 ± 1.5 and 16.9 ± 1.7%, respectively, during the first 12 months of teriparatide administration and 5.2 ± 0.8 and 6.2 ± 1.8%, respectively, during teriparatide retreatment (P < 0.001 and P = 0.001). Increases in osteocalcin (P < 0.001), N-terminal propeptide of type 1 collagen (P < 0.001), and N-telopeptide (P < 0.001) were greater during the first period of teriparatide administration.
Conclusion: The response to teriparatide is attenuated when readministered after a 12-month hiatus.
The response to re-treatment with teriparatide, after a 2-year course of therapy and a 1-year hiatus, is clearly diminished.
Osteoporosis affects over 20 million Americans and leads to about 1.5 million fractures each year in the United States (1). Once daily teriparatide [human PTH (1–34)] administration increases bone mineral density (BMD) in estrogen-deficient women (2,3,4,5) and in osteoporotic men (6,7,8) and reduces fracture risk in women with postmenopausal osteoporosis (4). The effect of teriparatide on BMD and bone turnover is exuberant during the first 6–12 months of administration and then begins to wane (2,3,6,7,9,10,11,12,13,14,15,16). Therefore, whereas teriparatide can completely reverse bone loss in osteopenic rodents (17,18,19,20), its anabolic effect on bone has been more modest in humans. In clinical practice, patients are typically treated with teriparatide for 2 yr and then placed on a bisphosphonate (21). Although this approach increases BMD substantially, it rarely normalizes BMD. Therefore, alternative approaches are needed if PTH is to reverse osteoporosis completely. One strategy to enhance the anabolic potential of PTH that seemed intuitively attractive, combining it with a potent antiresorptive agent like alendronate, actually reduces its anabolic effect instead of enhancing its action (5,6,13,22). Another strategy to reverse osteoporosis with PTH would be to identify a way to restore the initial potent anabolic response to teriparatide that is seen during the initial 6–12 months of its use. To determine whether the initial exuberant response to teriparatide could be restored by prolonged withholding of the drug, we treated osteoporotic men and women with teriparatide for 24 months, withdrew teriparatide for 12 months, and then retreated them with teriparatide for an additional 12 months.
Subjects and Methods
Study subjects
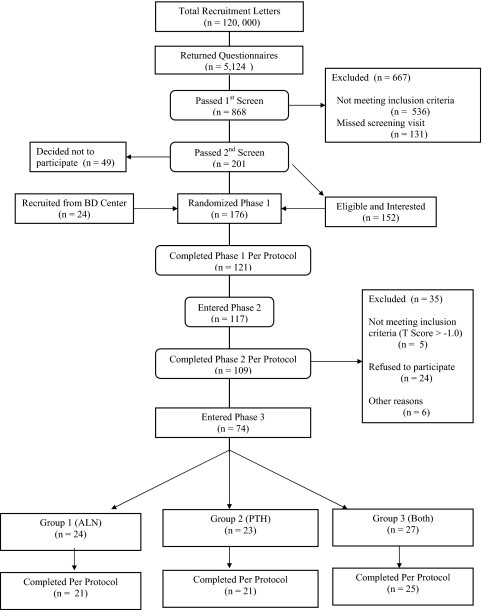
We mailed 120,000 recruitment letters to subjects within a 10-mile radius of Massachusetts General Hospital (Fig. 1). Of 5124 people who returned the questionnaire, 575 men and 293 women were interested and eligible for further screening. Of these 868 people, 506 were disqualified because their BMD was too high, 30 on the basis of screening blood tests, and 131 for not keeping their screening appointment, leaving 201 subjects who were eligible by BMD and screening laboratory criteria. Forty-nine subjects decided not to participate, and an additional 24 were recruited from our clinic or bone density center. Thus, the final cohort consisted of 176 subjects (93 women and 83 men).
Figure 1.
Flow of subjects through the protocol.
Participants were required to be men or postmenopausal women 46 to 85 yr old and to have BMD of the lumbar spine in the posterior-anterior or lateral projection or the femoral neck at least 2 sd values below the mean of gender-matched young adults. Subjects were excluded if they were currently using medications known to affect bone metabolism or if they had used bisphosphonates within the last year. The inclusion criteria have been published previously (6).
Study protocol
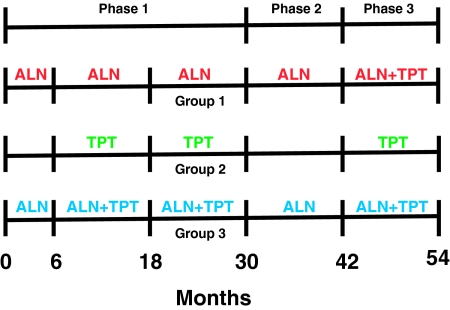
The study was conducted in three phases (Fig. 2). In phase 1, participants were randomly assigned by a computer-generated list to receive alendronate alone (10 mg orally once daily, group 1, n = 59), teriparatide alone (37 μg sc once daily, group 2, n = 58), or both (group 3, n = 59). Randomization of both men and women was stratified by age (above or below 65) and BMD T-score (above or below −2.5). Alendronate was begun at the baseline visit and continued for 30 months in groups 1 and 3. Teriparatide was begun at month 6 and continued for 24 months in groups 2 and 3. Treatment was not blinded. The results for phase 1 for the male subjects have been published previously (6,13). Subjects who completed phase 1 on their assigned treatment (n = 121) were eligible to continue into phase 2 (months 30–42), during which teriparatide was discontinued in groups 2 and 3. Alendronate was continued in groups 1 and 3 during phase 2. No other osteoporosis medications were permitted during phase 2. A total of 117 subjects (63 men and 54 women) entered phase 2. Subjects who completed phase 2 on their assigned treatment (n = 109) were eligible to continue into phase 3 (months 42–54), during which teriparatide was administered for 12 months to all three groups. To be eligible for phase 3, subjects were required to have a T-score below −1 at either the lumbar spine or proximal femur. Alendronate was continued in groups 1 and 3. Seventy-four subjects (43 men and 31 women) entered phase 3, of whom 24 were originally in group 1, 23 in group 2, and 27 in group 3. Calcium intake was estimated by a research dietitian and maintained at 1000 to 1200 mg daily through diet or supplements. All subjects received 400 IU of vitamin D daily. The effects of retreatment in individuals receiving teriparatide alone (group 2) are the subject of this report.
Figure 2.
Diagram of the study protocol. ALN, Alendronate; TPT, teriparatide.
Blood was collected at baseline and at 1, 2, 3, 6, 7, 8, 9, 12, 18, 24, 30, 36, 42, 43, 44, 45, 48, and 54 months to assess routine chemistries and biochemical markers of bone turnover including serum N-telopeptide, osteocalcin, and N-terminal propeptide of type 1 collagen (P1NP). For each subject, blood samples were collected at the same time (± 2 h) as the baseline sample. At each visit, serum calcium was measured before and 4 to 6 h after teriparatide injection. Twenty-four-hour urinary calcium excretion was measured at baseline and every 6 months, and also 1 month after starting teriparatide (months 7 and 43). BMD was measured by dual-energy x-ray absorptiometry at baseline and every 6 months. A standardized questionnaire was administered at each visit to assess side effects. Compliance with study medications was assessed by medication diaries and by counting residual medication. The study was approved by the Institutional Review Board of Massachusetts General Hospital, and all subjects provided written informed consent.
Teriparatide preparation and dose adjustments
Good Manufacturing Practices (GMP) grade synthetic human PTH (1–34) (Bachem, Inc., Torrence, CA) was vialed as a sterile lyophilized powder (with mannitol, U.S.P.) under GMP conditions by Ben-Venue Laboratories (Bedford, OH) or vialed as a sterile solution by our hospital pharmacy. Each vial was supposed to contain 40 μg of teriparatide. Amino acid and high-pressure liquid chromatography analysis of these preparations indicated that, due to losses during vialing, each vial actually contained 37 μg of the peptide and that the peptide was identical in phase 1 and phase 3.
The teriparatide dose was reduced by 25% if any serum calcium value was above 11.5 mg/dl or if the investigators felt that the subject was experiencing a side effect of therapy. If hypercalcemia or symptoms persisted, the teriparatide dose was reduced by another 25%. If hypercalcemia or symptoms persisted after two dose reductions, teriparatide was discontinued. If the 24-h urinary calcium excretion was above 400 mg/d, dietary calcium and/or sodium was reduced by 25 to 50%. If hypercalciuria persisted, the teriparatide dose was reduced by 25 to 50% as described above. If hypercalciuria persisted after a 50% dose reduction, teriparatide was discontinued. All subjects began the retreatment phase on the full dose of teriparatide as was given at month 6. If the teriparatide dose was reduced between months 6 and 18, similar reductions were scheduled between months 42 and 54 at the corresponding point of teriparatide administration, even if there was otherwise no indication for a dose change. For example, if the teriparatide dose was reduced by 25% due to hypercalcemia at month 9, it was also reduced by 25% at month 45, even if the blood calcium level was normal. Alternatively, some subjects developed an indication for reducing the teriparatide dose between months 42 and 54 that was not present during months 6 to 18.
Evaluation for teriparatide antibodies
Antibodies against PTH (1–34) were measured in subjects in group 2 at months 6, 30, 42, and 54 using both a commercially available antihuman PTH (1–34) ELISA (Immutopics Inc., San Clemente, CA) and by incubating HPLC-purified 125I(Tyr34) PTH (1–34)-amide with patient serum and then using dextran-coated charcoal to remove iodinated PTH not bound to anti-PTH (1–34) antibodies (23).
Measurements of BMD
BMD of the lumbar spine in the posterior-anterior and lateral projections, the proximal femur, the distal one third radius shaft, and total body was measured by dual-energy x-ray absorptiometry (QDR 4500A; Hologic, Waltham, MA). For the radius shaft, two measurements were made at each visit, and their mean was used in all analyses. Our short-term in vivo measurement sd values are 0.005, 0.014, 0.007, and 0.006 g/cm2 for posterior-anterior spine, lateral spine, femoral neck, and total hip, respectively. Daily measurements of an anthropomorphic spine phantom demonstrated long-term stability of the densitometer. Individual vertebrae with obvious deformities or areas of focal sclerosis were excluded from analyses. Total body scans were analyzed without the head region because it often contains artifacts (24). All bone density scans were analyzed by individuals blinded to study treatment.
Measurements of bone turnover
Serum N-telopeptide was measured using an enzyme-linked immunoassay (Wampole Laboratories, Princeton, NJ). Serum P1NP was measured using a RIA (Orion Diagnostica, Espoo, Finland). Serum osteocalcin was measured using an enzyme-linked immunoassay (ALPCO Diagnostics, Windham, NH). For each subject, all samples from months 0 to 30 were assayed together, as were all samples from months 30 to 54, unless an individual value needed to be repeated. Because the osteocalcin kit used to measure samples from months 0 to 30 was modified before the month 30–54 samples were assayed, month 30 samples were measured with both kits and a correction factor was applied to all osteocalcin measurements after month 30.
Statistical analysis
The predetermined primary end point was the difference in the change in posterior-anterior lumbar spine BMD during months 6 to 18 and months 42 to 54 in subjects receiving teriparatide alone (group 2). Secondary end points were differences in changes in BMD at other skeletal sites and changes in bone turnover markers during these months in group 2. By design, all subjects who entered phase 3 had completed phases 1 and 2 on their assigned therapy. Two of the 23 subjects in group 2 discontinued participation before any follow-up BMD measurements were made during phase 3. Their data are excluded because changes in BMD could not be calculated.
BMD data were first analyzed using all subjects who remained on teriparatide until at least month 48 of the retreatment phase (per protocol analysis, n = 21). One subject discontinued teriparatide at month 48; his data are included for months 6 through 12 and 42 through 48. Changes in BMD between months 6 and 18 and months 42 and 54 were compared using paired t tests. Because baseline BMD was different at months 6 and 42, absolute changes were compared, rather than percentage changes. For bone turnover markers, differences in the area under the curve from months 6 to 18 and months 42 to 54 were compared using paired t tests. BMD and bone marker data were also analyzed after excluding five subjects whose mean teriparatide dose was at least 20% lower during retreatment because of mandatory dose reductions that were not required during initial therapy (dose-matched analysis, n = 16). Because the results from both types of analysis were similar, only the per protocol analyses are presented. We chose not to present a true intention-to-treat analysis because it would include two subjects who stopped teriparatide after only 3 months of the retreatment phase. Including them would bias the results toward our conclusion that the response to teriparatide is reduced during retreatment. Analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC). All P values are two-sided. Unless otherwise noted, data are presented as the mean ± sd.
Results
Characteristics of the subjects
The baseline characteristics of the subjects are shown in Table 1. No subjects had previously used bisphosphonates or other prescription osteoporosis medications.
Table 1.
Baseline characteristics of subjects treated with teriparatide alone
| Characteristic | Teriparatide alone (n = 21) |
|---|---|
| Age (yr) | 60 ± 8 |
| No. of males/females | 12/9 |
| Height (cm) | 169.8 ± 8.8 |
| Weight (kg) | 73.4 ± 12.9 |
| Body mass index (kg/m2) | 25.5 ± 4.3 |
| Calcium intake (mg/d) | 993 ± 506 |
| 25-Hydroxyvitamin D (ng/ml)a | 24 ± 10 |
| PTH (pg/ml) | 36 ± 12 |
| Osteocalcin (ng/ml) | 29.8 ± 10.1 |
| P1NP (ng/ml) | 48.0 ± 11.0 |
| Serum N-telopeptide (nmol BCE) | 15.6 ± 4.1 |
| BMD (g/cm2) | |
| Posterior-anterior spine | 0.819 ± 0.113 |
| T-score | −2.3 ± 0.9 |
| Lateral spine | 0.637 ± 0.062 |
| T-score | −1.9 ± 0.9 |
| Femoral neck | 0.647 ± 0.088 |
| T-score | −2.1 ± 0.6 |
| Total hip | 0.812 ± 0.127 |
| T-score | −1.4 ± 0.9 |
| One third radius | 0.662 ± 0.119 |
| T-score | −1.7 ± 1.4 |
| Total body | 0.909 ± 0.132 |
| T-score | Not available |
Data are presented as the mean ± sd.
To convert to nanomoles per liter, multiply by 2.496.
Teriparatide compliance and dose matching
During months 42 to 54, all but two subjects took at least 90% of their prescribed teriparatide doses. The mean ± sd teriparatide dose was 30 ± 6 μg/d during months 6 to 18 and 26 ± 8 μg/d during months 42 to 54 (P = 0.02). Sixteen subjects had dose reductions.
Changes in BMD, bone turnover markers, and serum calcium during initial treatment and retreatment with teriparatide
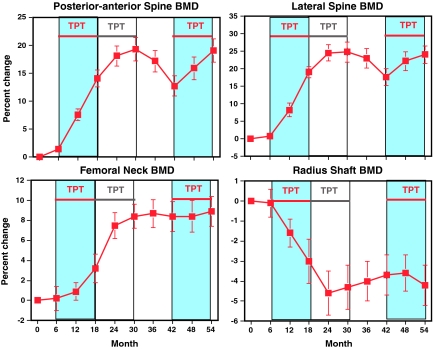
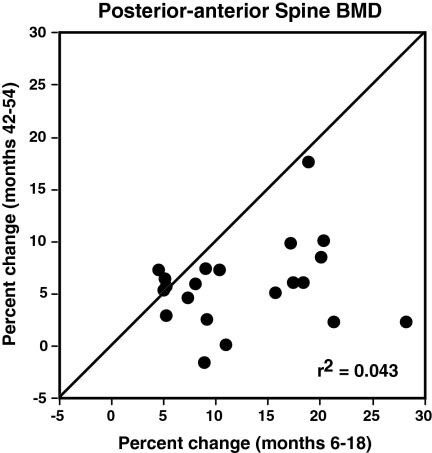
Figure 3 shows the percentage changes in BMD during the entire 54-month study. Posterior-anterior and lateral spine BMD increased sharply during months 6 to 30, declined modestly during months 30 to 42 after teriparatide was stopped, and increased again during teriparatide retreatment, although less than during initial teriparatide therapy. Femoral neck BMD increased during months 6 to 30 and then was stable after teriparatide was stopped and during teriparatide retreatment. In contrast, BMD of the radius shaft decreased during months 6 to 30, increased slightly after teriparatide was stopped, and remained stable during teriparatide retreatment. Absolute and percentage changes during the initial 12 months of teriparatide (months 6 to 18) and during teriparatide retreatment (months 42 to 54) are shown in Table 2. Compared with the initial 12 months of teriparatide administration, increases in posterior-anterior spine, lateral spine, femoral neck, and total body BMD were reduced by 53, 59, 93, and 87%, respectively, when teriparatide was readministered. At each skeletal site, the BMD at the end of the initial period of teriparatide therapy (month 30) and at the end of retreatment (month 54) were virtually identical (Table 2). The change in spine BMD during teriparatide retreatment was not related to the BMD at month 42 (R2 = 0.08, P = 0.21; and R2 = 0.001, P = 0.90 for posterior-anterior and lateral spine BMD, respectively). Figure 4 shows the percentage change in posterior-anterior spine BMD during months 6 to 18 and months 42 to 54 in each subject. There was no significant association between the change in spine BMD during phase 1 and phase 3 (R2 = 0.04), nor was there evidence for a bimodal distribution of responders and nonresponders. Increases in spine BMD were similar between months 18 and 30 and months 42 and 54 (0.05 ± 0.04 vs. 0.04 ± 0.04 g/cm2; P = 0.25).
Figure 3.
Change in BMD of the posterior-anterior spine, lateral spine, femoral neck, and radius shaft during initial teriparatide (TPT) treatment (months 6–30), TPT withdrawal (months 30–42), and TPT re-treatment (months 42–54). Periods being compared (months 6–18 and 42–54) are denoted by the blue shading and red horizontal lines. Data are presented as mean ± se.
Table 2.
Changes in BMD (g/cm2) during the first 12 months of teriparatide therapy (months 6 to 18) and 12 months of teriparatide retreatment (months 42 to 54) and BMD at the end of each treatment period in subjects receiving teriparatide alone
| Skeletal site | BMD change, months 6–18 | BMD change, months 42–54 | P value | BMD at end of month 30 | BMD at end of month 54 | P value |
|---|---|---|---|---|---|---|
| PA spine | 0.102 ± 0.011 (12.5 ± 1.5%) | 0.048 ± 0.008 (5.2 ± 0.8%) | <0.001 | 0.975 ± 0.028 | 0.969 ± 0.030 | 0.49 |
| Lateral spine | 0.111 ± 0.012 (16.9 ± 1.7%) | 0.045 ± 0.015 (6.2 ± 1.8%) | 0.001 | 0.797 ± 0.031 | 0.792 ± 0.030 | 0.75 |
| Femoral neck | 0.016 ± 0.008 (2.8 ± 1.3%) | 0.0004 ± 0.005 (0.2 ± 0.8%) | 0.08 | 0.703 ± 0.020 | 0.700 ± 0.020 | 0.59 |
| Radius shaft | −0.019 ± 0.007 (−2.8 ± 1.1%) | 0.0004 ± 0.002 (0.001 ± 0.4%) | 0.02 | 0.636 ± 0.029 | 0.641 ± 0.030 | 0.31 |
| Total body | 0.023 ± 0.007 (2.5 ± 0.8%) | 0.004 ± 0.003 (0.5 ± 0.4%) | <0.001 | 0.955 ± 0.033 | 0.958 ± 0.034 | 0.51 |
Data are presented as mean ± se (percentage change ± se). P values are for comparison of BMD change during months 6 to 18 vs. months 42 to 54 and for comparisons of BMD at the end of months 30 and 54.
Figure 4.
Percentage change in posterior-anterior spine BMD in each subject during months 6 to 18 and months 42 to 54. The diagonal line is the line of identity.
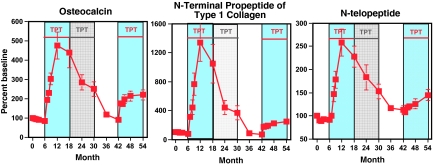
Figure 5 shows the changes in bone turnover markers during the 54-month study period. Bone turnover markers were similar at the beginning of phase 1 and phase 3. Serum P1NP (P < 0.001), osteocalcin (P < 0.001), and N-telopeptide (P < 0.001) all increased more during the initial 12 months of teriparatide treatment than during teriparatide retreatment. A similar blunting of the effect of teriparatide retreatment was observed in subjects also receiving alendronate (data not shown).
Figure 5.
Change in serum osteocalcin, P1NP, and N-telopeptide levels during initial teriparatide (TPT) treatment (months 6–30), TPT withdrawal (months 30–42), and TPT re-treatment (months 42–54). Periods being compared (months 6–18 and 42–54) are denoted by the blue shading and red horizontal lines. Data are presented as mean ± se.
Four hours after teriparatide injection at the periodic visits, serum calcium increased 0.25 ± 0.08 mg/dl at month 6 and 0.26 ± 0.06 mg/dl at month 42 (P = 0.91) and 0.55 ± 0.06 mg/dl at month 18 and 0.56 ± 0.09 mg/dl at month 54 (P = 0.95).
Adverse events
Hypercalcemia was not detected in any blood sample collected 24 h after teriparatide dosing but occurred in 23 of 268 blood samples collected 4 to 6 h after teriparatide dosing. Hypercalcemia in blood samples collected 4 to 6 h after teriparatide dosing was less common during teriparatide retreatment (7 of 130) than during initial therapy (16 of 138), probably because of automatic dose reductions if hypercalcemia or hypercalciuria had occurred during the initial period of teriparatide therapy. Urinary calcium excretion exceeded 400 mg/d in six of 87 collections during months 6 to 18 and in five of 81 collections during months 42 to 54.
Antiteriparatide antibodies
Antibodies against teriparatide were not detected in any subject.
Discussion
In this study, we found that skeletal responses to teriparatide were attenuated when it was readministered for 12 months after a 12-month hiatus. During the first course of teriparatide therapy, there was a substantial improvement in spine BMD, with most of the increase occurring during the first 12 months of therapy. When teriparatide was readministered, however, increases in BMD were 53 to 93% smaller. Teriparatide-induced increases in bone turnover markers were dramatically reduced when teriparatide was readministered.
Little is known about the effect of readministering teriparatide after a teriparatide-free interval. Six cycles of human PTH (1–38) plus etidronate disodium (3 wk of therapy followed by a 12-wk drug-free interval) had no effect on bone mass in eight osteoporotic women and men (25). Increases in serum alkaline phosphatase and osteocalcin were slightly attenuated when human PTH (1–38) was administered for 14 d, stopped for 86 d, and then readministered for 14 d (26). Cosman et al. (11) administered teriparatide daily for 15 months or for three 3-month cycles alternating with 3-month periods without teriparatide to women also taking alendronate. Spine BMD increased similarly in both groups. Markers of bone formation increased similarly during each of the three cycles of teriparatide therapy, whereas increases in urinary N-telopeptide, a marker of bone resorption, decreased progressively after the first cycle of teriparatide therapy (11). To our knowledge, the effects of readministering teriparatide after a standard 2-yr course of daily therapy have not been examined previously.
Teriparatide is the only anabolic agent currently approved for treatment of osteoporosis and is thus the only agent with the potential to cure osteoporosis. However, the exuberant stimulation of BMD and bone turnover that occurs during the first 6–12 months of teriparatide administration begins to wane despite continued therapy, thereby limiting its long-term potential (2,3,6,7,9,10,11,12,13,14,15,16). A similar pattern of responsiveness to teriparatide is seen in animals (20,27). Thus, if teriparatide is to reverse osteoporosis, modifications of its use are needed. One such modification, combining teriparatide with a potent antiresorptive agent, actually diminishes its anabolic effect (6,13,22). The current study demonstrates that another seemingly logical approach, readministering teriparatide after a “drug holiday,” also fails to normalize BMD.
The reason(s) why the stimulation of bone turnover by teriparatide begins to wane after 6 to 12 months of daily therapy and why the response to a second course of therapy is diminished is unknown. Although the development of blocking antibodies would explain both phenomena, we did not detect antiteriparatide antibodies in our subjects. Second, the absorption of teriparatide might decrease during long-term therapy. Studies are currently being conducted to test that hypothesis. Third, prolonged daily teriparatide administration might down-regulate PTH receptors. The acute rise in serum calcium was similar at the beginning and the end of each treatment period, however, arguing against receptor down-regulation. Fourth, it is possible that there is a maximal skeletal response to teriparatide, which was achieved during the initial 2 yr of therapy and then reached again during the second course of therapy after a period of bone loss had temporarily reduced bone mass below this theoretical ceiling. The absence of an inverse association between BMD at the start of retreatment and the change in BMD argues against a ceiling effect. Finally, teriparatide target cells, including mature osteoblasts, osteoblast precursors, bone lining cells, or osteoclast precursors, could become depleted during long-term teriparatide therapy leading to a progressive decrease in responsiveness to teriparatide during long-term therapy. If the population of teriparatide target cells were not fully replenished after 1 yr without teriparatide therapy, responsiveness to a repeat course of teriparatide therapy would also be attenuated.
Teriparatide is approved by the U.S. Food and Drug Administration (FDA) to treat men and women with osteoporosis for up to 2 yr. A recent study reported that within the first 2 yr of teriparatide therapy, the risk of nonspine fractures decreased as the duration of therapy increased (28). Because the efficacy and safety of teriparatide beyond 2 yr have not been well studied, however, treatment with teriparatide for more than 2 yr is not recommended. Our data demonstrate that the response of teriparatide is clearly diminished when given for a third year, after a 1-yr interruption of therapy. Moreover, retreating with teriparatide after a 1-yr hiatus merely served to restore BMD to the level achieved at the end of 2 yr of continuous therapy, although it is possible that BMD might have increased further if retreatment had been continued even longer. Thus, we agree with the recommendations of others that teriparatide should be administered for no more than 2 yr, after which an antiresorptive agent should be given to maintain teriparatide-induced gains in BMD (29,30,31), and we do not recommend repeat courses of therapy in patients who have already received teriparatide for 2 yr.
Certain limitations of this study deserve mention. First, the average teriparatide dose was approximately 30 μg/d, a dose midway between the doses administered in the Fracture Prevention Trial (4), whereas the FDA-approved dose is 20 μg/d. It is possible that the effects of a lower dose would be different. Second, we withheld teriparatide for 1 yr. Results could differ with shorter or longer periods of drug withdrawal. Third, our cohort included both men and women. The effects of teriparatide on BMD in men and women are indistinguishable, however (4,8). Fourth, because the original study was designed to compare the effects of teriparatide, alendronate, or both on BMD and bone turnover, a group receiving calcium and vitamin D alone was not included, although the teriparatide-alone group did receive only calcium and vitamin D for the first 6 months and all parameters were stable during that time. Fifth, the average BMD at the start of retreatment in our subjects was higher than is often seen in patients treated with teriparatide. It is possible that the response to retreatment would be different in subjects with lower BMD, although there was no association between the BMD at the start of retreatment and the magnitude of the subsequent response. Sixth, it is unclear whether the relatively high rate of discontinuation before phase 3 might have influenced the results. Finally, because the sample size was small, it was not feasible to explore factors or subgroups of subjects that may be related to the attenuation of teriparatide responsiveness.
In summary, the response to teriparatide is attenuated when it is readministered for 1 yr after 2 yr of therapy and a 1-yr period without teriparatide. Although this approach does restore BMD to its before “drug holiday” level, it fails to increase BMD further. Understanding the reasons for this phenomenon could lead to strategies to improve responsiveness to teriparatide.
Acknowledgments
We thank Dr. Henry M. Kronenberg for his critical advice regarding this protocol; Ms. Annmarie Hayes, Ms. Kate Gibson, Ms. Melissa Davis, and Mrs. Carol Shea for their meticulous administration of the study protocol; Ms. Robbin Cleary, Ms. Sarah Zhang, and Ms. Annmarie Swarcz for performing the bone density measurements; and the nursing and dietary staff of the Mallinckrodt General Clinical Research Center for their dedicated care of the patients.
Footnotes
This work was supported by National Institutes of Health Grants 5 P50 AR44855, K24 DK02759, and RR-1066.
This trial was registered at ClinicalTrials.gov: NCT00000400.
Disclosure Summary: The authors have nothing to declare.
First Published Online April 28, 2009
Abbreviations: BMD, Bone mineral density; P1NP, N-terminal propeptide of type 1 collagen.
References
- Finkelstein JS 2000 Osteoporosis. In: Goldman L, Bennett JC, eds. Cecil textbook of medicine. 21st ed. Philadelphia: W.B. Saunders Company; 1366–1373 [Google Scholar]
- Finkelstein JS, Klibanski A, Schaefer EH, Hornstein MD, Schiff I, Neer RM 1994 Parathyroid hormone for the prevention of bone loss induced by estrogen deficiency. N Engl J Med 331:1618–1623 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Klibanski A, Arnold AL, Toth TL, Hornstein MD, Neer RM 1998 Prevention of estrogen deficiency-related bone loss with human parathyroid hormone-(1–34): a randomized, controlled trial. JAMA 280:1067–1073 [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH 2001 Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441 [DOI] [PubMed] [Google Scholar]
- Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ 2003 The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM 2003 The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349:1216–1226 [DOI] [PubMed] [Google Scholar]
- Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP 2000 Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 85:3069–3076 [DOI] [PubMed] [Google Scholar]
- Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA 2003 The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18:9–17 [DOI] [PubMed] [Google Scholar]
- Body JJ, Gaich GA, Scheele WH, Kulkarni PM, Miller PD, Peretz A, Dore RK, Correa-Rotter R, Papaioannou A, Cumming DC, Hodsman AB 2002 A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1–34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 87:4528–4535 [DOI] [PubMed] [Google Scholar]
- Chen P, Satterwhite JH, Licata AA, Lewiecki EM, Sipos AA, Misurski DM, Wagman RB 2005 Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res 20:962–970 [DOI] [PubMed] [Google Scholar]
- Cosman F, Nieves J, Zion M, Woelfert L, Luckey M, Lindsay R 2005 Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med 353:566–575 [DOI] [PubMed] [Google Scholar]
- Ettinger B, San Martin J, Crans G, Pavo I 2004 Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19:745–751 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Leder BZ, Burnett SM, Wyland JJ, Lee H, de la Paz AV, Gibson K, Neer RM 2006 Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J Clin Endocrinol Metab 91:2882–2887 [DOI] [PubMed] [Google Scholar]
- Hodsman AB, Fraher LJ, Ostbye T, Adachi JD, Steer BM 1993 An evaluation of several biochemical markers for bone formation and resorption in a protocol utilizing cyclical parathyroid hormone and calcitonin therapy for osteoporosis. J Clin Invest 91:1138–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, Dempster D, Cosman F 1997 Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 350:550–555 [DOI] [PubMed] [Google Scholar]
- McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF 2005 Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165:1762–1768 [DOI] [PubMed] [Google Scholar]
- Kimmel DB, Bozzato RP, Kronis KA, Coble T, Sindrey D, Kwong P, Recker RR 1993 The effect of recombinant human (1–84) or synthetic human (1–34) parathyroid hormone on the skeleton of adult osteopenic ovariectomized rats. Endocrinology 132:1577–1584 [DOI] [PubMed] [Google Scholar]
- Liu CC, Kalu DN, Salerno E, Echon R, Hollis BW, Ray M 1991 Preexisting bone loss associated with ovariectomy in rats is reversed by parathyroid hormone. J Bone Miner Res 6:1071–1080 [DOI] [PubMed] [Google Scholar]
- Mitlak BH, Williams DC, Bryant HU, Paul DC, Neer RM 1992 Intermittent administration of bovine PTH-(1–34) increases serum 1,25-dihydroxyvitamin D concentrations and spinal bone density in senile (23 month) rats. J Bone Miner Res 7:479–484 [DOI] [PubMed] [Google Scholar]
- Wronski TJ, Yen CF, Qi H, Dann LM 1993 Parathyroid hormone is more effective than estrogen or bisphosphonates for restoration of lost bone mass in ovariectomized rats. Endocrinology 132:823–831 [DOI] [PubMed] [Google Scholar]
- Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ 2005 One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 353:555–565 [DOI] [PubMed] [Google Scholar]
- Ste-Marie LG, Schwartz SL, Hossain A, Desaiah D, Gaich GA 2006 Effect of teriparatide [rhPTH(1–34)] on BMD when given to postmenopausal women receiving hormone replacement therapy. J Bone Miner Res 21:283–291 [DOI] [PubMed] [Google Scholar]
- Young G, Marcus R, Minkoff JR, Kim LY, Segre GV 1987 Age-related rise in parathyroid hormone in man: the use of intact and midmolecule antisera to distinguish hormone secretion from retention. J Bone Miner Res 2:367–374 [DOI] [PubMed] [Google Scholar]
- Taylor A, Konrad PT, Norman ME, Harcke HT 1997 Total body bone mineral density in young children: influence of head bone mineral density. J Bone Miner Res 12:652–655 [DOI] [PubMed] [Google Scholar]
- Hesch RD, Heck J, Delling G, Keck E, Reeve J, Canzler H, Schober O, Harms H, Rittinghaus EF 1988 Results of a stimulatory therapy of low bone metabolism in osteoporosis with (1–38)hPTH and diphosphonate EHDP. Klin Wochenschr 66:976–984 [DOI] [PubMed] [Google Scholar]
- Hodsman AB, Fraher LJ 1990 Biochemical responses to sequential human parathyroid hormone (1–38) and calcitonin in osteoporotic patients. Bone Miner 9:137–152 [DOI] [PubMed] [Google Scholar]
- Mitlak BH, Burdette-Miller P, Schoenfeld D, Neer RM 1996 Sequential effects of chronic human PTH (1–84) treatment of estrogen-deficiency osteopenia in the rat. J Bone Miner Res 11:430–439 [DOI] [PubMed] [Google Scholar]
- Lindsay R, Miller P, Pohl G, Glass EV, Chen P, Krege JH 16 October 2008 Relationship between duration of teriparatide therapy and clinical outcomes in postmenopausal women with osteoporosis. Osteoporos Int 10.1007/s00198-008-0766-0 [DOI] [PubMed] [Google Scholar]
- Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR 2006 Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Arnold AL 1999 Increases in bone mineral density after discontinuation of daily human parathyroid hormone and gonadotropin-releasing hormone analog administration in women with endometriosis. J Clin Endocrinol Metab 84:1214–1219 [DOI] [PubMed] [Google Scholar]
- Rittmaster RS, Bolognese M, Ettinger MP, Hanley DA, Hodsman AB, Kendler DL, Rosen CJ 2000 Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab 85:2129–2134 [DOI] [PubMed] [Google Scholar]