Abstract
Context: Primary pigmented nodular adrenocortical disease (PPNAD) results in most cases from mutations of the protein kinase A (PKA) regulatory subunit 1A (PRKAR1A) gene. Patients with PPNAD exhibit a paradoxical increase in cortisol secretion in response to dexamethasone.
Objective: The aim was to investigate the mechanism of the action of dexamethasone on adrenocortical cells removed from patients with PPNAD and a transgenic model of PPNAD [Tg(tTA/X2AS) mice].
Design and Setting: We performed an in vitro study in an academic research laboratory.
Patients: Eleven patients with histologically proven PPNAD were included in the study.
Intervention: Cultured PPNAD cells were incubated with dexamethasone in the presence of various modulators of the cAMP/PKA pathway and the glucocorticoid receptor antagonist RU486.
Main Outcome Measure: Cortisol and corticosterone were measured by radioimmunological assays in cell culture supernatants.
Results: Dexamethasone stimulated in vitro cortisol secretion from PPNAD tissues in six patients. The stimulatory effect of dexamethasone on cortisol release was not reduced by the adenylyl cyclase inhibitor SQ22536 or potentiated by the phosphodiesterase inhibitor IMBX and the cAMP analog 8Br-cAMP. Conversely, the PKA inhibitor H89 and RU486 inhibited the cortisol response to dexamethasone. Dexamethasone had no effect on cortisol production from normal human adrenocortical cells but stimulated corticosteroidogenesis in the presence of RU486. Similarly, dexamethasone failed to influence corticosterone release by adrenocortical cells removed from Tg(tTA/X2AS) mice but stimulated corticosteroidogenesis in the presence of RU 486.
Conclusions: These results indicate that, in human PPNAD tissues, dexamethasone paradoxically stimulates cortisol release through a glucocorticoid receptor-mediated effect on PKA catalytic subunits.
In adrenal tissues removed from patients with primary pigmented nodular adrenocortical disease, dexamethasone paradoxically stimulates cortisol secretion through activation of protein kinase A.
Primary pigmented nodular adrenocortical disease (PPNAD) is a rare cause of ACTH-independent Cushing’s syndrome that may be isolated or occur as part of the complex of spotty skin pigmentation, myxomas, and endocrine overactivity, also referred to as Carney complex (1). Histologically, PPNAD is characterized by the presence of black or brown adrenocortical micronodules, the internodular cortex being usually atrophic. PPNAD is a congenital disorder inherited in an autosomal dominant manner (1). In most patients, the disease is caused by germline mutations of the protein kinase A (PKA) regulatory subunit 1A (PRKAR1A) gene (2,3,4,5). In addition, mutations of the phosphodiesterase (PDE) 11A and PDE8 genes have been described in patients with PPNAD or nonpigmented variants of the disease (6,7). All these genetic events lead to constitutive activation of the cAMP/PKA pathway that secondarily favors glucocorticoid hypersecretion and adrenocortical hyperplasia (5,8).
It has been shown that patients with PPNAD exhibit a paradoxical increase in cortisol secretion in response to Liddle’s test; i.e. administration of dexamethasone at doses of 2 mg/d for 2 d followed by 8 mg/d for 2 d (9). This abnormal cortisol response is now used as a biological criterion for the diagnosis of the disease (10). An increase in urinary free cortisol (UFC) excretion on the second day of high-dose dexamethasone administration higher than 50% of the basal level usually supports the diagnosis of PPNAD (9). However, the paradoxical cortisol response to dexamethasone is not pathognomonic of the disease. In fact, such observation has been reported in only 69% of patients with PPNAD (9) and can be observed in patients with cortisol-producing adrenocortical adenomas (11). In this latter group of patients, the sensitivity of the tumor tissues to dexamethasone has been attributed to the occurrence of somatic PRKAR1A mutations (11).
The molecular mechanisms involved in dexamethasone-induced cortisol secretion from PPNAD tissues are not fully elucidated. A recent study has shown that the plasma cortisol response to dexamethasone observed in vivo in PPNAD patients can be reproduced in vitro, confirming therefore the occurrence of a direct stimulatory action of the drug on hyperplastic adrenocortical tissues (12). Conversely, dexamethasone was found to have no effect on cortisol release by tissue explants derived from ACTH-independent macronodular adrenal hyperplasias causing Cushing’s syndrome (12). Immunohistochemical studies revealed the presence of the glucocorticoid receptor (GR) in the PPNAD micronodules, whereas no immunoreactivity was detected in the internodular cortex. In addition, the receptor appears to be overexpressed in PPNAD tissues in comparison with normal adrenals, suggesting that the abnormal stimulatory action of dexamethasone on cortisol release is a GR-mediated phenomenon (12). Because it is established that GR can interact with PKA through protein/protein interaction (13), it was conceivable that the paradoxical increase in cortisol secretion induced by dexamethasone in adrenocortical PPNAD tissues could be the consequence of a GR-mediated action of dexamethasone on the constitutively activated cAMP/PKA pathway.
In the present study, we have investigated the mechanism of action of dexamethasone on cortisol production by cultured adrenocortical cells derived from patients with histologically proven PPNAD undergoing bilateral adrenalectomy for Cushing’s syndrome. In particular, we have examined whether dexamethasone-stimulated cortisol production could be the result of an action of the glucocorticoid at the different steps of the cAMP/PKA pathway. We have also verified whether the effect of dexamethasone is actually mediated by GR in both human PPNAD tissues and adrenal glands removed from a transgenic mouse model of PPNAD.
Patients and Methods
Human PPNAD tissue studies
Patients
Eleven patients with PPNAD or PPNAD with Carney complex who underwent bilateral adrenalectomy for ACTH-independent Cushing’s syndrome were enrolled in the study. In all patients, the diagnosis of PPNAD was assessed on the basis of the histological criteria previously established by Carney and Young (14), i.e. the presence of multiple pigmented nodules disseminated in the cortex of the two adrenal glands generally associated with internodular atrophy. The main clinical characteristics of the patients are presented in Table 1. Six patients (P2, P3, P4, P6, P9, and P11) exhibited a paradoxical cortisol response to dexamethasone in vivo. Normal adrenal tissues were removed from patients undergoing expanded nephrectomy for kidney cancer. The experimental protocol was institutional review board reviewed and approved, and all subjects gave their informed consent.
Table 1.
Clinical and molecular characteristics of patients with PPNAD
| Patient | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | F | F | M | M | F | F | F | M | M | F |
| Age (yr) | 27 | 20 | 24 | 25 | 15 | 27 | 28 | 12 | 17 | 4 | 55 |
| UFC (μg/d) (N < 80) | 286 | 39 | 171 | 934 | 750 | 498 | 6924 | 154 | 271 | 877 | 76 |
| Plasma cortisol 0800 h (μg/liter) (N = 90–308) | 340 | 69 | 334 | 210 | 149 | 243 | 650 | 163 | 188 | 359 | 176 |
| Evening cortisol (μg/liter) | 286a | 68a | 288b | 220a | 291b | 271a | 700a | 5.0c | 269b | ND | 173a |
| Plasma ACTH 0800 h (pg/ml) (N = 20–60) | 3 | 12 | 2 | 2 | 5 | <5 | 2 | 6 | 7 | <5 | 7 |
| Liddle’s test (% variation UFC vs. bl; positive if > 50%) | −31% negative | +118% positive | +68% positive | +384% positive | ND | +51% positive | ND | +32% negative | +79% positive | ND | +350% positive |
| Presentation of adrenal glands at CT scan | Normal | Normal | Normal | MacroN | MicroN | MicroN | MRI: normal | MicroN | Normal | Normal | MicroN |
| Mutation in PRKAR1A gene | c709-7del6 (TTTTTA) | c709-7del6 (TTTTTA) | WT | c. 502 + 1G>T | c.708 +1G>T | c709-7del6 (TTTTTA) | WT | c709-7del6 (TTTTTA) | c709-7del6 (TTTTTA) | WT | WT |
| Consequence in protein sequence | Splice variant | Splice variant | NA | Splice variant | Truncated | Splice variant | NA | Splice variant | Splice variant | NA | NA |
| In vitro basal cortisol production (pg/106 cells/24 h) | 3,500 ± 58 | 3,810 ± 42 | 3,071 ± 26 | 3,025 ± 59 | 3,188 ± 24 | 87,235 ± 61 | 13,032 ± 50 | 11,932 ± 33 | 5,520 ± 60 | 719 ± 13 | 403 ± 12 |
| In vitro cortisol | +51 ± 0.5, | +40.7 ± 2.0, | +25.4 ± 0.6, | +298 ± 14, | +25.2 ± 7.1, | +5.7 ± 1.2, | +2.4 ± 1.0, | −5.2 ± 1.1, | +9.8 ± 1.4, | −2.3 ± 5.9, | +711 ± 53, |
| response to dexamethasone (variation % bl) | P < 0.05 | P < 0.05 | P < 0.05 | P < 0.01 | P < 0.05 | NS | NS | NS | NS | NS | P < 0.001 |
| Substances tested in vitro | 8Br-cAMP, H89 | SQ22536, IBMX, 8Br-cAMP, H89, RU486 | IBMX, H89, RU486 | SQ22536, IBMX, 8Br-cAMP, H89, RU486 | SQ22536, IBMX, 8Br-cAMP, H89, RU486 | NA | NA | NA | NA | NA | H89, RU486 |
Conversion factors: cortisol, nanomoles = micrograms × 2.76; ACTH, picomoles per liter = picograms per milliliter × 0.22; F, Female; M, male; MacroN, macronodular; MicroN, micronodular; WT, wild type; ND, not done; bl, basal level; NA, not applicable; NS, not significant; MRI, magnetic resonance imaging.
Plasma cortisol 2000 h.
Plasma cortisol 2400 h.
Salivary cortisol 2400 h.
Genetic studies
The search for germline PRKAR1A gene mutations was performed as previously reported (4). Briefly, DNA was extracted from peripheral blood leukocytes using the Wizard Genomic DNA purification kit (Promega, Charbonnières-les-Bains, France), and the 12 exons and the flanking intronic sequences of the PRKAR1A gene were separately PCR amplified using the primers and the conditions formerly described. Both strands of the amplified products were directly sequenced on an automatic sequencer. Nucleotides were numbered in accordance with the reference sequence for PRKAR1A (GenBank accession no. NM_002734). The PRKAR1A gene mutations characterized in the patients are given in Table 1. A search for germline mutations of the PDE11A gene was also carried out in the 11 patients, as previously published (15). No mutation disrupting the expression of the gene was detected in any patient.
Adrenocortical cell culture
All patients with PPNAD underwent bilateral adrenalectomy. Normal and PPNAD adrenal tissues were obtained at surgery and immediately immersed in culture medium until cell dissociation. Briefly, adrenocortical explants were enzymatically dispersed as previously described (16). Adrenocortical cells were cultured at 37 C in 5% CO2. Incubation experiments of cells were conducted for 24 h after 2 d in culture with fresh DMEM (control experiments) or DMEM with dexamethasone in the presence or absence of various modulators of the cAMP/PKA pathway [SQ22536, 3-isobutyl-1-methylxanthine (IBMX), and H89], a cAMP analog (8Br-cAMP), and the GR antagonist RU486. The compounds tested in each patient tissue are indicated in Table 1. All reagents were obtained from Sigma (Saint-Quentin Fallavier, France). Cells were incubated with each secretagogue for 24 h at 37 C. Cortisol concentrations in culture medium were measured using a formerly reported RIA procedure (17). Cross-reactivity of cortisol antibodies with dexamethasone was less than 0.01%. Results are expressed as mean ± se, and statistical significance was assessed by Student’s t test and Bonferroni test after one-way ANOVA.
Transgenic mouse tissue studies
Transgenic mouse PPNAD model
The transgenic mouse model of PPNAD used in the present study has been previously described (18). These mice carry an antisense transgene for PRKAR1A exon 2 under the control of a tetracycline-responsive promoter [the Tg(tTA/X2AS) line]. They develop several features of human PPNAD, including adrenocortical hyperplasia and hyperglucocorticoidism associated with an increase in adrenal type II PKA total activity (18).
Adrenocortical cell culture
Animals were killed, and the adrenal tissues were immediately immersed in culture medium until cell dispersion. The tissues were then processed as human PPNAD tissues for cell culture studies. Incubation experiments of cells were conducted for 24 h after 2 d in culture with fresh DMEM (control experiments) or DMEM with dexamethasone in the presence or absence of the GR antagonist RU486. Cells were incubated with each secretagogue for 24 h at 37 C. Corticosterone concentrations in culture medium were measured using a formerly reported RIA procedure. Cross-reactivity of corticosterone antibodies with dexamethasone was less than 0.01%. Results are expressed as mean ± se, and statistical significance was assessed by Bonferroni test after one-way ANOVA.
Results
Effect of dexamethasone on cortisol production from cultured human PPNAD cells
Basal cortisol secretion from cultured PPNAD cells was very variable from one tissue to the other (Table 1). Dexamethasone induced a significant increase in cortisol production by cells removed from six of the 11 patients, i.e. P1-5 and P11, but did not affect cortisol secretion by cells derived from patients 6 and 9, who were positive for the Liddle’s test (Table 1). Interestingly, a stimulatory effect of dexamethasone was observed in patient P1 who did not exhibit any paradoxical response to the Liddle’s test in vivo. The maximum in vitro cortisol responses (efficacy) to dexamethasone ranged from +25% (P3 and P5) to +711% (P11).
Role of cAMP in the cortisol response to dexamethasone from cultured PPNAD cells
We have examined whether dexamethasone may activate cortisol secretion from PPNAD cells by enhancing cAMP production or potentiating its stimulatory action on PKA. We have therefore investigated the effect of various synthetic compounds either inhibiting cAMP production/degradation or mimicking its action on PKA, on the cortisol response to dexamethasone by cells removed from patients P1-5. Typical profiles illustrating the effects of these pharmacological agents are presented in Fig. 1. The adenylyl cyclase inhibitor SQ22536 (5 × 10−4 m) was found to have no significant influence on dexamethasone-evoked cortisol release by P2 (Fig. 1A) and P4–5 cells. IBMX (10−4 m), an inhibitor of PDEs, stimulated basal cortisol production (+83.5 ± 26.3%) by P2, P3 (Fig. 1B), P4, and P5 cells but did not potentiate the cortisol response to dexamethasone. Similarly, the cAMP analog 8Br-cAMP stimulated cortisol secretion (+183.1 ± 69.7%) from P1, P2, P4 (Fig. 1C), and P5 cells but failed to enhance the stimulatory action of dexamethasone.
Figure 1.

Typical profiles illustrating the action of modulators of the cAMP/PKA pathway on dexamethasone-induced cortisol secretion by PPNAD cells. A, Effect of the adenylyl cyclase inhibitor SQ22536 on basal and dexamethasone-induced cortisol secretion by cultured P2 cells. Dexamethasone (10−6 m, 24 h) stimulated by 40 ± 4% and 31 ± 8% cortisol secretion (P = 0.37) in the absence and presence of SQ22536 (5 × 10−4 m; 24 h), respectively. B, Effect of the PDE inhibitor IBMX on basal and dexamethasone-induced cortisol secretion from cultured P3 cells. Dexamethasone (10−6 m, 24 h) stimulated by 26 ± 6% and 39 ± 4% cortisol secretion (P = 0.12) in the absence and presence of IBMX (10−4 m; 24 h), respectively. C, Effect of the cAMP analog 8Br-cAMP on basal and dexamethasone-induced cortisol secretion from cultured P4 cells. Dexamethasone (10−6 m; 24 h) stimulated by 297 ± 14% and 264 ± 154% cortisol secretion (P = 0.84) in the absence and presence of 8Br-cAMP (10−4 m; 24 h), respectively. Data values represent mean ± sem from four experiments. NS, Not significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Role of PKA catalytic subunits in the cortisol response to dexamethasone from cultured PPNAD cells
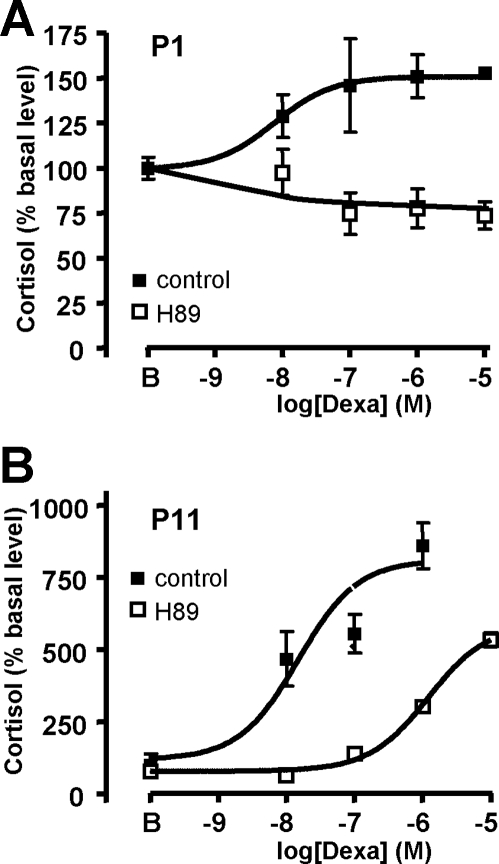
It was also conceivable that dexamethasone may stimulate cortisol secretion by PPNAD cells through a direct action on PKA catalytic subunits. To verify this hypothesis, we have investigated the influence of the PKA inhibitor H89 on the cortisol response to dexamethasone by cells removed from the patients. In all cases, H89 was found to decrease basal cortisol production (mean, −46 ± 8%). In addition, H89 (10−5 m) inhibited basal cortisol production (mean, −45.1 ± 8.4%). In P1–3 cells, H89 totally blocked dexamethasone-evoked cortisol release (Fig. 2A and Table 2), whereas it partially inhibited the cortisol response to dexamethasone by shifting the dose-response curve to the right in P4–5 and P11 cells (Fig. 2B and Table 2).
Figure 2.
Typical profiles illustrating the action of the PKA inhibitor H89 on the cortisol response to dexamethasone by PPNAD cells. Effect of graded concentrations of dexamethasone (10−9 to 10−5 m) on cortisol secretion by cultured cells derived from P1 (A) and P11 (B) cells in the absence (▪) and presence (□) of the PKA inhibitor H89 (10−5 m). Data values represent mean ± sem from four experiments. For evaluation of the effect of dexamethasone in the presence of H89, basal level was calculated as the mean cortisol production by cultured cells incubated with H89 alone.
Table 2.
Efficacy and potency of dexamethasone on PPNAD cells
| Patient no. | Control
|
H89
|
||
|---|---|---|---|---|
| Emax (% bl) | −log EC50 (m) | Emax (% bl) | −log EC50 (m) | |
| P1 | 151.0 ± 0.5 | −8.11 ± 0.03 | NC | NC |
| P2 | 140.7 ± 2.0 | −8.46 ± 0.17 | NC | NC |
| P3 | 125.4 ± 0.6 | −8.84 ± 0.14 | NC | NC |
| P4 | 397.8 ± 14 | −7.04 ± 0.08 | 1013 ± 19.3 | −6.1 ± 0.04 |
| P5 | 125.2 ± 7.1 | −7.77 ± 0.26 | 126.2 ± 8.4 | −6.7 ± 0.8 |
| P11 | 811.2 ± 53.5 | −7.82 ± 0.19 | 563 ± 22.8 | −5.91 ± 0.07 |
NC, Not calculable; bl, basal level.
Role of the GR in the cortisol response to dexamethasone from cultured PPNAD cells
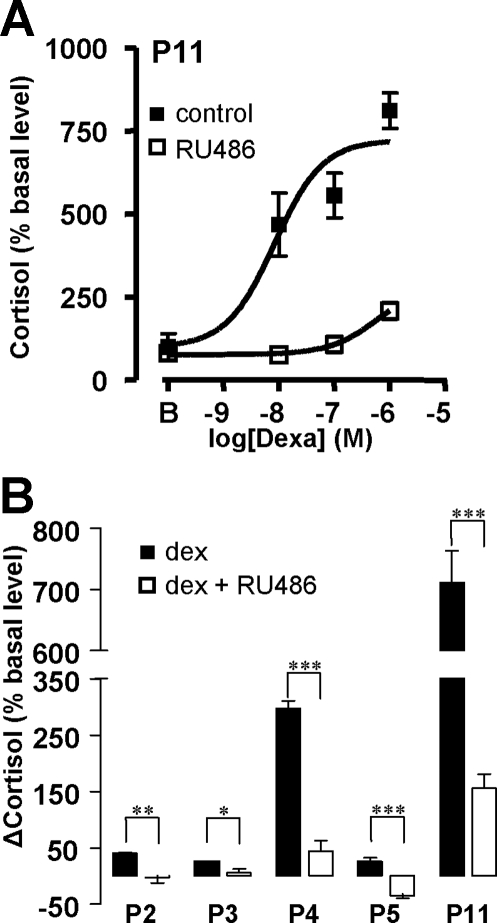
The role of GR in the action of dexamethasone on PPNAD tissues was investigated by evaluating the action of the GR antagonist RU486 on dexamethasone-induced cortisol secretion by cells removed from patients P2-5 and P11. RU486 (10−6 m) provoked a significant decrease in cortisol production (−21.7 ± 3.7%; P < 0.05) and inhibited the cortisol response to dexamethasone (Fig. 3, A and B).
Figure 3.
Effect of the GR antagonist RU486 on the cortisol response to dexamethasone by PPNAD cells. A, Effect of graded concentrations of dexamethasone (10−9 to 10−6 m) on cortisol secretion by cultured cells derived from P11 cells in the absence (▪) and presence (□) of RU486 (10−6 m). B, Effect of RU486 (10−6 m) on the cortisol responses to dexamethasone (10−6 m) by PPNAD cells derived from patients P2–5 and P11. Data values represent mean ± sem from four experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Effect of dexamethasone on cortisol production from cultured human and mouse adrenocortical cells: role of the GR
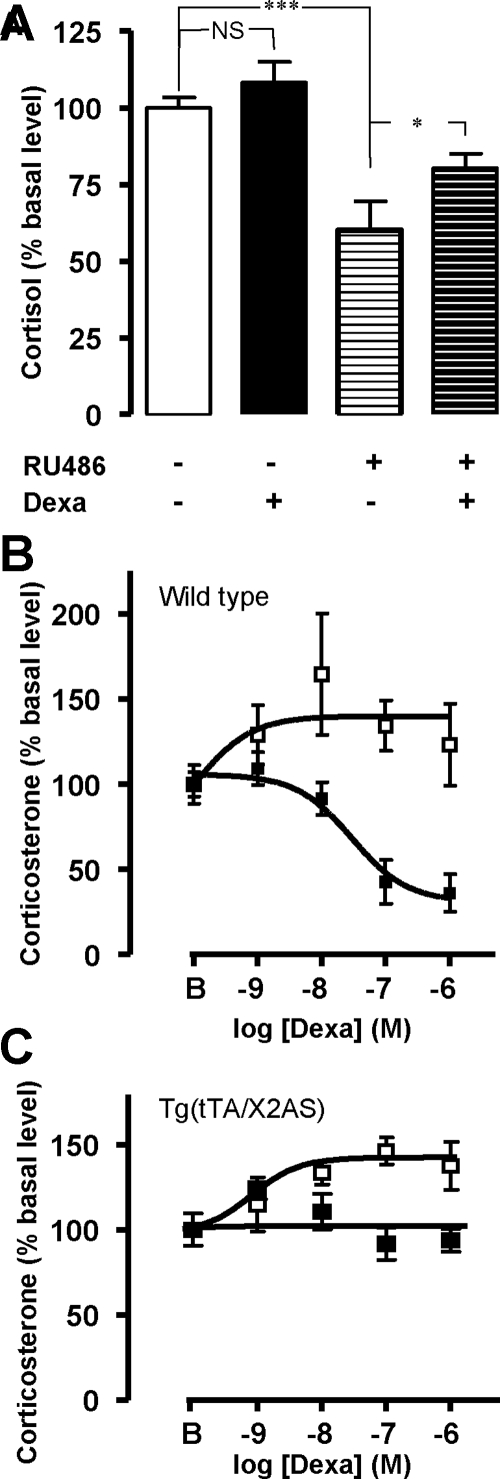
Dexamethasone (10−6 m) had no effect on cortisol secretion from normal human adrenocortical cells. Conversely, preincubation of the cells with RU486 (10−6 m) reduced cortisol production and revealed a stimulatory effect of dexamethasone (Fig. 4A). Dexamethasone (10−10 to 10−6 m) decreased corticosterone secretion from adrenocortical cells removed from wild-type mice (maximum inhibition, −60.2 ± 1.9%) but induced a dose-dependent increase in corticosterone release in the presence of RU486 [maximum efficacy (Emax), +41.6 ± 2.5%; Fig. 4B]. In contrast, dexamethasone failed to influence corticosterone release by adrenocortical cells removed from Tg(tTA/X2AS) mice but stimulated corticosteroidogenesis (Emax, +42.4 ± 0.6%) in the presence of RU 486 (Fig. 4C).
Figure 4.
Action of dexamethasone on glucocorticoid production from human and mouse adrenocortical cells. A, Effect of dexamethasone (10−6 m, 24 h) on cortisol secretion by cultured normal human adrenocortical cells obtained from four different subjects in the absence and presence of RU486 (10−6 m). Dexamethasone did not affect corticosteroidogenesis but stimulated cortisol secretion by 39 ± 15% (P = 0.03) in the presence of RU486. B and C, Effect of graded concentrations of dexamethasone (10−9 to 10−6 m) on corticosterone secretion by cultured wild-type (B) and transgenic Tg(tTA/X2AS) (C) mice adrenocortical cells in the absence (▪) and presence (□) of RU486 (10−6 m). Data values represent mean ± sem from four experiments. NS, Not significant. *, P < 0.05; ***, P < 0.001.
Discussion
The PKA holoenzyme is a heterotetramer composed of two dimers formed by regulatory subunits and inactive catalytic subunits, respectively (8). Binding of cAMP to regulatory subunits provokes the dissociation of the complex and the subsequent release of catalytic subunits that phosphorylate, in turn, a wide variety of proteins. There are four genes that encode the different regulatory subunits (RIα, RIβ, RIIα, and RIIβ) and three encoding the catalytic subunits (Cα, Cβ, and Cγ) (8). A large majority of cases of PPNAD and Carney complex are related to mutations of RIα subunit (5). These mutations are thought to prevent RIα binding to catalytic subunits and therefore to increase the cytosolic concentration of free catalytic subunits in adrenocortical cells leading to adrenal hyperplasia and glucocorticoid hypersecretion (8). PPNAD patients usually exhibit a paradoxical increase in plasma cortisol levels after dexamethasone administration (9). The stimulatory effect of dexamethasone on PPNAD tissues has also been observed in vitro, indicating that the drug exerts a direct action on adrenocortical cells (12). In the present study, dexamethasone was found to activate in vitro cortisol production from adrenocortical cells in six out of a series of 11 patients with PPNAD. It is interesting to notice that the in vitro cortisol responses to dexamethasone were not strictly correlated with the results of the Liddle’s test. For instance, the stimulatory effect of dexamethasone was observed in one patient, i.e. patient P1, that did not exhibit any paradoxical cortisol response to dexamethasone in vivo, indicating that the Liddle’s test may lack sensitivity in some patients for the detection of abnormal stimulation of cortisol release by dexamethasone. This observation may result from accelerated metabolism of dexamethasone, as formerly described in some subjects (19,20). Conversely, dexamethasone was found to have no influence on cortisol secretion by cells removed from patients P6 and P9 who previously responded to the Liddle’s test. It is conceivable that expression of the GR, which is heterogeneous in PPNAD tissues (12), may be low in the adrenal samples used for cell culture experiments, explaining this apparent discrepancy. The amplitude of the cortisol response to dexamethasone was highly variable from one patient to another. This variability as well as the lack of effect of dexamethasone in some patients may be the consequence of the fact that the genetic defects involved in the disease were different among the subjects tested. However, the series of patients included in the present study was too limited to have a chance to demonstrate any significant correlation between dexamethasone-induced increase in cortisol production and genotype.
Because PPNAD is characterized by constitutive activation of PKA in the adrenocortical tissue, we have postulated that the paradoxical stimulatory effect of dexamethasone on cortisol secretion may be due to an interaction of the drug with the cAMP/PKA pathway. We have therefore attempted to influence the cortisol response to dexamethasone from PPNAD cells by use of various pharmacological agents acting at the different steps of the cAMP/PKA pathway. We observed that IBMX, an inhibitor of PDE, and/or the cAMP analog 8Br-cAMP induced a significant increase in basal cortisol secretion from P2–5 cells, indicating that PKA activity can be stimulated by cAMP, as already reported in PPNAD cells harboring PRKAR1A mutations (11). This result can be explained by the fact that, in these cells, inactivation of R1α subunit by germline genetic defects is counteracted by overexpression of other PKA regulatory subunits, such as the RIIβ subunit (18). Conversely, IBMX, as well as the adenylyl cyclase inhibitor SQ22536, did not modify dexamethasone-evoked cortisol secretion, indicating that the stimulatory action of dexamethasone on PPNAD cells is not mediated by cAMP. Similarly, a possible potentiation of the stimulatory action of cAMP on corticosteroidogenesis by dexamethasone could be excluded because the stimulatory effects of dexamethasone and the cAMP analog 8Br-cAMP on cortisol secretion were only additive. Another possibility was that dexamethasone may directly activate PKA catalytic subunits, as previously shown in cell line models (13). We could confirm this assumption by showing that the PKA inhibitor H89, which blocks the activity of PKA catalytic subunits, inhibited the corticotropic effect of dexamethasone on PPNAD cells. We also observed that H89 was able to decrease basal cortisol production, indicating that, in PPNAD cells, basal steroidogenesis is dependent on the cAMP/PKA pathway, as previously observed in macronodular adrenocortical tissues (16). Collectively, our data show that dexamethasone-induced paradoxical increase in cortisol secretion from PPNAD tissues observed in vivo and in vitro results from an interaction of the drug with PKA catalytic subunits in adrenocortical cells. The previous demonstration that GR is overexpressed in PPNAD cells (12) prompted us to verify whether the stimulatory effect of dexamethasone on cortisol release was actually GR-dependent. Consistent with this hypothesis, we noticed that the GR antagonist RU486 totally inhibited the dexamethasone-induced cortisol response. Interestingly, RU486 was found to decrease basal glucocorticoid production, indicating that cortisol exerts an ultrashort positive feedback on its own production in PPNAD cells. These data indicate that dexamethasone stimulates PKA catalytic subunits in PPNAD cells through a GR-mediated mechanism that may theoretically involve either a genomic effect leading to up-regulation of PKA catalytic subunit genes or a nongenomic action resulting in direct activation of PKA catalytic subunits via protein/protein interaction (13,21). The recent observation that PKA catalytic subunits are not overexpressed in PPNAD tissues (unpublished data from the transcriptomic analysis presented in Ref. 22) suggests that the action of dexamethasone on PKA is rather the consequence of direct GR/PKA catalytic subunit interaction. In addition, it is physiopathologically relevant to notice that the GR-mediated stimulatory effect of glucocorticoids on cortisol synthesis, together with overexpression of GR and PKA constitutive activation, is likely to constitute a local amplification loop that may explain the high secretory activity of PPNAD cells.
The comparison of the results obtained in PPNAD tissues with those achieved in normal human and mouse adrenals is also of great interest for the comprehension of the mechanisms involved in the action of dexamethasone on corticosteroid secretion. As previously shown in rats (23), dexamethasone was found to inhibit corticosterone production by wild-type mouse adrenocortical cells, whereas the glucocorticoid had no effect on cortisol release from normal human adrenocortical cells. In addition, incubation of both normal human and mouse adrenocortical cells with RU486 unmasked a stimulatory effect of dexamethasone on cortisol and corticosterone release that corresponds therefore to a GR-independent and consequently nongenomic action. It is possible that this unexpected effect may be mediated by an unknown membrane receptor and involve second messengers, as formerly proposed in other tissue models (24). Because dexamethasone alone has no influence on glucocorticoid production from normal human adrenocortical tissues, we postulate that, in physiological conditions, the GR-independent stimulatory action of dexamethasone on glucocorticoid production is counteracted by an inhibitory GR-mediated effect of the drug through an unidentified cross-talk at the PKA level. Apparently, the GR-mediated inhibitory action of dexamethasone is predominant in mouse adrenocortical tissues. In human PPNAD, the increase in PKA catalytic subunit intracellular content resulting from PRKAR1A gene mutations seems to favor a GR-dependent stimulatory action of dexamethasone. In the transgenic mouse model of PPNAD, no effect of dexamethasone is observed, suggesting therefore that inactivation of the PRKAR1A gene reduces the GR-dependent inhibitory action of dexamethasone. Conversely, the observation that dexamethasone stimulates corticosterone release in the presence of RU486 as efficiently as it does in wild-type mouse adrenocortical tissues indicates that the PRKAR1A gene mutation does not modify the GR-independent stimulatory effect on glucocorticoid production. Taken together, our data suggest that the effects of dexamethasone on adrenocortical cells are complex and involve several intracellular processes in which PKA plays a pivotal role.
In conclusion, the present study shows that, in human PPNAD tissues, dexamethasone paradoxically stimulates cortisol release through a GR-mediated effect on PKA catalytic subunits. In addition, our results indicate that the action of dexamethasone on adrenocortical cells also involves GR-independent nongenomic mechanisms.
Acknowledgments
We are indebted to Dr. P. Grise for his collaboration. We also thank M. Gras and H. Lemonnier for technical assistance.
Footnotes
This work was supported by INSERM U413/EA4310, IFRMP 23, the Carney Complex Network, the U.S. National Institutes of Health [National Institute of Child Health and Human Development Intramural Project Z01-HD-000642-04 (to C.A.S.)], the CHU de Rouen, the Réseau COMETE, the Assistance Publique-Hôpitaux de Paris, and the Conseil Régional de Haute-Normandie.
Disclosure Summary: E.L., V.P., K.J.G., R.L., S.C., B.F., J.Y., and L.G. have nothing to declare. C.A.S. is a U.S. Government employee, and all related rules apply. J.B. received grants from Agence Nationale de la Recherche (ANR) (ANR-08-GENOPAT-007). H.L. received grants from ANR (ANR-08-GENOPAT-007) and Programme Hospitalier de Recherche Clinique (PHRC) (AOM 06 179).
First Published Online April 21, 2009
Abbreviations: Emax, Maximum efficacy; GR, glucocorticoid receptor; IBMX, 3-isobutyl-1-methylxanthine; PDE, phosphodiesterase; PKA, protein kinase A; PPNAD, primary pigmented nodular adrenocortical disease; PRKAR1A, PKA regulatory subunit 1A gene; UFC, urinary free cortisol.
References
- Stratakis CA, Kirschner LS 1998 Clinical and genetic analysis of primary bilateral adrenal diseases (micro- and macronodular disease) leading to Cushing syndrome. Horm Metab Res 30:456–463 [DOI] [PubMed] [Google Scholar]
- Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA 17 March 2009 Mutations in regulatory subunit type 1A of cyclic AMP-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab 10.1210/jc.2008–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groussin L, Horvath A, Jullian E, Boikos S, Rene-Corail F, Lefebvre H, Cephise-Velayoudom FL, Vantyghem MC, Chanson P, Conte-Devolx B, Lucas M, Gentil A, Malchoff CD, Tissier F, Carney JA, Bertagna X, Stratakis CA, Bertherat J 2006 A PRKAR1A mutation associated with primary pigmented nodular adrenocortical disease in 12 kindreds. J Clin Endocrinol Metab 91:1943–1949 [DOI] [PubMed] [Google Scholar]
- Groussin L, Kirschner LS, Vincent-Dejean C, Perlemoine K, Jullian E, Delemer B, Zacharieva S, Pignatelli D, Carney JA, Luton JP, Bertagna X, Stratakis CA, Bertherat J 2002 Molecular analysis of the cyclic AMP-dependent protein kinase A (PKA) regulatory subunit 1A (PRKAR1A) gene in patients with Carney complex and primary pigmented nodular adrenocortical disease (PPNAD) reveals novel mutations and clues for pathophysiology: augmented PKA signaling is associated with adrenal tumorigenesis in PPNAD. Am J Hum Genet 71:1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratakis CA, Boikos SA 2007 Genetics of adrenal tumors associated with Cushing’s syndrome: a new classification for bilateral adrenocortical hyperplasias. Nat Clin Pract Endocrinol Metab 3:748–757 [DOI] [PubMed] [Google Scholar]
- Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, Keil M, Heyerdahl S, Matyakhina L, Libè R, Fratticci A, Kirschner LS, Cramer K, Gaillard RC, Bertagna X, Carney JA, Bertherat J, Bossis I, Stratakis CA 2006 A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet 38:794–800 [DOI] [PubMed] [Google Scholar]
- Horvath A, Mericq V, Stratakis CA 2008 Mutation in PDE8B, a cyclic AMP-specific phosphodiesterase in adrenal hyperplasia. N Engl J Med 358:750–752 [DOI] [PubMed] [Google Scholar]
- Bossis I, Voutetakis A, Bei T, Sandrini F, Griffin KJ, Stratakis CA 2004 Protein kinase A and its role in human neoplasia: the Carney complex paradigm. Endocr Relat Cancer 11:265–280 [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Sarlis N, Kirschner LS, Carney JA, Doppman JL, Nieman LK, Chrousos GP, Papanicolaou DA 1999 Paradoxical response to dexamethasone in the diagnosis of primary pigmented nodular adrenocortical disease. Ann Intern Med 131:585–591 [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Kirschner LS, Carney JA 2001 Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab 86:4041–4046 [DOI] [PubMed] [Google Scholar]
- Bertherat J, Groussin L, Sandrini F, Matyakhina L, Bei T, Stergiopoulos S, Papageorgiou T, Bourdeau I, Kirschner LS, Vincent-Dejean C, Perlemoine K, Gicquel C, Bertagna X, Stratakis CA 2003 Molecular and functional analysis of PRKAR1A and its locus (17q22-24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res 63:5308–5319 [PubMed] [Google Scholar]
- Bourdeau I, Lacroix A, Schürch W, Caron P, Antakly T, Stratakis CA 2003 Primary pigmented nodular adrenocortical disease: paradoxical responses of cortisol secretion to dexamethasone occur in vitro and are associated with increased expression of the glucocorticoid receptor. J Clin Endocrinol Metab 88:3931–3937 [DOI] [PubMed] [Google Scholar]
- Doucas V, Shi Y, Miyamoto S, West A, Verma I, Evans RM 2000 Cytoplasmic catalytic subunit of protein kinase A mediates cross-repression by NF-κB and the glucocorticoid receptor. Proc Natl Acad Sci USA 97:11893–11898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JA, Young WF 1992 Primary pigmented nodular adrenocortical disease and its associated conditions. Endocrinologist 2:6–21 [Google Scholar]
- Libé R, Fratticci A, Coste J, Tissier F, Horvath A, Ragazzon B, Rene-Corail F, Groussin L, Bertagna X, Raffin-Sanson ML, Stratakis CA, Bertherat J 2008 Phosphodiesterase 11A (PDE11A) and genetic predisposition to adrenocortical tumors. Clin Cancer Res 14:4016–4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louiset E, Contesse V, Groussin L, Cartier D, Duparc C, Barrande G, Bertherat J, Vaudry H, Lefebvre H 2006 Expression of serotonin7 receptor and coupling of ectopic receptors to protein kinase A and ionic currents in adrenocorticotropin-independent macronodular adrenal hyperplasia causing Cushing’s syndrome. J Clin Endocrinol Metab 91:4578–4586 [DOI] [PubMed] [Google Scholar]
- Lefebvre H, Contesse V, Delarue C, Feuilloley M, Hery F, Grise P, Raynaud G, Verhofstad AA, Wolf LM, Vaudry H 1992 Serotonin-induced stimulation of cortisol secretion from human adrenocortical tissue is mediated through activation of a serotonin4 receptor subtype. Neuroscience 47:999–1007 [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Kirschner LS, Matyakhina L, Stergiopoulos SG, Robinson-White A, Lenherr SM, Weinberg FD, Claflin ES, Batista D, Bourdeau I, Voutetakis A, Sandrini F, Meoli EM, Bauer AJ, Cho-Chung YS, Bornstein SR, Carney JA, Stratakis CA 2004 A transgenic mouse bearing an antisense construct of regulatory subunit type 1A of protein kinase A develops endocrine and other tumours: comparison with Carney complex and other PRKAR1A induced lesions. J Med Genet 41:923–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizenga NA, Koper JW, de Lange P, Pols HA, Stolk RP, Grobbee DE, de Jong FH, Lamberts SW 1998 Interperson variability but intraperson stability of baseline plasma cortisol concentrations, and its relation to feedback sensitivity of the hypothalamo-pituitary-adrenal axis to a low dose of dexamethasone in elderly individuals. J Clin Endocrinol Metab 83:47–54 [DOI] [PubMed] [Google Scholar]
- Yanovski JA, Cutler Jr GB, Chrousos GP, Nieman LK 1998 The dexamethasone-suppressed corticotropin-releasing hormone stimulation test differentiates mild Cushing’s disease from normal physiology. J Clin Endocrinol Metab 83:348–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann S, Steuer B, Alonso A, Kinzel V, Pyerin W 1996 Promoter of the gene encoding the bovine catalytic subunit of cAMP-dependent protein kinase isoform C β2. Biochim Biophys Acta 1309:211–220 [DOI] [PubMed] [Google Scholar]
- de Reyniès A, Assié G, Rickman DS, Tissier F, Groussin L, René-Corail F, Dousset B, Bertagna X, Clauser E, Bertherat J 2009 Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol 27:1108–1115 [DOI] [PubMed] [Google Scholar]
- Carsia RV, Malamed S 1983 Glucocorticoid control of steroidogenesis in isolated rat adrenocortical cells. Biochim Biophys Acta 763:83–89 [DOI] [PubMed] [Google Scholar]
- Song IH, Buttgereit F 2006 Non-genomic glucocorticoid effects to provide the basis for new drug developments. Mol Cell Endocrinol 246:142–146 [DOI] [PubMed] [Google Scholar]