Abstract
Context: Increasing serum 25-hydroxyvitamin D (25-OHD) in adults may enhance calcium absorption (Ca-abs). There are few similar pediatric data leading to uncertainty about the optimal target for 25-OHD to maximize Ca-abs.
Objective: Our objective was to evaluate the relationship between 25-OHD and Ca-abs in a large cohort of school-age children and adolescents.
Design: We evaluated data from 439 Ca-abs measurements performed using dual-tracer stable isotope techniques conducted at our center over a 15-yr period in 251 healthy children, 4.9–16.7 yr of age.
Results: Serum 25-OHD ranged from 28 to 197 nmol/liter (mean 85 ± 2 nmol/liter) (sem). Total Ca-abs (intake times fractional absorption) were significantly correlated to 25-OHD in the whole population (r = 0.16, P = 0.001). This relationship was closer in the 197 studies in early puberty (Tanner 2 or 3, r = 0.35, P < 0.001) and not significant in pre- or late pubertal subjects. For the whole population, fractional Ca-abs adjusted for calcium intake were slightly but significantly higher at 25-OHD of 28–50 nmol/liter (0.344 ± 0.019) compared with 25-OHD of 50–80 nmol/liter (0.280 ± 0.014) or 25-OHD greater than 80 nmol/liter (0.297 ± 0.015, P < 0.01 for each), suggesting adaptation to moderately low 25-OHD values.
Conclusion: There is no consistent pattern of relationship between 25-OHD and either fractional or total calcium absorption in school-age children. However, there appears to be a modest calcium absorptive response to higher 25-OHD during early puberty.
There is no consistent pattern of relationship between the serum 25-hydroxyvitamin D concentration and calcium absorption in school-age children.
Considerable evidence exists for a role for vitamin D in a range of health outcomes in addition to its classic role of promoting active calcium absorption (Ca-abs). However, determination of dietary recommendations for vitamin D, especially in children, may be based in large part on identifying a target level of serum 25-hydroxyvitamin D concentrations (25-OHD) that optimizes Ca-abs, ultimately leading to greater bone mineral acquisition (1). In adults, it has been reported that Ca-abs linearly increases as 25-OHD increases from approximately 20 to 80 nmol/liter (2). However, this relationship was not identified by other recent studies in adults that found minimal effects of 25-OHD on Ca-abs unless the 25-OHD was extremely low (below ∼15 nmol/liter) (3,4).
Pediatric data regarding the relationship between 25-OHD and Ca-abs are extremely sparse. In older children and adolescents, two recent reports have failed to find a relationship between Ca-abs and 25-OHD across a broad range of 25-OHD above approximately 25 nmol/liter (5,6). It is likely that very low 25-OHD leading to poor calcium absorption is a major part of the etiology of rickets, especially among children in the first 3 yr of life. However, even in this case, it has been impossible to identify a specific 25-OHD associated with rickets (7). The combination of a very low calcium intake and vitamin D status may be associated with many cases of rickets, rather than any specific threshold low level of 25-OHD being causative (7,8).
Numerous individuals have advocated reevaluation of the current dietary requirements for vitamin D (9,10,11,12). It has been widely recommended that a goal of achieving a 25-OHD greater than 75–80 nmol/liter be set for all individuals, including children (13). Achieving this goal, especially in the absence of substantial sunshine exposure, would require the use of vitamin D supplementation or fortification strategies beyond those currently in place (1,12,13). Because these strategies would not be simple to implement, continuing evaluation of the basis for these recommendations is necessary.
Over the last 15 yr, we have used the dual-tracer stable isotope technique to assess calcium absorption in healthy children on their usual diet (14,15,16,17,18). In many of these studies, we obtained 25-OHD values using RIA techniques. Our purpose in this investigation was to pool the calcium absorption and 25-OHD data we had obtained in school-age children and adolescents. Our database of more than 400 such studies is among the largest database of pediatric calcium absorption studies in existence. We hypothesized that we would not find a significant relationship between vitamin D status, as assessed by 25-OHD, and Ca-abs in healthy children who did not have very low 25-OHD or calcium intakes.
Subjects and Methods
Subjects and research studies
We pooled data from 440 separate stable isotope calcium absorption studies conducted at the Children’s Nutrition Research Center and the General Clinical Research Center of Texas Children’s Hospital between 1991 and 2005. Studies were included in which both calcium absorption and 25-OHD were measured, and in which the study was performed without any specific dietary intervention. In one of these 440 studies, the 25-OHD was very low (18 nmol/liter) and far below the lowest value of the other 439 studies (28 nmol/liter). Total Ca-abs in that study was 240 mg/d and not out of the range of the other studies, but because this was an extreme outlier for 25-OHD and we did not want to suggest that our data reflect 25-OHD values below the general range of values, we chose to exclude this single study from the analysis.
The 439 absorption studies included in this study were part of four separate clinical research studies of calcium absorption in 251 children and adolescents (5,14,15,16,17,18,19). Characteristics of these four studies are shown in Table 1. Results relating fractional calcium absorption to 25-OHD values were previously published for 43 subjects from one of these studies (5). None of the other 396 absorption studies in this analysis have previously had this relationship reported.
Table 1.
Individual studies composing this analysis
| Study | References | Study years | Subjects | Number of calcium absorption measurements | Mean age (yr) | Mean 25-OHD (nmol/liter) | Calcium intake (mg/d) | Calcium absorption (%) | Total calcium absorption (mg/d) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 14,16 | 1991–1994 | 82 F | 93 F | 11.3 ± 3.2 | 37 ± 12 | 910 ± 260 | 32.3 ± 11.2 | 291 ± 130 |
| 2 | 15 | 1994–1996 | 13 F/12 M | 25 (13 F/12 M) | 11.7 ± 1.5 | 45 ± 13 | 1310 ± 82 | 25.9 ± 8.9 | 338 ± 134 |
| 3 | 17,18 | 1996–1999 | 53 F | 182 F | 9.0 ± 0.9 | 37 ± 14 | 1205 ± 194 | 31.8 ± 10.7 | 384 ± 131 |
| 4 | 5,19 | 2000–2005 | 103 (48 F/55 M) | 139 (70 F/69 M) | 11.8 ± 1.1 | 28 ± 7 | 896 ± 400 | 31.8 ± 10.4 | 282 ± 132 |
All data are mean ± sd. M, Male; F, female.
Subjects for each study were selected to approximately match the ethnic distribution of the greater Houston area. Subjects received a physical examination including Tanner staging before inclusion in the study for all of the studies except for study 1 (Table 1) in which self-reporting was used (a total of 93 of the studies involved self-reporting; the others all had physical examinations). To be enrolled, subjects had to be healthy and not using any medications or multivitamins.
Breast Tanner stage was used for females and penile Tanner stage for males. Of the studies, 184 were conducted when the subject was Tanner 1, 129 when Tanner 2, 68 when Tanner 3, 39 when Tanner 4, and 19 when Tanner 5. Due to the self-reporting of Tanner staging in one of the studies and the small number of Tanner 5 subjects, we combined Tanner 2 and 3 and these studies are described as being done when the subjects were early pubertal. Similarly, we combined Tanner 4 and 5 and these are described as late pubertal. Subjects who were Tanner 1 are described as prepubertal.
Written informed consent was obtained from a parent or legal guardian for each subject; assent was obtained from all of the study subjects. The Institutional Review Board of Baylor College of Medicine and Affiliated Hospitals approved each protocol.
Dietary methods
At the screening visit, the study dietitian asked subjects what they usually ate on a normal day and food preferences were obtained. Inpatient menus for the overnight study visit were based on the usual daily calcium intake. All foods and beverages during the inpatient and outpatient visits were pre- and postweighed to accurately determine intake. Subjects were instructed to keep food records for at least 2 d after the Ca-abs measurement. In most cases weighed food records were obtained, but in about one third of the studies, only nonweighed food diaries were obtained. To reflect the marketplace changes in dietary food contents during the study, dietary intake data were collected using Nutrition Data System for Research software, developed by the Nutrition Coordinating Center (University of Minnesota, Minneapolis, MN). No dietary supplements of any type were given to the study subjects.
Calcium absorption and analytical methods
For the calcium absorption measurements, each subject was given a breakfast containing approximately one third of their daily intake of calcium. Toward the end of breakfast, the subjects were given a stable isotope of calcium that had been mixed with 240 ml of calcium-fortified orange juice or milk. After breakfast, a different calcium stable isotope was infused over 2 min. Beginning with breakfast, a complete 24-h urine collection was obtained.
Urine samples were prepared for thermal ionization mass spectrometric analysis as previously described using an oxalate precipitation technique. Urine samples obtained were analyzed to determine their calcium isotope ratios using a Finnigan MAT 261 (Bremen, Germany) magnetic sector thermal ionization mass spectrometer.
Fractional absorption of calcium was calculated as the relative recovered oral vs. iv isotope in a 24-h urine specimen collected at the time of the tracer administration as previously described (5). Total calcium absorption was calculated as the product of calcium intake and fractional calcium absorption.
Serum 25-OHD was measured using commercial RIA kits available from DiaSorin Inc. (Stillwater, MN, previously known as Incstar). The intra- and interassay coefficients of variation were 10%–15%.
Statistical methods
The relationship between fractional and total Ca-abs and 25-OHD was evaluated by both linear regression analysis and general linear modeling in which Ca-abs was the dependent variable and the effects of gender, race, pubertal status, calcium intake, and 25-OHD were covariates. Fisher’s least significant differences correction was used for multiple comparisons, and all data were analyzed using SPSS (version 16.0; SPSS, Inc., Chicago, IL). Data are reported as mean ± sd except as specifically noted.
Results
Study population
Results for the study subjects are shown based on pubertal status in Table 2. Most subjects had one or two studies each. A total of 45 of the subjects had three to five studies performed. All studies in any individual were separated by at least 6 months, usually 12 months. Of note is the decrease in 25-OHD during puberty.
Table 2.
Subject demographics
| Prepubertal (n = 184) | Early pubertal (n = 197) | Late pubertal (n = 58) | P valuea | |
|---|---|---|---|---|
| Age (yr) | 9.0 ± 1.5 | 11.0 ± 1.4 | 13.7 ± 1.7 | <0.001b |
| Weight (kg) | 29.7 ± 7.9 | 40.7 ± 9.6 | 53.9 ± 12.2 | <0.001b |
| Serum 25-OHD (nmol/liter) | 95 ± 33 | 80 ± 28 | 75 ± 30 | <0.001c |
| Calcium intake (mg/d) | 1093 ± 245 | 1039 ± 324 | 952 ± 279 | 0.004d |
| Total Ca-abs (mg/d) | 324 ± 115 | 344 ± 158 | 292 ± 136 | 0.04e |
| Ca-abs fraction | 0.301 ± 0.90 | 0.330 ± 0.102 | 0.314 ± 0.124 | 0.02f |
| Urinary calcium (mg/kg · d) | 2.8 ± 2.0 | 2.2 ± 1.5 | 2.0 ± 1.2 | 0.001g |
| Alkaline phosphatase activity (IU/liter) | 227 ± 58 | 266 ± 75 | 176 ± 100 | <0.001b |
All data are mean ± sd.
Shown are overall P values. Subsequent pairwise comparisons were performed after adjustment by Fisher’s least significant differences for multiple comparisons. Significant pairwise comparisons (P < 0.05) are noted as below.
Each pairwise comparison (P < 0.001).
Pairwise comparison: pre- vs. early pubertal and pre- vs. late pubertal (P < 0.001).
Pairwise comparison: pre- vs. late pubertal (P = 0.001), early vs. late pubertal (P = 0.04).
Pairwise comparison: early vs. late pubertal (P = 0.01).
Pairwise comparison: early vs. prepubertal (P = 0.005).
Pairwise comparison: pre- vs. early pubertal (P = 0.004), pre- vs. late pubertal (P = 0002).
The mean 25-OHD for all studies was 85 ± 2 nmol/liter (sem). A total of 224 studies were conducted in Caucasians, 100 in African-Americans, 98 in Hispanics, and 17 in Asians. We did not have available the season of measurement for many of the studies in reviewing the data. However, because of the age of the school children and availability for in-patient stays, the majority of studies were conducted during the summer months.
Relationship between 25-OHD and calcium absorption
Total absorption
When 25-OHD, gender, race, and pubertal status were included in a general linear model, a significant effect on total Ca-abs was seen for each (P < 0.05 for gender, P < 0.01 for race, pubertal status, and 25-OHD, and for the interaction of 25-OHD and pubertal status was significant, P < 0.001). The r value for the whole model was 0.35 (P < 0.001). Other interactions were not significant including those involving race and 25-OHD.
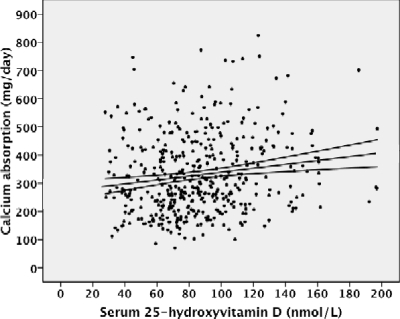
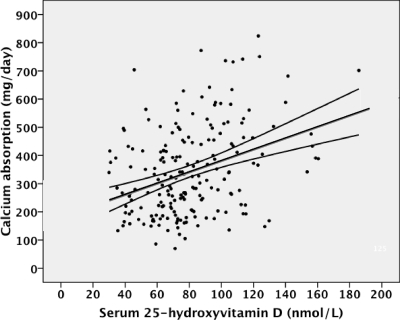
We evaluated the effects of 25-OHD on total Ca-abs based on pubertal status by linear regression analysis. For the whole group, this relationship was significant (r = 0.16, P < 0.001; Fig. 1). For early pubertal subjects, serum 25-OHD was highly significantly related to total Ca-abs (r = 0.35, P < 0.001; Fig. 2). They were not significantly related for pre- or late pubertal subjects (supplemental Figs. 1 and 2, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://jcem.endojournals.org).
Figure 1.
Relationship between total Ca-abs and 25-OHD for all 439 studies (y = 1.73 × +270, r = 0.16, P = 0.001).
Figure 2.
Relationship between total Ca-abs and 25-OHD for studies in early pubertal (Tanner 2 and 3) subjects (y = 5.0 × + 184, r = 0.35, P < 0.001).
We considered the possibility that some of this effect was due to a positive relationship between calcium intake and 25-OHD. This is likely because much of the vitamin D intake in children is from milk, which is also a major source of calcium in the diet of children and adolescents. We found that serum 25-OHD was significantly related to calcium intake (r = 0.27, P < 0.001).
Although this indicates that only approximately 7% of the variability in 25-OHD was associated with calcium intake, this relationship could confound the interpretation of the relationship between total Ca-abs and 25-OHD. One way of evaluating this issue is to consider fractional Ca-abs using calcium intake as a covariate as well as looking at results at both low and high calcium intakes as groups.
Fractional absorption
We evaluated the relationship between fractional Ca-abs and 25-OHD in all subjects as well as separately in subjects with calcium intake less than 1000 mg/d and those 1000 mg/d or greater. Because 25-OHD primarily stimulates active calcium absorption at low intakes, we would expect that if 25-OHD was increasing calcium absorption, we would find a relationship such that increased 25-OHD was more closely associated with higher Ca-abs at lower compared with calcium intakes.
For the whole population (n = 439), there was no close relationship between 25-OHD and fractional absorption (r = 0.03, P = 0.46). In a general linear model with calcium intake, pubertal status, gender, and race as covariates, fractional absorption was not significantly related to 25-OHD (P = 0.34) but was significantly (negatively) associated with calcium intake (P = 0.03).
Using linear regression analysis, for the 182 subject with calcium intake less than 1000 mg/d, the relationship between 25-OHD and fractional Ca-abs was not significant (r = 0.07, P = 0.35) and for the 257 subjects with intakes 1000 mg/d or greater (r < 0.01, P = 0.95). Using other cut points for calcium intake such as 600 or 800 mg/d gave similar results. Thus, we did not see a greater effect of 25-OHD on calcium absorption fraction at low calcium intakes and overall found no significant relationship between 25-OHD and fractional Ca-abs.
Analysis by 25-OHD groups
Evaluation of vitamin D status is often done by categorizing individuals based on their 25-OHD (1,5,9,10,11,12,13). We compared values less than 50 nmol/liter, values of 50–80 nmol/liter, and values greater than 80 nmol/liter. These values are arbitrary but are consistent with current guidelines targeting values in children of at least 50 nmol/liter to avoid deficiency or insufficiency and the recent literature in adults targeting values greater than 80 nmol/liter as being optimal (9,10,11,12,13).
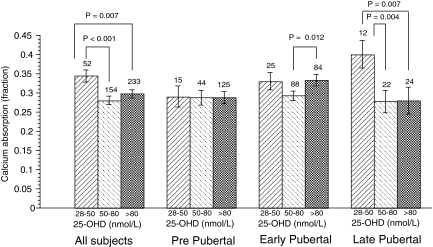
We evaluated the relationship between fractional Ca-abs and 25-OHD group without correction for race, gender, pubertal status, and calcium intake (Table 3) and after adjustment for these (Fig. 3). Because of the significant interaction of vitamin D group and pubertal status, we looked at the relationships at each pubertal status. Of note was that the interaction between gender and vitamin D group was not significant in this model (P = 0.14), and the interaction of race and vitamin D group was not significant (P = 0.28). These group analyses demonstrate a variable pattern of relationship between calcium absorption fraction and vitamin D group. The high values seen in postpubertal girls for the lowest levels should be evaluated with caution due to the small number of subjects in those groups.
Table 3.
Relationship between serum 25-OHD and Ca-ab fraction not adjusted for dietary calcium intake
| Vitamin D group | Serum 25-OHD range | All subjects | Prepubertal | Early pubertal | Late pubertal |
|---|---|---|---|---|---|
| Group 1 | 28–50 nmol/liter | 0.362 ± 0.125 (54) | 0.319 ± 0.093 (15) | 0.347 ± 0.114 (25) | 0.444 ± 0.151 (12) |
| Group 2 | 50–80 nmol/liter | 0.302 ± 0.092 (154) | 0.302 ± 0.090 (44) | 0.307 ± 0.092 (88) | 0.280 ± 0.097 (22) |
| Group 3 | >80 nmol/liter | 0.315 ± 0.100 (233) | 0.299 ± 0.092 (125) | 0.349 ± 0.105 (84) | 0.279 ± 0.088 (24) |
| P value | 0.001 | 0.72 | 0.02 | 0.001 |
All data shown are fractional Ca-ab (mean ± sd) with sample size given in parentheses. P value is for overall comparison. Subsequent group pairwise comparisons were performed after adjustment by Fisher’s least significant differences for multiple comparisons. Significant pairwise comparisons (P < 0.05) are noted as follows: all subjects: group 1 vs. group 2, P < 0.001, group 1 vs. group 3, P = 0.003; early pubertal: group 2 vs. group 3, P = 0.008; late pubertal: group 1 vs. group 2, P < 0.001, group 1 vs. group 3, P < 0.001.
Figure 3.
Relationship between fractional Ca-abs and 25-OHD. Covariates in each analysis were gender, race, and calcium intake. Pubertal status was also included as a covariate in the analysis of all subjects. Overall differences were significant (P < 0.05) for all except prepubertal subjects. Pair-wise comparisons (values shown on graph for the paired P values), were performed after adjustment by Fisher’s least significant differences for multiple comparisons.
Discussion
We found that both fractional and total calcium absorption were inconsistently related to 25-OHD, especially during early puberty. We did not find evidence that, in the presence of calcium intakes greater than approximately 600 mg/d, values of 25-OHD that have been considered insufficient (13) (28–50 nmol/liter) had a significant negative association with fractional calcium absorption. We found no significant effect of race or gender on our results, although this may reflect inadequate sample size, especially for race or the challenges of identifying populations based on race and ethnicity in our region.
The overall magnitude of effect of 25-OHD on total calcium absorption was small. For the whole group, approximately 3% of the variation in total calcium absorption was related to 25-OHD, and during early puberty this value increased to about 12%. These values may be influenced somewhat by the significant relationship between calcium intake and 25-OHD. When fractional absorption was used with calcium intake as a covariate, no such significant relationship was seen. Taken as a whole, our data demonstrate an inconsistent relationship between 25-OHD and calcium absorption that was primarily positive during early puberty.
We have previously shown that calcium absorption increases during early puberty (16,18). It is likely that this is primarily hormonally driven and not primarily vitamin D dependent (20). However, vitamin D-dependent calcium absorption may also be increased during puberty as suggested by the previously reported significant relationship between 1,25 dihydroxyvitamin D and calcium absorption (5). It may also be related to different effects of the vitamin D receptor (VDR) during puberty. Calcium absorption during puberty is related to Fok1 gene polymorphisms (21,22). The polymorphism with the lowest calcium absorption, ff, was also the one with subjects with the highest 25-OHD. This paradoxical finding could indicate an interaction of genotype and vitamin D status such that depending on VDR genotype, during puberty, higher levels of vitamin D are needed to fully stimulate calcium absorption. Much more information is needed, however, on the interrelationship of puberty, VDR genotypes, serum vitamin D, and calcium absorption before this information can by used to make specific nutritional recommendations.
The finding of higher fractional absorption in subjects with 25-OHD of 28–50 nmol/liter compared with 50–80 nmol/liter (Fig. 3) is likely related to compensatory increases in PTH and 1,25 dihydroxyvitamin D. Further evaluation in additional subjects at a lower range of 25-OHD would be needed to define the lowest value of 25-OHD at which such compensation might occur. Long-term negative consequences of this adaptation including effects on bone of mild hyperparathyroidism might exist but have not been clearly demonstrated in populations of healthy children across the range of 25-OHD in this study.
Our findings are consistent with those of Weaver et al. (6), who did not find a relationship between either calcium absorption or calcium retention and 25-OHD in 158 studies conducted in young female adolescents. Mean 25-OHD as well as the range of values was similar to those in our population.
There are very few studies relating vitamin D levels to outcomes in school age children. In Finland, pubertal girls with severe hypovitaminosis D had lower bone mineral density and accrual of bone mineral during puberty (23). There are no intervention studies of children in this age group in which calcium absorption has been assessed before and after vitamin D supplementation. It is important that data in adults not be assumed to be applicable to pediatric populations. Our data demonstrate these distinctions and that childhood is not a single time period in terms of vitamin D and calcium metabolism.
This study is limited in that it represented a pooling of separate studies conducted over a 15-yr period conducted in populations at low risk for rickets or severe calcium and vitamin D deficiency. However, we did not change our basic investigative protocol or the population base from which we conducted studies in this time period. Some subjects had multiple studies. However, as with the studies in adults (24) and adolescents (6), studies done at different time periods can reasonably be pooled together. Our study did not provide either PTH or 1,25 dihydroxyvitamin D data with which to further interpret the calcium absorption results.
Our subjects were not severely vitamin D deficient using accepted standards for these distinctions (13). This study cannot be considered as representative of children in all populations and was not intended as a study of the usual distribution of 25-OHD values across the United States or globally. Results in populations at higher latitudes generally demonstrate lower mean 25-OHD and a greater proportion of children with low 25-OHD (25).
Our findings may not apply to subjects with low calcium intakes. This study was conducted across a range of calcium intakes but did not include values less than about 600 mg/d. In theory, the effects of vitamin D level on calcium absorption are defined at a constant calcium intake, with low calcium intakes favoring vitamin D-dependent absorption. However, such constant intakes do not exist in free-living children or adolescents, and calcium intake is tightly linked to vitamin D intake in most children in the United States due to the presence of both calcium and vitamin D in milk. Assessment of calcium (and vitamin D) intake is limited in accuracy in free-living individuals and highly variable day to day. Our analysis of fractional calcium absorption corrected for calcium intake may be the best way to account for these variations and to consider the effects of 25-OHD on calcium absorption in free-living populations. The weak relationship between calcium intake and fractional absorption indicates that intake was not a major factor in the relationships we described.
Targeting of specific 25-OHD for the entire population has been advocated recently (9,10,11,12,13). Although a range of health benefits for such targeting are possible, in children, there are currently few data related to outcomes other than those related to bone. Although severe vitamin D deficiency may directly affect bone, the effect of vitamin D on calcium absorption remains important in developing nutritional guidelines.
Misra et al. (13) on behalf of the Lawson Wilkins Pediatric Endocrine Society have described a 25-OHD less than 37.5 nmol/liter as deficiency and a value 37.5–50.0 nmol/liter as insufficiency. Data supporting these values include increased PTH at low 25-OHD (25) and changes in bone turnover markers with lower 25-OHD (26,27,28). Caution, however, should be used in interpreting both elevations of PTH and bone turnover markers in pediatric populations.
A recent study in Finland (29) found a benefit to some measures of bone mineral mass after 1 yr of 400 IU/d vitamin D supplementation. However, neither these data nor those of Cashman et al. (28) from Ireland clearly identify a cutoff value of 25-OHD for vitamin D sufficiency. Some benefit to supplementation with vitamin D in young adolescent Lebanese girls was found, although no significant effect was seen on whole-body (excluding the head) bone mineral content (30). Of note is that the studies showing a benefit to higher 25-OHD values are primarily those conducted during puberty, consistent with the findings in this study.
In summary, we found no consistent pattern of relationship between 25-OHD and either fractional or total calcium absorption across the age range of approximately 5- to 16-yr-old children. However, we did identify a modest positive effect of higher 25-OHD on calcium absorption primarily during early puberty. Calcium absorptive benefits for early pubertal children from any targeted value of 25-OHD would require further evaluation with controlled trials of vitamin D supplementation. Such studies would need to account for gender and race as well as specific genetic markers (e.g. the Fok1 polymorphism of the VDR) that are known to affect calcium absorption.
Supplementary Material
Footnotes
This work is a publication of the U.S. Department of Agriculture (USDA)/Agricultural Research Service (ARS) Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, and Texas Children’s Hospital (Houston, TX). This project has been funded in part with federal funds from the USDA/ARS under Cooperative Agreement 58-6250-6-001, National Center for Research Resources General Clinical Research for Children Grant RR00188, and National Institutes of Health Grant AR43740. Contents of this publication do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 21, 2009
Abbreviations: 25-OHD, 25-Hydroxyvitamin D; Ca-abs, calcium absorption; VDR, vitamin D receptor.
References
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Institute of Medicine (U.S.) 1997 DRI, dietary reference intakes: for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press [Google Scholar]
- Heaney RP, Dowell MS, Hale CA, Bendich A 2003 Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 22:142–146 [DOI] [PubMed] [Google Scholar]
- Need AG, O'Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE 2008 Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res 23:1859–1863 [DOI] [PubMed] [Google Scholar]
- Need AG, Nordin BE 2008 Misconceptions—vitamin D insufficiency causes malabsorption of calcium. Bone 42:1021–1024 [DOI] [PubMed] [Google Scholar]
- Abrams SA, Griffin IJ, Hawthorne KM, Gunn SK, Gundberg CM, Carpenter TO 2005 Relationships among vitamin D levels, PTH, and calcium absorption in young adolescents. J Clin Endocrinol Metab 90:5576–5581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CM, McCabe LD, McCabe GP, Braun M, Martin BR, Dimeglio LA, Peacock M 2008 Vitamin D status and calcium metabolism in adolescent black and white girls on a range of controlled calcium intakes. J Clin Endocrinol Metab 293:3907–3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranney A, Weiler HA, O'Donnell S, Puil L 2008 Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr 88:513S–519S [DOI] [PubMed] [Google Scholar]
- Oramasionwu GE, Thacher TD, Pam SD, Pettifor JM, Abrams SA 2008 Adaptation of calcium absorption during treatment of nutritional rickets in Nigerian children. Br J Nutr 100:387–392 [DOI] [PubMed] [Google Scholar]
- Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, Norman AW, Scragg R, Whiting SJ, Willett WC, Zittermann A 2007 The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 85:649–650 [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R 2006 Estimates of optimal vitamin D status. Osteoporos Int 16:713–716 [DOI] [PubMed] [Google Scholar]
- Hollis BW 2005 Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr 135:317–322 [DOI] [PubMed] [Google Scholar]
- Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition 2008 Prevention of rickets and vitamin d deficiency in infants, children, and adolescents. Pediatrics 122:1142–1152 [DOI] [PubMed] [Google Scholar]
- Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society 2008 Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 122:398–417 [DOI] [PubMed] [Google Scholar]
- Abrams SA, Stuff JE 1994 Calcium metabolism in girls: current dietary intakes lead to low rates of calcium absorption and retention during puberty. Am J Clin Nutr 60:739–743 [DOI] [PubMed] [Google Scholar]
- Abrams SA, Grusak MA, Stuff J, O'Brien KO 1997 Calcium and magnesium balance in 9–14-y-old children. Am J Clin Nutr 66:1172–1177 [DOI] [PubMed] [Google Scholar]
- Bronner F, Abrams SA 1998 Development and regulation of calcium metabolism in healthy girls. J Nutr 128:1474–1480 [DOI] [PubMed] [Google Scholar]
- Abrams SA, Copeland KC, Gunn SK, Stuff JE, Clarke LL, Ellis KJ 1999 Calcium absorption and kinetics are similar in 7- and 8-year-old Mexican-American and Caucasian girls despite hormonal differences. J Nutr 129:666–671 [DOI] [PubMed] [Google Scholar]
- Abrams SA, Copeland KC, Gunn SK, Gundberg CM, Klein KO, Ellis KJ 2000 Calcium absorption, bone accretion and kinetics increase during early pubertal development in girls. J Clin Endocrinol Metab 85:1805–1808 [DOI] [PubMed] [Google Scholar]
- Abrams SA, Griffin IJ, Hawthorne KM, Liang L, Gunn SK, Darlington G, Ellis KJ 2005 A combination of prebiotic short-and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am J Clin Nutr 82:471–476 [DOI] [PubMed] [Google Scholar]
- Abrams SA, Strewler GJ 2007 Adolescence: how do we increase intestinal calcium absorption to allow for bone mineral mass accumulation? Bonekey Osteovision 4:147–157 [Google Scholar]
- Ames SK, Ellis KJ, Gunn SK, Copeland KC, Abrams SA 1999 Vitamin D receptor gene Fok1 polymorphism predicts calcium absorption and bone mineral density in children. J Bone Miner Res 14:740–746 [DOI] [PubMed] [Google Scholar]
- Abrams SA, Griffin IJ, Hawthorne KM, Chen Z, Gunn SK, Wilde W, Darlington G, Shypailo R, Ellis K 2005 Vitamin D receptor Fok1 polymorphisms affect calcium absorption, kinetics and bone mineralization rates during puberty. J Bone Miner Res 20:945–953 [DOI] [PubMed] [Google Scholar]
- Lehtonen-Veromaa MKM, Mottonen TT, Nuotio IO, Irjala KMA, Leino AE, Viikari JSA 2002 Vitamin D and attainment of peak bone mass among peripubertal Finnish girls: a 3-y prospective study. Am J Clin Nutr 76:1446–1453 [DOI] [PubMed] [Google Scholar]
- Abrams SA, Yergey AL, Heaney RP 1994 Relationship between balance and dual tracer isotopic measurements of calcium absorption and excretion. J Clin Endocrinol Metab 79:965–969 [DOI] [PubMed] [Google Scholar]
- Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ 2004 Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 158:531–537 [DOI] [PubMed] [Google Scholar]
- Jones G, Blizzard C, Riley MD, Parameswaran V, Greenaway TM, Dwyer T 1999 Vitamin D levels in prepubertal children in Southern Tasmania: prevalence and determinants. Eur J Clin Nutr 53:824–829 [DOI] [PubMed] [Google Scholar]
- Jones G, Dwyer T, Hynes KL, Parameswaran V, Greenaway TM 2005 Vitamin D insufficiency in adolescent males in Southern Tasmania: prevalence, determinants, and relationship to bone turnover markers. Osteoporos Int 16:636–641 [DOI] [PubMed] [Google Scholar]
- Cashman KD, Hill TR, Cotter AA, Boreham CA, Dubitzky W, Murray L, Strain JJ, Flynn, Robson PJ, Wallace JMW, Kiely M 2008 Low vitamin D status adversely affects bone health parameters in adolescents. Am J Clin Nutr 87:1039–1044 [DOI] [PubMed] [Google Scholar]
- Viljakainen HT, Natri AM, Kärkkäinen M, Huttunen MM, Palssa A, Jakobsen J, Cashman KD, Mølgaard C, Lamberg-Allardt C 2006 A positive dose-response effect of vitamin D supplementation on site-specific bone mineral augmentation in adolescent girls: a double-blinded randomized placebo-controlled 1-year intervention. J Bone Miner Res 21:836–844 [DOI] [PubMed] [Google Scholar]
- El-Hajj Fuleihan G, Nabulsi M, Tamim H, Maalouf J, Salamoun M, Khalife H, Choucair M, Arabi A, Vieth R 2006 Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab 91:405–412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.