Abstract
Context: It is uncertain how between-meal variations in energy availability and physiological changes in ghrelin, leptin, and insulin affect appetite.
Objective: The aim of the study was to examine the influence on human appetite of the meal size and its nutrient content or changes in energy availability and concentrations of ghrelin, leptin, and insulin.
Design: We conducted a crossover study manipulating meal size and energy availability through exercise energy expenditure and iv nutrient replacement (TPN).
Setting: The study was performed at a Clinical Research Center.
Participants: Ten healthy postmenopausal women (age, 59.7 ± 1.5 yr; mean body mass index, 26 kg/m2) were studied.
Interventions: We conducted trials based on different morning meal size (418 vs. 2090 KJ), presence or absence of exercise energy expenditure (2273 to 2361 KJ), energy replacement by TPN (1521 to 1538 KJ), and a midday ad libitum meal.
Main Outcome Measures: Changes in hunger, fullness, midday ad libitum food consumption, and concentrations of ghrelin, leptin, insulin, and metabolic fuels were measured. We also performed midday meal tests for the presence of caloric compensation.
Results: Appetite was influenced by the size and energy content of the meals, but not by variation in energy availability which also did not trigger consummatory compensation. Exercise reduced hunger and increased fullness. Ghrelin, leptin, and insulin responded to changes in energy availability but not to meal size. Appetite was unaffected by physiological changes in ghrelin, leptin, or insulin.
Conclusions: During rest, appetite is influenced by the size and energy content of meals, but it bears no homeostatic relationship to between-meal changes in energy availability due to small meals, exercise, or TPN, or concentrations of ghrelin, leptin, and insulin.
Study results indicate that between meals, human appetite is influenced by signals generated by food passage through the mouth and the gastrointestinal tract, rather than by changes in energy availability, or in concentrations of ghrelin, leptin, and insulin.
How energy availability affects human appetite is of great interest in view of the recent rise in obesity and associated morbidities. Two homeostatic negative feedback mechanisms are thought to control human appetite and animal and human food intake. First, mechanical stimuli and gastrointestinal hormones control meal size by inducing satiation (1,2). Duodenal hormone cholecystokinin (CCK) and lower intestinal hormones glucagon-like peptide-1 (GLP-1) (2) and peptide YY (3) induce satiation, whereas pharmacological blockade or deletion of hormone receptors prevents the effect (4). Hunger stimulus is less clearly defined, with the stomach hormone ghrelin a potential mediator (5). Ghrelin and the satiation hormones communicate with the hindbrain through the afferent vagus (6). Brain can also initiate feeding because it can directly sense reduction in energy availability (7,8), as can nonhomeostatic circadian and social factors, whereas satiation may be the principal negative feedback in meal eating (4).
A second negative feedback loop responds to changes in body fat. The mediators are the adipocyte hormone leptin and pancreatic insulin. They reach the hypothalamic arcuate nucleus through the circulation. A proportional relationship between body fat mass and basal levels of leptin and insulin in humans, decreased feeding in response to intrahypothalamic administration of the two hormones, and increased feeding in the absence of their hypothalamic receptors in rodents support this concept (9,10,11). In humans, congenital absence of leptin leads to significant hyperphagia and morbid obesity, which can be reversed by leptin administration (12).
The role for leptin and insulin in the normal daily control of feeding behavior is less well established. Least well understood is the possible influence on human appetite of bioenergetic events of intermediate duration that occur between a single meal and cumulative changes in body fat. Increased nutrient load after large meals and decreased energy availability after exercise over time affect body fat level. These changes in energy availability could influence human appetite through the known interactions between insulin or leptin and short-term controls such as CCK (13,14). Ghrelin, leptin, and insulin also respond to between-meal changes in energy availability in humans: during fasting ghrelin concentration rises (15), whereas leptin and insulin decline. Reverse changes occur during feeding (10) or overfeeding (16). Insulin secretion is suppressed by sympathetic activation during exercise. Aerobic exercise energy expenditure has equivocal effects on secretion of ghrelin and leptin because it causes ghrelin to decline (17), remain unchanged (18), or increase (19) and leptin to remain unchanged (20) or gradually decline (21).
Little empirical evidence supports direct influence of between-meal energy changes on human appetite. Intravenous infusions of nutrients providing between 836 (22) and 20,064 KJ/d (23) do not satiate or abolish periodic hunger. Exercise does not affect appetite at low intensities and volumes but suppresses it as both increase (24), at least in the short term. Moreover, energy cost of exercise is not compensated over hours and days after exercise (25).
The present study was designed to address whether changes in appetite and intestinal hormone levels could be affected by the size and energy content of a meal or by short-term energy perturbation induced by exercise or iv administration of nutrients.
Subjects and Methods
Subjects
Ten healthy postmenopausal women provided informed consent approved by the University of Michigan Medical School Institutional Review Board. A health questionnaire was administered, and general laboratory chemistries and thyroid function were obtained. Weight, height, and body fat were measured by dual-energy x-ray absorptiometry (Prodigy; Lunar Radiation Corporation, Madison, WI) and by bioimpedance (RJL Quantum II, Clinton, MI). To determine relative effort in individuals differing in aerobic fitness, maximal effort was assessed with indirect calorimetry (Physio-Dyne, Quoque, NY) during a treadmill test consisting of 2% slope increases every 3 min at 4.5 km/h until the respiratory quotient of 1 was achieved.
Study protocol (Table 1)
Table 1.
Energy content of meals and TPN and energy cost of exercise
| Group | Energy consumed at 0600 h (KJ) | Energy infused from 0600 to 1100 h (KJ) | Energy cost of exercise from 1000 to 1200 h (KJ) | Energy consumed at 1300 h (KJ) |
|---|---|---|---|---|
| SED-AL | 2090 | 0 | 0 | 3337 |
| SED-R | 418 | 0 | 0 | 3433 |
| SED-R-TPN | 418 | 1521 | 0 | 3359 |
| EX | 2090 | 0 | −2361 | 3156 |
| EX-TPN | 2090 | 1538 | −2273 | 3409 |
Five trials were assigned in random order and separated by at least 1 wk: a sedentary trial with large (ad libitum) breakfast and no exercise (SED-AL); small-meal (restricted) sedentary trials with either saline infusion (SED-R) or iv nutrient replacement (SED-R-TPN); and exercise trials with a large breakfast and saline (EX) or TPN infusion (EX-TPN). After admission to the Clinical Research Center at 1800 h, a standardized meal was provided at 1900 h. At 0545 h the following morning, two indwelling Teflon catheters were inserted into contralateral forearm veins, one for infusions and the other for blood withdrawal. A breakfast was provided at 0600 h and an ad libitum meal at 1300 h. Exercise took place between 1000 and 1200 h. The trial ended at 1700 h.
Meals
All meals contained 60% carbohydrates, 25% fat, and 15% protein. Morning meals consisted of a bagel with peanut butter and jelly and provided either 418 KJ (SED-R trial) or 2090 KJ (SED-AL and EX trials). At 1300 h, unlimited quantities of food that consisted of baked chicken breast, cooked rice and corn, salad with ranch salad dressing, potato roll, margarine, banana, vanilla ice cream with chocolate icing, and a beverage containing dextrose, were provided in all trials to allow for compensatory eating. The food provided and any portions that remained uneaten were weighed to determine the energy content.
Intravenous nutrients
TPN solution contained 1672 KJ derived from 60% dextrose (465 mOsm), 25% Liposyn triglycerides (735 mOsm), and 15% Aminosyn amino acids (200 mOsm) emulsified in 750 ml with 0.45% saline. TPN was infused between 0600 and 1100 h.
Exercise
Subjects walked on a level treadmill between 1000 and 1200 h at 46 ± 1.4% of maximum oxygen uptake (VO2max). Exercise duration and intensity were chosen to match the energy withheld from morning meal and provided in TPN. Shorter duration of higher intensity exercise is less easily tolerated.
Psychophysical ratings
Ratings of appetite were assessed hourly during waking hours and at 30-min intervals between 1000 and 1400 h, with the 100-mm visual analog scales for hunger and fullness anchored with zero and extreme valuation at the two ends (26).
Metabolic measurements
Exercise metabolism was measured by indirect calorimetry (Physio-Dyne) during min 0 to 30 and 60 to 90 of exercise.
Blood collection
Blood was collected hourly and at 15- and 30-min intervals at the start of meals and exercise. Blood tubes were EDTA-coated and contained 250 KIU of Aprotinin (Sigma Chemical, St. Louis, MO) and 10 μl dipeptidyl peptidase-4 inhibitor (Millipore Corp., Linco Research, St. Louis, MO) per milliliter of blood. Plasma was kept frozen at −80 C.
Hormone and metabolite measurements
Concentrations of total ghrelin, leptin, and insulin were measured with RIAs (Millipore Corp., Linco Research). Intra- and interassay coefficients of variation were, respectively, 2.2 and 20% for insulin, 10.1 and 11.8% for ghrelin, and 9.1 and 14.2% for leptin. Lincoplex immunoassay kit with the sensitivity of 0.2 pg/ml was used for glucose insulinotropic peptide (GIP), a gut peptide that served as a marker of gastrointestinal food transit (27). Intra- and interassay coefficients of variation were 11 and 19%, respectively. Plasma glucose, nonesterified fatty acid (FFA), and β-hydroxybutyrate concentrations were quantified with enzymatic spectrophotometric methods from, respectively, Thermo DMA (Arlington, TX), WACO Diagnostics (Richmond, VA), and Randox Laboratories (Oceanside, CA).
Statistics
Data are presented as means and se. Hormone, metabolite, and psychophysical data were analyzed by repeated-measures mixed model ANOVA with SAS software 9.1 (SAS Institute, Cary, NC). Three periods of dependent variable change were selected for analysis: morning nutritional manipulation, exercise, and the period after the midday meal. For ghrelin, leptin, FFAs, and β-hydroxybutyrate that responded to changes in energy availability, the times were 0600 to 0930 h, 1000 to 1400 h, and 1430 to 1700 h. Postprandial times were used for insulin and glucose: 0600 to 0930 h, 1000 to 1300 h, and 1315 to 1700 h; and for GIP, 0600 to 1300 h and 1315 to 1700 h. Psychophysical data were analyzed between 0630 and 0930, 1000 and 1200, and 1315 and 1700 h.
Energy availability or balance consisted of energy eaten and infused less energy expended during exercise and was analyzed with ANOVA before and after the midday meal. The meal size and energy availability were correlated with changes in dependent variables. The same time periods as were used in ANOVA served for regression analyses performed on areas under the curve calculated by the trapezoidal rule for hormones and metabolites and on averaged psychophysical data. Bonferroni correction was used for multiple comparisons. α value of 0.05 served as a criterion of significance.
Results
Subjects
Subjects were 59.7 ± 1.5 yr of age, with body mass of 73.7 ± 2.9 kg and body mass index of 25.6 ± 0.8 kg/m2. Body fat was 37.4 ± 1.5% by dual-energy x-ray absorptiometry and 36.3 ± 1.5% by bioimpedance.VO2max was 2.0 l O2/min or 27.7 ± 1.2 ml O2/kg · min.
Exercise
Exercise intensity was 46 ± 1.4% of VO2max. Energy cost of exercise was 2361.3 ± 112.9 KJ in EX trial and 2272.7 ± 117 KJ in the EX -TPN trial.
Intravenous nutrients
Infusion of 1521.5 ± 16.7 KJ in TPN replaced 91% of withheld food calories in the SED-R-TPN trial, whereas 1538.2 ± 21 KJ in TPN replaced 68% of the energy cost of exercise in the EX-TPN trial.
Appetite ratings
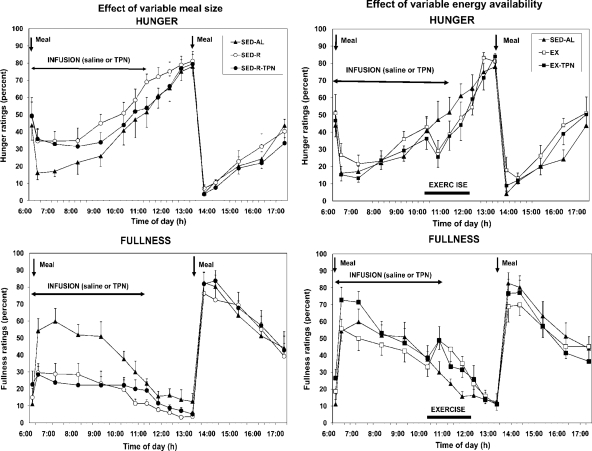
Hunger was significantly greater after small meals than it was after large meals (Fdf 4,36 = 39.3; P < 0.0001; Fig. 1, top left) and was negatively correlated with the morning (r = −0.511; F = 14.81; P < 0.0005) but not with the afternoon meal size (r = 0.008). Fullness was significantly higher after large meals than after small meals (Fdf 4,36 = 115.3; P < 0.0001; Fig. 1, bottom left) and was positively correlated with both the morning (r = 0.608; F = 24.56; P < 0.0001) and afternoon meal size (r = 0.521; F = 15.69; P < 0.0005). TPN had no effect on appetite ratings. Hunger and fullness were not correlated with energy availability either before (r = 0.02 and r = −0.341, respectively) or after (r = −0.194 and r = 0.314, respectively) the midday meal.
Figure 1.
The effects of variable meal size (left) and energy availability (right) on the psychophysical ratings of hunger (top) and fullness (bottom) in 10 postmenopausal women subjected to a sedentary trial with a large morning meal (SED-AL), or a small morning meal (SED-R), 2 h of moderate intensity after a large morning meal (EX), and iv nutrient infusion (TPN) as a replacement of energy withheld from a morning meal (SED-R-TPN) or expended through exercise (EX-TPN). Meal size had a negative effect on hunger (Fdf4,36 = 39.3; P < 0.0001) and a positive effect on fullness (Fdf4,36 = 115.3; P < 0.0001). Exercise energy expenditure had a negative effect on hunger (Fdf4,36 = 25.5; P < 0.0001) and a positive effect on fullness (Fdf4,36 = 42.8; P < 0.0001). TPN had no effect on the psychophysical ratings.
During exercise, hunger was significantly and similarly suppressed in EX and EX-TPN trials compared with the two small-meal trials (Fdf 4,36 = 25.5; P < 0.0001; Fig. 1, top right). Fullness was significantly and similarly higher during the EX and EX-TPN trials than during the small-meal trials (Fdf 4,36 = 42.8; P < 0.0001; Fig. 1, bottom right). TPN had no effect on appetite ratings. Hunger (r = 0.02) and fullness (r = −0.341) were not correlated with energy availability before the midday, indicating an absence of exercise effect.
Compensatory eating during midday meal
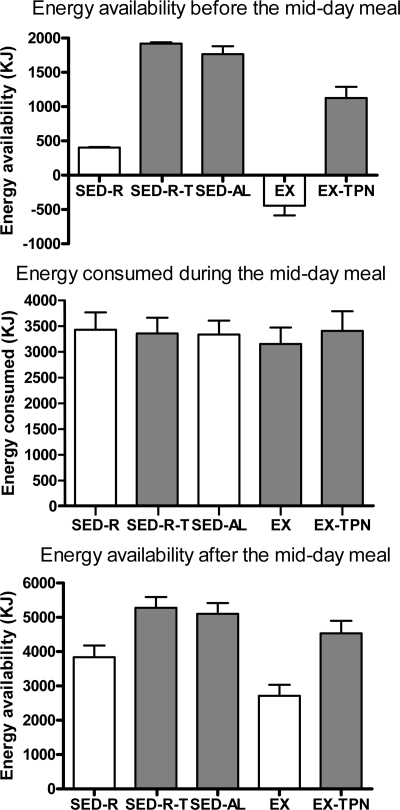
Energy balance before the midday meal was significantly lower after a small meal (SED-R) and exercise (EX) trials than after the three high-energy trials (Fdf 4,45 = 77.13; P < 0.0001; Fig. 2, top). The ad libitum midday food consumption ranged between 3156 ± 321 KJ (EX trial) and 3433 ± 337 KJ (SED-R trial) (Fig. 2, center) and did not compensate for reduced energy availability in SED-R and EX trials. Likewise, the order of trials did not affect caloric consumption, which was 3193 ± 247, 3400 ± 305, 3335 ± 276, 3241 ± 410, and 3273 ± 447 KJ in trials 1 through 5, respectively (P = 0.948). Energy balance after the midday meal remained significantly lower in SED-R and EX trials than in the other three trials (Fdf 4,45 = 10.17; P < 0.0001; Fig. 2, bottom).
Figure 2.
Effects of the morning energy availability (top) on the energy consumed during the midday meal (center) and the residual postmeal energy balance (bottom) in 10 women subjected to small (SED-R) or large (SED) morning meals, exercise (EX), and TPN (SED-R-TPN and EX-TPN). Midday meal did not compensate for the significantly lower energy balance in SED-R and EX trials (Fdf4,45 = 77.13; P < 0.0001), which remained uncorrected after the meal (Fdf4,45 = 10.17; P < 0.0001).
Responses of insulin, GIP, and glucose to variable meal size and energy availability
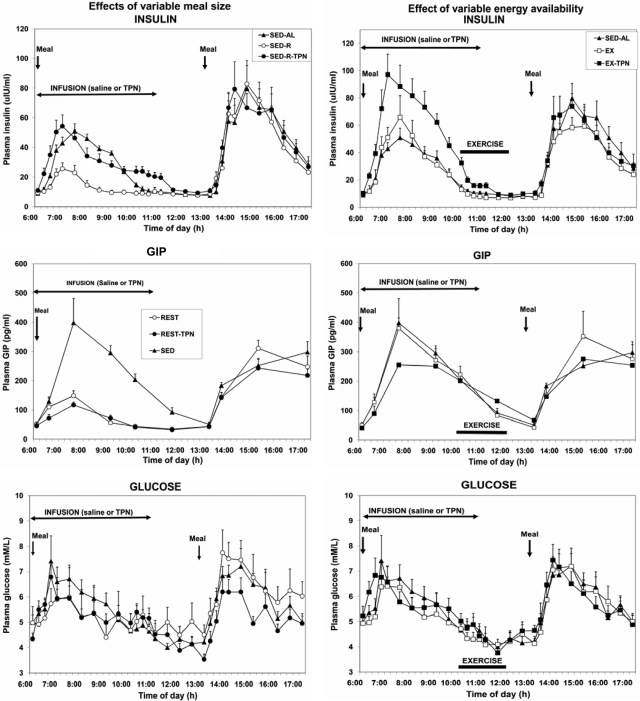
Between 0630 and 0930 h, insulin concentration was proportional to the energy content of the meal and TPN infusate administered before the start of exercise (Fig. 3; Fdf 4,36 = 25.71; P < 0.0001). Insulin concentration was unaffected by exercise. Insulin concentration was significantly higher in the EX-TPN than in the SED-AL trial between 0645 and 0830 h, and in EX-TPN than in EX trial at 0700 h and from 0800 through 0900 h. Insulin was significantly correlated with both morning (r = 0.451; F = 12.29; P < 0.001) and afternoon (r = 0.700; F = 40.35; P < 0.0001) energy content of the meal and with energy availability both before (r = 0.448; F = 12.05; P = 0.0011) and after (r = 0.591; F = 22.53; P < 0.0001) the midday meal.
Figure 3.
The effects of variable meal size (left) and energy availability (right) on the plasma concentrations of insulin (top), GIP (center), and glucose (bottom) under the experimental conditions described in Fig. 1. Insulin and glucose showed significant postprandial increases to meal size (left) and TPN (right) (Fdf4,36 = 25.71; P < 0.0001), whereas GIP responded only to meal size (Fdf4,45 = 42.29; P < 0.0001). Neither hormone responded to exercise energy expenditure. Postprandial increases in glucose concentration were unaffected by meal size or exercise.
GIP concentration predominantly varied with morning meal size (Fdf 4,36 = 42.29; P < 0.0001; Fig. 3, center left) and was unaffected by exercise (Fig. 3, center right). GIP was unaffected by TPN in sedentary trials (Fig. 3, center left) and was only suppressed at 0730 h in the EX-TPN trial (Fdf 9,81 = 15.9; P < 0.0001). GIP was significantly correlated with both morning (r = 0.803; F = 76.31; P < 0.0001) and midday (r = 0.479; F = 14.28; P < 0.001) meal size, but not with either morning (r = 0.074) or afternoon (r = 0.190) energy availability. Postprandial increases in plasma glucose concentration (Fdf 9,81 = 7.25; P < 0.0001) did not differ between trials (Fig. 3, bottom).
Responses of ghrelin and leptin to variable meal size and energy availability
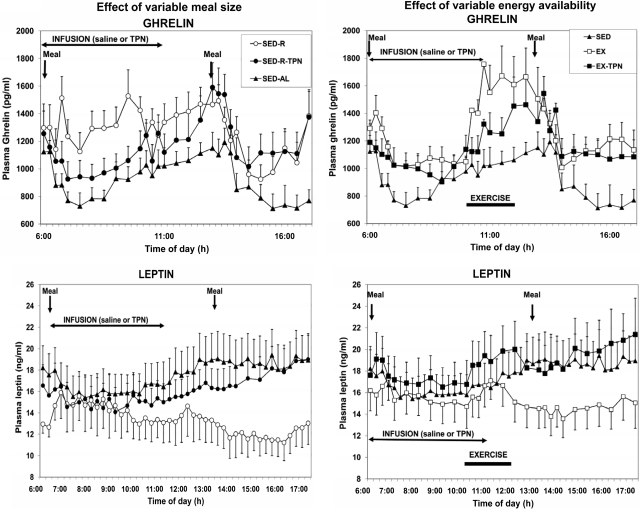
Before exercise, plasma ghrelin concentration was significantly affected by both the experimental treatments (Fdf 4,36 = 13.23; P < 0.0001) and time (Fdf 9,81 = 2.61; P = 0.011). Ghrelin responded more to reduced energy availability in small meals than to exercise energy expenditure. TPN abolished a significant 0645 h increase in plasma ghrelin in the SED-R compared with the SED-AL trial (Fig. 4, top left). Higher plasma ghrelin concentrations in SED-R trial persisted until the midday meal but did not reach statistical significance. Treatment effects also were significant during the exercise period (Fdf 4,36 = 5.28; P < 0.002) as were the changes with time (Fdf 12,108 = 4.35; P < 0.0001), but higher plasma ghrelin concentrations in EX relative to SED-AL and EX-TPN trials did not reach statistical significance, as was also the case after the midday meal (Fig. 4, right). Ghrelin area under the curve was significantly positively correlated with the energy cost of exercise (r = 0.473; F = 5.178; P = 0.035).
Figure 4.
The effects of variable meal size (left) and energy availability (right) on the plasma concentrations of total ghrelin (top) and leptin (bottom) under the experimental conditions described in Fig. 1. Plasma ghrelin concentration was rapidly influenced by energy availability as the increases in the small-meal (Fdf4,36 = 13.23; P < 0.0001) and exercise trials (Fdf4,36 = 5.28; P < 0.002) were abolished by TPN. Plasma leptin concentration slowly and progressively increased in response to reduced energy availability caused by small meal (Fdf4,36 = 48.06; P < 0.0001) and exercise (Fdf4,36 = 39.08; P < 0.0001), and this response was abolished by TPN.
Leptin declined in equal measure, but after a delay of several hours, to reduced energy availability caused by small meals and exercise (Fig. 4, center left and right, respectively). A significant decline in SED-R compared with SED-AL and SED-R-TPN trials started at 1230 h, about 6 h after the intake of a small meal (Fdf 4,36 = 48.06; P < 0.0001), and it progressively increased until 1400 h and remained through the remainder of the study (Fdf 4,36 = 39.1; P < 0.0001; Fig. 4, center). A significant decline in plasma leptin in the EX trial (Fdf 4,36 = 12.12; P < 0.0001) started at 1330 h after a 1.5-h delay (Fig. 4, center right) and progressively increased (Fdf 4,36 = 39.08; P < 0.0001) until 1400 h and remained through the remainder of the study. Declines in leptin were prevented by TPN.
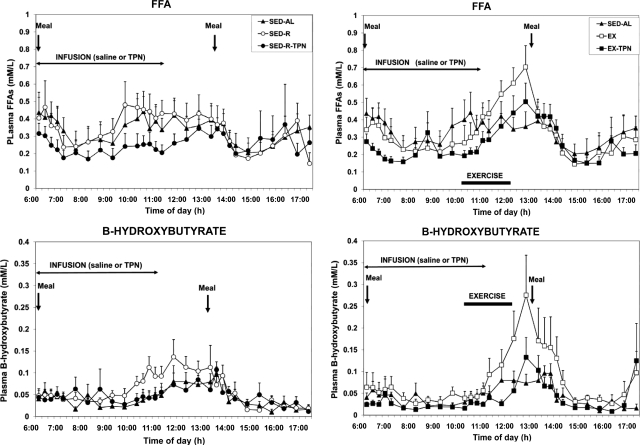
Responses of FFAs and β-hydroxybutyrate to variable meal size and energy availability
Plasma FFAs increased both before (effect of trials Fdf 4,36 = 7.9, P = 0.0001; and Fdf 9,81 = 2.59, P < 0.011, respectively) and during exercise (Fdf 4,36 = 10.1, P < 0.0001; and Fdf 12,108 = 5.12, P < 0.0001), and these increases were prevented by TPN. Likewise, ketone body concentrations increased both before (effect of trials Fdf 4,36 = 3.69; P = 0.013) and during exercise (effect of trials Fdf 4,36 = 19.23; P < 0.0001 effect of time), and these increases also were prevented by TPN (Fig. 5).
Figure 5.
The effects of variable meal size (left) and energy availability (right) on the plasma concentrations of FFAs (top) and β-hydroxybutyrate (bottom) under the experimental conditions described in Fig. 1. FFA and ketone body concentrations increased to exercise energy expenditure (Fdf4,36 = 10.1, P < 0.0001; and Fdf4,36 = 19.23, P < 0.0001, respectively), but not to the different meal size, and these increases were abolished by TPN.
Discussion
This study produced three important findings. The first is that human appetite in sedentary state is primarily influenced by passage of food through the mouth and the gastrointestinal tract because the magnitude of fullness ratings was proportional to the size and energy content of the meal and to changes in the digestive hormone GIP. Our data are consistent with the observations showing that intragastric, intraduodenal, or intrailial nutrient infusions significantly increase or advance the onset of satiation in response to as few as 142 KJ (28) or as many as 4180 KJ of energy (29,30,31,32). These psychophysical changes to intragastric nutrients were associated with increased secretion of gut peptides CCK, GIP (31), and GLP-1 (28,31) and with declines in the rate of gastric emptying (32). Collectively, these data indicate that processing of nutrient energy through the mouth and the gastrointestinal tract is necessary to influence satiation. Furthermore, perception of satiation depends on vagal transmission via brain stem to the higher brain centers of the information produced by food during its gastrointestinal transit because vagal deletion prevents gastrointestinal hormone influence in the modulation of feeding (33,34).
Our studies do not provide an unequivocal answer to what influences hunger. Hunger ratings in sedentary state were disinhibited by small meals, but they also reached identical high values at the same point in time in response to very different levels of energy balance. This would suggest operation of an all-or-none meal initiation signal that responds to the absence of food in the gastrointestinal tract, to brain detection of energy deficit (7,8), and probably also to circadian timing.
Our second important finding is that appetite in the sedentary state is not influenced by between-meal changes in energy availability. Neither hunger nor fullness ratings were affected by iv restoration of energy that was either withheld from meals or expended during exercise. This suggests, and supports the position of others (4), that between-meal increases in circulating nutrient load and exercise energy expenditure are not under homeostatic feedback control. Absence of compensatory increases in food intake in response to negative energy balance caused by a small meal or the energy cost of exercise, also reflect an absence of homeostatic feedback control. Absence of homeostatic metering of available energy is represented even more clearly by decreased hunger ratings and increased ratings of fullness during exercise in the face of rising negative energy balance (25). Although we do not have information on the possible influence of the satiation hormones CCK, GLP-1, and peptide YY, exercise-associated appetite suppression could not be attributed to increased satiation signals from the gut hormone GIP, which was unaffected by exercise. The answer to this puzzling phenomenon requires further study and may reflect sympathetic suppression of afferent vagal signaling regarding nutrient digestion and absorption during exercise.
Our third important finding is that physiological changes in the concentrations of ghrelin, leptin, and insulin respond in slightly different ways to changes in energy availability but have no influence on human appetite. The well-known insulin response to circulating nutrients was present and changed in synchrony with changes in plasma glucose and energy content of the meals and TPN. Insulin’s failure to respond to the energy cost of exercise can be explained by the sympathetic inhibition of its release during exercise. Ghrelin increased rapidly to reduced energy availability in the small meals more than to the energy cost of exercise, and both responses were abolished by TPN infusions. Our data confirm rapid responsiveness of ghrelin to negative energy balance (15) and its tendency to rise in parallel with hunger and to decline after ingestion of a meal (1). Our data, however, do not support the proposition that physiological changes in plasma ghrelin stimulate hunger in humans as was suggested by infusion of supraphysiological concentrations of ghrelin (35). We also confirm delayed leptin response to negative energy balance produced by nutritional manipulations (36) or exercise (21). Leptin response correctly reflected reduced energy availability throughout the study and, unlike ghrelin, was unaffected by the ad libitum midday meal.
The limitations of this study are in imperfect matching of the magnitude of reductions in energy availability through food restriction and exercise and restoration of withheld or expended energy through TPN. Exercise expended between 2274 (EX-TPN) and 2362 (EX) KJ, while the small meals withheld 1672 KJ. Thus exercise-induced reduction in energy availability was between 36 and 41% greater than after dietary restriction. Likewise, TPN replaced 1522 KJ or 91% of the 1672 KJ withheld in small meals, but only 68% (1538 KJ) of the energy expended in exercise. Despite these deficiencies, TPN infusion corrected the effects of reduced energy availability on responses by ghrelin, leptin, insulin, FFAs, and β-hydroxybutyrate.
In summary, we have found a strong association between satiation and energy content of food passing through the mouth and the gastrointestinal tract. Between meals, human appetite is unaffected by increased circulating nutrient load or energy cost of exercise. Such changes in energy availability do not trigger compensatory food intake. During exercise energy expenditure, the absence of homeostatic negative energy feedback is underscored by paradoxical decreases in hunger and increases in fullness. In contrast to the lack of their effect on appetite, changes in energy availability caused by food restriction, exercise, and TPN exert a significant influence over plasma concentrations of ghrelin, leptin, and insulin. Physiological responses of ghrelin, leptin, and insulin to changes in energy availability do not influence appetite or consummatory compensation but undoubtedly have other significant physiological and metabolic roles. Our data therefore do not support the concept of homeostatic between-meal metering of changes in energy availability but indicate instead that human appetite is influenced by signals generated by food passage through the mouth and the gastrointestinal tract.
Acknowledgments
The authors thank the study volunteers and Michigan Clinical Research Unit nursing staff and dieticians for their help in the execution of the study, Dr. D. A. Lawrence for the use of Luminex equipment, Jean Hunt for help with illustrations, and Kathleen Welch for statistical analyses.
Footnotes
This study was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant 1R15-DK066286 (to K.T.B.), National Institutes of Health (NIH) Grant M01-RR00042 (to the University of Michigan Clinical Research Unit), NIH Grant DK20572 (to the Michigan Diabetes Research and Training Center), and by a grant from the Tanita Healthy Weight Community Fund (to K.T.B.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 28, 2009
Abbreviations: CCK, Cholecystokinin; EX, exercise; FFA, nonesterified fatty acid; GIP, glucose insulinotropic peptide; GLP-1, glucagon-like peptide-1; SED, sedentary; TPN, iv nutrient replacement; VO2max, maximum oxygen uptake.
References
- Cummings DE, Overduin J 2007 Gastrointestinal regulation of food intake. J Clin Invest 117:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ 1998 Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J CIin Invest 101:515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR 2002 Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418:650–654 [DOI] [PubMed] [Google Scholar]
- Woods SC 2004 Gastrointestinal satiety signals. An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol 286:G7–G13 [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS 2001 A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50:1714–1719 [DOI] [PubMed] [Google Scholar]
- Berthoud HR 2008 Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 20(Suppl 1):64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alquier T, Kawashima J, Tsuji Y, Kahn BB 2007 Role of hypothalamic adenosine 5′-monophosphate-activated protein kinase in the impaired counterregulatory response induced by repetitive neuroglucopenia. Endocrinology 148:1367–1375 [DOI] [PubMed] [Google Scholar]
- He W, Lam TK, Obici S, Rossetti L 2006 Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci 9:227–233 [DOI] [PubMed] [Google Scholar]
- Flier JS 2004 Obesity wars: molecular progress review confronts an expanding epidemic. Cell 116:337–350 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG 2000 Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW 2006 Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S 1999 Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341:879–884 [DOI] [PubMed] [Google Scholar]
- Matson CA, Wiater MF, Kuijper JL, Weigle DS 1997 Synergy between leptin and cholecystokinin (CCK) to control daily caloric intake. Peptides 18:1275–1278 [DOI] [PubMed] [Google Scholar]
- Riedy CA, Chavez M, Figlewicz DP, Woods SC 1995 Central insulin enhances sensitivity to cholecystokinin. Physiol Behav 58:755–760 [DOI] [PubMed] [Google Scholar]
- Ravussin E, Tschöp M, Morales S, Bouchard C, Heiman ML 2001 Plasma ghrelin concentrations and energy balance: overfeeding and negative energy balance studies in twins. J Clin Endocrinol Metab 86:4547–4551 [DOI] [PubMed] [Google Scholar]
- Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF 1996 Response of leptin to short-term and prolonged overfeeding in humans. J Clin Endocrinol Metab 81:4162–4165 [DOI] [PubMed] [Google Scholar]
- Toshinai K, Kawagoe T, Shimbara T, Tobina T, Nishida Y, Mondal MS, Yamaguchi H, Date Y, Tanaka H, Nakazato M 2007 Acute incremental exercise decreases plasma ghrelin level in healthy men. Horm Metab Res 39:849–851 [DOI] [PubMed] [Google Scholar]
- Kraemer RR, Durand RJ, Acevedo EO, Johnson LG, Kraemer GR, Hebert EP, Castracane VD 2004 Rigorous running increases growth hormone and insulin like growth factor-I without altering ghrelin. Exp Biol Med 229:240–246 [DOI] [PubMed] [Google Scholar]
- Erdmann J, Tahbaz R, Lippl F, Wagenpfeil S, Schusdziarra V 2007 Plasma ghrelin levels during exercise. Effects of intensity and duration. Regul Pept 143:127–135 [DOI] [PubMed] [Google Scholar]
- Weltman A, Pritzlaff CJ, Wideman L, Considine RV, Fryburg DA, Gutgesell ME, Hartman ML, Veldhuis JD 2000 Intensity of acute exercise does not affect serum leptin concentrations in young men. Med Sci Sports Exerc 32:1556–1561 [DOI] [PubMed] [Google Scholar]
- Essig DA, Alderson NL, Ferguson MA, Bartoli WP, Durstine JL 2000 Delayed effects of exercise on the plasma leptin concentration. Metabolism 49:395–399 [DOI] [PubMed] [Google Scholar]
- Murray CD, le Roux CW, Gouveia C, Bassett P, Ghatei MA, Bloom SR, Emmanuel AV, Gabe SM 2006 The effect of different macronutrient infusions on appetite, ghrelin, and peptide YY in parenterally fed patients. Clin Nutr 25:626–633 [DOI] [PubMed] [Google Scholar]
- Jordan HA, Moses 3rd H, MacFayden Jr BV, Dudrick SJ 1974 Hunger and satiety in humans during parenteral hyperalimentation. Psychosom Med 36:144–155 [DOI] [PubMed] [Google Scholar]
- King NA, Burley VJ, Blundell JE 1994 Exercise-induced suppression of appetite: effects on food intake and implications for energy balance. Eur J Clin Nutr 48:715–724 [PubMed] [Google Scholar]
- King NA, Lluch A, Stubbs RJ, Blundell JE 1997 High dose exercise does not increase hunger or energy intake in free living males. Eur J Clin Nutr 51:478–483 [DOI] [PubMed] [Google Scholar]
- Hill AJ, Blundell JE 1982–1983 Nutrients and behaviour: research strategies for the investigation of taste characteristics, food preferences, hunger sensations and eating patterns in man. J Psychiatr Res 17:203–212 [DOI] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ 2007 Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157 [DOI] [PubMed] [Google Scholar]
- Feltrin KL, Little TJ, Meyer JH, Horowitz M, Smout AJ, Wishart J, Pilichiewicz, AN, Rades T, Chapman IM, Feinle-Bisset C 2004 Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol 287:R524–R533 [DOI] [PubMed] [Google Scholar]
- Cecil JE, Castiglione K, French S, Francis J, Read NW 1998 Effects of intragastric infusions of fat and carbohydrate on appetite ratings and food intake from a test meal. Appetite 30:65–77 [DOI] [PubMed] [Google Scholar]
- French SJ, Conlon CA, Mutuma ST, Arnold M, Read NW, Meijer G, Francis J 2000 The effects of intestinal infusion of long-chain fatty acids on food intake in humans. Gastroenterology 119:943–948 [DOI] [PubMed] [Google Scholar]
- Lavin JH, Wittert GA, Andrews J, Yeap B, Wishart JM, Morris HA, Morley JE, Horowitz M, Read NW 1998 Interaction of insulin, glucagon-like peptide 1, gastric inhibitory polypeptide, and appetite in response to intraduodenal carbohydrate. Am J Clin Nutr 68:591–598 [DOI] [PubMed] [Google Scholar]
- Welch I, Saunders K, Read NW 1985 Effect of ileal and intravenous infusions of fat emulsions on feeding and satiety in human volunteers. Gastroenterology 89:1293–1297 [DOI] [PubMed] [Google Scholar]
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR 2005 The inhibitory effects of peripheral administration of peptide YY (3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044:127–131 [DOI] [PubMed] [Google Scholar]
- le Roux CW, Neary NM, Halsey TJ, Small CJ, Martinez-Isla AM, Ghatei MA, Theodorou NA, Bloom SR 2005 Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab 90:4521–4524 [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR 2001 Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86:5992–5995 [DOI] [PubMed] [Google Scholar]
- van Aggel-Leijssen DP, van Baak MA, Tenenbaum R, Campfield LA, Saris WH 1999 Regulation of average 24 h human leptin level; the influence of exercise and physiological changes in energy balance. Int J Obes Relat Metab Disord 23:151–158 [DOI] [PubMed] [Google Scholar]