Abstract
Context and Objective: Of the recently identified type 2 diabetes mellitus (T2D) susceptibility loci, transcription factor 7-like 2 (TCF7L2) confers the greatest relative risk for T2D and significantly predicts conversion to T2D in persons with impaired glucose tolerance. TCF7L2 is, therefore, also a strong candidate gene for polycystic ovary syndrome (PCOS), a common endocrine disorder characterized by androgen excess and menstrual irregularities and associated with insulin resistance and a 7-fold increased risk for T2D.
Research Design and Methods: We tested for association between 58 single nucleotide polymorphisms mapping to TCF7L2 and PCOS in 624 index (PCOS) cases and 553 control women of European ancestry. Furthermore, in the women with PCOS, we tested for association with seven reproductive and metabolic quantitative traits.
Results: Although we did not detect evidence for association between the previously described TCF7L2 T2D locus, the proinsulin:insulin molar ratio, a marker of pancreatic β-cell dysfunction, was strongly associated with this locus (P = 2.1 × 10−4). We also observed evidence for association between PCOS and two single nucleotide polymorphisms, rs11196236 (P = 9.0 × 10−4) and rs11196229 (P = 0.0027) mapping more than 100 kb centromeric to the previously published T2D susceptibility loci.
Conclusions: We have observed evidence of association with two independent TCF7L2 loci in a PCOS cohort: 1) association between the proinsulin:insulin molar ratio and the T2D locus; and 2) association with reproductive PCOS phenotype and a novel locus. This study suggests that variation in different regions of a susceptibility gene contributes to distinct phenotypes.
Two independent TCF7L2 loci are associated with glucose tolerance in women with PCOS and with PCOS, respectively.
Polycystic ovary syndrome (PCOS) is a common endocrine disorder affecting 5–10% of reproductive-age women in Western societies (1) and is characterized by hyperandrogenemia and irregular menses. Furthermore, PCOS is also associated with insulin resistance, pancreatic β-cell dysfunction, and obesity, abnormalities that confer a substantially increased risk for metabolic syndrome and type 2 diabetes mellitus (T2D) (reviewed in Ref. 2). These reproductive and metabolic abnormalities are heritable, and male as well as female first-degree relatives are at risk for metabolic syndrome and T2D (3,4,5,6,7).
Despite its phenotypic overlap with T2D and obesity, PCOS has a number of unique features (e.g. hyperandrogenism, disordered gonadotropin secretion, postbinding defect in insulin signaling). This observation raises the fundamental question: is PCOS a genetically distinct disorder or do the same obesity/T2D susceptibility genes interact with additional genetic or environmental factors to result in the PCOS phenotype? Although PCOS is likely the most common T2D subphenotype (8), this question is also relevant to other T2D subgroups such as gestational diabetes mellitus (reviewed in Ref. 9). Multiple obesity/T2D candidate genes have been investigated in PCOS (reviewed in Ref. 10). However, studies with sufficiently large sample sizes have failed to detect significant association between the potential T2D susceptibility loci calpain 10 and PCOS (11).
To date the locus with strongest and most reproducible evidence for association with T2D is the transcription factor 7-like 2 (TCF7L2) gene, and more specifically the single nucleotide polymorphisms (SNPs), rs7903146 and rs12255372, which map to introns 3 and 4 of TCF7L2, respectively (12). TCF7L2, also known as TCF4, is a transcription factor in the wnt-signaling pathway, and it functions as a nuclear receptor for β-catenin. The wnt- signaling pathway is critical for cell proliferation, motility, and embryogenesis including the development of the pancreas and islets. The risk allele T of rs7903146 has been shown to be a strong predictor of future T2D and is associated with increased pancreatic-cell TCF7L2 expression, decreased insulin secretion, and increased proinsulin:insulin ratio (13,14,15,16). Also depletion of TCF7L2 in cultured human pancreatic islets results in increased pancreatic β-cell apoptosis, decreased β-cell proliferation, and decreased glucose-stimulated insulin secretion (17).
Only one study has examined the role of genetic variation in TCF7L2 in PCOS (18). This study found no evidence for association between rs7903146 or rs12255372, the SNPs that are associated with T2D, and PCOS in two large Caucasian cohorts. Limiting the study to these SNPs is based on the a priori assumption that the same variants that are associated with T2D will also be associated with PCOS. We know that this assumption is not true in genes causing Mendelian disorders. For instance, mutations in the gene for lamin a/c can result in such diverse phenotypes as Hutchison-Gilford progeria, Dunnigan-type familial partial lipodystrophy, or Emery-Dreyfuss muscular dystrophy, depending on the location within the gene of the disease-associated mutation or variant (reviewed in Ref. 19). Different mutations in the cystic fibrosis gene, CFTR, are associated with pancreatic insufficiency, with impaired lung function, or with congenital bilateral absence of the vas deferens (reviewed in Ref. 20). Among complex traits, examples of phenotypic heterogeneity include the apolipoprotein E locus, which is associated with Alzheimer’s, Parkinson’s, and cardiovascular disease among others (reviewed in Ref. 21) and the CDKN2A/B locus on chr9p21.3, which is associated with T2D and myocardial infarction (22,23).
We performed this study to test the hypothesis that variation in the TCF7L2 gene is associated with PCOS or related metabolic traits. In light of the genetic heterogeneity observed at TCF7L2 (12,24), we examined the role of genetic variation in the entire approximately 260-kb genomic region encompassing TCF7L2.
Subjects and Methods
Subjects
This study was approved by the Institutional Review Boards of the Brigham and Women’s Hospital, Northwestern University Feinberg School of Medicine, Pennsylvania State University College of Medicine, and University of Pennsylvania Medical Center. Written informed consent was obtained from all participants. We studied 624 index cases (probands) with PCOS, and 553 control women (81 intensively phenotyped and 472 minimally phenotyped from a DNA repository) of European Caucasian ancestry. Phenotypic characteristics of cases and controls are given in Table 1.
Table 1.
Phenotypic characteristics of study participants
| PCOS (n = 624)
|
Minimally phenotyped controls (n = 472)
|
Intensively phenotyped controls (n = 81)
|
||||
|---|---|---|---|---|---|---|
| n | Median (range) | n | Median (range) | n | Median (range) | |
| Age (yr) | 624 | 28 (14–48)a | 472 | 35 (18–45) | 81 | 30 (18–55) |
| BMI (kg/m2) | 624 | 35.0 (16.5–64.5)b | 463 | 23.2 (16.7–68.1) | 81 | 27.3 (18.0–53.5) |
| Waist circumference (cm) | 408 | 101 (58–170) | ND | ND | 70 | 85 (63–134) |
| T (ng/dl) [(mmol/liter)] | 624 | 73 (29–337)c [2.5 (1.0–11.7)c] | ND | ND | 81 | 85 (63–134) [1.0 (0.2–1.7)] |
| uT (ng/dl) [(mmol/liter)] | 621 | 24 (1.7–109)c [0.8 (0.06–3.8)c] | ND | ND | 81 | 6.0 (1–16.0) [0.2 (0.03–0.6)] |
| DHEAS (ng/ml) [(μmol/liter)] | 616 | 2089 (50–13,336)c [5.7 (0.1–36.2)c] | ND | ND | 79 | 1,341 (102–3,484) [3.6 (0.3–9.5)] |
| SHBG (nmol) | 514 | 56 (12–426)c | ND | ND | 39 | 124 (46–331) |
| Fasting insulin (μU/ml) [(pmol/liter)] | 601 | 22 (3–152) [132 (18–912)] | ND | ND | 74 | 11 (4–29) [66 (24–174)] |
| Proinsulin (pmol) | 560 | 14.9 (2.0–114.4)c | ND | ND | 66 | 9.1 (3.8–20.4) |
| Fasting glucose (mg/dl) [(mmol/liter)] | 607 | 88 (58–189) [4.9 (3.2–10.5)] | ND | ND | 80 | 90 (72–130) [5.0 (4.0–7.2)] |
| 2-h glucose (mg/dl) [(mmol/liter)] | 259 | 128 (68–324)c [7.1 (3.8–18.0)c] | ND | ND | 80 | 108 (54–204) [6.0 (3.0–11.3)] |
| HDL (mg/dl) [(mmol/liter)] | 577 | 40 (15–107)c [1.03 (0.39–2.77)c] | ND | ND | 66 | 50 (27–88) [1.29 (0.70–2.28)] |
| TTG (mg/dl) [(mmol/liter)] | 578 | 137 (35–2,427)c [3.54 (0.91–62.76)c] | ND | ND | 66 | 76 (37–306) [1.97 (0.96–7.91)] |
Conversion factors: uT and T (ng/dl to mmol/liter), multiply by 0.03467; DHEAS (ng/ml to μmol/liter), multiply by 0.002714; insulin (μU/ml to pmol/liter), multiply by 6.0; glucose (mg/dl to mmol/liter), multiply by 0.05551; HDL (mg/dl to mmol/liter), multiply by 0.02586; and TTG (mg/dl to mmol/liter), multiply by 0.01129. ND, Not determined; TTG, total triglycerides.
P < 0.01 vs. intensively phenotyped controls.
P < 0.0001 vs. intensively phenotyped controls.
P < 0.0001 vs. intensively phenotyped controls after adjusting for BMI and age.
PCOS cases
PCOS was defined according to the classic National Institute of Child Health and Human Development (NICHD) criteria and as previously implemented by us (5,6,7,25). In brief, all women with PCOS had hyperandrogenemia and chronic anovulation with the exclusion of specific disorders of the ovaries, adrenal, or pituitary (5,6,7,25) and therefore, fulfill the NICHD, Rotterdam, Androgen Excess Society criteria for the diagnosis of PCOS (26,27,28).
Controls
Intensively phenotyped reproductively normal control women (n = 81) were phenotyped as previously reported (5,6) with normal androgen levels and regular menses of similar age, weight, and ethnicity to the PCOS cases. To increase the number of control subjects, we used minimally phenotyped women (n = 472) selected from NUgene, a large scale GenBank (http://www.nugene.org) that combines a centralized genomic DNA sample collection and storage system with the ability to update participants’ health status with periodic data uploads from electronic medical records. Such population-based controls have been used successfully in multiple studies, including genome-wide association studies, because they are relatively easy and inexpensive to collect and can be used as controls for multiple phenotypes (29). To decrease the likelihood of including women with PCOS, we excluded women with known diabetes based on a questionnaire and preferentially included women with documented pregnancies. Of the 472 subjects, 272 had at least one pregnancy (average, 2.2 pregnancies; range, 1 to 11). Women who had undergone in vitro fertilization were excluded. We, therefore, expect that our control cohort will include fewer women with PCOS than the population prevalence of PCOS (5–10%) (1). Nevertheless, we assumed that the control population would have the population prevalence of PCOS in our power calculations, which had minimal impact on power (see Power analysis).
Study protocols
None of the subjects were receiving medications known to alter reproductive hormone levels or glucose homeostasis for at least 1 month before study. Contraceptive steroids were stopped at least 3 months before study. Anthropometric measurements (blood pressure, waist circumference, weight, and height) were taken as reported (30). A 75-g oral glucose tolerance test (OGTT) was carried out as previously described (7) after a 300-g carbohydrate diet and overnight fast in 259 women with PCOS (7). After a baseline blood sample for fasting reproductive and metabolic hormones, blood samples were obtained for insulin and glucose levels at 0 and 2 h after glucose challenge.
Biochemical assays
Circulating levels of glucose, insulin, proinsulin, total testosterone (T), non-SHBG-bound testosterone (uT), dehydroepiandrosterone sulfate (DHEAS), SHBG, high-density lipoprotein (HDL) cholesterol, and triglyceride were determined as previously reported (5,25).
Genotyping
We genotyped SNPs mapping to 258 kb that encompass TCF7L2 and 20 kb of genomic sequence upstream and downstream of TCF7L2 (Fig. 1). The SNPs were selected using the HAPMAP Tagger function (http://www.hapmap.org/cgi-perl/gbrowse/hapmap_B35/) to tag the entire genomic segment in Caucasians (i.e. the CEPH trios genotyped by HAPMAP) at an r2 of ≥ 0.8. SNPs were genotyped using the Illumina Goldengate array system (Illumina, San Diego, CA) according to the manufacturers’ recommendations.
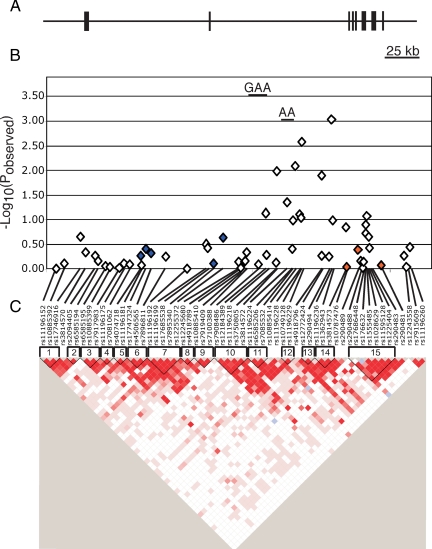
Figure 1.
Schematic of TCF7L2 association study in PCOS. A, TCF7L2 genomic region. The horizontal line indicates the genomic region encompassed in our genetic analysis. The vertical lines indicate the relative positions of the exons of the TCF7L2 gene. B, Association results. The −Log10(pobserved) values are plotted along the y-axis. The relative location of each SNP is indicated along the x-axis. The diamonds correspond to association results of individual SNPs. The short horizontal line corresponds to the haplotype with statistically significant evidence for association with PCOS. Blue diamonds correspond to SNPs that are in LD (D’ > 0.4) with SNPs that are associated with T2D susceptibility SNPs in Caucasians in the Grant et al. study (12), whereas the orange diamonds correspond to variants in LD with SNPs associated with T2D in the Taiwanese cohort (24). C, Pairwise LD (D’) plot. Pairwise D’ results were plotted with Haploview. Dark red indicates strong LD, whereas white indicates no LD. The location and number of each haplotype block are shown above the pairwise LD plot.
Data analyses: secondary phenotypes
Metabolic syndrome
The metabolic syndrome phenotype was assigned according to the American Heart Association criteria (31). Women were considered affected if they fulfilled any three of the following five criteria: systolic blood pressure of at least 130 mm Hg and/or diastolic blood pressure of at least 85 mm Hg; waist circumference at least 88 cm; fasting glucose above 100 mg/dl (>5.6 mmol/liter); HDL above 50 mg/dl (<1.42 mmol/liter); and triglycerides of at least 150 mg/dl (≥1.69 mmol/liter). A total of 457 PCOS cases had complete data to assign metabolic syndrome affected status: 227 women with PCOS had metabolic syndrome, and 230 women with PCOS were unaffected.
Glucose intolerance phenotype in women with PCOS
Glucose intolerance was defined as postchallenge glucose of at least 140 mg/dl (≥7.8 mmol/liter) (n = 99), or a fasting glucose above 100 mg/dl (>5.6 mmol/liter) (n = 53) in women who did not have an OGTT. A total of 149 women with PCOS had glucose intolerance, and 422 had normal glucose tolerance. The cases with normal glucose tolerance are henceforth referred to as normoglycemic, and the women with glucose intolerance are referred to as dysglycemic. We estimated steady-state β-cell function (%B) and the homeostatic index of insulin resistance (HOMA-IR) with the HOMA Calculator v2.2.2 (http://www.dtu.ox.ac.uk/index.php?maindoc=/homa) in 563 women with PCOS. Clinical characteristics of women with PCOS and intensively phenotyped controls were compared using the Student’s t test.
Power analysis
We used the Genetic Power Calculator package to calculate the power to detect an association between rs12255372 and PCOS in our cohort (32). The parameters used for this analysis were: 553 controls, 624 cases, genotypic relative risk for GT of 1.64, genotypic risk for TT of 3.29, and rs12255372 allele T frequencies of 0.386 for cases and 0.260 for controls (see Ref. 12 for U.S. genotypic risk and allele frequencies in Grant et al.) and using unselected controls with a 5% population prevalence of PCOS. Assuming these parameters, we had more than 97% power to detect an effect at P < 1 × 10−5. We also calculated our power for average Caucasian (U.S., Danish, and Icelandic) allele frequencies (cases = 0.362, controls = 0.268) and had more than 75% power to detect an effect at P = 1 × 10−4. We, therefore, had sufficient power to detect a relevant effect in our cohort.
We calculated power for our metabolic syndrome cohort (227 affected, 230 unaffected) using the same parameters as for the PCOS cohort. We had more than 76% power for the U.S. allele frequencies and P = 1 × 10−3. We had more than 60% power given the Caucasian allele frequencies and P = 1 × 10−2. Therefore, we also had reasonable if reduced power for the much smaller metabolic syndrome cohort.
Similarly, power analyses for quantitative traits were carried out for 624 PCOS probands using the program CaTSQT2 (Skol, A., personal communications and Ref. 33) assuming an additive model. We had 61% power to detect a variant that explains 2% of the variance of the quantitative trait analysis and 83% power to detect a variant that explains 3% of the variance of the quantitative trait analysis.
Genetic analysis
We tested for association between 58 SNPs and 60 haplotypes belonging to 15 haplotype blocks with two dichotomous traits: PCOS and metabolic syndrome as defined above. These analyses were implemented using Haploview 4.0 (haploview@broad.mit.edu/haploview/haploview-downloads) (34). We corrected for multiple testing by generating corrected significance levels by carrying out 10,000 permutations by swapping case control labels. Pairwise linkage disequilibrium (LD) plots of D’ were generated using Haploview 4.0.
We also assessed the impact of genetic variation at the SNPs on the distribution of seven quantitative traits in the subjects with PCOS. The traits tested were body mass index (BMI) (n = 624), T (n = 624), DHEAS (n = 616), SHBG (n = 514), proinsulin:insulin ratio (n = 556), HOMA-IR (n = 562), and HOMA%B (n = 562). These analyses were implemented using PLINK 0.99 (http://pngu.mgh.harvard.edu/purcell/plink/) (35). We corrected for multiple testing by generating corrected significance levels by carrying out at least 100,000 permutations by swapping subject IDs. All quantitative trait analyses were carried out only in the PCOS subjects.
We used genomic control (36,37) to control for possible population stratification. The genomic inflation factor λ was calculated from 91 SNPs. These SNPs were selected from 384 SNPs genotyped in the cohort studied in this report and were selected to be maximally informative for differentiating between continental groups based on the Hapmap Phase 1 populations (Caucasians = CEU, Han Chinese from Beijing = CHB, Japanese from Tokyo = JPT, and Africans from the Yoruban tribe of Ibadan, Nigeria = YRI). Allele frequencies are not in LD with each other (r2 < 0.2) and do not map to or were in LD with suspected PCOS susceptibility loci. λ was calculated as median χ2 value of the 91 genomic control SNPs divided by 0.456. Genomic control corrected study statistic Y2 equals χ2/λ.
Definition of TCF7L2 T2D loci
The original TCF7L2 T2D locus (12) mapped to introns 3 and 4 of the gene and is delimited by the SNPs rs4506565, rs7896811, rs11196192, rs11196199, rs17685538, rs7895340, and rs12255372 in our study. We designated this region the “Caucasian” T2D locus. Similarly, we designated the region at the 3′end of the gene identified in a Taiwanese cohort (24) as the “Taiwanese” T2D locus.
Results
Clinical features
Clinical features of the PCOS cases and both control cohort are shown in Table 1. Women with PCOS were significantly younger than the intensively phenotyped controls. As expected, women with PCOS also had significantly higher BMI, larger waist circumference, elevated T, elevated uT, elevated DHEAS, and lower SHBG than the intensively phenotyped controls. Furthermore, women with PCOS had significantly elevated 2-h glucose levels, proinsulin levels, and TTG levels and lower HDL levels. Fasting insulin and fasting glucose levels did not differ significantly between women with PCOS and the intensively phenotyped controls.
Genotyping
All of the genotyped SNPs passed all quality control measures including Hardy Weinberg equilibrium values of at least 0.001, minimum genotyping frequency of at least 75%, and minimum minor allele frequency of at least 0.001 (Table 2 and Fig. 1). Average call rates were above 99% for each SNP and each DNA sample.
Table 2.
Results of dichotomous trait analysis
| Name | Locationa | HW | Call rate | Casesb MAF | Controlsb MAF | PCOS
|
Metabolic syndrome
|
||
|---|---|---|---|---|---|---|---|---|---|
| Y2c | P valued | Y2c | P valued | ||||||
| rs11196152 | 114676795 | 0.466 | 99.3 | 0.509 | 0.499 | 0.001 | 0.976 | 0.191 | 0.662 |
| rs10885392 | 114683123 | 0.687 | 99.4 | 0.265 | 0.270 | 0.083 | 0.773 | 1.374 | 0.241 |
| rs17746916 | 114694771 | 0.363 | 99.4 | 0.075 | 0.061 | 1.486 | 0.223 | 0.000 | 1.000 |
| rs3814570 | 114698500 | 0.540 | 99.2 | 0.263 | 0.288 | 0.532 | 0.466 | 0.610 | 0.435 |
| rs2094405 | 114705679 | 0.997 | 99.4 | 0.287 | 0.267 | 0.387 | 0.534 | 0.312 | 0.576 |
| rs6585194 | 114707461 | 0.903 | 99.4 | 0.287 | 0.271 | 0.165 | 0.685 | 0.077 | 0.781 |
| rs6585195 | 114712944 | 0.166 | 99 | 0.135 | 0.137 | 0.023 | 0.879 | 0.022 | 0.882 |
| rs10885399 | 114716167 | 0.04 | 99.4 | 0.216 | 0.217 | 0.009 | 0.924 | 0.021 | 0.885 |
| rs7917983 | 114722872 | 0.790 | 99.6 | 0.488 | 0.486 | 0.003 | 0.958 | 0.509 | 0.476 |
| rs11196175 | 114726604 | 0.527 | 99.4 | 0.282 | 0.282 | 0.079 | 0.779 | 4.012 | 0.045 |
| rs7081062 | 114730735 | 0.658 | 99.4 | 0.369 | 0.366 | 0.062 | 0.803 | 0.180 | 0.671 |
| rs4074718 | 114738607 | 0.771 | 99.4 | 0.475 | 0.464 | 0.390 | 0.532 | 0.109 | 0.741 |
| rs11196181 | 114739008 | 0.307 | 99.4 | 0.071 | 0.068 | 0.044 | 0.834 | 0.124 | 0.725 |
| rs17747324 | 114742493 | 0.458 | 99.4 | 0.226 | 0.233 | 0.751 | 0.386 | 1.701 | 0.192 |
| rs4506565 | 114746031 | 0.991 | 99.1 | 0.309 | 0.319 | 0.511 | 0.475 | 0.940 | 0.332 |
| rs7896811 | 114756707 | 0.798 | 99.2 | 0.153 | 0.15 | 0.318 | 0.573 | 0.807 | 0.369 |
| rs11196192 | 114772277 | 0.898 | 99.6 | 0.070 | 0.076 | 0.000 | 1.000 | 0.369 | 0.544 |
| rs11196199 | 114786107 | 0.792 | 98.7 | 0.171 | 0.159 | 1.011 | 0.315 | 0.783 | 0.376 |
| rs17685538 | 114787461 | 0.192 | 99.7 | 0.111 | 0.120 | 0.808 | 0.369 | 1.819 | 0.177 |
| rs7895340 | 114791515 | 0.645 | 99.8 | 0.468 | 0.464 | 0.088 | 0.767 | 0.003 | 0.958 |
| rs12255372 | 114798892 | 1 | 99.6 | 0.273 | 0.295 | 1.438 | 0.230 | 0.977 | 0.323 |
| rs12245680 | 114810181 | 0.543 | 99.2 | 0.095 | 0.104 | 0.132 | 0.716 | 0.497 | 0.481 |
| rs4918789 | 114811797 | 0.172 | 99.3 | 0.464 | 0.464 | 0.003 | 0.958 | 1.118 | 0.290 |
| rs10885410 | 114814463 | 1 | 99.3 | 0.262 | 0.268 | 0.130 | 0.718 | 1.693 | 0.193 |
| rs7919409 | 114814966 | 0.503 | 99.2 | 0.111 | 0.120 | 0.153 | 0.696 | 0.646 | 0.422 |
| rs7100388 | 114815803 | 0.698 | 99.4 | 0.107 | 0.094 | 0.554 | 0.457 | 0.037 | 0.847 |
| rs7908486 | 114824488 | 0.444 | 99.1 | 0.354 | 0.365 | 0.066 | 0.797 | 0.786 | 0.375 |
| rs12184389 | 114829760 | 0.404 | 99.4 | 0.172 | 0.139 | 3.188 | 0.074 | 0.087 | 0.768 |
| rs11196218 | 114830484 | 0.261 | 99.5 | 0.278 | 0.261 | 0.402 | 0.526 | 0.436 | 0.509 |
| rs3750805 | 114837133 | 0.186 | 99.5 | 0.117 | 0.120 | 0.118 | 0.731 | 0.007 | 0.933 |
| rs3814572 | 114837713 | 0.256 | 99.4 | 0.133 | 0.176 | 6.515 | 0.0106 | 0.092 | 0.762 |
| rs11196224 | 114845387 | 0.673 | 99.6 | 0.318 | 0.273 | 4.036 | 0.045 | 0.012 | 0.913 |
| rs6585206 | 114849241 | 1 | 99 | 0.185 | 0.171 | 0.729 | 0.393 | 0.208 | 0.648 |
| rs7085532 | 114849453 | 0.053 | 99.8 | 0.321 | 0.288 | 2.611 | 0.106 | 0.533 | 0.465 |
| rs10885414 | 114851294 | 0.055 | 99 | 0.277 | 0.335 | 6.975 | 0.0083 | 0.330 | 0.566 |
| rs11196228 | 114854287 | 0.478 | 99.2 | 0.096 | 0.077 | 3.067 | 0.080 | 0.202 | 0.653 |
| rs10749128 | 114855479 | 0.376 | 93.5 | 0.257 | 0.22 | 2.814 | 0.093 | 0.332 | 0.564 |
| rs11196229 | 114856162 | 0.327 | 99.5 | 0.207 | 0.269 | 9.005 | 0.0027 | 0.001 | 0.976 |
| rs4918796 | 114870332 | 0.231 | 99.4 | 0.25 | 0.206 | 6.155 | 0.013 | 0.013 | 0.909 |
| rs12772424 | 114870541 | 0.146 | 99.3 | 0.401 | 0.387 | 0.377 | 0.539 | 0.300 | 0.584 |
| rs290494 | 114875861 | 0.363 | 99 | 0.159 | 0.171 | 0.339 | 0.560 | 0.128 | 0.721 |
| rs11196236 | 114877712 | 0.175 | 99.4 | 0.246 | 0.189 | 10.973 | 0.0009 | 0.000 | 1.000 |
| rs1362943 | 114878598 | 0.480 | 99.7 | 0.310 | 0.345 | 2.611 | 0.106 | 0.006 | 0.938 |
| rs3814573 | 114888083 | 0.032 | 99.7 | 0.340 | 0.367 | 2.119 | 0.145 | 0.126 | 0.723 |
| rs10787476 | 114888904 | 0.251 | 99.4 | 0.061 | 0.058 | 0.011 | 0.916 | 0.828 | 0.363 |
| rs290489 | 114897045 | 0.987 | 99.1 | 0.235 | 0.243 | 0.689 | 0.407 | 3.279 | 0.070 |
| rs290488 | 114898972 | 0.003 | 82.9 | 0.061 | 0.066 | 0.164 | 0.686 | 0.113 | 0.727 |
| rs17686448 | 114900402 | 1 | 99.5 | 0.048 | 0.040 | 0.006 | 0.938 | 0.627 | 0.428 |
| rs176632 | 114901069 | 0.817 | 99.8 | 0.139 | 0.149 | 0.131 | 0.717 | 0.222 | 0.638 |
| rs1555485 | 114902524 | 0.312 | 99 | 0.190 | 0.211 | 1.732 | 0.188 | 0.470 | 0.493 |
| rs1028629 | 114902646 | 0.679 | 99.4 | 0.160 | 0.186 | 2.336 | 0.126 | 0.931 | 0.335 |
| rs11595128 | 114903468 | 0.715 | 99.4 | 0.142 | 0.165 | 2.967 | 0.085 | 1.740 | 0.187 |
| rs1225404 | 114904655 | 0.102 | 98.9 | 0.337 | 0.364 | 1.485 | 0.223 | 0.209 | 0.648 |
| rs290483 | 114905204 | 0.336 | 99.5 | 0.382 | 0.401 | 0.801 | 0.371 | 0.321 | 0.571 |
| rs290481 | 114913815 | 0.672 | 99.8 | 0.159 | 0.156 | 0.037 | 0.847 | 2.148 | 0.143 |
| rs12243558 | 114930492 | 0.145 | 99.2 | 0.217 | 0.203 | 0.373 | 0.541 | 1.758 | 0.185 |
| rs7915609 | 114932355 | 0.077 | 99.6 | 0.225 | 0.222 | 0.012 | 0.913 | 0.435 | 0.510 |
| rs11196260 | 114934868 | 0.570 | 99.8 | 0.036 | 0.029 | 0.810 | 0.368 | 1.005 | 0.316 |
MAF, Minor allele frequency; HW, Hardy Weinberg Equilibrium.
Location on chromosome based on dbSNP build 125.
Minor allele defined based on control frequency.
Corrected for population stratification using genomic control λ = 1.07.
Observed P value.
Genomic control
From a panel of 384 SNPs, 91 SNPs were not in significant LD (r2 < 0.2) and were not in LD with a PCOS susceptibility locus and maximally differed in allele frequency in the HAPMAP Phase 1 continental populations. Average δ for the 91 SNPs was 0.182 CEU vs. CHB, 0.170 CEU vs. JPT, 0.222 CEU vs. YRI, 0.049 CHB vs. JPT, 0.201 CHB vs. YRI, and 0.201 JPT vs. YRI. The genomic control inflation factor for the subjects studied in this analysis λ = median χ2/0.456 = 1.07. These findings show that there is some population stratification between the cases and controls in our study, so all test statistics in the case control analyses are corrected for population stratification (Y2 = χ2/λ). Although our method of selection of ancestry informative markers primarily differentiates populations based on continental origin, these markers do also differentiate to a lesser degree between European populations. Average δ for the 91 ancestry informative markers between a population of northwestern European origins (HAPMAP CEU) and a population of southern European origin (HAPMAP TSI–Tuscans in Italy) was 0.04. Genomic control correction was not needed for the quantitative trait analyses because those analyses were limited to PCOS cases.
Association testing
Of the markers we genotyped, six variants mapped to the T2D region identified by Grant et al. (12) and the whole genome association studies for T2D (rs4506565, rs7896811, rs11196192, rs11196199, rs17685538, rs7895340, and rs12255372; Fig. 1 and Table 2). None of these markers showed evidence for association with PCOS (624 cases, 553 controls) or in the women with PCOS with metabolic syndrome (227 cases, 230 controls; Table 2) after correction for multiple testing.
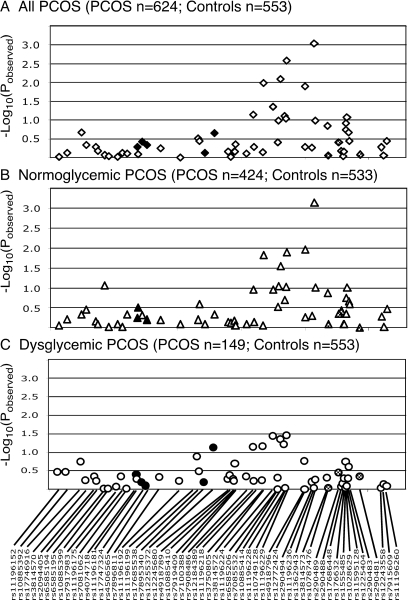
The preliminary evidence for association between TCF7L2 and PCOS was found approximately 100 kb downstream of the Caucasian T2D locus. We will refer to this locus as the TCF7L2 PCOS locus to differentiate it from the T2D locus. This region includes six SNPs and six haplotypes with nominally significant evidence for association with PCOS (Table 1 and Fig. 1). After correction for multiple testing and population stratification, one SNP (rs11196236, allele G, pobserved = 9.0 × 10−4; pcorrected = 0.047) remains statistically significant, and one locus approaches statistical significance (rs11196229, allele G, pobserved = 0.0027; pcorrected = 0.14). Similarly, two haplotype (block 11, haplotype GAA, pobserved = 2.9 × 10−4, pcorrected = 0.016; block 12, haplotype AA, pobserved = 0.0009, pcorrected = 0.049) reached statistical significance.
The strongest evidence for association with the metabolic syndrome was with rs11196175, although this finding did not remain statistically significant after correction for multiple testing (pobserved = 0.045; pcorrected = 0.08). However, sample sizes for the metabolic syndrome phenotype (227 affected, 230 unaffected) are significantly smaller that those for the PCOS phenotype and, consequently, have substantially lower power. Larger sample sizes are needed to establish the role of rs11196175 in the metabolic syndrome in women with PCOS.
Our second major finding is that the proinsulin:insulin molar ratio (a marker of pancreatic β-cell dysfunction) showed statistically significant evidence for association in the PCOS subjects (rs4506565; pobserved = 2.1 × 10−4; pcorrected = 0.010) even after correction for multiple testing. Interestingly, this association maps to the Caucasian TCF7L2 T2D locus (see Fig. 3A). Other metabolic measures, including BMI (rs4506565; pobserved = 0.0036; pcorrected = 0.14), SHBG (rs7081062; pobserved = 0.014; pcorrected = 0.45; rs7917983; pobserved = 0.046; pcorrected = 0.83), HOMA-IR (rs17747324; pobserved = 0.021; pcorrected = 0.58; rs11196175; pobserved = 0.036; pcorrected = 0.77), HOMA%B (rs4074718; pobserved = 0.0038; pcorrected = 0.15), fasting insulin levels (rs4506565; pobserved = 0.023; pcorrected = 0.60; rs11196175; pobserved = 0.035; pcorrected = 0.75; rs7081062; pobserved = 0.047; pcorrected = 0.84), and waist circumference (rs17747324; pobserved = 0.023; pcorrected = 0.61), also showed nominal evidence for association with SNPs in this region, although these findings did not remain significant after correction for multiple testing. Neither T nor DHEAS levels showed even nominal evidence for association in this region (data not shown). However, these androgen levels did show nominal evidence for association with the PCOS specific locus (T, rs37500805; pobserved = 0.021; pcorrected = 0.57; DHEAS, rs6585206, pobserved = 0.011; pcorrected = 0.38). These findings did not remain significant after correction for multiple testing. All the quantitative trait analyses were carried only in the PCOS subjects.
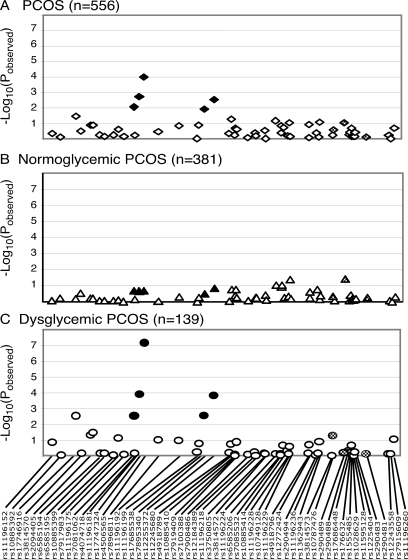
Figure 3.
Quantitative trait analysis of proinsulin:insulin ratio in the complete PCOS cohort (A), normoglycemic women with PCOS (B), and glucose-intolerant women with PCOS (C). Solid black shading corresponds to SNPs that are in LD (D’ > 0.4) with SNPs that are associated with T2D susceptibility SNPs in Caucasians in the Grant et al. study (12), whereas the stippled shading corresponds to variants in LD with SNPs associated with T2D in the Taiwanese cohort (24). The −Log10(pobserved) value for each association test is shown along the y-axis, and the location of each SNP is indicated along the x-axis.
Association testing of PCOS cohort stratified based on glucose tolerance
To determine whether the proinsulin:insulin molar ratio-associated locus is restricted to a subset of women with PCOS, we tested for association in normoglycemic and dysglycemic PCOS. Virtually all the evidence for association with PCOS phenotype was found in the normoglycemic PCOS (Fig. 2B), and with only nominal evidence for association with PCOS in women with PCOS and glucose intolerance (Fig. 2C), implying that the TCF7L2 locus has a different role in women with PCOS and normal glucose tolerance than in women with PCOS who have evidence for impaired glucose tolerance. Henceforth, we will refer to this locus as the PCOS locus. Conversely, after stratification by glucose tolerance, there was no evidence for association between the proinsulin:insulin ratio and any of the SNPs tested in normoglycemic PCOS (Fig. 3B). In contrast, there is a very robust association with rs4506565 (pobserved = 9.8 × 10−5; pcorrected = 1.7 × 10−4) in PCOS with glucose intolerance, even in a relatively small cohort (n = 139; Fig. 3C).
Figure 2.
Association results of PCOS trait in the complete PCOS cohort (A), normoglycemic women with PCOS (B), and glucose-intolerant women with PCOS (C). Black symbols correspond to SNPs that are in LD (D’ > 0.4) with SNPs that are associated with T2D susceptibility SNPs in Caucasians in the Grant et al. study (12), whereas stippled symbols correspond to variants in LD with SNPs associated with T2D in the Taiwanese cohort (24). The −Log10(pobserved) value for each association test is shown along the y-axis, and the location of each SNP is indicated along the x-axis.
Discussion
In a detailed screen for association between PCOS and PCOS-associated quantitative traits and 58 SNPs mapping to the TCF7L2 gene, we identified two independent TCF7L2 loci with potential evidence for association. The first locus mapped approximately 100 kb downstream of the previously identified Caucasian T2D locus and was associated with PCOS. The second locus was associated with proinsulin:insulin ratio in women with PCOS and mapped to the previously identified Caucasian T2D susceptibility locus.
TCF7L2 confers the greatest risk for T2D (odds ratio = 1.37) of any susceptibility locus identified to date (38). Because of the overlap in the T2D and PCOS phenotypes, TCF7L2 is a very plausible candidate gene for PCOS. Barber et al. (18) found no evidence for association between the Caucasian T2D locus, mapping to rs7903146 and rs12255372, and PCOS in two cohorts: 1) 369 PCOS cases and 2577 controls of U.K. British/Irish origin; and 2) 540 women with symptoms of PCOS and 1083 controls from the Northern Finland Birth Cohort of 1966. In contrast to our study, Barber et al. (18) did not investigate the association between measures of glucose homeostasis and this TCF7L2 Caucasian in their PCOS cohort.
We studied the entire genomic region of TCF7L2 because we were investigating a distinct phenotype, PCOS, rather than typical T2D. Furthermore, studies have already demonstrated genetic heterogeneity within TCF7L2 in T2D cohorts of different ethnicities with the identification of a novel T2D locus in a Taiwanese cohort that maps to the 3′ end of the TCF7L2 gene (12,24). We also found no evidence for association between the rs7903146/rs12255372 region and PCOS in our cohort. However, we did observe preliminary evidence for association with PCOS in a region approximately 100 kb downstream of the Caucasian T2D locus. This PCOS susceptibility region was not in significant LD with the rs7903146/rs12255372 region and, therefore, was not due to an indirect effect of the Caucasian T2D locus. Although these findings remain significant after correction for population stratification and multiple testing, they will need to be replicated in an independent cohort to be firmly established as a new PCOS locus.
The putative “PCOS” locus was predominantly associated with the complete PCOS phenotype rather than PCOS subphenotypes because the evidence for association with PCOS (hyperandrogenemia and menstrual irregularities) was stronger than with androgen levels per se (total T or DHEAS). Our assignation of the PCOS phenotype required that a PCOS subject have both hyperandrogenemia and menstrual irregularities. Furthermore, a PCOS subject is considered hyperandrogenic by having either elevated T or elevated uT. Association with any given androgen therefore is not identical to association with PCOS. Furthermore, the quantitative trait analyses used to assess the association with androgen levels was carried out only in the PCOS patients, therefore by definition limiting the analysis to the subjects with elevated androgen levels. The evidence for association might be significantly stronger if the entire spectrum of androgen levels were included in the analysis. Although these findings reach statistical significance after correction for multiple testing even in a relatively small cohort, the association between PCOS and the TCF7L2 PCOS locus will need to be replicated in an independent cohort.
All evidence for association at the “T2D” locus with the proinsulin:insulin ratio was completely accounted for by the subset of PCOS women with glucose intolerance. Stratification of the PCOS cohort by glucose tolerance and examination of quantitative metabolic phenotypes suggested that, whereas PCOS per se was not associated with the Caucasian T2D locus, among a subset of women with PCOS (i.e. those with glucose intolerance), the Caucasian T2D locus was strongly associated with the proinsulin:insulin molar ratio, a marker of pancreatic β-cell dysfunction (39,40). Under normal circumstances, the majority of proinsulin is processed into the mature insulin peptide, and only a small percentage is secreted into the circulation (39). However, in subjects with T2D and individuals at high risk of T2D, there appears to be decreased processing resulting in increased circulating proinsulin:insulin molar ratios (41). Our findings of an association between the proinsulin:insulin ratio in PCOS women with abnormal glucose tolerance and the TCF7L2 Caucasian T2D locus are consistent with evidence from other studies that demonstrate that variation in the TCF7L2 gene is associated with defects in insulin secretion (13,14,42), perhaps by altering conversion of proinsulin to insulin by the pancreatic β-cell (42). Lyssenko et al. (13) found that the T2D-associated alleles at rs7903146 and rs12255372 are associated with defect in arginine-stimulated insulin secretion in women with abnormal glucose tolerance but not in women with normal glucose tolerance (13). Furthermore, Kirchhoff et al. (42) found that in a cohort of Germans from Tubingen at high risk of developing diabetes based on a family history of T2D, the TCF7L2 rs7903146 risk allele was positively associated with the proinsulin:insulin ratio. Similarly, González-Sánchez et al. (43) found evidence for association between rs7903146 and proinsulin:insulin ratio after 2-h OGTT in a nondiabetic Spanish cohort, although they did not find any evidence for association with the fasting proinsulin:insulin ratio. Although it is probable that our cohort of PCOS women with normal glycemia also included a small number of women with undiagnosed glucose intolerance because post-challenge testing was not performed in all PCOS, this misclassification would modestly reduce our power to detect an association, but it would not generate a false-positive finding.
Interestingly, the T2D-associated locus and the PCOS-specific locus are inherited independently (i.e. the two loci are not in LD) and are associated with different biochemical features of PCOS. Further detailed genetic studies of the TCF7L2 genomic region in multiple related disorders (T2D, gestational diabetes, and PCOS) and multiple ethnicities are required to fully dissect the functional regions of the gene and their impact on specific phenotypes.
Acknowledgments
We thank all the women and their families for participating in this study and Dr. Deborah Driscoll for sharing with us DNA from her PCOS cohort.
Footnotes
This work was supported by National Institutes of Health Grants P50 HD44405 (to M.U. and A.D.), U54 HD34449 (to A.D.), M01 RR00048 [to Northwestern University General Clinical Research Center (GCRC)], M01 RR10732 and C06 RR016499 (to Pennsylvania State University GCRC), and M01 RR02635 (to Brigham and Women’s Hospital GCRC).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 7, 2009
Abbreviations: %B, β-Cell function; BMI, body mass index; DHEAS, dehydroepiandrosterone sulfate; HDL, high-density lipoprotein; HOMA-IR, homeostatic index of insulin resistance; LD, linkage disequilibrium; OGTT, oral glucose tolerance test; PCOS, polycystic ovary syndrome; SNP, single nucleotide polymorphism; T, total testosterone; TCF7L2, transcription factor 7-like 2; T2D, type 2 diabetes mellitus; uT, non-SHBG-bound testosterone.
References
- Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R 2001 Prevalence of polycystic ovary syndrome (PCOS) in first degree relatives of patients with PCOS. Fertil Steril 75:53–58 [DOI] [PubMed] [Google Scholar]
- Cooper HE, Spellacy WN, Prem KA, Cohen WD 1968 Hereditary factors in Stein-Leventhal syndrome. Am J Obstet Gynecol 100:371–387 [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Yarali H, Oguz H, Bayraktar M 2003 Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab 88:2031–2036 [DOI] [PubMed] [Google Scholar]
- Yilmaz M, Bukan N, Ersoy R, Karakoça, Yetkin I, Ayvaz G, Cakir N, Arslan M 2005 Glucose intolerance, insulin resistance and cardiovascular risk factors in first degree relatives of women with polycystic ovary syndrome. Hum Reprod 20:2414–2420 [DOI] [PubMed] [Google Scholar]
- Sam S, Legro RS, Bentley-Lewis R, Dunaif A 2005 Dyslipidemia and metabolic syndrome in the sisters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 90:4797–4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam S, Legro RS, Essah PA, Apridonidze T, Dunaif A 2006 Evidence for metabolic and reproductive phenotypes in mothers of women with polycystic ovary syndrome. Proc Nat Acad Sci USA 103:7030–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam S, Sung YA, Legro RS, Dunaif A 2008 Evidence for pancreatic β-cell dysfunction in brothers of women with polycystic ovary syndrome. Metabolism 57:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dodson WC, Dunaif A 1999 Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84:165–169 [DOI] [PubMed] [Google Scholar]
- Robitaille J, Grant AM 2008 The genetics of gestational diabetes mellitus: evidence for relationship with type 2 diabetes mellitus. Genet Med 10:240–250 [DOI] [PubMed] [Google Scholar]
- Urbanek M 2007 The genetics of the polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab 3:103–111 [DOI] [PubMed] [Google Scholar]
- Haddad L, Evans JC, Gharani N, Robertson C, Rush K, Wiltshire S, Frayling TM, Wilkin TJ, Demaine A, Millward A, Hattersley AT, Conway G, Cox NJ, Bell GI, Franks S, McCarthy MI 2002 Variation within the type 2 diabetes susceptibility gene calpain-10 and polycystic ovary syndrome. J Clin Endocrinol Metab 87:2606–2610 [DOI] [PubMed] [Google Scholar]
- Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K 2006 Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 38:320–323 [DOI] [PubMed] [Google Scholar]
- Lyssenko V, Lupi R, Marchetti P, Del Guerra S, Orho-Melander M, Almgren P, Sjögren M, Ling C, Eriksson KF, Lethagen AL, Mancarella R, Berglund G, Tuomi T, Nilsson P, Del Prato S, Groop L 2007 Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 117:2155–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez JC, Jablonski KA, Bayley N, Pollin TI, de Bakker PI, Shuldiner AR, Knowler WC, Nathan DM, Altshuler D 2006 TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med 355:241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Gianniny L, Burtt NP, Lyssenko V, Giuducci C, Sjögren M, Florez JC, Almgren P, Isomaa B, Orho-Melander M, Lindblad U, Daly MJ, Tuomi T, Hirschhorn JN, Ardlie KG, Groop LC, Altshuler D 2006 Common single nucleotide polymorphisms in TCF7L2 are reproducibly associated with type 2 diabetes and reduce the insulin response to glucose in nondiabetic individuals. Diabetes 55:2890–2895 [DOI] [PubMed] [Google Scholar]
- Loos RJ, Franks PW, Francis RW, Barroso I, Gribble FM, Savage DB, Ong KK, O’Rahilly S, Wareham NJ 2007 TCF7L2 polymorphisms modulate proinsulin levels and β-cell function in a British Europid population. Diabetes 56:1943–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu L, Sauter NS, Schulthess FT, Matveyenko AV, Oberholzer J, Maedler K 2008 Transcription factor 7-like 2 regulates β-cell survival and function in human pancreatic islets. Diabetes 57:645–653 [DOI] [PubMed] [Google Scholar]
- Barber TM, Bennett AJ, Groves CJ, Sovio U, Ruokonen A, Martikainen H, Pouta A, Hartikainen AL, Elliott P, Wass JA, Järvelin MR, Zeggini E, Franks S, McCarthy MI 2007 Disparate genetic influences on polycystic ovary syndrome (PCOS) and type 2 diabetes revealed by a lack of association between common variants within the TCF7L2 gene and PCOS. Diabetologia 50:2318–2322 [DOI] [PubMed] [Google Scholar]
- Worman HJ, Bonne G 2007 “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res 313:2121–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowntree RK, Harris A 2003 The phenotypic consequences of CFTR mutations. Ann Hum Genet 67:471–485 [DOI] [PubMed] [Google Scholar]
- Jofre-Monseny L, Minihane AM, Rimbach G 2008 Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutri Food Res 52:131–145 [DOI] [PubMed] [Google Scholar]
- Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, Jonasdottir A, Sigurdsson A, Baker A, Palsson A, Masson G, Gudbjartsson DF, Magnusson KP, Andersen K, Levey AI, Backman VM, Matthiasdottir S, Jonsdottir T, Palsson S, Einarsdottir H, Gunnarsdottir S, Gylfason A, Vaccarino V, Hooper WC, Reilly MP, Granger CB, Austin H, Rader DJ, Shah SH, Quyyumi AA, Gulcher JR, Thorgeirsson G, Thorsteinsdottir U, Kong A, Stefansson K 2007 A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science 316:1491–1493 [DOI] [PubMed] [Google Scholar]
- Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, et al 2007 Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316:1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Chang TJ, Jiang YD, Kuo SS, Lee KC, Chiu KC, Chuang LM 2007 Association study of the genetic polymorphisms of the transcription factor 7-like 2 (TCF7L2) gene and type 2 diabetes in the Chinese population. Diabetes 56:2631–2637 [DOI] [PubMed] [Google Scholar]
- Legro RS, Driscoll D, Strauss 3rd JF, Fox J, Dunaif A 1998 Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA 95:14956–14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadski JK, Dunaif A 1992 Diagnostic criteria for polycystic ovary syndrome. In: Givens J, Haseltine F, Merriman G, eds. The polycystic ovary syndrome. Cambridge, MA: Blackwell Scientific; 377–384 [Google Scholar]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2004 Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19:41–47 [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF 2006 Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 91:4237–4245 [DOI] [PubMed] [Google Scholar]
- McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JP, Hirschhorn JN 2008 Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet 9:356–369 [DOI] [PubMed] [Google Scholar]
- Legro RS, Bentley-Lewis R, Driscoll D, Wang SC, Dunaif A 2002 Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab 87:2128–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith Jr SC, Spertus JA, Costa F 2005 Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752 [DOI] [PubMed] [Google Scholar]
- Purcell S, Cherny SS, Sham PC 2003 Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 19:149–150 [DOI] [PubMed] [Google Scholar]
- Skol AD, Scott LJ, Abecasis GR, Boehnke M 2007 Optimal designs for two-stage genome-wide association studies. Genet Epidemiol 31:776–788 [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ 2005 Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265 [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC 2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin B, Roeder K 1999 Genomic control for association studies. Biometrics 55:997–1004 [DOI] [PubMed] [Google Scholar]
- Bacanu SA, Devlin B, Roeder K 2000 The power of genomic control. Am J Hum Genet 66:1933–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, et al 2008 Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 40:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PW, Abbasi F, Carantoni M, Chen YD, Azhar S, Reaven GM 1997 Insulin resistance does not change the ratio of proinsulin to insulin in normal volunteers. J Clin Endocrinol Metab 82:3221–3224 [DOI] [PubMed] [Google Scholar]
- Tura A, Pacini G, Kautzky-Willer A, Ludvik B, Prager R, Thomaseth K 2003 Basal and dynamic proinsulin-insulin relationship to assess β-cell function during OGTT in metabolic disorders. Am J Physiol Endocrinol Metab 285:E155–E162 [DOI] [PubMed] [Google Scholar]
- Røder ME, Porte Jr D, Schwartz RS, Kahn SE 1998 Disproportionately elevated proinsulin levels reflect the degree of impaired B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 83:604–608 [DOI] [PubMed] [Google Scholar]
- Kirchhoff K, Machicao F, Haupt A, Schäfer SA, Tschritter O, Staiger H, Stefan N, Häring HU, Fritsche A 2008 Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia 51:597–601 [DOI] [PubMed] [Google Scholar]
- González-Sánchez J, Martínez-Larrad M, Zabena C, Pérez-Barba M, Serrano-Ríos M 2008 Association of variants of the TCF7L2 gene with increases in the risk of type 2 diabetes and the proinsulin:insulin ratio in the Spanish population. Diabetologia 51:1993–1997 [DOI] [PubMed] [Google Scholar]