Abstract
Context: Osteoporosis primarily affects postmenopausal women. However, young women with estrogen deficiency also are at increased risk for low bone density.
Objective: The aim of the study was to assess bone density and associated risk factors for reduced bone density in young, estrogen-deficient women using primary ovarian insufficiency (POI) as the disease model.
Design and Setting: We conducted a cross-sectional study at a tertiary care research center.
Participants: We studied women with POI (n = 442), concurrent controls (n = 70), and matched controls from NHANES III (n = 353).
Primary Outcome Measure: We measured bone mineral density (BMD) using dual-energy x-ray absorptiometry.
Results: Patients on average had 2–3% lower BMD at L1–L4, femoral neck, and total hip (P < 0.01 at all sites). The modifiable risk factors for BMD below the expected range for age (Z-score <−2) were: more than 1-yr delay in diagnosis of estrogen deficiency (P = 0.018), low (<32 ng/ml) vitamin D levels (P = 0.002), estrogen replacement nonadherence (P = 0.002), low calcium intake (P = 0.005), and lack of exercise (P = 0.005). As compared to Caucasians, African-American and Asian women with POI were 3.18 and 4.34 times more likely, respectively, to have Z-scores below −2 (P = < 0.0001 for both). Race was an overall risk factor, but on regression modeling, not an independent predictor of low bone density.
Conclusions: Women with POI have lower bone density compared to regularly menstruating women. Compared to Caucasians, minority women with estrogen deficiency are more likely to have BMD below the expected range for age. This racial disparity appears to be related to a combined effect of several modifiable risk factors. Delay in diagnosis of POI also contributes to reduced bone density by delaying proper therapy.
Factors significantly associated with lower bone density in estrogen-deficient young women include delay in diagnosis, menstrual irregularity before age 20, vitamin D insufficiency, lack of exercise, and non-compliance with hormone replacement therapy.
Osteoporosis is a major public health problem, affecting millions of people worldwide. Estrogens have important anticatabolic and anabolic effects on bone, and estrogen deficiency plays a central role in the development of osteoporosis (1). Hypogonadism is a well-recognized risk factor for impaired bone density. Although osteoporosis primarily affects postmenopausal and elderly women, younger women with hypogonadism are at increased risk and may have even greater health implications due to their longer remaining life span.
The World Health Organization (WHO) defined osteoporosis as “a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fractures.” T-scores were originally designed to predict fracture risk in postmenopausal women (2). Age is an important predictor of fracture risk. At the same bone mineral density (BMD), an older woman is more likely to have a fracture compared with a younger woman of reproductive age, most likely because of lower bone quality (3). Therefore, T-scores do not predict the same fracture risk in younger women of reproductive age as they do in older, postmenopausal women. A position statement by the International Society for Clinical Densitometry recommends that Z-scores, not T-scores, should be preferred in women before menopause and males under age 50 (4). In these individuals, a Z-score of −2.0 or lower is defined as “below the expected range for age,” and a Z-score above −2.0 is “within the expected range for age.” We thus assess risk factors related to BMD below the expected range for age in these young women (Z ≤−2).
In young women, irregular menstruation and amenorrhea are presenting symptoms of estrogen deficiency related to hypogonadism. Amenorrhea is a common condition, affecting as many as 3–5% of young women (5). Most studies assessing the effects of hypogonadism on BMD in young women are confounded by factors such as nutritional deficiency, X chromosomal haploinsufficiency, associated malignancy, chemotherapy, or excessive exercise (6,7,8,9). Here we investigate spontaneous 46,XX primary ovarian insufficiency (POI) as a model condition of hypogonadism to study effects of estrogen deficiency on bone health in young women who do not have these confounding variables (10).
Spontaneous 46,XX POI (11) (also known as “premature ovarian failure” and “premature menopause”) affects 1 in 250 women by age 35 and 1 in 100 women by age 40 (12). POI is a state of hypergonadotropic hypogonadism, characterized by oligo-amenorrhea, infertility, estrogen deficiency, and its associated symptoms such as hot flashes, vaginal dryness, dysparunia, and insomnia. The objective of this study was to assess the degree of bone deficit and its associated risk factors in young, estrogen-deficient women using spontaneous POI as the disease model. A priori study hypotheses were that estrogen-deficient young women have lower BMD compared with normal controls and delay in diagnosis of POI contributes to reduced bone density in these young women.
Subjects and Methods
Study population
In this cross-sectional study, from January 2000 to December 2005, we recruited consecutively 442 women with spontaneous POI and 70 control women who had normal ovarian function. Patients were recruited by letters to physicians, notices in medical journals, and through the internet. Concurrent controls were recruited by local advertisement and through the internet. We also compared this consecutive series of women with POI to a matched control group from the National Health and Nutrition Examination Survey (NHANES III), a national probability sample (13). The Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (Bethesda, MD), approved the study.
Women with POI
Inclusion criteria were: 1) diagnosis of POI (i.e. at least 4 months of oligomenorrhea or amenorrhea and two FSH levels in the menopausal range, as defined by the local laboratory, confirmed on two separate occasions at least 1 month apart before age 40 yr); 2) current age between 18 and 42 yr; and 3) no iatrogenic cause of POI or known chromosomal abnormality.
Concurrent controls
Concurrent control women were healthy, had a body mass index (BMI) of 19 to 30 kg/m2, were between ages 18 and 40 yr, were not pregnant, and were regularly menstruating (cycles between 21 and 35 d). They reportedly did not smoke or abuse alcohol (fewer than two drinks a day). They were taking no chronic medications and were not using hormonal contraception. Participants were compensated according to National Institutes of Health (NIH) guidelines.
NHANES controls
The NHANES is sponsored by the National Center for Health Statistics and is designed to assess the health and nutritional status of adults in the United States. The survey is unique in that it combines interviews, physical examinations, and bone density measurements. NHANES III was conducted from October 1988 through October 1994 in two phases, each of which comprised a national probability sample of the total civilian, noninstitutionalized population (13).
The NHANES control group was selected from 10,649 healthy women as a probability-weighted control group to 353 women with POI matched for age (within 1 yr), race, and BMI (within 1 kg/m2). Exclusion criteria were FSH above 10 IU/liter, current pregnancy, smoking more than two cigarettes per day, or any serious medical condition.
Study procedures
The evaluation included a complete medical history and physical examination, laboratory screening to detect any accompanying endocrine failures, as well as other clinically occult autoimmune disorders (14). These tests included thyroid function (TSH, free T4, total T3), adrenal (cortisol, androstenedione, dehydroepiandrosterone sulfate, renin, aldosterone), ovarian (free and total testosterone, FSH, LH, estradiol, progesterone, SHBG), pituitary (prolactin, TSH, FSH, LH, ACTH, IGF), parathyroid (PTH, 25-hydroxyvitamin D [25(OH)D], calcium and ionized calcium, 24-h urine collection for calcium and phosphorous), and tests for autoimmunity (antinuclear antibodies, antithyroid antibodies, antiadrenal antibodies, rheumatoid factor, antiparietal cell antibodies, and antiendomysial antibodies). Women with POI were required to be withdrawn from any estrogen/progestin hormone therapy for at least 2 wk before study. For control women, blood samples were drawn during the midfollicular phase of their menstrual cycle (d 5–12). Admission staff recorded self-designated race/ethnicity using the following categories: White, African-American, Hispanic, Asian/Pacific Islander, and other race/ethnicity. In addition, women with POI completed structured questionnaires to assess daily calcium intake (15), depression (16), and other lifestyle risk factors for osteoporosis such as exercise, use of contraceptives, hormone replacement therapy (HRT), smoking, alcohol intake, and reproductive history.
BMD
NIH measurements
We measured areal BMD (g/cm2) with a Hologic QDR4500 instrument (Hologic, Inc., Bedford, MA) dual-energy x-ray absorptiometer at the posterior anterior lumbar spine (vertebrae L1–L4), femoral neck, and total hip. We calculated T-score from mean peak BMD and sd and Z-scores from age-specific mean BMD and sd, both derived from healthy women (normative data from Hologic database for lumbar spine and NHANES III for femoral neck and total hip). T- and Z-scores were calculated using ethnic-specific databases. Daily scans of a lumbar spine tissue equivalent phantom obtained over a 6-month period gave a less than 0.04% coefficient of variation (CV) for the posterior anterior L1–L4 measurement.
NHANES III measurements
Bone area, bone mineral content, and BMD were measured in the proximal femur by dual-energy x-ray absorptiometry with Hologic QDR1000 instrument at the femur neck, trochanter, intertrochanter, Ward’s triangle, and total femur (13). BMD measurements for spine were not available for the NHANES control group. A rigorous quality-control program, including use of anthropomorphic phantoms and review of each quality control and respondent scan at a central site, was used throughout the study to ensure data quality.
Concurrent controls were a good control group because BMD assessment was performed using the same densitometer as the POI women and they were recruited in the same time frame as women with POI. So temporal effects of confounding factors such as awareness of vitamin D and calcium deficiency and supplementation are not affected by temporal change in medical practice. The only problem was that they were not matched and, hence, differed for key variables such as age, weight, and race. In contrast, the NHANES group was a good control group in that it was matched for key variables, but bone density assessment was performed with a different densitometer. However, both densitometers, the Hologic QDR 1000 and 4500, were calibrated to the same “gold standard” at the factory, and measurements in spine phantoms give results with less than 0.5% difference (17).
Hormonal assessments
Blood samples were drawn from fasted subjects at 0800 h; serum was separated within 1 h and frozen. FSH and LH were measured by microparticle enzyme immunoassay (AxSYM System; Abbott Diagnostics, Abbott Park, IL). FSH intraassay CV was 4.9%, and the interassay CV was 6.5%; for LH, these were 5.8 and 6.4%, respectively. Estradiol was measured by competitive chemiluminescence immunoassay (Immulite 2000 analyzer; Diagnostic Products Corporation, Los Angeles, CA); intraassay and interassay CVs were less than 11.0%.
We measured serum total testosterone by RIA after extraction chromatography (Esoterix Endocrinology, Calabasas Hills, CA) and serum free testosterone concentrations using equilibrium dialysis (18). The minimum reportable free fraction is 0.1%. We measured 25(OH)D (Clinical Center Labs at NIH, Bethesda, MD) (SI, nmol/liter = 2.496 × ng/ml) (19) and PTH (Nichols Institute, San Juan Capistrano, CA) by chemiluminescent immunoassay (SI, ng/liter = 1.0 × pg/ml). Interassay CV and intraassay CV were 5.5 and 3.6% for PTH and 5.8 and 6.6% for 25(OH)D, respectively.
Statistical analysis
We used Statistical Analysis Software (SAS Institute, Cary, NC) to analyze the data. We compared continuous clinical characteristics and hormones using the Wilcoxon rank-sum test. To compare race, we used Fisher’s exact test for independence provided in StatXact 4. We tested correlation using Spearman rank correlation coefficient. All P values are two-tailed. Results are presented as mean ± sd. We used multiple regression analysis to adjust for simultaneous influential variables. Prevalence proportional ratio (PPR) was used as a measure of association with Z-score less than −2 because it is directly interpretable and applicable regardless of disease prevalence. PPR is also conservative and consistent compared with the odds ratio, especially when the prevalence of the disease is high (20). In this manuscript, BMD is presented at individual sites (lumbar spine, femoral neck, and total hip), and a subject is considered to have BMD below the expected range for age if her Z-score is less than −2 at any site.
Results
Women with POI have reduced BMD
Table 1 shows demographic and reproductive characteristics of women with spontaneous POI (n = 442) and concurrent controls (n = 70). Compared with concurrent controls, the study group was slightly older, was more often Caucasian, and had a higher BMI. BMD remained lower in women with POI compared with concurrent controls even after adjusting for race, age, and BMI in a regression model. As expected, women with POI were less likely to have been pregnant or have children and had higher gonadotropin levels and lower sex hormone levels. There was no difference in the age at menarche, height, or education level between cases and concurrent controls.
Table 1.
Characteristics of women with POI (n = 442) and concurrent controls (n = 70)
| POI | Control | P value | |
|---|---|---|---|
| Demographics | |||
| Age (yr)a | 32.3 (5.5) | 30.1 (7.2) | <0.01 |
| Race, n (%) | 0.02 | ||
| White | 348 (79) | 50 (71) | |
| African-American | 48 (11) | 11 (16) | |
| Asian | 18 (4) | 8 (11) | |
| Hispanic | 28 (6) | 1 (1) | |
| Height (cm)a | 164.9 (7.2) | 164.4 (6.0) | 0.67 |
| Weight (kg)a | 66.4 (13.3) | 62.3 (10.1) | 0.04 |
| BMI (kg/m2)a | 24.4 (4.6) | 22.9 (2.8) | 0.04 |
| Reproductive characteristics | |||
| Age at menarche (yr)a | 12.8 (1.5) | 12.7 (1.3) | 0.72 |
| Nulligravida, n (%) | 290 (66) | 35 (50) | 0.02 |
| Nullipara, n (%) | 358 (81) | 40 (57) | <0.0001 |
| FSH (IU/liter)a | 75.3 (39.9) | 6.3 (2.0) | <0.0001 |
| LH (IU/liter)a | 47.6 (24.6) | 7.9 (5.8) | <0.0001 |
| Estradiol (pg/ml)a | 46.9 (53.5) | 102.7 (97.2) | <0.0001 |
| Free testosterone (pg/ml)a | 2.5 (1.3) | 3.5 (1.5) | <0.0001 |
Data are reported as mean (sd).
Women with POI had a mean (sd) age of onset of first menstrual irregularity of 25.0 (8.1) yr, an age of diagnosis of 28.9 (6.4) yr, and duration of POI after diagnosis of 2.9 (4.0) yr. Approximately one third (32.3%; 143 of 442) of women developed irregular menstruation before age 20. There was a mean delay of 4.4 (5.4) yr in the diagnosis of POI after onset of menstrual irregularity. From the onset of menstrual irregularity until they were seen in our institute, women had an average time of not taking any HRT of 2.8 (3.3) yr. This was due to a variety of reasons, such as nonadherence, delay in the diagnosis, and confusion regarding use of hormone replacement. Some women were taking oral contraceptive pills (OCP) during the diagnostic delay.
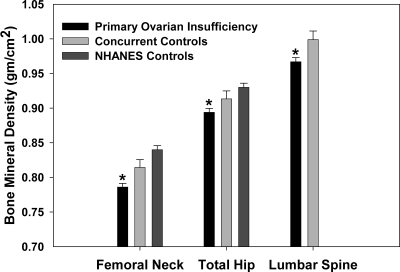
Women with POI had significantly lower BMD at all sites compared with concurrent controls and NHANES controls (Fig. 1). BMD of concurrent controls was not statistically significantly different compared with NHANES controls. These results did not change when we analyzed data excluding 29 women who had past history of receiving any medication that might affect bone metabolism (12 women on testosterone, nine on bisphosphonates, five on prednisone, one on raloxifene, and two on dehydroepiandrosterone sulfate). Of the women with POI, 61 (15%) had Z-scores below the expected range for age as defined by a Z-score less than −2 compared with only two (3%) concurrent controls (P = 0.005). There were 33 women (8%) with POI who had bone density in the osteoporotic range (T-score <−2.5), compared with none of the concurrent controls (P < 0.009). Alcohol intake did not differ between women with POI and controls. Women with POI had significantly higher mean (sd) calcium intake of 1667 (757) g/d compared with concurrent controls intake of 1286 (452) g/d (P < 0.001). This was primarily due to more women with POI taking supplements (70%) compared with controls (7.8%).
Figure 1.
BMD (g/cm2) of women with POI (n = 353), concurrent controls (n = 70), and NHANES controls (n = 353). *, P < 0.001 compared with either control group indicates statistical significance. The concurrent and NHANES control groups did not differ significantly (P = 0.07). Multiple regression analysis was used to adjust for the effect on bone density of age, BMI, and race. Error bars represent sem.
Risk factors affecting BMD in women with POI
Risk factors related to onset of POI
As shown in Table 2, women with POI who had onset of menstrual irregularity before age 20 were 2.72 times more likely to have Z-score below −2 compared with women who had onset of menstrual irregularity at age 20 or later (P < 0.0001). Also, women who had a delay in diagnosis of more than 1 yr from the time of onset of menstrual irregularity were 1.96 times more likely to have this degree of reduced bone density than women diagnosed sooner (P = 0.018). In addition, the amount of time not on HRT was a significant risk factor for reduced bone density (P = 0.002). When these three factors were analyzed with multiple regression, each remained independently significant with P < 0.05.
Table 2.
Risk factors for Z-score <−2 at any site as assessed by PPR in 442 women with POI
| Risk factors | PPR | 95% CI | P |
|---|---|---|---|
| Factors related to POI | |||
| Onset of menstrual irregularity before age 20 yr | 2.72 | 1.74, 4.33 | <0.0001 |
| Delay in diagnosis >1 yr | 1.96 | 1.14, 3.35 | 0.018 |
| Modifiable risk factors (proportion of women with risk factor) | |||
| Serum 25(OH)D <32 ng/ml (80 nmol/liter) (58%) | 2.89 | 1.47, 5.69 | 0.002 |
| No regular exercise (23%) | 1.93 | 1.22, 3.06 | 0.005 |
| Weight <55 kg (18%) | 2.8 | 1.47, 5.36 | 0.002 |
| Daily calcium intake <1000 mg (49%) | 2.8 | 1.37, 5.75 | 0.005 |
| Smoking >2 cigarettes/day (10%) | 0.91 | 0.25, 3.39 | 0.84 |
PPR of 2.72 in the first row means that if a woman with POI had onset of menstrual irregularity before age 20, her chance of having Z-score below −2 is 2.72 times higher than a woman with POI with onset of menstrual irregularity after age 20.
Modifiable risk factors
Of 442 women with POI, 58% had vitamin D levels below 32 ng/ml, 49% had calcium intake less than 1000 mg/d, 28% were noncompliant with estrogen replacement therapy, 23% were not exercising regularly, and 10% were currently smoking. As shown in Table 2, factors that were significantly associated with a Z-score below −2 were low vitamin D levels (<32 ng/ml), low body weight (<55 kg), lack of regular exercise, not taking hormone replacement, and inadequate calcium intake (<1000 mg/d) (all P values < 0.005).
We investigated the relationship between type of prior hormone therapy (which varied greatly) and bone density. We classified the type of replacement therapy as: 1) OCP (n = 68); 2) conventional HRT (n = 184); 3) combined (either first took HRT followed by OCP or vice versa over the years; n = 61); 4) other, such as progesterone only (n = 19); or 5) no replacement (n = 92). The BMD at femoral neck (P = 0.17), lumbar spine (P = 0.36), and total hip (P = 0.14) and Z-score below −2 at any site (P = 0.89) were not different for these groups or when compared between only OCP and HRT groups. The use of OCP or HRT did not differ by race (P = 0.43).
Racial differences
As shown in Table 3, compared with Caucasians, African-American and Asian women with POI were 3.18 and 4.34 times, respectively, more likely to have Z-scores less than −2 (P < 0.0001 for both). The mean delay of diagnosis from time of onset of menstrual irregularity, compared with Caucasians, did not differ significantly by racial or ethnic group, and ranged from 4.3 to 5.1 yr. As shown in Table 4, Asians were less likely to be compliant with HRT compared with Caucasians (44% vs. 75%; P = 0.01), had lower vitamin D levels (17.1 vs.33.6 ng/ml; P < 0.001), and had lower calcium intake (1258 vs. 1947 mg/d; P = 0.016). Vitamin D level and calcium intake were also significantly lower in African-Americans and Hispanic women compared with Caucasians. Race became nonsignificant when multiple regression analysis was performed including race, HRT compliance, delay in diagnosis, duration of nonadherence to HRT, calcium intake, 25(OH)D, exercise, and history of depression.
Table 3.
Race/ethnicity and risk for Z-score <−2 at any site as assessed by PPR in women with POI (n = 442)
| Race/ethnicity | PPR | 95% CI | P |
|---|---|---|---|
| Caucasian | — | — | — |
| African-American | 3.18 | 1.86, 5.43 | <0.0001 |
| Asian | 4.34 | 2.37, 7.96 | <0.0001 |
| Hispanic | 1.49 | 0.70, 3.17 | 0.2989 |
Table 4.
Serum 25(OH)D levels, calcium intake, and compliance to HRT segregated by race/ethnicity and compared to Caucasian
| Race/ethnicity | Serum 25(OH)D (ng/ml)
|
Calcium intake (mg/d)
|
HRT compliance
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| n (429) | Mean (sem) | P | n (250) | Mean (sem) | P | n (423) | Compliant(%) | P | |
| Caucasian | 337 | 33.6 (0.8) | — | 205 | 1947 (49) | — | 336 | 75 | — |
| African-American | 48 | 19.3 (2.3) | <0.001 | 25 | 1716 (144) | <0.001 | 44 | 66 | 0.20 |
| Asian | 18 | 17.1 (3.7) | <0.001 | 7 | 1258 (142) | 0.016 | 18 | 44 | 0.01 |
| Hispanic | 26 | 24.8 (3) | 0.01 | 13 | 1803 (241) | <0.001 | 25 | 64 | 0.24 |
For 25(OH)D, nmol/liter = 2.496 × ng/ml.
Effect of race and serum 25(OH)D level in estrogen-deficient women
African-American women with 25(OH)D levels less than 32 ng/ml (80 nmol/liter) were 6.7 times more likely to have Z-score less than −2 compared with African-American women with 25(OH)D level greater than 32 ng/ml (P < 0.001). Asian women with 25(OH)D levels less than 32 ng/ml were 9.07 times more likely to have Z-scores below −2 compared with Asian women with 25(OH)D levels above 32 ng/ml (P < 0.001). In estrogen-deficient Caucasian women, there was only a trend toward interaction between 25(OH)D deficiency and Z-score below −2 (PPR = 1.94; P = 0.09) (Table 5).
Table 5.
Within-race risk for BMD Z-score <−2 based on 25(OH)D levels in women with POI (n = 442)
| Race/ethnicity | PPR | 95% CI | P |
|---|---|---|---|
| Caucasian | 1.94 | 0.88, 4.28 | 0.099 |
| African-American | 6.74 | 3.17, 14.3 | <0.0001 |
| Asian | 9.07 | 4.1, 20.04 | <0.0001 |
| Hispanic | 3.88 | 1.35, 11.2 | 0.012 |
Data represent serum 25(OH)D deficiency (<32 ng/ml) and risk for Z-score <−2 by race/ethnicity. For 25(OH)D, nmol/liter = 2.496 × ng/ml.
Other factors related to BMD
In this cross-sectional study, risk factors related to ovarian insufficiency such as serum FSH (PPR = 1.005; P = 0.097), LH (PPR = 1.004; P = 0.386), estradiol (PPR = 0.993; P = 0.057) and free testosterone (PPR = 1.036; P = 0.68) levels as well as ovarian volume (PPR = 0.946; P = 0.08) were not significantly related to Z-score below −2 in women with POI. Women with lower estradiol level and lower ovarian volume showed a trend toward a risk for lower bone density. Levels of serum PTH (PPR = 1.0; P = 0.97) and alkaline phosphatase (PPR = 1.002; P = 0.87), depression (PPR = 1.18; P = 0.078), and history of thyroid disease (PPR = 1.00; P = 0.99) were not associated with Z-score below −2.
Discussion
Women with POI who have a normal karyotype provide a unique opportunity to study the effects of estrogen deficiency on bone health in young women without interplay of complicating factors such as X-chromosome haploinsufficiency, eating disorders, extreme exercise, or chemotherapy.
Compared with controls, women with POI had significantly lower BMD at all sites (by approximately 2–3%). They were more likely to have a BMD below the expected range for age (Z-score ≤−2.0) and a T-score below −2.5 compared with concurrent and NHANES control women. Furthermore, they were more likely to have lower bone density if they had more than 1 yr delay in diagnosis, age of onset of menstrual irregularity before age 20 yr, low vitamin D levels (<32 ng/ml), low body weight (<55 kg), lack of regular exercise, low calcium intake, and noncompliance for HRT. Furthermore, our findings suggest that minority women are more likely to have Z-scores below −2 compared with Caucasian women. Race was an overall risk factor but, on regression modeling, not an independent predictor of low bone density. This stresses the importance of correcting these risk factors in minority women.
Women who had more than 1 yr in delay of diagnosis of POI after the onset of menstrual irregularities were more likely to have lower BMD compared with women who were diagnosed within 1 yr of onset of the symptoms. In a prior study of women with POI, 25% reported that more than 5 yr elapsed from the onset of a disordered menstrual pattern until the diagnosis of POI was established. Attributing the loss of menstrual regularity to overall stress or a recent stressful event is tempting; however, this approach can delay the detection of significant pathology that can have long-term health consequences (21). To our knowledge, this is the first study to report an association between delay in diagnosis of an estrogen-deficient state and reduced bone density. It should be noted that, as with eating disorders, the osteopenia may not be completely reversible (22). Studies are needed to determine whether HRT will restore BMD of young women with POI to normal.
In our study, African-American race was associated with lower Z-scores in women with POI. Delay in diagnosis and HRT compliance were not different in African-Americans compared with Caucasians. African-Americans were more likely to be 25(OH)D deficient and have low calcium intake compared with Caucasians. Asians were more likely to be noncompliant with HRT, to be 25(OH)D deficient, and to have low calcium intake and more likely to have Z-scores below −2. When race was analyzed together with modifiable risk factors in multiple regression, it became nonsignificant. This suggests that associations of race with low Z-scores are a consequence of the lower vitamin D levels, low calcium intake, and lower compliance with HRT in women of minority races. Overall, in the United States, minority race provides an important global indicator of risk factors for bone health. Modifiable factors need to be addressed in all women, especially in minority women. The role of calcium and vitamin D in maintaining bone health has been well studied in postmenopausal women, but there is limited evidence in young women (23). Very few vitamin D intervention studies with changes in BMD or fracture outcomes have been reported in minority women (24,25).
Here we show that women who develop an estrogen-deficient state before age 20 have a higher risk of having reduced bone density. Late menarche and menstrual irregularity are important risk factors for the development of osteoporosis and fractures later in life (26). An accurate menstrual history is important in making a diagnosis. Clinicians, parents, and adolescents should view the menstrual cycle as a vital sign. This will minimize the risks of delay in the diagnosis of estrogen deficiency, maximize a young woman’s chance of achieving a healthy peak bone mass, and reduce the long-term risk of osteoporosis and its associated morbidity (27).
We show that time not on hormone replacement is significantly related to lower bone density in young women with estrogen insufficiency. This is really important in view of the relatively high noncompliance rate and the fear about estrogen use related to the Women’s Health Initiative (WHI) study (28). A secondary analysis of WHI randomized controlled trials of hormone therapy showed that there were no significant increases in risk due to hormone therapy for any outcome including coronary heart disease, stroke, and global index events at ages 50 to 59 yr, and there was a reduction in total mortality in this age group [hazard ratio, 0.70; 95% confidence interval (CI), 0.51–0.96] (29). In this specific patient population of younger, estrogen-deficient women, benefits of HRT likely outweigh risks, although there are no randomized trials that address this issue over an extended period of time.
There are limitations to our study. The concurrent control group was slightly different from the study group with regard to age, race, and BMI. These factors might have an influence on bone density. Therefore, we adjusted for these factors using multiple regression analysis. Differences in bone density remained significant after adjustment. We also compared the study group with an age-, race-, and BMI-matched control group from the NHANES III study, which confirmed our findings. The BMD in the concurrent control group was not different from the NHANES III control group. Other limitations include the cross-sectional design and assessment of risk factors that were self-reported and based on recall. However, we used structured, validated questionnaires to collect data when possible, such as for calcium intake and screening for depression. We did not have information on vitamin D and PTH levels in controls. The strength of this study is the large number of young estrogen-deficient women with a well-characterized condition who lack confounding factors such as nutritional deficiency, chemotherapy, etc. Long-term prospective studies on fracture rates are needed to establish a true causal link between estrogen deficiency and fracture risk in this population.
Conclusions
Women with POI have reduced bone density compared with regularly menstruating women. Compared with Caucasian women, minority women with estrogen deficiency are more likely to have Z-scores below −2. This difference appears to be related to a combined effect of vitamin D deficiency, low calcium intake, and noncompliance to HRT. Delay in diagnosis of POI contributes to reduced bone density by delaying proper therapy. Therefore, our findings demonstrate the need to investigate loss of menstrual cycle regularity in a timely manner.
Acknowledgments
We thank Ms. Brenda Hanning for her editorial help, Ms. June Ventura for extensive help with organizing the database, and Ms. Mary Ryan for help with literature review.
Footnotes
Address requests for reprints to: Lawrence M. Nelson, M.D., Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, CRC, Room 1-3330, 10 Center Drive, MSC-103, Bethesda, Maryland 20892. E-mail: Lawrence_Nelson@nih.gov.
This work was supported by Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. V.H.V., N.G.S., and L.M.N. are Commissioned Officers in the U.S. Public Health Service.
L.M.N. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: The authors have nothing to declare.
First Published Online April 28, 2009
Abbreviations: BMD, Bone mineral density; CI, confidence interval; CV, coefficient of variation; HRT, hormone replacement therapy; OCP, oral contraceptive pills; 25(OH)D, 25-hydroxyvitamin D; POI, primary ovarian insufficiency; PPR, prevalence proportional ratio.
References
- Raisz LG 2005 Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115:3318–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1994 Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 843:1–129 [PubMed] [Google Scholar]
- Kanis JA 2002 Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936 [DOI] [PubMed] [Google Scholar]
- Leslie WD, Adler RA, El-Hajj Fuleihan G, Hodsman AB, Kendler DL, McClung M, Miller PD, Watts NB 2006 Application of the 1994 WHO classification to populations other than postmenopausal Caucasian women: the 2005 ISCD Official Positions. J Clin Densitom 9:22–30 [DOI] [PubMed] [Google Scholar]
- Bachmann GA, Kemmann E 1982 Prevalence of oligomenorrhea and amenorrhea in a college population. Am J Obstet Gynecol 144:98–102 [DOI] [PubMed] [Google Scholar]
- Misra M, Klibanski A 2006 Anorexia nervosa and osteoporosis. Rev Endocr Metab Disord 7:91–99 [DOI] [PubMed] [Google Scholar]
- Mincey BA 2003 Osteoporosis in women with breast cancer. Curr Oncol Rep 5:53–57 [DOI] [PubMed] [Google Scholar]
- Keay N, Fogelman I, Blake G 1997 Bone mineral density in professional female dancers. Br J Sports Med 31:143–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalov VK, Axelrod L, Baron J, Hanton L, Nelson LM, Reynolds JC, Hill S, Troendle J, Bondy CA 2003 Selective reduction in cortical bone mineral density in Turner syndrome independent of ovarian hormone deficiency. J Clin Endocrinol Metab 88:5717–5722 [DOI] [PubMed] [Google Scholar]
- Nelson LM 2009 Clinical practice. Primary ovarian insufficiency. N Engl J Med 360:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright F, Smith PH, Fraser R 1942 A syndrome characterized by primary ovarian insufficiency and decreased stature. Am J Med Sci 204:625–648 [Google Scholar]
- Coulam CB, Adamson SC, Annegers JF 1986 Incidence of premature ovarian failure. Obstet Gynecol 67:604–606 [PubMed] [Google Scholar]
- Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston Jr CC, Lindsay R 1998 Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8:468–489 [DOI] [PubMed] [Google Scholar]
- Kim TJ, Anasti JN, Flack MR, Kimzey LM, Defensor RA, Nelson LM 1997 Routine endocrine screening for patients with karyotypically normal spontaneous premature ovarian failure. Obstet Gynecol 89:777–779 [DOI] [PubMed] [Google Scholar]
- Sebring NG, Denkinger BI, Menzie CM, Yanoff LB, Parikh SJ, Yanovski JA 2007 Validation of three food frequency questionnaires to assess dietary calcium intake in adults. J Am Diet Assoc 107:752–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Kroenke K, Hornyak R, McMurray J 2000 Validity and utility of the PRIME-MD patient health questionnaire in assessment of 3000 obstetric-gynecologic patients: the PRIME-MD Patient Health Questionnaire Obstetrics-Gynecology Study. Am J Obstet Gynecol 183:759–769 [DOI] [PubMed] [Google Scholar]
- Bouyoucef SE, Cullum ID, Ell PJ 1996 Cross-calibration of a fan-beam x-ray densitometer with a pencil-beam system. Br J Radiol 69:522–531 [DOI] [PubMed] [Google Scholar]
- Nelson JC, Tomei RT 1988 Direct determination of free thyroxin in undiluted serum by equilibrium dialysis/radioimmunoassay. Clin Chem 34:1737–1744 [PubMed] [Google Scholar]
- Lensmeyer GL, Wiebe DA, Binkley N, Drezner MK 2006 HPLC method for 25-hydroxyvitamin D measurement: comparison with contemporary assays. Clin Chem 52:1120–1126 [DOI] [PubMed] [Google Scholar]
- Thompson ML, Myers JE, Kriebel D 1998 Prevalence odds ratio or prevalence ratio in the analysis of cross sectional data: what is to be done? Occup Environ Med 55:272–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzubaidi NH, Chapin HL, Vanderhoof VH, Calis KA, Nelson LM 2002 Meeting the needs of young women with secondary amenorrhea and spontaneous premature ovarian failure. Obstet Gynecol 99:720–725 [DOI] [PubMed] [Google Scholar]
- Gulekli B, Davies MC, Jacobs HS 1994 Effect of treatment on established osteoporosis in young women with amenorrhoea. Clin Endocrinol (Oxf) 41:275–281 [DOI] [PubMed] [Google Scholar]
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O'Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D, the Women’s Health Initiative Investigators 2006 Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med [Erratum (2006) 354:1102] 354:669–683 [Google Scholar]
- Bell RA, Quandt SA, Spangler JG, Case LD 2002 Dietary calcium intake and supplement use among older African American, white, and Native American women in a rural southeastern community. J Am Diet Assoc 102:844–847 [DOI] [PubMed] [Google Scholar]
- Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA 2002 Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 76:187–192 [DOI] [PubMed] [Google Scholar]
- Davies MC, Hall ML, Jacobs HS 1990 Bone mineral loss in young women with amenorrhoea. BMJ 301:790–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard PJ, Nelson LM 2003 Adolescent girls, the menstrual cycle, and bone health. J Pediatr Endocrinol Metab 16(Suppl 3):673–681 [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J 2002 Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML 2007 Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297:1465–1477 [DOI] [PubMed] [Google Scholar]