Abstract
Context: Testosterone (T) plus progestin combinations are the most promising hormonal male contraceptives. Nestorone (NES), a progestin without estrogenic or androgenic activity, when combined with T may be an excellent candidate for male contraception.
Objective: Our objective was to determine the effect of transdermal NES gel alone or with T gel on gonadotropin suppression.
Design and Setting: The randomized, unblinded clinical trial was conducted at two academic medical centers.
Participants: A total of 140 healthy male volunteers participated.
Interventions: One hundred subjects were randomized initially (20 per group) to apply NES gel 2 or 4 mg, T gel 10 g, or T gel 10 g plus NES gel 2 or 4 mg daily for 20 d. Because only about half of the subjects in T plus NES 4 mg group suppressed serum gonadotropins to 0.5 IU/liter or less (suboptimal suppression), two additional groups of 20 men were randomized to apply daily T gel 10 g plus NES gel 6 or 8 mg.
Main Outcome Variable: Suppression of serum LH and FSH concentrations to 0.5 IU/liter or less after treatment was the main outcome variable.
Results: A total of 119 subjects were compliant with gel applications with few study-related adverse events. NES alone reduced gonadotropins significantly but less than T gel alone. Combined T gel 10g plus NES gel 6 or 8 mg suppressed both serum gonadotropins to 0.5 IU/liter or less in significantly more men than either gel alone.
Conclusion: Transdermal NES gel alone had gonadotropin suppression activity. Combined transdermal NES (6 or 8 mg) plus T gel demonstrated safe and effective suppression of gonadotropins, justifying a longer-term study of this combination for suppression of spermatogenesis.
Combined transdermal nestorone and testosterone gel demonstrated safe and effective suppression of gonadotropins, justifying a longer term study of this combination for suppression of spermatogenesis.
Hormonal methods to suppress sperm production in men should be effective, safe, reliable, affordable, and reversible (1,2,3). Administration of testosterone (T) alone and T plus a progestin in men have shown contraceptive efficacy similar to female contraceptive methods where pregnancy rates in the partner had been reported to be very low (4,5,6,7). Recent retrospective analyses of available data from most hormonal male contraceptive studies showed that addition of a progestin to T increased the rate and extent of the suppression of spermatogenesis and younger men with lower initial sperm concentration and serum T levels showed faster suppression of spermatogenesis (8). Recovery of spermatogenesis occurred in all men with a predictable time course after stopping the administration of T and progestins. The rate of recovery was faster with shorter duration of treatment and short-acting T preparations (9).
Injectable esters such as injectable T ester and implants effectively suppress spermatogenesis at doses that produce relatively high serum T levels (4,6,7,10). Because progestins enhance the rate and extent of suppression of spermatogenesis compared with T alone, many combinations of T and progestins have been tested to seek the optimal combinations of specific hormones, formulations, dosages, and routes of administration (11,12,13). Injectable progestins including depo-medroxyprogesterone acetate and norethindrone enanthate and implants and oral preparations containing levonorgestrel and etonogestrel are shown to enhance the suppression of spermatogenesis by T injections or implants (14,15,16,17,18,19,20,21,22).
When transdermal T patches were administered with oral levonorgestrel, the suppression of spermatogenesis to severe oligozoospermia occurred in a quarter of participants (23,24). This limited suppression of sperm concentrations was attributed to lower serum T levels achieved with the patch. Daily application of T gel results in a dose-proportional increase in steady serum T levels in hypogonadal men and produces sustained relief of symptoms (25,26,27,28). When transdermal T gel was administered together with depo-medroxyprogesterone acetate in a male contraceptive trial, over 90% of the men became severely oligozoospermic (22).
Nestorone (NES, 16-methylene-17α-acetoxy-19-norpregn- 4-ene-3, 20-dione, formerly referred to as ST 1435) is a 19-nor-progesterone derivative without androgen or estrogen receptor binding activity. It is 2.9-fold more potent than progesterone in relative receptor binding activity and 90-fold more potent in inhibiting ovulation in female rats (29,30). Vaginal NES rings have been tested for female contraception (31,32,33). Unlike other progestins that have not been developed into a transdermal delivery system, NES gel as a potential contraceptive has been studied in women but not in men (34).
Our goal is to develop user-friendly transdermal hormonal male contraceptives. We investigated whether NES gel alone suppressed gonadotropins and whether NES gel had any additive effect to transdermal T gel on gonadotropin suppression, a surrogate marker of suppression of spermatogenesis, in a prospective, randomized, open-label, two-center trial.
Subjects and Methods
Subjects
Initially, 100 healthy male volunteers between the ages 18 and 50 yr (32.3 ± 0.78 yr, mean ± sem) were recruited and enrolled at two academic health centers in Los Angeles and Seattle. Subjects underwent a screening phase to determine that they had normal baseline blood biochemistry, hematology, urinalysis, fasting lipid profiles, hormones, and semen analysis (sperm concentration >20 million/ml) according to the World Health Organization Manual for the Examination of Human Semen (35). Eligible subjects were randomized using computer-generated numbers to five groups of 20 subjects each. Because in the initial study, T gel plus NES 2 or 4 mg groups failed to suppress both serum LH and FSH to 0.5 IU/liter or less in more than 50% of men, an additional 40 subjects were recruited and randomized in an extension study where T gel was administered with 6 and 8 mg/d NES gel. The protocol was approved by the Institutional Review Board of all three institutions (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center; University of Washington; and the Rockefeller University for Population Council).
Study medications
T gel 10 g (Testim) was provided by Auxilium (Malvern, PA) in tubes containing 5 g T gel and applied to both upper arms and shoulders. The formulation is a hydroalcoholic gel containing 1% T. Ten grams of Testim applied to the skin contains 100 mg T and delivers approximately 10 mg T to the systemic circulation (25). NES gel was formulated and developed by the Population Council (New York, NY). The doses of 2, 4, and 6 mg/d NES were supplied as 1, 2, and 3 ml of 2 mg NES per gramg gel. For the 8-mg dose, 3 ml of 2.7 mg NES per gram gel were used. The NES gel was aspirated into syringes by the study staff and dispensed to the subjects who applied the gel daily to an area of 15 cm2 on the abdomen.
Study design
The initial study was a prospective, randomized, open-label, two-center trial. A total of 100 subjects were enrolled and randomized to one of five groups (20 per group with 10 per site): group 1, 2 mg NES/d; group 2, 4 mg NES/d; group 3, 10 g T gel/d; group 4, 10 g T gel plus 2 mg NES/d; and group 5, 10 g T gel plus 4 mg NES/d. Because the suppression of both gonadotropins to 0.5 IU/liter or less was suboptimal and lesser than anticipated, two additional groups of 20 men were randomized to receive higher dose of NES: group 6, 10 g T gel plus 6 mg NES gel/d, and group 7, 10 g T gel plus 8 mg NES/d. The total duration of treatment was about 20 d followed by a recovery period of about 4 wk (end of study visit on d 49 ± 2). Compliance was determined by counting all empty and unused tubes or syringes and a diary on gel application was maintained by each subject.
Serum samples were obtained on d 1, 21, and 49 for SHBG, T, and free T. On d 1 and 21, five samples of blood were drawn at 15-min intervals for measurement of serum LH and FSH. On d 21, the serial samples for LH and FSH were drawn approximately 24 h after last application of the gel. These serial blood samples were collected to determine whether the pulsatile secretion of both gonadotropins was suppressed by treatment. Fasting blood samples for safety laboratory analyses were collected at screening and end of treatment visits. All serum samples were stored at −20 C until analyses and all samples from a subject were measured in one assay.
Hormone assays and safety laboratory tests
The safety laboratory tests were analyzed at the local laboratories. The screening semen analyses were performed according to standard methods (35). Serum samples for NES concentration were measured by the Population Council using a validated RIA (32,34). Serum T, free T, and SHBG were measured by sensitive and specific RIA assays as previously described (36). The adult male range for serum T was 9.4–30.9 nmol/liter, free T 0.127–0.576 nmol/liter, and SHBG 10.8–46.6 nmol/liter. The intraassay coefficient of variations for LH and FSH fluoroimmunometric assays were 3.9 and 5.4%, respectively; and the interassay variations for LH and FSH were 6.8 and 9.8%, respectively (adult normal male ranges, LH 1.3–8.1 U/liter and FSH 1.4–9.5 U/liter); and the lower limit of quantification was determined to be 0.1 IU/liter (26,36).
Statistical analyses
This was a dose-ranging study to measure the degree of gonadotropin suppression after daily self-application of T and NES gels. The primary efficacy outcome was the percentage of efficacy-evaluable men with both LH and FSH suppressed to 0.5 IU/liter or less after 20 d treatment. Secondary outcomes included mean percent changes from d 1–21 in LH, FSH, T, free T, and SHBG concentrations; percentage of efficacy-evaluable men with both LH and FSH less than or equal to 1.0 IU/liter at d 21; and mean NES concentration at d 21 in those receiving NES). Five samples of blood were drawn for serum FSH and LH to increase the likelihood that this might reflect the mean serum LH and FSH levels. Serum LH and FSH concentrations were means of five samples taken on d 1 and 21, and other hormone concentrations were from a single serum sample.
A participant was defined as efficacy evaluable (compliant with study medications) if he 1) administered at least 80% of the medication doses during treatment, i.e. on at least 16 d; 2) had blood samples drawn within 48 h of the last administration; and 3) had detectable serum NES (≥27 pmol/liter) at d 21 if he was in a NES group. Statistical assessments were performed with SAS version 9.1.3 (Cary, NC). Hormone levels and percent changes were summarized with medians and first to third quartile due to skewness. T plus NES groups were pairwise compared with the T-only group for these outcomes using Kruskal-Wallis tests, and the overall associations between these outcomes and dose were determined with Spearman correlations (37). Fisher’s exact test was used for group comparisons for the percentage of men with low gonadotropins. Changes in safety laboratory parameters from screening to d 21 were summarized with medians using all 140 enrolled subjects and assessed for significance with Wilcoxon signed-rank tests. Numbers of men outside safety parameter reference ranges were compared between screening and d 21 using Mantel-Haenszel statistics (37).
The study size was based on the desired precision and lower bound for the percentage of men with suppressed gonadotropins that would warrant a subsequent study of spermatogenesis suppression with longer duration of treatment. For each treatment group, the 95% confidence bounds for a percentage of gonadotropin suppression would not exceed ±25%. Anticipated longer-term spermatogenesis suppression in men with gonadotropin suppression at approximately 21 d was estimated from recently completed longer studies of T alone or in combination with levonorgestrel or with the GnRH antagonist, acyline (19,22,24,36,38,39). In these studies combined, 96% (92%) of men who achieved suppression of both gonadotropins to less than 0.5 IU/liter (<1.0 IU/liter) after 3–4 wk treatment later achieved suppression of sperm concentration to 1 million/ml or less, the defined goal for male contraceptive clinical trials (40).
Results
Characteristics of study participants
Table 1 categorizes the reasons for excluding 21 subjects from efficacy analyses (top) and summarizes the pretreatment characteristics for the 119 remaining evaluable men (bottom). Sixteen subjects were considered not efficacy evaluable because they did not meet the criteria for being compliant with study medications. Another five subjects were withdrawn from the study: one stopped treatment after experiencing panic attacks (classified as mild and possibly related to study medication in the T plus NES 8 mg group), one after acne (classified as moderate and probably related to study medications in the T alone group), one after nausea and diarrhea, one subject withdrew consent on advice of a family member, and one subject was withdrawn after taking a double dose of NES. Median rate of compliance, as ascertained by returned tubes and syringes, was 95% (first to third quartiles, 91–95%), with 82% of men taking at least 90% of scheduled doses. These rates did not differ significantly among the treatment groups. The 140 randomized men were 77% Caucasian and had median age, weight, and body mass index of 30 yr (range 18–50), 82.7 kg, and 26.1, respectively. There were no significant differences in these characteristics among the treatment groups although the T plus NES 6 mg group tended to weigh less.
Table 1.
Reasons for exclusion of subjects for analyses and baseline characteristics of evaluable subjects
| Group
|
All subjects | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| T gel dose (g) | 0 | 0 | 10 | 10 | 10 | 10 | 10 | |
| NES gel dose (mg) | 2 | 4 | 0 | 2 | 4 | 6 | 8 | |
| Randomized (n) | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 140 |
| Withdrawn (n) | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 5 |
| <80% compliant (n) | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 4 |
| Sample >48 h after dose (n) | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 4 |
| Nondetectable serum NES levels (n) | 2 | 2 | NA | 1 | 1 | 1 | 1 | 8 |
| Efficacy evaluable (n) | 16 | 16 | 19 | 19 | 15 | 18 | 16 | 119 |
| Age (yr)a | 32 (23–37) | 30 (23–36) | 35 (26–42) | 34 (24–42) | 35 (28–46) | 29 (26–39) | 29 (26–34) | 31 (25–39) |
| Caucasian (n) | 13 (81%) | 15 (94%) | 13 (68%) | 16 (84%) | 11 (73%) | 12 (67%) | 13 (81%) | 93 (78%) |
| Weight (lbs)a | 185 (155–203) | 181 (169–201) | 175 (164–194) | 193 (165–207) | 191 (160–207) | 162 (141–177) | 189 (164–209) | 179 (162–202) |
| BMI (kg/m2)a | 26.9 (23–29) | 25.0 (24–27) | 27.1 (24–29) | 25.5 (25–28) | 27.7 (24–30) | 24.9 (21–26) | 27.2 (24–29) | 25.9 (24–29) |
| Serum T (nmol/liter)a | 14.2 (10.1–18.4) | 16.1 (12.2–17.3) | 15.9 (13.2–20.3) | 16.2 (13.0–21.3) | 15.5 (13.5–19.1) | 19.6 (15.4–21.7) | 16.8 (13.4–19.8) | 16.3 (13.2–19.8) |
| Serum Free T (nmol/liter)a | 0.32 (0.25–0.41) | 0.32 (0.26–0.38) | 0.37 (0.29–0.47) | 0.33 (0.30–0.41) | 0.32 (0.25–0.36) | 0.31 (0.20–0.45) | 0.32 (0.21–0.36) | 0.33 (0.25–0.41) |
| Serum SHBG (nmol/liter)a | 30.1 (18.9–36.8) | 31.0 (23.4–41.0) | 24.5 (19.9–45.8) | 32.4 (27.2–41.4) | 35.6 (21.8–54.1) | 30.3 (20.0–42.1) | 21.5 (15.8–32.9) | 28.6 (20.8–41.5) |
BMI, Body mass index; NA, not applicaple.
Results are shown as median (first to third quartiles).
Effect on serum gonadotropins
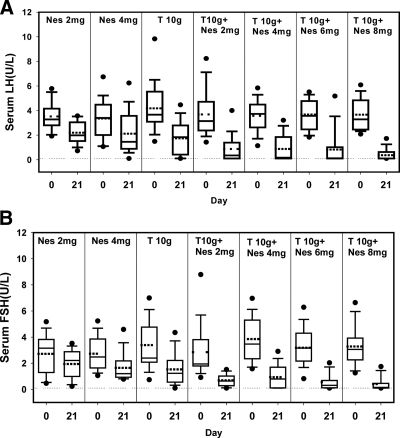
There were significant decreases in LH and in FSH in all groups (P < 0.05) (Fig. 1, A and B). Significantly greater decreases in LH and FSH were seen (P < 0.0001) as NES was added in increasing doses, ultimately attaining over 90% median decreases of both gonadotropins with T gel plus 6 or 8 mg NES gel (Table 2). The percentage of men with both LH and FSH less than 0.5 or 1 IU/liter was less than 13% for NES gel-only groups and less than 32% in T gel-only group but increased significantly (P < 0.0001) as NES gel was added in increasing doses to 68.8 and 81.3%, respectively, in the 6- and 8-mg/d NES plus T gel groups. The additive effect of NES gel, when used in combination with T gel 10 g compared with T 10 g alone, was observed only when the NES dose was at least 6 mg. When NES 6 or 8 mg/d NES gel was added to 10 g T gel, a greater percent decrease in serum gonadotropin levels (P < 0.05) and more subjects suppressed serum gonadotropin levels to less than 0.5 or 1 IU/liter (P < 0.05) when compared with T gel alone (Table 2). In the T gel plus Nes 8 mg group, serum LH and FSH levels were below the lower limit of quantification in 11 of 16 subjects, and spikes of LH secretion were observed in only two of the remaining five subjects who had measurable LH levels.
Figure 1.
Serum LH (A) and FSH (B) concentrations before and after transdermal application of NES and T gel for 20 d. The dotted line represents the lower limit of quantification of serum LH and FSH, respectively. Box and whiskers plots are used in all figures where the dark line in the box is the median and the dotted line is the mean concentration. The lower and upper box boundaries represent the 25th and 75th percentiles, the whiskers are the 10th and 90th percentiles, and the dots are values below the 10th or above the 90th percentiles.
Table 2.
Effect of NES and T gel on serum gonadotropins
| Group
|
|||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| T gel dose (g) | 0 | 0 | 10 | 10 | 10 | 10 | 10 |
| NES gel dose (mg) | 2 | 4 | 0 | 2 | 4 | 6 | 8 |
| Evaluable (n) | 16 | 16 | 19 | 19 | 15 | 18 | 16 |
| d-1 LH (IU/liter)a | 3.3 (2.8–4.1) | 3.4 (2.1–4.5) | 3.7 (3.1–5.5) | 3.2 (2.4–4.7) | 3.7 (2.6–4.5) | 3.6 (2.5–4.7) | 3.3 (2.5–4.8) |
| LH, % decreaseb | 41 (18–54) | 56 (13–65) | 71 (32–85) | 85 (55–96) | 91 (57–97) | 95 (83–97) | 96 (89–97) |
| d-1 FSH (IU/liter)a | 3.2 (1.4–3.8) | 2.5 (1.7–3.8) | 2.4 (2.1–4.8) | 2.0 (1.8–3.8) | 3.5 (2.4–5.3) | 3.2 (2.2–4.3) | 3.1 (2.4–3.9) |
| FSH, % decreaseb | 27 (21–35) | 37 (29–54) | 52 (22–80) | 75 (60–89) | 76 (42–96) | 92 (74–95) | 93 (82–96) |
| Men with d-21 LH and FSH ≤0.5 IU/literc | 0, 0 (0–21) | 0, 0 (0–21) | 4, 21.1 (6–46) | 7, 36.8 (16–62) | 6, 40.0 (16–68) | 11, 61.1 (36–83) | 11, 68.8 (41–89) |
| Men with d-21 LH and FSH ≤1 IU/literc | 1, 6.3 (0–30) | 2, 12.5 (2–38) | 6, 31.6 (13–57) | 11, 57.9 (34–80) | 8, 53.3 (27–79) | 13, 72.2 (47–90) | 13, 81.3 (54–96) |
Results shown as median (first to third quartiles).
Results shown as median (first to third quartiles) for percent decrease from d 1–21; P < 0.05 for percent decreases in all groups.
Results shown as number, percentage, and 95% confidence interval for percentage.
Serum NES concentrations
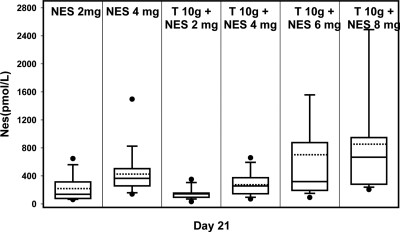
When applied with T 10 g, median serum NES levels at d 21 significantly (P < 0.0001) increased with application of increasing amounts of NES gel (Fig. 2), showing a dose-related response. Higher serum NES levels at d 21 were significantly associated with greater percent reductions in both LH (Spearman correlation = 0.50; P < 0.0001) and FSH (Spearman correlation = 0.60; P < 0.0001).
Figure 2.
Serum NES levels in the subjects administering NES gel for 20 d.
Serum total and free T and SHBG
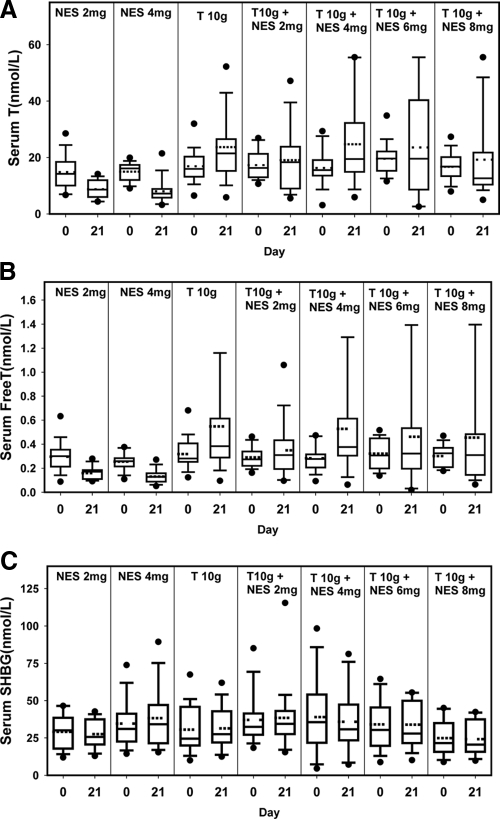
Large inter-individual variations in serum total and free T concentrations were observed after T gel application (Fig. 3, A and B). There were no significant differences in serum total and free T and SHBG among treatment groups at d 1 (Table 1). Both NES gel-only groups showed significant decreases in serum total and free T concentrations (P < 0.05) from d 1–21as a result of the suppression of gonadotropins. The T gel-only group and T gel plus NES gel 4 mg group showed significant increases from d 1–21 in serum total T levels (P < 0.05). There was no trend of changes in serum T with increasing NES dosage (P = 0.46; Fig. 3A). Serum SHBG changes were minor and nonsignificant for all treatment groups (Fig. 3C).
Figure 3.
Serum total T (A), free T (B), and SHBG (C) concentrations before and after transdermal application of NES and T gel for 20 d.
Safety laboratory monitoring parameters
Twenty-three percent of men had at least a 1-kg loss in body weight, and 19% had at least a 1-kg gain, with a median nonsignificant decrease of 0.2 kg (P = 0.39). No significant changes from screening to d 21 were observed for prostate-specific antigen, hemoglobin and hematocrit, liver transaminases, alkaline phosphatase, urea nitrogen, creatinine, and triglycerides (supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Median total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol concentrations were significantly (P < 0.01) decreased by 7, 5, and 10%, respectively, and total to HDL cholesterol ratio significantly increased by 4% (P = 0.002) in all groups combined. The decreases in HDL were significant and more than 9.5% in all of the five groups receiving T gel but were less than 4% and nonsignificant in the two groups receiving only NES gel. At screening, 50 of 140 subjects had serum HDL cholesterol below the reference range compared with 82 of 140 subjects at d 21 (or end of study visit for those who dropped from the study), whereas 17 and five subjects had serum HDL levels above the reference ranges at screening and d 21, respectively (P < 0.0001, supplemental Table 3).
Adverse events
There were no serious adverse events. Vital signs and physical examination did not change significantly during the study. Acne was reported in 10% of the men, and only one was in a NES-only group. Pruritis was reported as possibly and probably related to study medication in 4.6% of subjects both in the T and the NES groups. Only 2.1% of subjects had erythema at the application site with both T and NES gels. One subject discontinued because of acne. Other common adverse events were headache in 5% and nausea in 5% of subjects. Most adverse events occurred in all treatment groups except subject-reported decreased libido (4.3%), which was reported only in the two NES gel-alone groups and probably related to the suppressed serum T levels. There was one subject in the T gel plus NES 4 mg group whose serum LH and FSH concentrations remained less than 1 IU/liter 1 month after stopping application of the gels, although his serum T was within the reference range. No other safety parameters or semen samples were collected at the end of study visit.
Discussion
NES is not active orally but shows a high antiovulatory effect in female hormonal contraception studies when given via nonoral routes such as transdermal gel and vaginal ring (32,33,34,41). In contrast to the androgenic progestins, levonorgestrel or desogestrel, NES does not bind to either the androgen receptor or SHBG. It has neither estrogenic nor glucocorticoid activity. In female rabbits and rats, NES was more potent than levonorgestrel or desogetrel in standard tests for progestational activity. In castrated male rats, NES has no detectable androgenic actions on the prostate and the levator ani muscle compared with levonorgestrel. In male rats, NES failed to show significant suppression of serum LH compared with T and levonorgestrel (29). We tested NES (using about 2- to 4-fold the doses used in females) in healthy volunteers with the goal of developing transdermal NES gel together with T gel as a user-controlled, provider-independent (self-administered) method of male contraception. We elected to conduct this trial before performing a spermatogenesis suppression study to determine the effective dose of NES for gonadotropin suppression and for an additive effect when administered together with the selected T gel dose.
We showed that in men, transdermal NES gel at about two to four times the dose used in females suppressed both serum LH and FSH significantly although to a lesser extent than T gel alone. In the NES gel-alone groups, the decrease in gonadotropins resulted in significant decreases in serum T levels and self-reported decreased libido in six subjects. When increasing doses of NES were administered together with T transdermally, serum NES showed a dose-proportional rise, resulting in a dose-proportional increase in the suppression of both gonadotropins. At higher doses tested (NES 6 or 8 mg/d) in combination with T gel, the suppression of gonadotropin levels and the number of men that suppressed to very low levels were significantly greater than T gel alone. Many of these men had nondetectable serum LH and FSH. It should be noted that the suppression of gonadotropins was assessed about 24 h after the application of the gels when the negative feedback effects of the T and NES may not be maximal. The data provide evidence that although NES on its own has weak to moderate gonadotropin suppressive activity in men, when used in combination with T, the additive effect is significant.
In this study, T gel alone suppressed gonadotropins to very low levels (≤0.5 or 1 IU/liter) in 20–30% of men. Serum T concentrations achieved with transdermal T preparations were rather variable and significantly increased in only two of the five groups of men that received T gel. In a previous male contraceptive study using T gel 10 g/d together with depo-medroxyprogesterone acetate, serum T levels were variable and reached rather high levels in some subjects (22). The dose of the T gel used (10 g T gel delivers about 10 mg of T per day to the body) is at the upper limit of the daily T production rate in healthy adult men (42). We chose this dose of T gel to show that even with a high replacement dose of T we could demonstrate a significant enhancement of gonadotropin suppression by the addition of NES; we therefore speculate that the combined treatment of 10 g T gel plus at least 6 mg or more NES gel per day will have major suppressive effects on spermatogenesis. Once this dose of T plus NES gel showed efficacy in suppression of sperm production, then the dose of the T gel might be lowered to avoid high serum T levels observed in some subjects that might result in increased hemoglobin/hematocrit and decreased serum HDL cholesterol levels.
The combined treatment had few adverse effects. Mild acne developed in 10% of subjects; one subject (T gel-alone group) had moderately severe acne and stopped participating in the study. Mild local erythema and pruritis occurred in a small number of subjects, which did not result in subject discontinuation. Serum total and LDL cholesterol decreased by 5–7%, but HDL cholesterol decreased significantly by 10%. The decrease in HDL cholesterol levels occurred in all T gel treatment groups but less in the NES-alone groups suggesting that the decrease is likely secondary to the T administered. These changes are small and may not be clinically relevant. Longer-term studies are required to confirm the decrease in HDL cholesterol which was also noted in hypogonadal men treated with T gel (28). Whether the decreases in HDL cholesterol in the presence of smaller but concomitant decrease LDL cholesterol levels would increase the risk of cardiovascular risks remains controversial (43,44).
In conclusion, in this study, we demonstrated that NES alone had a moderate gonadotropin-suppressive activity in men. When combined with a relatively high dose of T gel, NES (at doses of >6 mg/d) had additive effect on the suppression of gonadotropins. Previous studies showed suppression of gonadotropins within 3–4 wk after administration of T and progestins equate severe suppression of spermatogenesis when the combination was used for a longer period of time. We are currently planning to conduct a spermatogenesis suppression study using T gel 10 g with 8 mg or higher doses of NES. If the combination proves efficacious, future studies will aim to develop this combination into a new provider-independent method using a daily combined androgen and progestin transdermal preparation for male contraception.
Supplementary Material
Acknowledgments
We thank the study coordinators, the Endocrine Research Laboratory at Los Angeles Biomedical Research Institute, Health Decisions, and Laura Hull, M.A., for their assistance with the conduct of the study, hormone assays, data management, and preparation of the manuscript, respectively.
Footnotes
Results from this work were presented in part at the 90th Annual Meeting of The Endocrine Society, San Francisco, California, 2008.
This study was supported by the National Institute of Child Health and Human Development through the Contraceptive Clinical Network Centers (Male Area) HHSN275200403369I to Los Angeles Biomedical Research Institute and HHSN275200403370I to the University of Washington). The study in Los Angeles was supported by the General Clinical Research Center and its core laboratory (MO1 RR00425).
Disclosure Summary: The authors have nothing to declare.
First Published Online April 14, 2009
Abbreviations: HDL, High-density lipoprotein; LDL, low-density lipoprotein; NES, nestorone; T, testosterone.
References
- Page ST, Amory JK, Bremner WJ 2008 Advances in male contraception. Endocr Rev 29:465–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieschlag E, Henke A 2005 Hopes for male contraception. Lancet 365:554–556 [DOI] [PubMed] [Google Scholar]
- Liu PY, McLachlan RI 2008 Male hormonal contraception: so near and yet so far. J Clin Endocrinol Metab 93:2474–2476 [DOI] [PubMed] [Google Scholar]
- Gu YQ, Wang XH, Xu D, Peng L, Cheng LF, Huang MK, Huang ZJ, Zhang GY 2003 A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy Chinese men. J Clin Endocrinol Metab 88:562–568 [DOI] [PubMed] [Google Scholar]
- Turner L, Conway AJ, Jimenez M, Liu PY, Forbes E, McLachlan RI, Handelsman DJ 2003 Contraceptive efficacy of a depot progestin and androgen combination in men. J Clin Endocrinol Metab 88:4659–4667 [DOI] [PubMed] [Google Scholar]
- 1990 Contraceptive efficacy of testosterone-induced azoospermia in normal men. World Health Organization Task Force on methods for the regulation of male fertility. Lancet 336:955–959 [PubMed] [Google Scholar]
- 1996 Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril [Erratum (1996) 65:1267] 65:821–829 [PubMed] [Google Scholar]
- Liu PY, Swerdloff RS, Anawalt BD, Anderson RA, Bremner WJ, Elliesen J, Gu YQ, Kersemaekers WM, McLachlan RI, Meriggiola MC, Nieschlag E, Sitruk-Ware R, Vogelsong K, Wang XH, Wu FC, Zitzmann M, Handelsman DJ, Wang C 2008 Determinants of the rate and extent of spermatogenic suppression during hormonal male contraception: an integrated analysis. J Clin Endocrinol Metab 93:1774–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C 2006 Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet 367:1412–1420 [DOI] [PubMed] [Google Scholar]
- Handelsman DJ, Conway AJ, Boylan LM 1992 Suppression of human spermatogenesis by testosterone implants. J Clin Endocrinol Metab 75:1326–1332 [DOI] [PubMed] [Google Scholar]
- Meriggiola MC, Farley TM, Mbizvo MT 2003 A review of androgen-progestin regimens for male contraception. J Androl 24:466–483 [DOI] [PubMed] [Google Scholar]
- Nieschlag E, Zitzmann M, Kamischke A 2003 Use of progestins in male contraception. Steroids 68:965–972 [DOI] [PubMed] [Google Scholar]
- Wang C, Swerdloff RS 2004 Male hormonal contraception. Am J Obstet Gynecol 190:S60–S68 [DOI] [PubMed] [Google Scholar]
- Gu YQ, Tong JS, Ma DZ, Wang XH, Yuan D, Tang WH, Bremner WJ 2004 Male hormonal contraception: effects of injections of testosterone undecanoate and depot medroxyprogesterone acetate at eight-week intervals in Chinese men. J Clin Endocrinol Metab 89:2254–2262 [DOI] [PubMed] [Google Scholar]
- Handelsman DJ, Conway AJ, Howe CJ, Turner L, Mackey MA 1996 Establishing the minimum effective dose and additive effects of depot progestin in suppression of human spermatogenesis by a testosterone depot. J Clin Endocrinol Metab 81:4113–4121 [DOI] [PubMed] [Google Scholar]
- Kamischke A, Ploger D, Venherm S, von Eckardstein S, von Eckardstein A, Nieschlag E 2000 Intramuscular testosterone undecanoate with or without oral levonorgestrel: a randomized placebo-controlled feasibility study for male contraception. Clin Endocrinol (Oxf) 53:43–52 [DOI] [PubMed] [Google Scholar]
- Kamischke A, Venherm S, Ploger D, von Eckardstein S, Nieschlag E 2001 Intramuscular testosterone undecanoate and norethisterone enanthate in a clinical trial for male contraception. J Clin Endocrinol Metab 86:303–309 [DOI] [PubMed] [Google Scholar]
- Meriggiola MC, Costantino A, Saad F, D'Emidio L, Morselli Labate AM, Bertaccini A, Bremner WJ, Rudolph I, Ernst M, Kirsch B, Martorana G, Pelusi G 2005 Norethisterone enanthate plus testosterone undecanoate for male contraception: effects of various injection intervals on spermatogenesis, reproductive hormones, testis, and prostate. J Clin Endocrinol Metab 90:2005–2014 [DOI] [PubMed] [Google Scholar]
- Wang C, Wang XH, Nelson AL, Lee KK, Cui YG, Tong JS, Berman N, Lumbreras L, Leung A, Hull L, Desai S, Swerdloff RS 2006 Levonorgestrel implants enhanced the suppression of spermatogenesis by testosterone implants: comparison between Chinese and non-Chinese men. J Clin Endocrinol Metab 91:460–470 [DOI] [PubMed] [Google Scholar]
- Wu FC, Aitken RJ 1989 Suppression of sperm function by depot medroxyprogesterone acetate and testosterone enanthate in steroid male contraception. Fertil Steril 51:691–698 [DOI] [PubMed] [Google Scholar]
- Mommers E, Kersemaekers WM, Elliesen J, Kepers M, Apter D, Behre HM, Beynon J, Bouloux PM, Costantino A, Gerbershagen HP, Gronlund L, Heger-Mahn D, Huhtaniemi I, Koldewijn EL, Lange C, Lindenberg S, Meriggiola MC, Meuleman E, Mulders PF, Nieschlag E, Perheentupa A, Solomon A, Vaisala L, Wu FC, Zitzmann M 2008 Male hormonal contraception: a double-blind, placebo-controlled study. J Clin Endocrinol Metab 93:2572–2580 [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Anawalt BD, Irwig MS, Brockenbrough AT, Matsumoto AM, Bremner WJ 2006 Testosterone gel combined with depomedroxyprogesterone acetate is an effective male hormonal contraceptive regimen and is not enhanced by the addition of a GnRH antagonist. J Clin Endocrinol Metab 91:4374–4380 [DOI] [PubMed] [Google Scholar]
- Buchter D, von Eckardstein S, von Eckardstein A, Kamischke A, Simoni M, Behre HM, Nieschlag E 1999 Clinical trial of transdermal testosterone and oral levonorgestrel for male contraception. J Clin Endocrinol Metab 84:1244–1249 [DOI] [PubMed] [Google Scholar]
- Gonzalo IT, Swerdloff RS, Nelson AL, Clevenger B, Garcia R, Berman N, Wang C 2002 Levonorgestrel implants (Norplant II) for male contraception clinical trials: combination with transdermal and injectable testosterone. J Clin Endocrinol Metab 87:3562–3572 [DOI] [PubMed] [Google Scholar]
- Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R 2003 AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab 88:2673–2681 [DOI] [PubMed] [Google Scholar]
- Swerdloff RS, Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Longstreth J, Berman N 2000 Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab 85:4500–4510 [DOI] [PubMed] [Google Scholar]
- Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T, Berman N 2000 Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. Testosterone Gel Study Group. J Clin Endocrinol Metab 85:2839–2853 [DOI] [PubMed] [Google Scholar]
- Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Berman N, Hull L, Swerdloff RS 2004 Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab 89:2085–2098 [DOI] [PubMed] [Google Scholar]
- Kumar N, Koide SS, Tsong Y, Sundaram K 2000 Nestorone: a progestin with a unique pharmacological profile. Steroids 65:629–636 [DOI] [PubMed] [Google Scholar]
- Li F, Kumar N, Tsong YY, Monder C, Bardin CW 1997 Synthesis and progestational activity of 16-methylene-17α-hydroxy-19-norpregn-4-ene-3,20-dione and its derivatives. Steroids 62:403–408 [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R 2006 New progestagens for contraceptive use. Hum Reprod Update 12:169–178 [DOI] [PubMed] [Google Scholar]
- Fraser IS, Weisberg E, Brache V, Alvarez F, Massai R, Mishell Jr DR, Apter D, Gale J, Tsong YY, Sivin I 2005 Serum Nestorone and ethinyl estradiol levels, and ovulation inhibition in women using three different dosage combinations of a Nestorone progestogen-ethinyl estradiol contraceptive vaginal ring on a bleeding-signaled regimen. Contraception 72:40–45 [DOI] [PubMed] [Google Scholar]
- Sivin I, Mishell Jr DR, Alvarez F, Brache V, Elomaa K, Lahteenmaki P, Massai R, Miranda P, Croxatto H, Dean C, Small M, Nash H, Jackanicz TM 2005 Contraceptive vaginal rings releasing Nestorone and ethinylestradiol: a 1-year dose-finding trial. Contraception 71:122–129 [DOI] [PubMed] [Google Scholar]
- Fraser IS, Weisberg E, Kumar N, Kumar S, Humberstone AJ, McCrossin L, Shaw D, Tsong YY, Sitruk-Ware R 2007 An initial pharmacokinetic study with a metered dose transdermal system for delivery of the progestogen Nestorone as a possible future contraceptive. Contraception 76:432–438 [DOI] [PubMed] [Google Scholar]
- World Health Organization 1999 Laboratory manual for the examination of human semen and sperm cervical mucus interaction. 4th ed. Cambridge, UK: Cambridge University Press [Google Scholar]
- Qoubaitary A, Meriggiola C, Ng CM, Lumbreras L, Cerpolini S, Pelusi G, Christensen PD, Hull L, Swerdloff RS, Wang C 2006 Pharmacokinetics of testosterone undecanoate injected alone or in combination with norethisterone enanthate in healthy men. J Androl 27:853–867 [DOI] [PubMed] [Google Scholar]
- Agresti A 2002 Categorical data analysis. New York: John Wiley & Sons [Google Scholar]
- Amory JK, Anawalt BD, Bremner WJ, Matsumoto AM 2001 Daily testosterone and gonadotropin levels are similar in azoospermic and nonazoospermic normal men administered weekly testosterone: implications for male contraceptive development. J Androl 22:1053–1060 [DOI] [PubMed] [Google Scholar]
- Anawalt BD, Amory JK, Herbst KL, Coviello AD, Page ST, Bremner WJ, Matsumoto AM 2005 Intramuscular testosterone enanthate plus very low dosage oral levonorgestrel suppresses spermatogenesis without causing weight gain in normal young men: a randomized clinical trial. J Androl 26:405–413 [DOI] [PubMed] [Google Scholar]
- Aaltonen P, Amory JK, Anderson RA, Behre HM, Bialy G, Blithe D, Bone W, Bremner WJ, Colvard D, Cooper TG, Elliesen J, Gabelnick HL, Gu YQ, Handelsman DJ, Johansson EA, Kersemaekers W, Liu P, MacKay T, Matlin S, Mbizvo M, McLachlan RI, Meriggiola MC, Mletzko S, Mommers E, Muermans H, Nieschlag E, Odlind V, Page ST, Radlmaier A, Sitruk-Ware R, Swerdloff R, Wang C, Wu F, Zitzmann M 2007 10th Summit Meeting consensus: recommendations for regulatory approval for hormonal male contraception. J Androl 28:362–363 [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R 2004 Pharmacological profile of progestins. Maturitas 47:277–283 [DOI] [PubMed] [Google Scholar]
- Wang C, Catlin DH, Starcevic B, Leung A, DiStefano E, Lucas G, Hull L, Swerdloff RS 2004 Testosterone metabolic clearance and production rates determined by stable isotope dilution/tandem mass spectrometry in normal men: influence of ethnicity and age. J Clin Endocrinol Metab 89:2936–2941 [DOI] [PubMed] [Google Scholar]
- Liu PY, Death AK, Handelsman DJ 2003 Androgens and cardiovascular disease. Endocr Rev 24:313–340 [DOI] [PubMed] [Google Scholar]
- Wu FC, von Eckardstein A 2003 Androgens and coronary artery disease. Endocr Rev 24:183–217 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.