Abstract
Context: Adult women with polycystic ovary syndrome (PCOS) have decreased GnRH pulse generator sensitivity to progesterone (P)-mediated slowing. This defect is androgen mediated because it is reversed with androgen receptor blockade. Adolescent hyperandrogenism often precedes PCOS.
Objective: The aim of the study was to evaluate GnRH pulse generator sensitivity to P-mediated slowing in normal and hyperandrogenic girls.
Design: We conducted a controlled interventional study.
Setting: The study was conducted in a general clinical research center.
Participants: A total of 26 normal control (NC) and 26 hyperandrogenic (HA) girls were studied.
Intervention: Frequent blood sampling was performed for 11 h to assess LH pulse frequency before and after 7 d of oral estradiol and P.
Main Outcome Measure: We measured the slope of the percentage reduction in LH pulse frequency as a function of d 7 P (slope).
Results: Overall, Tanner 3-5 HA subjects were less sensitive to P-mediated slowing than Tanner 3-5 NC (slope, 4.7 ± 3.4 vs. 10.3 ± 7.7; P = 0.006). However, there was variability in the responses of HA subjects; 15 had P sensitivities within the range seen in NC, whereas nine were relatively P insensitive. The two groups had similar testosterone levels. Fasting insulin levels were higher in P-insensitive HA girls (39.6 ± 30.6 vs. 22.2 ± 13.9 μIU/ml; P = 0.02), and there was an inverse relationship between fasting insulin and P sensitivity in HA girls (P = 0.02). Tanner 1-2 NC had lower testosterone levels and were more P sensitive than Tanner 3-5 NC (slope, 19.3 ± 5.8; P = 0.04).
Conclusions: Hyperandrogenism is variably associated with reduced GnRH pulse generator sensitivity to P-mediated slowing during adolescence. In addition to androgen levels, insulin resistance may modulate P sensitivity.
Hyperandrogenism is variably associated with reduced GnRH pulse generator sensitivity to progesterone-mediated slowing during adolescence; insulin, in addition to androgens, may modulate progesterone sensitivity.
Polycystic ovary syndrome (PCOS) is a common clinical disorder affecting 6–7% of women during their reproductive years (1,2,3). Although clinically heterogeneous, it is defined by hyperandrogenism (HA) and ovulatory dysfunction, with or without polycystic ovary morphology (4), and is associated with obesity, insulin resistance, and subfertility (5). PCOS is also characterized by neuroendocrine abnormalities, including a persistently rapid GnRH pulse frequency. This signals the pituitary gonadotropes to preferentially synthesize and secrete LH over FSH (6,7), resulting in the elevated plasma LH and LH:FSH ratios typical of PCOS (8). Excess LH stimulates ovarian androgen production, contributing to HA, whereas the relative deficit of FSH leads to impaired follicular development. In adults, the persistently rapid GnRH pulse frequency is in part explained by impaired hypothalamic sensitivity to progesterone (P)-mediated slowing (9,10). This defect can be corrected with the use of the androgen receptor blocker flutamide, indicating that it is caused by excess androgens (11).
Adolescent HA is thought to be a precursor to adult PCOS. HA girls have increased LH concentrations and pulse frequency as well as accelerated maturation of GnRH pulse patterns during pubertal development (12,13). We previously evaluated 20 girls [nine normal controls (NC), 11 HA] and found that HA was variably associated with decreased hypothalamic P sensitivity in adolescence (14). Approximately half of the HA girls had impaired P sensitivity similar to that seen in adult women with PCOS, whereas the others maintained normal P sensitivity despite equivalent degrees of HA. This study was not powered to assess differences within the HA population; however, we hypothesized that there were hormonal and/or demographic differences between the P-sensitive and P-insensitive subgroups that contributed to the differences in hypothalamic P sensitivity. Given that abnormal neuroendocrine control of GnRH secretion is thought to play a central role in the development of PCOS in adolescence, understanding the factors that mediate variable P sensitivity in HA girls may have important implications for screening and prevention. In the present study, we aimed to confirm our previous findings of variable hypothalamic P sensitivity in an expanded population and to identify factors that could account for the differences in P sensitivity seen in girls with similar androgen levels.
Given that androgens modulate hypothalamic P sensitivity in adult women, we have proposed that testosterone (T) may play a similar role in the normal maturation of the hypothalamic-pituitary-ovarian axis in girls (15,16). Free T concentrations rise 4- to 8-fold during the course of normal female puberty (17,18), and we have hypothesized that this rise in free T leads to a progressive decline in hypothalamic sensitivity to estradiol (E2) and P feedback. Therefore, we also aimed to compare the androgen levels and hypothalamic P sensitivity of normal girls in early puberty to those in later puberty and to assess the overall relationship between androgen levels and hypothalamic P sensitivity during adolescence.
Subjects and Methods
Subjects and study procedures
Volunteers were recruited from the pediatric and adult endocrinology clinics at the University of Virginia (UVA) and the University of California-San Diego (UCSD) as well as through advertisements. Twenty-six girls were classified as HA based on either biochemical (free T >2.5 sd values above the mean for normal weight control subjects of the same Tanner stage) or clinical (hirsutism) evidence of HA. Twenty-six girls without evidence of HA served as NC. Data on 20 of these subjects (nine NC and 11 HA) have been reported previously (14). Participants were taking no drugs known to affect the reproductive axis and had not used hormonal medications for at least 90 d before starting the study.
Study procedures were approved by the Institutional Review Boards at UVA and UCSD. Informed assent and consent were obtained from the study participants and parents, respectively. Each volunteer underwent a detailed history and physical exam including assessment of pubertal stage using the Tanner scale for breast development (19). A fasting laboratory evaluation was performed, including LH, FSH, estrone (E1), E2, P, T, SHBG, androstenedione, dehydroepiandrosterone sulfate (DHEAS), 17-hydroxyprogesterone, prolactin, TSH, β-human chorionic gonadotropin, insulin, glucose, complete blood count, chemistry panel, and liver panel. With the exception of T and insulin levels in HA subjects, all laboratory values were within normal limits.
NC subjects were initially admitted to the General Clinical Research Center (GCRC) between cycle d 8 and 10 (to best approximate the hormonal milieu in PCOS). HA subjects were initially admitted either between d 8 and 10 of the cycle or more than 60 d after the last episode of menstrual bleeding, depending on the frequency of their cycles. Subjects were admitted to the GCRC at 1700 h on d 0. A forearm iv line was placed, and hematocrit and β-human chorionic gonadotropin were obtained to exclude anemia and pregnancy. Frequent blood samples were obtained between 1900 and 0700 h, every 10 min for LH, every 1 h for FSH, and every 2 h for E2, P, and T. After fasting for 8 h, a sample was drawn for measurement of insulin, SHBG, E1, and androstenedione. Lights were extinguished at 2300 h to facilitate sleep, and the subjects were offered meals at standard GCRC mealtimes.
On the day of GCRC discharge, subjects began oral E2 (Estrace, 1 mg/d; Warner Chilcott, Rockaway, NJ) and micronized P suspension (PharmaTek, Huntington, NY; or Spectrum, New Brunswick, NJ; suspension formulated by institutional research pharmacy) (25–125 mg at 0700, 1500, and 2300 h). In earlier studies in adult women, E2 alone did not significantly alter LH pulsatility in either NC or subjects with PCOS (10); given limitations in recruitment, E2-only controls were not repeated in this study. The dose of P was chosen by taking into account body weight and aiming to achieve mean plasma P levels in the range of 2–8 ng/ml. Subjects were instructed to take the P with a small, fat-containing snack to improve consistency of absorption. On d 3 and 5 of the study, E2 and P levels were measured at 1700 h to assess absorption and compliance. On d 7, the subjects returned to the GCRC for a second overnight admission identical to the first, except that oral E2 and P were continued and the subjects did not fast secondary to the snack given with the 2300 h dose of P. After the second admission, the E2 and P were discontinued, and subjects were given iron supplementation (ferrous gluconate, 325 mg, one to two times daily depending on weight) for 30 d.
Hormonal measurements
Blood was allowed to clot, and serum was removed and stored at −20 C until analysis. All samples were analyzed at the UVA Center for Research in Reproduction Ligand Core Laboratory. Samples from each subject were analyzed in duplicate in the same assay for each hormone.
Manufacturer, assay sensitivity, intraassay coefficient of variation (CV), and interassay CV are shown subsequently for each hormone. LH and FSH were measured using chemiluminescence [Diagnostic Products Corp. (DPC), Los Angeles, CA; 0.1 and 0.05 IU/liter, respectively; 1.9–5.0%; 4.8–6.9%). E2, P, and total T were measured by RIA (either Diagnostic Systems Laboratories (DSL), Inc., Webster, TX, or DPC; 1.5 pg/ml, 0.05 ng/ml, and 5 ng/ml, respectively; 2.9–6.9%; 4.1–15.2%). SHBG and DHEAS were measured using chemiluminescence (DPC; 0.2 nmol/liter and 7.0 μg/dl, respectively; 2.0–6.2%; 3.8–7.8%). E1, androstenedione, and insulin were measured by RIA (DSL; 15.0 pg/ml, 0.1 ng/ml, and 2.6 μU/ml, respectively; 3.3–8.6%, 5.7–14.9%). Samples with measured values below assay sensitivity were assigned the value of the assay’s sensitivity.
Free T was calculated from total T and SHBG using the following equation: FT = [T-(N)(FT)]/[(KT)(SHBG) − (KT)(T) + (N)(KT)(FT)]/3.467, where FT is free T concentration (pg/ml), T is total T concentration (ng/dl), KT is association constant of SHBG for T (1.0 × 109), SHBG is SHBG concentration (nmol/liter), and N is (KA)(CA) + 1; KA is the association constant for albumin for T (3.6 × 104), and CA is the albumin concentration (assumed to be 4.3 g/dl) (20).
Formulas for converting from conventional to SI units are: P × 3.18 (pmol/liter); total T × 3.47 (pmol/liter); free T × 3.47 (pmol/liter); E2 × 3.67 (pmol/liter); DHEAS × 0.0027 (μmol/liter); and insulin × 7.18 (pmol/liter).
Data and statistical analysis
LH pulses were detected using the computer algorithm Cluster 7 (21). Analysis parameters were a test nadir and peak size of 2 × 2 with a t statistic of 2.45 for both up- and downstrokes. Missing LH values made up less than 0.1% of the total and were ignored. As described previously, LH pulses detected by the Cluster 7 program were accepted only if the pulse amplitude exceeded the range of LH intraassay variability: specifically, amplitude greater than 0.1 IU/liter for pulses with peaks 0.1–0.5 IU/liter, greater than 0.25 IU/liter for peaks 0.5–1 IU/liter, greater than 0.5 IU/liter for peaks 1–5 IU/liter, and greater than 1 IU/liter for peaks greater than 5 IU/liter (22).
All data are presented as mean ± sd unless otherwise indicated. All hypothesis tests were two-sided and were conducted at the 0.05 level of significance. Hormone levels for d 0 and 7 are the means of measurements performed during each admission. Given the small number of subjects, we employed nonparametric statistical tests that are based on ranks of observations and require no assumptions about the underlying distribution of data. Wilcoxon rank-sum tests were used for the majority of comparisons. When comparison involved at least 10 observations from each sample, the method of normal approximation was employed; otherwise, exact tests were performed. Because all of the HA subjects were Tanner stage 3-5, the primary comparisons were made between the Tanner 3-5 NC and Tanner 3-5 HA subjects. The slope of the percentage reduction in LH pulses per 11 h as a function of d 7 P concentration was used as a measure of hypothalamic P sensitivity and will be referred to as “slope.” P sensitivities (slopes) in the NC and HA subjects were compared, as well as various hormonal and clinical parameters. To further evaluate the variability in hypothalamic P sensitivity in the HA population, we defined two groups: HA girls who fell within the range of responses seen in Tanner 3-5 NC subjects (delineated by the outlined area in Fig. 1B) were defined as “P-sensitive,” whereas those who did not were defined as “P-insensitive.” We compared hormonal and clinical parameters in the P-sensitive and P-insensitive subgroups. Comparisons were also made between the Tanner 1-2 and Tanner 3-5 NC subjects. Fisher’s exact tests were used to assess the associations between P-sensitivity status and both study site (UVA vs. UCSD) and Hispanic ethnicity. Spearman rank correlation was used to evaluate the relationship between free T and P sensitivity (slope) in all subjects and between fasting insulin levels and P sensitivity (slope) in the HA subjects.
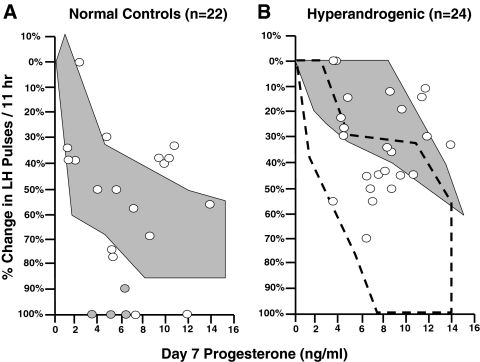
Figure 1.
The percentage change in LH pulse frequency per 11 h after 7 d of oral E2 and P in NC (A) and HA (B) adolescent girls. The data are plotted as a function of mean plasma P on d 7. The white circles represent the Tanner 3-5 girls. The shaded circles represent the Tanner 1-2 girls. The shaded areas represent the range of responses to 7 d of oral E2 and P in NC adult women (A) and adult women with PCOS (B). The outlined area in panel B represents the range of responses in NC adolescent girls.
Due to a combination of pharmacy and patient error, four subjects did not receive the 2300 h P dose during the d 7 admission. Given data in adult women that there is continued suppression of LH pulsatility at least 7 d after completion of a 10-d course of P (22), we felt that it was unlikely that this missed final dose had an impact on the d 7 LH pulse frequency. However, the missed dose may have falsely lowered their mean d 7 P level compared with those who took the 2300 h P dose. Therefore, we extrapolated mean d 7 P levels for these subjects using their mean 1700–2300 P level and an equation derived from subjects who took the full study medication regimen. The r2 value for the equation describing the relationship between 1700–2300 P level and mean d 7 level was 0.90.
Results
Baseline clinical and hormonal characteristics for the 46 subjects included in the data analysis are shown in Table 1. Six subjects (two HA and four NC) were excluded from the final analysis. Five had d 7 P levels above 15 ng/ml (range, 21.5–33.4 ng/ml); P levels above 15 ng/ml suppress LH pulse frequency in all adult women, including those with PCOS (10). One subject was noncompliant with the study medications.
Table 1.
Baseline clinical and hormonal characteristics of NC and HA subjects
| Tanner 1-2 NC (n = 4) | Tanner 3-5 NC (n = 18) | Tanner 3-5 HA (n = 24) | |
|---|---|---|---|
| Age (yr) | 9.7 ± 1.6 (0.8, 9.5)b | 13.9 ± 1.9 (0.5, 13.9) | 16.1 ± 1.7 (0.3, 16.0)b |
| Years postmenarche | NA | 1.5 ± 1.9 (0.4, 0.7) | 3.6 ± 1.8 (0.4, 3.0)b |
| BMI (kg/m2) | 20.2 ± 6.2 (3.1, 17.2) | 22.0 ± 3.9 (0.9, 21.4) | 33.9 ± 7.6 (1.6, 33.3)c |
| BMI-for-age percentile | 68 ± 21 (10, 62) | 67 ± 28 (7, 79) | 92 ± 17 (3, 98)c |
| Total T (ng/dl) | 5.4 ± 1.9 (0.9, 5.1)c | 22.1 ± 8.6 (2.0, 22.5) | 51.3 ± 17.1 (3.5, 49.4)c |
| SHBG (nmol/liter) | 26.5 ± 14.6 (7.3,26.6) | 36.2 ± 16.5 (3.9, 32.9) | 16.8 ± 9.7 (2.0, 13.6)c |
| Free T (pg/ml) | 1.1 ± 0.4 (0.2,1.1)b | 4.0 ± 2.0 (0.5, 3.2) | 13.5 ± 5.2 (1.1, 13.4)c |
| DHEAS (mg/dl) | 53.8 ± 39.0 (19.5, 47.5)a | 122.8 ± 59.4 (14.4, 112.0) | 138.8 ± 68.3 (14.0, 125.5) |
| Androstenedione (ng/ml) | 0.7 ± 0.3 (0.1, 0.7)b | 1.5 ± 0.5 (0.1, 1.5) | 2.2 ± 1.0 (0.2, 1.9)b |
| E1 (pg/ml) | 29.0 ± 10.8 (5.4, 29.6) | 39.3 ± 19.8 (4.7, 34.8) | 55.5 ± 20.7 (4.2, 53.8)a |
| E2 (pg/ml) | 37.8 ± 21.1 (10.6, 43.9) | 57.0 ± 16.9 (4.0, 57.0) | 63.3 ± 18.1 (3.7, 61.7) |
| P (ng/ml) | 0.4 ± 0.1 (0.1, 0.3) | 0.4 ± 0.2 (0.0, 0.4) | 0.6 ± 0.2 (0.0, 0.6)b |
| LH (IU/liter) | 1.5 ± 1.6 (0.8, 1.1)b | 5.6 ± 3.2 (0.8, 4.7) | 7.9 ± 4.4 (0.9, 7.3) |
| FSH (IU/liter) | 2.0 ± 1.2 (0.6, 2.1)b | 4.6 ± 1.5 (0.4, 4.4) | 3.9 ± 0.8 (0.2, 3.8) |
| Fasting insulin (mIU/ml) | 17.2 ± 21.3 (10.6, 9.1) | 21.5 ± 12.0 (2.8, 18.3) | 28.7 ± 22.8 (4.6, 22.7) |
Data are presented as mean± sd (sem, median). NA, Not applicable.
P < 0.05;
P < 0.01;
P < 0.001 vs. Tanner 3-5 NC.
Tanner 1-2 NC were younger and had lower total T, free T, androstenedione, DHEAS, LH, and FSH than the Tanner 3-5 NC. Tanner 3-5 HA subjects were older, both in chronological and gynecological (years postmenarche) age, than Tanner 3-5 NC subjects. They had higher body mass index (BMI) and BMI-for-age percentiles. In addition to higher total T, HA subjects had lower SHBG levels, resulting in free T levels that were 3.4-fold higher than the NC. Androstenedione and E1 levels were higher in the HA subjects. P levels were also higher in the HA subjects, although no subject had a baseline P greater than 1.0 ng/ml. No other differences were found in baseline hormonal parameters.
The results before and after 7 d of E2 and P in the NC and HA adolescent subjects are shown numerically in Table 2 and graphically in Fig. 1, with comparison to historical data in NC adults and adult women with PCOS (10). In the Tanner 3-5 subjects, the HA adolescents as a whole were less sensitive to the effects of P than the NC (slope, 4.7 ± 3.4 vs. 10.3 ± 7.7; P = 0.006). Although the adolescent NC behaved similarly to their adult counterparts, the HA subjects had considerably more variability in their responses to E2 and P than the adult women with PCOS. In fact, 15 HA girls had slopes that fell within the range of responses seen in the adolescent NC (defined by the outlined area in Fig. 1B).
Table 2.
Effects of 7 d of oral E2 and P in NC and HA adolescent girls
| Tanner 1-2 NC (n = 4) | Tanner 3-5 NC (n = 18) | Tanner 3-5 HA
|
|||
|---|---|---|---|---|---|
| All (n = 24) | P-sensitive (n = 15) | P-insensitive (n = 9) | |||
| Day 0 | |||||
| E2 (pg/ml) | 38 ± 21 (11, 44) | 57 ± 17 (4, 57) | 63 ± 18 (4, 62) | 67 ± 14 (4, 66) | 57 ± 24 (8, 54) |
| P (ng/ml) | 0.4 ± 0.1 (0.1, 0.3) | 0.4 ± 0.2 (0.0, 0.4) | 0.6 ± 0.2 (0.0, 0.6)b | 0.6 ± 0.1 (0.0, 0.6) | 0.6 ± 0.2 (0.1, 0.6) |
| FSH (IU/liter) | 2.0 ± 1.2 (0.6, 2.1)b | 4.6 ± 1.5 (0.4, 4.4) | 3.9 ± 0.8 (0.2, 3.8) | 3.8 ± 0.5 (0.1, 3.8) | 4.0 ± 1.1 (0.4, 3.5) |
| LH (IU/liter) | 1.5 ± 1.6 (0.8, 1.1)b | 5.6 ± 3.2 (0.8, 4.7) | 7.9 ± 4.4 (0.9, 7.3) | 7.7 ± 3.7 (1.0, 7.5) | 8.4 ± 5.5 (1.8, 6.4) |
| LH pulses/11 h | 5.3 ± 3.0 (1.5, 5.0) | 6.9 ± 1.7 (0.4, 7.0) | 9.5 ± 1.4 (0.3, 9.5)c | 10.0 ± 1.0 (0.3, 10.0) | 8.8 ± 1.6 (0.6, 8.0)d |
| Mean LH amplitude (IU/liter) | 1.4 ± 1.0 (0.5, 1.7)b | 4.3 ± 2.0 (0.5, 3.8) | 3.3 ± 2.0 (0.4, 2.8)a | 3.0 ± 1.5 (0.4, 2.9) | 3.7 ± 2.7 (0.9, 2.7) |
| Day 7 | |||||
| E2 (pg/ml) | 145 ± 50 (25, 148) | 166 ± 103 (24, 139) | 122 ± 47 (10, 117) | 128 ± 49 (13, 114) | 114 ± 44 (15, 123) |
| P (ng/ml) | 5.3 ± 1.2 (0.6, 5.7) | 6.8 ± 3.8 (0.9, 6.3) | 7.7 ± 2.9 (0.6, 7.9) | 6.9 ± 2.0 (0.5, 7.6) | 8.9 ± 3.9 (1.3, 9.7) |
| FSH (IU/liter) | 0.1 ± 0.2 (0.1, 0.0)c | 2.8 ± 1.7 (0.4, 2.3) | 3.2 ± 0.8 (0.2, 3.0) | 3.1 ± 0.5 (0.1, 3.0) | 3.4 ± 1.1 (0.4, 2.9) |
| LH (IU/liter) | 0.1 ± 0.1 (0.0, 0.1)c | 5.2 ± 4.7 (1.1, 5.0) | 6.6 ± 3.1 (0.6, 6.0) | 6.4 ± 3.1 (0.8, 6.4) | 7.0 ± 3.3 (1.1, 5.1) |
| LH pulses/11 h | 0.3 ± 0.5 (0.3, 0.0)b | 3.5 ± 2.0 (0.5, 4.0) | 6.3 ± 1.5 (0.3, 6.5)c | 5.7 ± 1.4 (0.4, 6.0) | 7.3 ± 0.9 (0.3, 8.0)e |
| Mean LH amplitude (IU/liter) | 0.3g | 7.9 ± 5.8 (1.4, 6.3) | 5.3 ± 2.9 (0.6, 4.3) | 5.7 ± 3.2 (0.8, 4.5) | 4.6 ± 2.4 (0.8, 3.9) |
| Reduction in LH pulses | |||||
| Absolute | 5.0 ± 2.6 (1.3, 5.0) | 3.4 ± 1.4 (0.3, 3.0) | 3.3 ± 1.9 (0.4, 3.5) | 4.3 ± 1.2 (0.3, 5.0) | 1.4 ± 1.3 (0.4, 1.0)f |
| Percent | 97 ± 6 (3, 100)b | 51 ± 26 (6, 45) | 33 ± 18 (4, 33)a | 43 ± 12 (3, 44) | 15 ± 12 (4, 14)f |
| % Reduction LH pulses/d 7 P | 19.3 ± 5.8 (2.9, 17.8)a | 10.3 ± 7.7 (1.8, 8.2) | 4.7 ± 3.4 (0.7, 4.6)b | 6.7 ± 2.8 (0.7, 5.9) | 1.5 ± 1.0 (0.3, 1.5)f |
Data are presented as mean± sd (sem, median).
P < 0.05 vs. Tanner 3-5 NC;
P < 0.01 vs. Tanner 3-5 NC;
P < 0.001 vs. Tanner 3-5 NC;
P < 0.05 vs. HA P-sensitive;
P < 0.01 vs. HA P-sensitive;
P < 0.001 vs. HA P-sensitive;
Only one pulse was present in the Tanner 1-2 NC population on d 7.
To evaluate further the variability in hypothalamic P sensitivity in the HA girls, these 15 subjects were defined as P-sensitive, whereas the nine subjects who fell outside of the range of responses seen in NC subjects were defined as P-insensitive. Baseline clinical and hormonal characteristics of the P-sensitive and P-insensitive HA subgroups are shown in Table 3. The P-sensitive subgroup had greater reductions in LH pulse frequency than the P-insensitive subgroup, in both absolute and percentage terms, despite achieving similar d 7 P levels (6.9 ± 1.9 vs. 8.9 ± 3.9 ng/ml). Therefore, the slope in the P-sensitive subjects was steeper (6.7 ± 2.8 vs. 1.5 ± 1.0; P < 0.0001). Peak P levels measured on d 3 and d 5 confirmed that the P-insensitive subjects were compliant with study medications. Baseline free T was not different in the two subgroups and, if anything, tended to be higher in the P-sensitive HA subjects (14.3 ± 4.7 vs. 12.0 ± 6.1 pg/ml). Baseline fasting insulin levels were lower in the P-sensitive subgroup (22.2 ± 13.9 vs. 39.6 ± 30.6 μIU/ml; P = 0.02) despite similar BMI values (34.1 ± 8.1 vs. 33.4 ± 7.0 kg/m2; BMI-for-age percentile 93 ± 10 vs. 89 ± 24). No other differences were found in the baseline hormonal parameters. The two subgroups were similar in age and years postmenarche. There was a trend toward increased Hispanic subjects in the P-sensitive group; only one of the nine P-insensitive subjects was Hispanic, whereas eight of the 15 P-sensitive subjects were Hispanic (P = 0.08). P-sensitive subjects were more likely to have been studied at UCSD than UVA (P = 0.03).
Table 3.
Clinical and hormonal characteristics of Tanner 3-5 HA P-sensitive and P-insensitive subjects
| HA P-sensitive (n = 15) | HA P-insensitive (n = 9) | |
|---|---|---|
| Age (yr) | 16.0 ± 1.5 (0.4, 16.1) | 16.2 ± 2.1 (0.7, 16.0) |
| Years postmenarche | 3.7 ± 1.7 (0.4, 3.0) | 3.6 ± 2.0 (0.7, 0.9) |
| BMI (kg/m2) | 34.1 ± 8.1 (2.1, 31.8) | 33.4 ± 7.0 (2.3, 33.6) |
| BMI-for-age percentile | 93 ± 10.3 (3, 96) | 89 ± 24 (8, 98) |
| Ethnicity (no. of subjects) | ||
| Hispanic | 8 | 1 |
| Non-Hispanic | 7 | 8 |
| Baseline | ||
| Total T (ng/dl) | 55.8 ± 16.2 (4.2, 59.0) | 43.7 ± 16.5 (5.5, 49.0) |
| SHBG (nmol/liter) | 16.6 ± 8.5 (2.2, 15.1) | 16.9 ± 12.0 (4.0, 12.3) |
| Free T (pg/ml) | 14.3 ± 4.7 (1.2, 12.6) | 12.0 ± 6.1 (2.0, 14.0) |
| DHEAS (μg/dl) | 137 ± 72 (21, 126) | 153 ± 75 (25, 162) |
| Androstenedione (ng/ml) | 2.3 ± 1.1 (0.3, 2.0) | 1.9 ± 0.6 (0.2, 1.7) |
| E1 (pg/ml) | 57.7 ± 20.2 (5.2, 61.0) | 51.7 ± 22.1 (7.4, 38.6) |
| E2 (pg/ml) | 66.9 ± 13.6 (3.5, 65.9) | 57.3 ± 23.6 (7.9, 54.2) |
| P (ng/ml) | 0.6 ± 0.1 (0.0, 0.6) | 0.6 ± 0.2 (0.1, 0.6) |
| LH (IU/liter) | 7.7 ± 3.7 (1.0, 7.5) | 8.4 ± 5.5 (1.8, 6.4) |
| FSH (IU/liter) | 3.8 ± 0.5 (0.1, 3.8) | 4.0 ± 1.1 (0.4, 3.5) |
| Fasting insulin (μIU/ml) | 22.2 ± 13.9 (3.6, 18.1) | 39.6 ± 30.6 (10.2, 29.4)a |
| Day 7 | ||
| Total T (ng/dl) | 50.8 ± 14.4 (3.7, 50.1) | 46.6 ± 19.0 (6.3, 44.0) |
| SHBG (nmol/liter) | 19.4 ± 10.1 (2.6, 17.9) | 18.9 ± 13.2 (4.4, 14.7) |
| Free T (pg/ml) | 12.2 ± 3.9 (1.0, 11.7) | 12.1 ± 6.4 (2.1, 11.7) |
| Androstenedione (ng/ml) | 1.9 ± 0.8 (0.2, 1.7) | 1.8 ± 0.6 (0.2, 1.7) |
| E1 (pg/ml) | 142 ± 52 (13, 146) | 161 ± 64 (22, 149) |
| E2 (pg/ml) | 128 ± 49 (13, 114) | 114 ± 44 (15, 123) |
| P (ng/ml) | 6.9 ± 2.0 (0.5, 7.3) | 8.9 ± 3.9 (1.3, 9.7) |
| LH (IU/liter) | 6.4 ± 3.1 (0.8, 6.4) | 7.0 ± 3.3 (1.1, 5.1) |
| FSH (IU/liter) | 3.1 ± 0.5 (0.1, 3.0) | 3.4 ± 1.1 (0.4, 2.9) |
Data are presented as mean ± sd (sem, median). Insulin levels on d 7 were not reported because subjects were not fasting secondary to snack given with 2300 h dose of P.
P = 0.02.
When the 14 HA subjects studied at UVA were analyzed separately, the association between P insensitivity and higher insulin levels persisted in the HA subjects, although it no longer achieved statistical significance (P = 0.06). No other differences emerged between the P-sensitive and P-insensitive subgroups.
The data were also reanalyzed using an alternative definition of P sensitivity. In this analysis, HA girls who fell within the range of responses seen in adult women with PCOS (shaded area in Fig. 1B) were defined as P-insensitive (n = 15), with the remaining subjects being defined as P-sensitive (n = 9). Again, the P-insensitive subjects had higher fasting insulin levels (P = 0.01), but no other differences were observed.
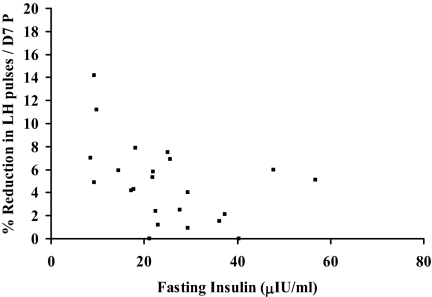
In the HA population as a whole, there was an inverse linear correlation between fasting insulin and P sensitivity (slope) (Spearman correlation coefficient, 0.48; P = 0.02) (Fig. 2).
Figure 2.
The relationship of fasting insulin concentration and the slope of the percentage reduction in LH pulse frequency as a function of d 7 P in HA girls. One outlier (fasting insulin 119 μIU/ml, slope 2.8) was excluded from the graph.
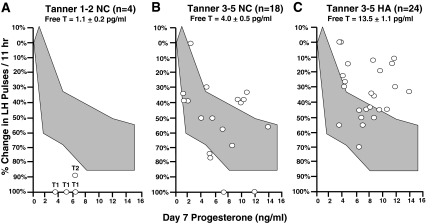
In the Tanner 1-2 NC subjects, the slope of the percentage reduction in LH pulses as a function of d 7 P was steeper than in the Tanner 3-5 NC (19.3 ± 5.8 vs. 10.3 ± 7.7; P = 0.04). All three of the Tanner 1 subjects had 100% suppression of their pulses after 7 d of E2 and P, whereas the Tanner 2 subject had 89% suppression. The d 7 P levels in the Tanner 1-2 subjects were similar to that seen in the older girls (5.3 ± 1.2 vs. 6.8 ± 3.8 ng/ml). The baseline free T levels in the Tanner 1-2 NC were less than one third of that seen in the Tanner 3-5 NC (P = 0.001).
Figure 3 illustrates the physiological decrease in hypothalamic P sensitivity associated with pubertal progression, as well as the further pathological decrease seen with HA. Given that free T rises during female puberty (17) and androgens mediate P insensitivity in adult women with PCOS (11), we examined the relationship between free T and P sensitivity. When all 46 subjects were analyzed, there was an inverse linear correlation between free T and P sensitivity (slope) (Spearman correlation coefficient, 0.41; P = 0.005).
Figure 3.
The percentage change in LH pulse frequency per 11 h after 7 d of oral E2 and P in Tanner 1-2 NC (A), Tanner 3-5 NC (B), and HA adolescent girls (C). The data are plotted as a function of mean plasma P on d 7. The Tanner 1-2 subjects are labeled with the Tanner stages of the individual subjects. The shaded areas represent the range of responses to 7 d of oral E2 and P in NC adult women.
Discussion
Unlike adult women with PCOS, who consistently demonstrate reduced sensitivity of the GnRH pulse generator to P-mediated suppression, this study provides further evidence for varied hypothalamic P sensitivity in adolescents with HA. These results suggest a continuous spectrum of P sensitivities within the HA adolescent population, ranging from normal to markedly impaired. Sixty-three percent of HA subjects had normal responses to luteal levels of P. The androgen levels in these subjects were equivalent to those seen in the HA subjects who were relatively resistant to P-mediated slowing, indicating that hyperandrogenemia is not the sole modulator of P sensitivity during adolescence.
In addition to supporting earlier findings of variable P sensitivity, this study found that P insensitivity was associated with elevated fasting insulin levels, suggesting that hyperinsulinemia may negatively impact hypothalamic P sensitivity. We previously reported that short-term treatment (5 wk) with the insulin-sensitizing agent metformin does not alter hypothalamic P sensitivity in adult women with PCOS despite some improvement in fasting insulin and T levels (23). Although there was no change in the PCOS population as a whole, a subset of subjects with lower baseline insulin levels and greater reductions in both fasting insulin and T while on metformin did have normalization of P sensitivity. It is unclear whether this improvement was due directly to a reduction in insulin levels or secondary to a reduction in insulin-augmented HA. Data on the neuroendocrine effects of hyperinsulinemia in adolescence are not yet available, and it is possible that insulin has effects on the developing hypothalamic-pituitary-ovarian axis that are absent in adulthood. The mechanisms by which insulin may act centrally to affect hypothalamic P sensitivity are unknown; however, given that insulin acts synergistically with LH to promote androgen production by the ovarian theca cells, it is possible that insulin acts through similar cellular pathways to alter P action at the level of the hypothalamus. Alternatively, given that HA may contribute to insulin resistance (24), the association between hyperinsulinemia and reduced P sensitivity in this study may indicate a phenotype characterized by increased sensitivity to the adverse effects of androgens on both hypothalamic P sensitivity and insulin resistance.
This study also found an association between P sensitivity and study site, with the P-sensitive subjects being more likely to have been studied at UCSD. This association may be partially explained by the trend toward Hispanic HA subjects having normal P sensitivity because the two sites recruit from local populations with different ethnic compositions: 82% of subjects studied at USCD were Hispanic vs. only 9% at UVA. The variability in hypothalamic P sensitivity and the association between P insensitivity and higher insulin levels persisted regardless of whether the subjects from UCSD were included in the analysis.
The clinical significance of the variable hypothalamic P sensitivity is unclear; however, it is possible that girls who maintain normal P sensitivity may fail to develop abnormalities of LH (GnRH) secretion and the full-blown PCOS phenotype. In a prevalence study in unselected blood donors in Spain, 14% of women not taking oral contraceptives had hyperandrogenemia with regular menses (1). Although detailed studies of ovulatory function were not performed, this suggests that a significant number of women with hyperandrogenemia do not develop PCOS by National Institutes of Health criteria (4). All of the HA P-sensitive girls described some degree of menstrual irregularity at the time of the study (range of 0–10 cycles a year). However, our ability to follow the natural course of their symptoms was limited by the fact that the majority of subjects initiated metformin or oral contraceptive pill treatment shortly after study completion.
We recognize some limitations to this study. The sample size was small in absolute terms, although relatively robust given the age of the subjects and the complexity of the study protocol. Given that the distribution of P sensitivities in the HA population appears to be continuous rather than dichotomous, any line defining P-sensitive and P-insensitive is somewhat arbitrary. However, we believe that using the results in NC adolescents as a reference is a reasonable approach, and the results were not substantively changed when an alternative definition of P insensitivity (using the results in adult women with PCOS as a reference) was employed. In addition, the inverse relationship between hypothalamic P sensitivity and fasting insulin levels in the entire HA population was confirmed using Spearman rank correlation. Our data are also limited in that we only have fasting insulin levels for these subjects. Fasting insulin is an imprecise measure of hyperinsulinemia and insulin resistance, and studies have varied widely as to its validity in obese pubertal populations (25,26). Formal studies with detailed measures of insulin sensitivity are needed to confirm a relationship between insulin resistance and hypothalamic P insensitivity.
The mechanisms that regulate the initiation and progression of puberty are not completely understood. Both central neural mechanisms and attenuation of sex steroid-mediated negative feedback have been postulated to underlie the pubertal augmentation in GnRH secretion (27,28). Although the sample size is small, the Tanner 1-2 subjects, all of whom had free T levels less than 2 pg/ml, were particularly sensitive to the effects of E2 and P on LH pulsatility. The finding that GnRH pulse generator sensitivity to negative sex steroid feedback decreases during pubertal progression is consistent with earlier human (29) and animal data (30,31) demonstrating decreased sex steroid-mediated suppression of tonic FSH and LH secretion during pubertal maturation. We have hypothesized that the rise in free T levels during puberty leads to a progressive decline in hypothalamic sensitivity to E2 and P feedback (15,16). In part, this could effect the evolution of the pattern of gonadotropin secretion from the slow, low amplitude pulses of childhood, to the diurnal pattern of early puberty (in which LH pulses are seen primarily during sleep before the early morning rise in P and later E2), to the continuous 24-h pulse pattern characteristic of late puberty and adulthood. If T does modulate hypothalamic sensitivity and pubertal maturation, one would expect the process to be exaggerated in girls with HA. In fact, girls with HA progress to the adult 24-h pattern of LH secretion approximately 2 yr before their normal counterparts (13). In addition, peripubertal obesity, which is associated with 2- to 9-fold elevations in free T (17,32), has been associated with earlier pubertal maturation (33,34). The findings of the present study, in which early pubertal girls have lower T concentrations and increased P sensitivity compared with girls in later puberty, are consistent with this hypothesis but require confirmation in larger numbers of early pubertal girls.
In conclusion, whereas there is an inverse relationship between androgen levels and the sensitivity of the GnRH pulse generator to P-mediated slowing during adolescence, impaired hypothalamic P sensitivity is not universally seen in HA girls. Additional factors, such as insulin resistance, may modulate hypothalamic P sensitivity and influence whether HA girls go on to develop the full clinical spectrum of PCOS.
Acknowledgments
We gratefully acknowledge Lauren Lockhart, M.P.H., Carrie C. Boyd, M.P.H., Quirine Lamberts Okonkwo, M.D., Chandan Chopra, and Amy Bellows, Ph.D., for research coordination; the nurses and staff of the General Clinical Research Center for implementation of the sampling protocols; and the Center for Research in Reproduction Ligand Core Laboratory for performance of all assays.
Footnotes
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement U54 HD28934, as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research through Grants U54-HD34179 (to UVA) and U54-HD12303 (to UCSD); General Clinical Research Center grants M01 RR00847 (to UVA) and M01 RR00827 (to UCSD); F32 HD055014 (to S.K.B.); K23 HD044742 (to C.R.M.); and T32 HD07383 (to K.D.H. and S.C.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 7, 2009
For editorial see page 2250
Abbreviations: CV, Coefficient of variation; DHEAS, dehydroepiandrosterone sulfate; E1, estrone; E2, estradiol; HA, hyperandrogenism; NC, normal control(s); P, progesterone; PCOS, polycystic ovary syndrome; T, testosterone.
References
- Asunción M, Calvo RM, San Millán JL, Sancho J, Avila S, Escobar-Morreale HF 2000 A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab 85:2434–2438 [DOI] [PubMed] [Google Scholar]
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO 2004 The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749 [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, Zapanti ED, Bartzis MI 1999 A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab 84:4006–4011 [DOI] [PubMed] [Google Scholar]
- Zawadski J, Dunaif A 1992 Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens J, Haseltine F, Merriam G, eds. Polycystic ovary syndrome. Oxford, UK: Blackwell Scientific; 377–384 [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF 2006 Position statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 91:4237–4245 [DOI] [PubMed] [Google Scholar]
- Gross KM, Matsumoto AM, Bremner WJ 1987 Differential control of luteinizing hormone and follicle-stimulating hormone secretion by luteinizing hormone-releasing hormone pulse frequency in man. J Clin Endocrinol Metab 64:675–680 [DOI] [PubMed] [Google Scholar]
- Spratt DI, Finkelstein JS, Butler JP, Badger TM, Crowley Jr WF 1987 Effects of increasing the frequency of low doses of gonadotropin-releasing hormone (GnRH) on gonadotropin secretion in GnRH-deficient men. J Clin Endocrinol Metab 64:1179–1186 [DOI] [PubMed] [Google Scholar]
- Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, Hall JE 1997 Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab 82:2248–2256 [DOI] [PubMed] [Google Scholar]
- Daniels TL, Berga SL 1997 Resistance of gonadotropin releasing hormone drive to sex steroid-induced suppression in hyperandrogenic anovulation. J Clin Endocrinol Metab 82:4179–4183 [DOI] [PubMed] [Google Scholar]
- Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC 1998 Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 83:582–590 [DOI] [PubMed] [Google Scholar]
- Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC 2000 Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 85:4047–4052 [DOI] [PubMed] [Google Scholar]
- Venturoli S, Porcu E, Fabbri R, Magrini O, Gammi L, Paradisi R, Flamigni C 1992 Longitudinal evaluation of the different gonadotropin pulsatile patterns in anovulatory cycles of young girls. J Clin Endocrinol Metab 74:836–841 [DOI] [PubMed] [Google Scholar]
- Apter D, Bützow T, Laughlin GA, Yen SS 1994 Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab 79:119–125 [DOI] [PubMed] [Google Scholar]
- Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC 2005 Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenemic adolescent girls. J Clin Endocrinol Metab 90:2810–2815 [DOI] [PubMed] [Google Scholar]
- Blank SK, McCartney CR, Marshall JC 2006 The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update 12:351–361 [DOI] [PubMed] [Google Scholar]
- Blank SK, McCartney CR, Helm KD, Marshall JC 2007 Neuroendocrine effects of androgens in adult polycystic ovary syndrome and female puberty. Semin Reprod Med 25:352–359 [DOI] [PubMed] [Google Scholar]
- McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S, Marshall JC 2007 Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab 92:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankarberg C, Norjavaara E 1999 Diurnal rhythm of testosterone secretion before and throughout puberty in healthy girls: correlation with 17β-estradiol and dehydroepiandrosterone sulfate. J Clin Endocrinol Metab 84:975–984 [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM 1969 Variations in pattern of pubertal changes in girls. Arch Dis Child 44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM 1999 A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML 1986 Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol 250:E486–E493 [DOI] [PubMed] [Google Scholar]
- McCartney CR, Gingrich MB, Hu Y, Evans WS, Marshall JC 2002 Hypothalamic regulation of cyclic ovulation: evidence that the increase in gonadotropin-releasing hormone pulse frequency during the follicular phase reflects the gradual loss of the restraining effects of progesterone. J Clin Endocrinol Metab 87:2194–2200 [DOI] [PubMed] [Google Scholar]
- Eagleson CA, Bellows AB, Hu K, Gingrich MB, Marshall JC 2003 Obese patients with polycystic ovary syndrome: evidence that metformin does not restore sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by ovarian steroids. J Clin Endocrinol Metab 88:5158–5162 [DOI] [PubMed] [Google Scholar]
- Dunaif A 1997 Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18:774–800 [DOI] [PubMed] [Google Scholar]
- Rössner SM, Neovius M, Montgomery SM, Marcus C, Norgren S 2008 Alternative methods of insulin sensitivity assessment in obese children and adolescents. Diabetes Care 31:802–804 [DOI] [PubMed] [Google Scholar]
- Conwell LS, Trost SG, Brown WJ, Batch JA 2004 Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care 27:314–319 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Fernandez DL 2001 Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev 22:111–151 [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- Kelch RP, Kaplan SL, Ghumbach MM 1973 Suppression of urinary and plasma follicle-stimulating hormone by exogenous estrogens in prepubertal and pubertal children. J Clin Invest 52:1122–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapisarda JJ, Bergman KS, Steiner RA, Foster DL 1983 Response to estradiol inhibition of tonic luteinizing hormone secretion decreases during the final stage of puberty in the rhesus monkey. Endocrinology 112:1172–1179 [DOI] [PubMed] [Google Scholar]
- Foster DL, Ryan KD 1979 Endocrine mechanisms governing transition into adulthood: a marked decrease in inhibitory feedback action of estradiol on tonic secretion of luteinizing hormone in the lamb during puberty. Endocrinology 105:896–904 [DOI] [PubMed] [Google Scholar]
- McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, Foster CM, Marshall JC 2006 The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab 91:1714–1722 [DOI] [PubMed] [Google Scholar]
- Wang Y 2002 Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics 110:903–910 [DOI] [PubMed] [Google Scholar]
- Anderson SE, Must A 2005 Interpreting the continued decline in the average age at menarche: results from two nationally representative surveys of U.S. girls studied 10 years apart. J Pediatr 147:753–760 [DOI] [PubMed] [Google Scholar]