Abstract
Seven-transmembrane-spanning receptors (7TMRs) are prominent drug targets. However, small-molecule ligands for 7-transmembrane-spanning receptors for which the natural ligands are large, heterodimeric glycoprotein hormones, like thyroid-stimulating hormone (TSH; thyrotropin), have only recently been reported, and none are approved for human use. We have used quantitative high-throughput screening to identify a small-molecule TSH receptor (TSHR) agonist that was modified to produce a second agonist with increased potency. We show that these agonists are highly selective for human TSHR versus other glycoprotein hormone receptors and interact with the receptor's serpentine domain. A binding pocket within the transmembrane domain was defined by docking into a TSHR homology model and was supported by site-directed mutagenesis. In primary cultures of human thyrocytes, both TSH and the agonists increase mRNA levels for thyroglobulin, thyroperoxidase, sodium iodide symporter, and deiodinase type 2, and deiodinase type 2 enzyme activity. Moreover, oral administration of the agonist stimulated thyroid function in mice, resulting in increased serum thyroxine and thyroidal radioiodide uptake. Thus, we discovered a small molecule that activates human TSHR in vitro, is orally active in mice, and could be a lead for development of drugs to use in place of recombinant human TSH in patients with thyroid cancer.
Keywords: 7TMR, G protein-coupled receptor, low-molecular-weight ligands, radioiodide uptake, TSH receptor
Small-molecule agonists and antagonists for 7-transmembrane-spanning receptors (7TMRs; G protein-coupled receptors) make up approximately 40% of the drugs in clinical use (1). The natural ligands for the 7TMRs for which drugs have been discovered are themselves primarily small molecules, such as norepinephrine, adenosine, and acetylcholine. Small-molecule ligands for the subclass of 7TMRs for which the natural ligands are 30-kDa heterodimeric glycoprotein hormones have recently begun to be described, but none have been approved for use in humans. For example, small-molecule agonists (2–5) and an antagonist (6) for the luteinizing hormone/chorionic gonadotropin receptor (LHCGR), and small-molecule agonists (7–9) and antagonists (10–12) for the FSH receptor (FSHR) have been reported. The thyrotropin (thyroid-stimulating hormone, TSH) receptor (TSHR) is the third member of the glycoprotein hormone receptor subfamily. We recently reported small-molecule agonists (13) and an antagonist (14) for human TSHR; however, the agonists were of low affinity and were shown to activate TSHRs only in a model cell system in which human TSHRs were ectopically over-expressed.
The physiologic role of TSHRs in the hypothalamic-pituitary-thyroid axis is well known. TSH, which is produced in the thyrotrophs of the anterior pituitary gland, is secreted into the circulation in response to TSH-releasing hormone stimulation and in turn stimulates the function of the thyroid follicular cells (i.e., thyrocytes) leading, in particular, to increases in size and number of thyrocytes, increases in iodide uptake, and biosynthesis and secretion of thyroid hormones. However, TSHRs are known to be expressed in multiple extrathyroidal tissues including bone, brain, kidney, testis, and cells of the immune system (15), but the role of TSHR in these tissues is not clear. For example, potential effects of TSHR activation on bone metabolism have been proposed (16, 17), but contradictory findings have been reported (18, 19) and a definitive conclusion has not been drawn (15). A small-molecule agonist that can be administered orally, diffuses out of the bloodstream easily, and can be produced at low cost may allow for any effect of TSHR activation on bone and the other tissues that express TSHRs to be definitively shown.
Recombinant human TSH (rhTSH; Thyrogen) is currently prescribed primarily as an adjunct for monitoring patients who are taking thyroid hormones after undergoing ablative surgery for thyroid cancer (20). rhTSH stimulates uptake of iodide and release of thyroglobulin (TG) from any residual thyroid cancer and thereby increases the sensitivity of measurements of radioiodine uptake or serum TG levels in these patients. However, rhTSH is difficult to produce, which leads to its high cost, and must be administered by injection. A small-molecule TSHR agonist would be worthwhile because it could produce the same beneficial effects as rhTSH but at a lower cost and with greater ease of oral administration, and may therefore be available for use in a larger patient population.
In this report, we describe a small-molecule TSHR agonist that can be used as a probe of TSHR function in animal studies and could serve as a lead for development of drugs that could be used in place of rhTSH in patients with thyroid cancer.
Results
Identification of Small-Molecule TSHR Agonists by Quantitative High-Throughput Screening.
We used quantitative high-throughput screening (qHTS) to identify TSHR agonists. This robust screening method allows in-depth analysis of the primary screening results and provides detailed information regarding potency, efficacy, and structure-activity relationships (21). We used a cell line that expresses both a cyclic nucleotide-gated cation channel (22) and TSHR (13). The qHTS of the optimized TSHR and null cellular assays across a 73,180-compound library was described elsewhere (13). Analysis of data from the combination of the target/counter-screen qHTS, along with data from other assays measuring cAMP-related responses, allowed for selection of 96 potential TSHR agonists from the primary screen. A secondary cAMP assay, which used a cAMP antibody and time-resolved fluorescence resonance energy transfer, further eliminated assay-related false-positive compounds. Forty-nine compounds were confirmed as true small-molecule TSHR agonists in these follow-up experiments.
We selected the 8 most potent compounds with an EC50 range from 0.6 to 12 μM and re-tested these for efficacy and selectivity. Compound 1 (Fig. 1A) was chosen for further study as it is a full agonist at TSHR by comparison with a maximally effective concentration of TSH (100% efficacy). Compound 1 activated TSHR-mediated cAMP accumulation with an EC50 of 660 nM. Furthermore, of the 8 most potent compounds from the qHTS, compound 1 was the most selective for TSHR, with no detectable agonist activity at the closely related LHCGR or FSHR (Fig. 1B).
Fig. 1.
Structures, selectivity, and potencies of compounds 1, 2, and 3. (A) Chemical structure of compound 1: (N-(4-(5-(3-(furan-2-ylmethyl)-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)-2-methoxybenzyloxy)phenyl)acetamide) [NCGC00168126–01]. Chemical structure of compound 2: (N-(4-(5-(3-benzyl-5-hydroxy-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)-2-methoxybenzyloxy)-phenyl)acetamide) [NCGC00161870–01]. Chemical structure of compound 3: 3-(furan-2-ylmethyl)-2-(4-methoxy-3-(phenoxymethyl)phenyl)-2,3-dihydroquinazolin-4(1H)-one [NCGC00165237–01]. (B) Cells stably expressing TSHRs, LHCGRs, or FSHRs were exposed to the noted concentrations of compounds 1 and 2 or to a maximally effective concentration of the cognate ligand, TSH (540 nM), LH (34 nM), or FSH (34 nM), respectively, in the presence of 1 mM isobutylmethylxanthine as described in Materials and Methods. After 60 min, the cells were lysed and cAMP levels were measured by ELISA. The data from 3 independent experiments with duplicate samples are shown as the percent of stimulation by the cognate hormone.
In an attempt to improve the potency of the small-molecule agonist, more than 100 analogues of compound 1 were synthesized and tested with bioassay AID 1401 (http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=1401&loc=ea_ras). Fig. 1A shows the structures of compound 1, the most potent analogue, compound 2, and an inactive analogue, compound 3. Compound 2 is a full agonist at TSHR with an EC50 of 40 nM (Fig. 1B) and, like compound 1, it has no activity at FSHR or LHCGR (data not shown). Compounds 1 and 2 contain an aminal, a functional group that is subject to hydrolysis and/or other degradation mechanisms. We therefore performed a hydrolytic stability study of these reagents at neutral, acidic (pH ≈2), and basic (pH ≈12) aqueous conditions. Compound 2 was surprisingly stable at neutral and basic conditions (t1/2 of ≈16 h) but was found to degrade at low pH (t1/2 of ≈3 h).
Compound 2 Binds to the Transmembrane Helical Bundle of TSHR.
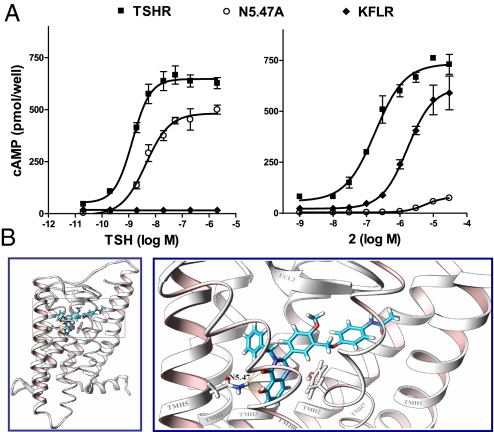
Based on our previous studies of 2 small-molecule ligands of TSHR, a partial agonist Org41841 (23) and an antagonist (14), we predicted that compound 2 would bind to the serpentine region of TSHR. To show that compound 2 binds to the serpentine domain, we tested the activation of a TSHR mutant in which the large amino-terminal ectodomain responsible for TSH binding was deleted. This truncated TSHR (KFLR) was shown by Vlaeminck-Guillem et al. (24) not to bind or be activated by TSH. Compound 2 exhibited 12% lower efficacy and lower potency (EC50 of 1.7 μM) at KFLR than TSHR (Fig. 2A). The finding that compound 2 activates a truncated TSHR receptor without the entire amino-terminal ectodomain provides experimental evidence that this small-molecule agonist binds to the serpentine region of TSHR.
Fig. 2.
Compound 2 activates TSHR by binding to the transmembrane helical bundle. (A) The effects of compound 2 or TSH on cAMP accumulation in cells expressing TSHR, TSHR-KFLR in which the large amino-terminal ectodomain, to which TSH binds, is deleted; or N5.47A, in which Asn at position 5.47 was mutated to Ala. The data from 2 independent experiments with duplicate samples are shown as the percent of stimulation of cAMP accumulation stimulated by TSH or compound 2. (B) Docking of compound 2 into the homology model of TSHR predicts that compound 2 binds within the transmembrane helical bundle (Left). Enlargement of the boxed region at Left (Right) shows an interaction of compound 2 with an Asn in TMH5 (N5.47).
We used a homology model of the serpentine region of TSHR based on opsin, the first structural template of an activated G protein-coupled receptor (25). Docking experiments predicted that compound 2 binds to the same cavity in the transmembrane helical bundle of TSHR as previously reported for a small-molecule partial agonist, Org41841, and an antagonist (14, 23), but compound 2 appears to have different sites of interaction (Fig. 2B). In particular, the model predicts that compound 2 forms a hydrogen bond with an Asn residue in TMH5 [N5.47 in the Ballesteros and Weinstein nomenclature (26); Fig. 2B]. To test the hypothesis that an interaction with N5.47 is necessary for activation of TSHR by compound 2, we constructed a site-specific mutant of TSHR in which N5.47 was substituted by Ala (N5.47A). The cell surface expression of N5.74A is 18% compared with TSHR, but this mutant is activated by TSH with a potency and efficacy similar to that of TSHR (Fig. 2A). In contrast, compound 2 has almost no activity at N5.47A; compound 1 is inactive at N5.47A as well (data not shown). These data support the conclusion that compound 2 binds in a pocket within the transmembrane helical bundle of TSHR.
Compound 2 Stimulates Thyroid-Specific Gene Expression in Primary Cultures of Human Thyrocytes.
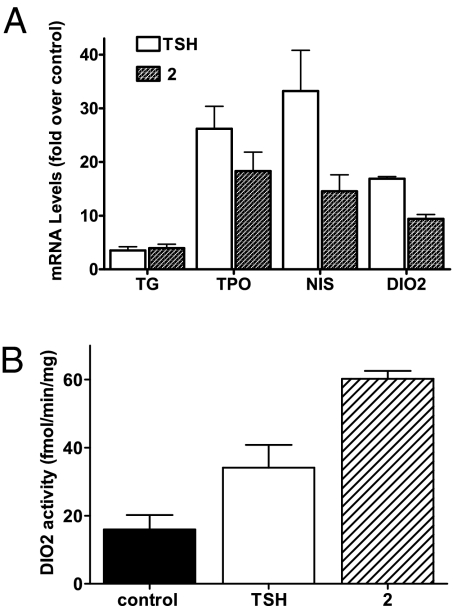
Because we identified these agonists using a model cell system, HEK293 cells stably over-expressing TSHR, we sought to confirm their activities in a more physiologically relevant cell system. We used human thyrocytes derived from 2 donors in primary cultures. As TSH specifically increases expression of several genes in thyrocytes (27), we tested the effects of compound 2 on expression of the mRNAs for TG, thyroperoxidase (TPO), sodium-iodide symporter (NIS), and deiodinase type 2 (DIO2; Fig. 3A). After 24 h, treatment of thyrocytes with compound 2 increased TG, TPO, NIS, and DIO2 mRNAs. Compound 2 increased TG mRNA as effectively as TSH and increased mRNAs for TPO, NIS, and DIO2 somewhat less effectively than TSH. We also measured the effects of compound 2 on DIO2 by assaying its enzymatic activity to de-iodinate T4 to tri-iodothyronine (28). Both TSH and compound 2 increased DIO2 protein activity (Fig. 3B).
Fig. 3.
Stimulation of the expression of the mRNAs for TG, TPO, NIS, and DIO2, and DIO2 protein by TSH or compound 2 in primary cultures of human thyrocytes. (A) Thyrocytes were incubated in media containing 2% FBS and TSH (18 nM) or compound 2 (30 μM) as described in Materials and Methods. After 24 h, the cells were lysed and the levels of the mRNAs were measured and normalized to GAPDH mRNA. The mRNA levels are presented as fold stimulation over control. The data from 3 independent experiments with duplicate samples are shown. (B) DIO2 enzymatic activity is presented as rates of thyroxine deiodination. The data from 3 independent experiments with duplicate samples are shown.
These results show that compound 2 is an effective agonist with activities similar to TSH in primary cultures of human thyrocytes that express TSHR at physiological levels.
Compound 2 Stimulates Thyroid Gland Function in Mice After i.p. Administration and by Esophageal Gavage.
To provide evidence that these small-molecule agonists could be leads for development of clinically useful drugs, it was necessary to show that they are effective in animal models. Before conducting studies in mice, we showed that compound 2 had similar potencies and efficacies in HEK cells overexpressing human or mouse TSHRs (data not shown). We then studied the effects of compound 2 on thyroid gland function in mice. To compare the effects of compound 2 versus those of TSH, we first administered compound 2 and TSH by i.p. injection (Fig. 4A). Compound 2 increased serum T4 to a level that was not statistically different from the increase with TSH, whereas the inactive compound 3 did not affect serum T4.
Fig. 4.
Stimulation by compound 2 of thyroxine (T4) secretion and thyroidal radioiodide uptake in mice. (A) Mice were given vehicle (PEG300, control), TSH (32 μg), compound 2 (0.5 mg), or the inactive analogue compound 3 (0.5 mg) by i.p. injection. Blood was withdrawn 2 h later and T4 was measured in the serum. The data from 2 independent experiments with 3 mice each are shown. (B) Vehicle (control) or compound 2 (2.5 mg) was given by esophageal gavage and serum T4 was measured 2 h later. The data from 4 independent experiments with 3 mice each are shown. (C) Vehicle (control) or compound 2 (2.5 mg) was given by esophageal gavage on d 1 and 2. On d 3, Na125I (20 μCi) was administered by esophageal gavage and the mice were killed 2 h later. The thyroid glands and small pieces of liver were excised and counted for 125I radioactivity. The data are presented as the ratio of radioactivity in the thyroid/liver. The data from 2 independent experiments with 2 and 4 mice each are shown.
For compound 2 to serve as a drug, it would be advantageous if it was active after oral administration. To simulate oral administration, we administered compound 2 to mice by esophageal gavage and showed that, via this route, compound 2 elevates serum T4 (Fig. 4B) and, perhaps more importantly if it were to be used as a lead drug, stimulated radioiodide uptake by the mouse thyroid gland (Fig. 4C). These data show that compound 2 stimulates thyroid cell function in mice after absorption from the gastrointestinal tract.
Discussion
This study was undertaken to identify small-molecule agonists for TSHR that are active in human thyrocytes and in animal models with the long-term goals of developing (i) useful probes to study the physiological role(s) of TSHR in extrathyroidal tissues and (ii) drugs that could be used as adjuncts to diagnose residual or recurrent tumor in patients following surgery for thyroid cancer. We accomplished these aims by extending our analysis of active compounds found by qHTS of drug-like molecules (21).
As qHTS yielded several classes of small molecules that showed activation of TSHR (13), the 8 most potent compounds were chosen for further analysis with TSHR and compared with the closely related LHCGR and FSHR. These experiments identified compound 1 as the most promising lead compound. Further chemical modification of compound 1 led to the identification of compound 2, an analogue with 16-fold improved potency. We found that compounds 1 and 2 have selectivities and efficacies for TSHR comparable to that of TSH in a model cell system over-expressing TSHRs. We then tested compound 2 in more physiologically relevant human thyrocytes, which express TSHRs endogenously and express the thyroid-specific genes TG, which is the precursor of thyroid hormones, and TPO, which couples iodide to tyrosyl residues of thyroglobulin (29). We also measured the effects of these agonists on the levels of 2 other genes important in thyroid gland function: NIS, which is the transporter that mediates iodide accumulation (30), and DIO2, which converts T4 to tri-iodothyronine (28). The small-molecule agonist increased the levels of mRNAs for TG, TPO, NIS, and DIO2, demonstrating the activity of compound 2 in a natural human target of TSH action. The increased enzyme activity of DIO2 in the thyrocytes after treatment with compound 2 suggested that DIO2 protein levels were increased as well. Moreover, we showed that compound 2 could stimulate thyroid gland function (increased secretion of T4 and thyroidal iodide uptake) in mice after administration by esophageal gavage and absorption from the gastrointestinal tract, which further supports its potential as a physiological probe and its use in patients.
We used 2 approaches to show that compound 2 binds to the serpentine domain of TSHR in contrast to the natural ligand TSH, which binds primarily to the large N-terminal ectodomain (31). We used a TSHR mutant that does not contain the N-terminal ectodomain and that was shown previously not to be activated by TSH (24) and showed that it was activated by compound 2. We also used a 3D homology model of TSHR (14, 23), which was based on the x-ray crystallographic structure of opsin (25), to predict the binding pocket for the small-molecule agonist and predict the amino acid residues within the pocket that interact with compound 2. By using a site-specific mutation within the putative TSHR binding pocket, we provided evidence that supports a transmembrane bundle site for the binding of compound 2. We predicted and provided experimental support for a similar transmembrane binding pocket for the small-molecule TSHR antagonist that we recently reported (14).
The most prominent potential clinical use of a small-molecule TSHR agonist is in a diagnostic test for metastasis and recurrence of thyroid cancer. Existing tests rely on rhTSH to stimulate 131I uptake in patients with differentiated thyroid cancer (20). However, hTSH binding to the TSHR is a moderate affinity interaction and, therefore, requires high doses of recombinant TSH that must be administered parenterally to be effective (32). Also, the procedure is cost-intensive because of the high cost of production of recombinant proteins. Thus, development of small-molecule agonists offer advantages of inexpensive production, oral administration, and potential increased activity versus recombinant TSH (33, 34).
Last, our results provide proof of principle for the effectiveness of small-molecule agonists for TSHR in human and murine cells expressing TSHRs endogenously. These findings support the potential use of compound 2 as a probe of TSHR function in tissues other than the thyroid gland that express TSHRs in animal models and perhaps in patients. Although the full efficacy and high selectivity of the new agonists described here are promising, chemical optimization of these new scaffolds supported by molecular modeling will be performed in an effort to further improve their potencies and therapeutic potential. It is anticipated that a complementary approach using rational modifications of these new small-molecule agonists will produce more potent molecules for testing in animal models and for future clinical development.
Materials and Methods
qHTS.
The qHTS was performed against a 73,180-member compound library using a cyclic nucleotide-gated channel-coupled cAMP assay (BD Biosciences). The parental cell line HEK293H-CNG and the cell line stably expressing TSHR were purchased from BD Biosciences. The compounds were serially diluted to measure dose-dependent receptor activation using a fully automated robotic screening system (Kalypsys). A detailed description of our qHTS protocol was recently described elsewhere (13).
Synthesis of Compounds 1, 2, and 3.
The chemical structures of compound 1 (NCGC00168126–01), compound 2 (NCGC00161870–01), and compound 3 (NCGC00165237–01) are illustrated in Fig. 1A. The chemical synthetic schemes are presented in the SI Appendix.
Cell Culture, Transient Transfection, and Generation of Stable Cell Lines Expressing TSHR, LHCGR, or FSHR.
HEK-EM 293 cells were grown in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 10 μg/mL streptomycin (Life Technologies) at 37 °C in a humidified 5% CO2 incubator. Cells were transiently transfected with WT TSHR and mutant receptor in 24-well plates (7.5 × 104 cells per well) with 0.2 μg DNA/well using FuGENE 6 reagent (Roche) according to the manufacturer's protocol. The generation of stable cell lines expressing TSHR, LHCGR, or FSHR was described elsewhere (14).
Site-Directed Mutagenesis of TSHR.
The N5.47A mutant was introduced into hTSHR-pcDNA3.1 via the QuikChange XL site-directed mutagenesis kit (Stratagene). The construct was verified by sequencing (MWG Biotech).
Determination of Intracellular cAMP Accumulation.
Transiently transfected HEK-EM293 cells or cells stably expressing TSHR, LHCGR, or FSHR were seeded into 96-well plates at a density of 50,000 cells/well in DMEM containing 10% FBS. Cells were cultured for 24 h before incubation for 1 h in serum-free DMEM containing 1 mM 3-isobutyl-1-methylxanthine (Sigma) and TSH, LH, FSH, or small-molecule ligands in a humidified 5% CO2 incubator at 37 °C. Following aspiration of the media, cells were lysed using lysis buffer of the cAMP-Screen Direct system (Applied Biosystems). The cAMP content of the cell lysate was determined using the method described in the manufacturer's protocol. The potencies (i.e., EC50) of the ligands were obtained from dose–response curves by data analysis with GraphPad Prism 4 software for Windows.
Determination of Cell Surface TSHR Expression.
After transfection, cells were cultured for 48 h, harvested using 1 mM EDTA/1 mM EGTA in PBS solution, and transferred to Falcon 2058 tubes. Cells were washed once with PBS solution containing 0.1% BSA and 0.1% NaN3 (binding buffer), incubated for 1 h with a 1:200 dilution of mouse anti-human TSHR antibody (Serotec) in binding buffer, washed twice, and incubated for 1 h in the dark with a 1:200 dilution of an Alexa Fluor 488-labeled F(ab′)2 fragment of goat anti-mouse IgG (Molecular Probes) in binding buffer. Before FACS analysis (FACSCalibur; BD Biosciences), cells were washed twice and fixed with 1% paraformaldehyde. Receptor expression was estimated by fluorescence intensity and transfection efficiency was estimated from the percentage of fluorescent cells.
Molecular Modeling and Docking Studies.
The homology model of the serpentine region of the human TSHR was generated with Sybyl 8.0 software (Tripos). In our previous studies on a low-molecular-weight antagonist for the TSHR (14), the structural template was based on the x-ray structure of the β2-adrenergic receptor containing an inverse agonist (35, 36). The reported low rmsd values between backbones of the transmembrane helices (TMHs) of the used structures and the previously solved x-ray structures of bovine rhodopsin (37–39) and adenosine receptor (40) support the reliability of the previous TSHR model. All these templates contain an inverse agonist that is stabilizing an inactive receptor conformation.
In contrast, for our docking studies of TSHR agonist in the present study, we used the recently solved x-ray structure of opsin (25) as a structural template for a TSHR model because this structure exhibits features likely of an active 7TMR conformation. In this new model, several TSHR-specific corrections were made, such as regular helix extensions in TMH2 and TMH5 of TSHR instead of structural bulges in the 2 helices of opsin, which are caused specifically by side chains that are not present in TSHR. Loops were refined by best fit and homology to fragments of other proteins from Protein Data Bank. Gaps of missing residues in the loops of the template structure were closed by the Loop Search tool implemented in Sybyl 8.0 software (Tripos). Conjugate gradient minimizations were performed until converging at a termination gradient of 0.05 kcal/(mol*Å) using the “force field” in Amber version 7.0 software (University of California, San Francisco). Quality and stability of the model were validated by checking the geometry by PROCHECK (41). For examination of the ligand binding site, several tools from the Tripos package, such as site ID and manual and automatic docking (Dock, FlexS, FlexX), were applied. The designation of the amino acids in the transmembrane domain was based on the Ballesteros and Weinstein nomenclature (26). Molecular graphic images were produced using the Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco.
Culture of Primary Human Thyrocytes.
Thyroid tissue samples were collected from normal thyroid tissue from patients undergoing total thyroidectomy for thyroid cancer at the National Institutes of Health Clinical Center. Patients provided informed consent on an institutional review board-approved protocol and materials were received anonymously via approval of research activity through the Office of Human Subjects Research. The specimens were maintained in Hanks' balanced salt solution (HBSS) on ice, and isolation of cells was initiated within 4 h after surgery. All manipulations were performed under sterile conditions. Tissue samples were minced into small pieces by fine surgical forceps and scissors in a 10-cm dish with a small volume of HBSS. Tissue pieces were transferred to a 15-mL tube (Falcon) and washed at least 3 times with HBSS. Afterward, tissue pieces were incubated with HBSS containing 3 mg/mL Collagenase Type IV (Gibco). Enzymatic digestion proceeded for 30 min or longer with constant shaking in a water bath at 37 °C until a suspension of isolated cells was obtained. After centrifugation for 5 min at 150 × g the supernatant was removed and cells were resuspended in 10 mL DMEM with 10% FBS. Cells were plated in 10-cm tissue culture dishes and incubated at 37 °C in a humidified 5% CO2 incubator. After 24 h, the supernatant containing non-adherent cells was removed. The primary cultures of thyroid cells formed a confluent monolayer within 5–7 d. For determination of TG, TPO, NIS, or DIO2 mRNA expression, thyrocytes were seeded into 24-well plates at a density of 0.6 × 104 cells/well 24 h before the experiment. For determination of DIO2 enzyme activity, cells were seeded in 6-well plates at a density of 2.5 × 105 cells/well 24 h before the experiment.
Quantitative RT-PCR.
Total RNA was purified using RNeasy Micro kits (Qiagen). First-strand cDNA was prepared using a High Capacity cDNA Archive Kit (Applied Biosystems). RT-PCR was performed in 25-μL reactions using cDNA prepared from 100 ng or less of total RNA and Universal PCR Master Mix (Applied Biosystems). Primers and probes were Assay-on-Demand (Applied Biosystems). Quantitative RT-PCR results were normalized to GAPDH to correct for differences in RNA input.
Deiodinase Assay.
125I-thyroxine (116 Ci/mmol; Perkin-Elmer) was purified using LH-20 columns (Amersham Pharmacia) before each experiment. Cells were homogenized in 0.25 M sucrose, 0.02 M Tris/HCl (pH 7.0), and 1 mM EDTA. After centrifugation at 1000 × g, the supernatant was collected and stored at –80 C. De-iodinase activity was measured according to previously described methods (42, 43). Each reaction was performed in the presence of 5 mM propylthiouracil (Sigma), an inhibitor of de-iodinase type 1. After counting the total radioactivity with a γ-counter, the 125I released was separated by ion-exchange chromatography in AG50W-X8 columns (Bio-Rad), and the effluent was measured in a γ-counter. Each data point was measured in triplicate; data were expressed as velocity of deiodination (femtomoles per minute per milligram protein) after correction for nonspecific deiodination.
Thyroid Function in Mice.
All experiments involving animals were approved by the institutional animal care and use committee at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Female C57BL/6 mice (Jackson Laboratory) were maintained on a standard diet (NIH-07; Zeigler Brothers). Water and food were available ad libitum. The age ranged from 9 to 18 weeks, and mean body weight was 25 g.
We compared the effects of compound 2 and TSH on serum T4 in mice. In the first series of experiments, we administered the compounds by i.p. injection to non-anesthetized mice. We administered TSH (32 μg in 0.2 mL saline solution), compound 2 (500 μg), or the inactive analogue compound 3 (500 μg) in 50 μL of PEG300 (vehicle). Serum was obtained from terminal retro-orbital bleeds from anesthetized mice 2 h after injection. Total T4 levels were measured with the GammaCoat Free T4 RIA (Diasorin) according to the manufacturer's protocol.
To determine whether compound 2 would be effective when given via the oral route, we administered compound 2 (2.5 mg) in 150 μL PEG300 by esophageal gavage; TSH could not be used as a positive control because it is not absorbed intact from the gastrointestinal tract. We measured serum T4 2 h after gavage administration by collecting 60 μL of blood from the tail vein; the control was PEG300 alone.
Because the current clinical use of rhTSH is to stimulate radioiodide uptake by residual/cancerous thyroid tissue, we measured the effect of compound 2 on 125I uptake by the thyroid glands of mice after administration of compound 2 by esophageal gavage in a protocol similar to that used in patients (44). We administered compound 2 (2.5 mg in 150 μL) or PEG300 alone to mice on d 1 and 2, and then administered Na125I (20 μCi in 300 μL of HBSS) via esophageal gavage on d 3. Two hours after administration of Na125I, the mice were euthanized and their thyroid glands were excised, as were small pieces of liver. The tissues were weighed and the 125I radioactivity in each tissue was counted. The data were analyzed as the ratio of 125I radioactivity in the thyroid to 125I radioactivity in liver.
Statistical Analysis.
Data are expressed as mean ± SE. The data were analyzed by Student t test or one-way ANOVA; P < 0.05 was considered significant.
Supplementary Material
Acknowledgments.
We thank Valentina Congedo for providing human thyroid tissue, Gilbert Vassart and Sabine Costagliola for providing the KFLR construct, and William Jou for technical assistance with the experiments in mice. We thank Bill Leister and Jeremy Smith for analytical support and Paul Shin for compound management support. This research was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases and the National Human Genome Research Institute, and the Molecular Libraries Initiative of the National Institutes of Health Roadmap for Medical Research, National Institutes of Health. Molecular graphic image production by the Chimera package (Resource for Biocomputing, Visualization, and Informatics, University of California, San Francisco) was supported by National Institutes of Health Grant P41 RR-01081.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904506106/DCSupplemental.
References
- 1.Filmore D. It's a GPCR world. Mod Drug Discov. 2004;11:24–28. [Google Scholar]
- 2.van Straten NC, et al. The first orally active low molecular weight agonists for the LH receptor: thienopyr(im)idines with therapeutic potential for ovulation induction. Chembiochem. 2002;3:1023–1026. doi: 10.1002/1439-7633(20021004)3:10<1023::AID-CBIC1023>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Heitman LH, et al. [3H]Org 43553, the first low-molecular-weight agonistic and allosteric radioligand for the human luteinizing hormone receptor. Mol Pharmacol. 2008;73:518–524. doi: 10.1124/mol.107.039875. [DOI] [PubMed] [Google Scholar]
- 4.Jorand-Lebrun C, et al. Identification, synthesis, and biological evaluation of novel pyrazoles as low molecular weight luteinizing hormone receptor agonists. Bioorg Med Chem Lett. 2007;17:2080–2085. doi: 10.1016/j.bmcl.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 5.Van Koppen CJ, et al. A signaling-selective, nanomolar potent allosteric low molecular weight agonist for the human luteinizing hormone receptor. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:503–514. doi: 10.1007/s00210-008-0318-3. [DOI] [PubMed] [Google Scholar]
- 6.Heitman LH, et al. Substituted terphenyl compounds as the first class of low molecular weight allosteric inhibitors of the luteinizing hormone receptor. J Med Chem. 2009;52:2036–2042. doi: 10.1021/jm801561h. [DOI] [PubMed] [Google Scholar]
- 7.Guo T, et al. Small molecule biaryl FSH receptor agonists. Part 1: lead discovery via encoded combinatorial synthesis. Bioorg Med Chem Lett. 2004;14:1713–1716. doi: 10.1016/j.bmcl.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Guo T, et al. Small molecule biaryl FSH receptor agonists. Part 2: lead optimization via parallel synthesis. Bioorg Med Chem Lett. 2004;14:1717–1720. doi: 10.1016/j.bmcl.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Yanofsky SD, et al. Allosteric activation of the follicle-stimulating hormone (FSH) receptor by selective, nonpeptide agonists. J Biol Chem. 2006;281:13226–13233. doi: 10.1074/jbc.M600601200. [DOI] [PubMed] [Google Scholar]
- 10.Wrobel J, et al. Synthesis of (bis)sulfonic acid, (bis)benzamides as follicle-stimulating hormone (FSH) antagonists. Bioorg Med Chem. 2002;10:639–656. doi: 10.1016/s0968-0896(01)00324-8. [DOI] [PubMed] [Google Scholar]
- 11.Arey BJ, et al. Identification and characterization of a selective, nonpeptide follicle-stimulating hormone receptor antagonist. Endocrinology. 2002;143:3822–3829. doi: 10.1210/en.2002-220372. [DOI] [PubMed] [Google Scholar]
- 12.van Straten NC, et al. Identification of substituted 6-amino-4-phenyltetrahydroquinoline derivatives: potent antagonists for the follicle-stimulating hormone receptor. J Med Chem. 2005;48:1697–1700. doi: 10.1021/jm049676l. [DOI] [PubMed] [Google Scholar]
- 13.Titus S, et al. Quantitative high-throughput screening using a live-cell cAMP assay identifies small-molecule agonists of the TSH receptor. J Biomol Screen. 2008;13:120–127. doi: 10.1177/1087057107313786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neumann S, et al. A low-molecular-weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology. 2008;149:5945–5950. doi: 10.1210/en.2008-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassett JH, Williams GR. Critical role of the hypothalamic-pituitary-thyroid axis in bone. Bone. 2008;43:418–426. doi: 10.1016/j.bone.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Abe E, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc Natl Acad Sci USA. 2008;105:4289–4294. doi: 10.1073/pnas.0712395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bassett JH, et al. A lack of thyroid hormones rather than excess thyrotropin causes abnormal skeletal development in hypothyroidism. Mol Endocrinol. 2008;22:501–512. doi: 10.1210/me.2007-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassett JH, et al. Thyroid hormone excess rather than thyrotropin deficiency induces osteoporosis in hyperthyroidism. Mol Endocrinol. 2007;21:1095–1107. doi: 10.1210/me.2007-0033. [DOI] [PubMed] [Google Scholar]
- 20.Duntas LH, Cooper DS. Review on the occasion of a decade of recombinant human TSH: prospects and novel uses. Thyroid. 2008;18:509–516. doi: 10.1089/thy.2007.0331. [DOI] [PubMed] [Google Scholar]
- 21.Inglese J, et al. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci USA. 2006;103:11473–11478. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinscheid RK, Kim J, Zeng J, Civelli O. High-throughput real-time monitoring of Gs-coupled receptor activation in intact cells using cyclic nucleotide-gated channels. Eur J Pharmacol. 2003;478:27–34. doi: 10.1016/j.ejphar.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 23.Jaschke H, et al. A low molecular weight agonist signals by binding to the transmembrane domain of thyroid-stimulating hormone receptor (TSHR) and luteinizing hormone/chorionic gonadotropin receptor (LHCGR) J Biol Chem. 2006;281:9841–9844. doi: 10.1074/jbc.C600014200. [DOI] [PubMed] [Google Scholar]
- 24.Vlaeminck-Guillem V, Ho SC, Rodien P, Vassart G, Costagliola S. Activation of the cAMP pathway by the TSH receptor involves switching of the ectodomain from a tethered inverse agonist to an agonist. Mol Endocrinol. 2002;16:736–746. doi: 10.1210/mend.16.4.0816. [DOI] [PubMed] [Google Scholar]
- 25.Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- 26.Ballesteros JA, Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations of G protein-coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- 27.Vassart G, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13:596–611. doi: 10.1210/edrv-13-3-596. [DOI] [PubMed] [Google Scholar]
- 28.Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumont JE. The action of thyrotropin on thyroid metabolism. Vitam Horm. 1971;29:287–412. doi: 10.1016/s0083-6729(08)60051-5. [DOI] [PubMed] [Google Scholar]
- 30.Dohan O, et al. The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24:48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- 31.Vassart G, Pardo L, Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci. 2004;29:119–126. doi: 10.1016/j.tibs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Kermode JC, Thompson BD, Edmonds CJ. Comparison of binding of bovine and human thyroid-stimulating hormone to receptor sites on human thyroid membranes. J Endocrinol. 1981;88:205–217. doi: 10.1677/joe.0.0880205. [DOI] [PubMed] [Google Scholar]
- 33.Leitolf H, Tong KP, Grossmann M, Weintraub BD, Szkudlinski MW. Bioengineering of human thyrotropin superactive analogs by site-directed “lysine-scanning” mutagenesis. Cooperative effects between peripheral loops. J Biol Chem. 2000;275:27457–27465. doi: 10.1074/jbc.M003707200. [DOI] [PubMed] [Google Scholar]
- 34.Szkudlinski MW. Recombinant human thyrotropins of the twenty-first century. Expert Opin Pharmacother. 2004;5:2435–2440. doi: 10.1517/14656566.5.12.2435. [DOI] [PubMed] [Google Scholar]
- 35.Cherezov V, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasmussen SG, et al. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 37.Palczewski K, et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 38.Salom D, et al. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc Natl Acad Sci USA. 2006;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Standfuss J, et al. Crystal structure of a thermally stable rhodopsin mutant. J Mol Biol. 2007;372:1179–1188. doi: 10.1016/j.jmb.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaakola VP, et al. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laskowski RA, Moss DS, Thornton JM. Main-chain bond lengths and bond angles in protein structures. J Mol Biol. 1993;231:1049–1067. doi: 10.1006/jmbi.1993.1351. [DOI] [PubMed] [Google Scholar]
- 42.St Germain DL. Dual mechanisms of regulation of type I iodothyronine 5′-deiodinase in the rat kidney, liver, and thyroid gland. Implications for the treatment of hyperthyroidism with radiographic contrast agents. J Clin Invest. 1988;81:1476–1484. doi: 10.1172/JCI113479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coppotelli G, Summers A, Chidakel A, Ross JM, Celi FS. Functional characterization of the 258 A/G (D2-ORFa-Gly3Asp) human type-2 deiodinase polymorphism: a naturally occurring variant increases the enzymatic activity by removing a putative repressor site in the 5′ UTR of the gene. Thyroid. 2006;16:625–632. doi: 10.1089/thy.2006.16.625. [DOI] [PubMed] [Google Scholar]
- 44.Pacini F, Castagna MG. Diagnostic and therapeutic use of recombinant human TSH (rhTSH) in differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2008;22:1009–1021. doi: 10.1016/j.beem.2008.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.