Abstract
Chromosomal rearrangements involving erythroblast transformation specific (ETS) family transcription factors were recently defined as the most common genetic alterations in human prostate cancer. Despite their prevalence, it is unclear what quantitative role they play in either initiation or progression of the disease. Using a lentiviral transduction and dissociated cell prostate regeneration approach, we find that acutely increased expression of ETS proteins in adult murine prostate epithelial cells is sufficient to induce the formation of epithelial hyperplasia and focal prostatic intraepithelial neoplasia (PIN) lesions, but not progression to carcinoma. However, combined expression of ERG with additional genetic alternations associated with human prostate cancer can lead to aggressive disease. Although ERG overexpression does not cooperate with loss of the tumor suppressor p53, it does collaborate with alterations in PI3K signaling, such as Pten knockdown or AKT up-regulation, to produce a well-differentiated adenocarcinoma. Most striking is our finding that overexpression of androgen receptor (AR) does not give rise to any hyperplastic lesions, but when combined with high levels of ERG, it promotes the development of a more poorly differentiated, invasive adenocarcinoma. These findings suggest that in human prostate cancer, the most potent function of ETS gene fusions may be to synergize with alternative genetic events and provide different pathways for carcinoma production and invasive behavior. Our results provide direct evidence for selective cooperating events in ERG-induced prostate tumorigenesis and offer a rational basis for combined therapeutic interventions against multiple oncogenic pathways in prostate cancer.
Keywords: AKT, androgen receptor, ERG, prostate cancer, PTEN
Human cancer is a multigene disorder in which several somatic mutations in cancer predisposition genes are required in a stepwise manner (1). The first genetic alteration usually confers a selective growth advantage and subsequently results in clonal expansion to initiate the neoplastic process. But the cells must accumulate several other rate-limiting mutations over a long period to become malignant (2). For prostate cancer (PCa), various oncogenes and tumor suppressor genes, including NKX3.1, PTEN, p27, and p53, and androgen receptor (AR) signaling have been implicated in the disease progression (3). However, the key initiating steps in prostate carcinogenesis remain uncertain, and most genetic alterations that can collaborate to drive tumor progression have yet to be elucidated.
Chromosomal translocations play critical roles in hematological malignancies (4). Several lines of evidence in epithelial cancers of distinct tissue origins suggest that acquired chromosomal rearrangements could also be the common genetic events in many, if not all, epithelial cancers (5). Recent studies showed that 50–80% of patients with PCa were found to harbor fusion transcripts between an androgen-regulated gene, TMPRSS2, and members of the erythroblast transformation specific (ETS) transcription factor family (ERG, ETV1, ETV4, or ETV5) (6–9). The TMPRSS2-ERG fusion at chromosome 21q22, via either interstitial deletion or chromosomal translocation, is the most predominant subtype (10, 11). This fusion results in overexpression of full-length or truncated ERG protein in prostate epithelial cells and was found to be associated with an aggressive phenotype of PCa (11, 12). However, these findings still could not distinguish a causative from correlative role for ETS family members in the development of PCa.
Furthermore, attempts by several independent groups to investigate the role of these gene fusions in PCa initiation by using transgenic approaches have produced conflicting results. Nelson and Vasioukhin and co-workers (13) generated a mouse strain with prostate-specific expression of the truncated ERG protein under the control of the modified probasin (ARR2PB) promoter, which displays significant prepubertal transcriptional activity (14, 15). These transgenic lines (129/sv background) developed epithelial hyperplasia and focal PIN lesions in the ventral lobes of the prostate as early as 5–6 months of age. Chinnaiyan and co-workers (16) reported similar observations by using the same strategy on the FVB background. However, Pandolfi and co-workers (17) and Sawyers and co-workers (18) described that their ERG transgenic mice (on the C57BL/6 or FVB background, respectively) possess little observable pathological findings with no increase in cell proliferation. The discrepancy in the reported phenotypes of ETS transgenic mice could be the result of distinct isoforms of ERG protein and different mouse genetic backgrounds used in these independent studies. Therefore, it is necessary to use alternative approaches to examine the definitive role of ETS family members in PCa evolution.
In this study, using a lentiviral transduction and tissue dissociation/regeneration approach (19), we demonstrate that high levels of ERG or ETV1 protein are individually sufficient to initiate neoplastic transformation of adult murine prostate epithelial cells and that ERG overexpression can collaborate with enhanced AR signaling and aberrant phosphoinositide 3-kinase (PI3K) pathway, resulting in the progression to invasive prostate adenocarcinoma. Our findings not only reveal the functional consequences of overexpression of ETS transcription factors in the development of PCa and provide direct evidence for alternative cooperating events in ERG-induced prostate tumorigenesis, but also offer experimental rationales for combined therapies against these signaling pathways in ETS-overexpressing PCa.
Results
ERG Overexpression in Adult Murine Prostate Cells Results in Epithelial Hyperplasia and Focal PIN Lesions.
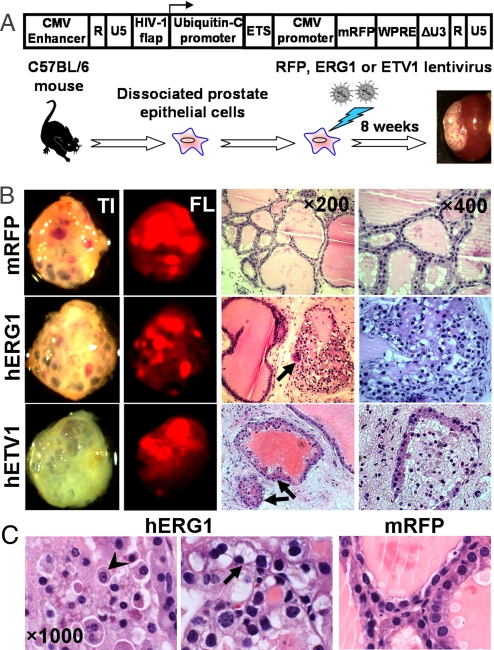
Dissociated prostate cells from 8–12 weeks C57BL/6 mice were transduced with lentivirus carrying human full-length ERG coding sequence and subsequently combined with urogenital sinus mesenchymal cells and engrafted into the subrenal capsules of male immunodeficient mice for 8–10 weeks (Fig. 1A). Histological examination of the ERG-overexpressing prostate regenerated tissues revealed the development of epithelial hyperplasia and focal PIN lesions (Fig. 1B), but no progression to carcinoma. These observations are similar to the phenotype of ERG transgenic mice recently reported by Klezovitch et al. and Tomlins et al. (13, 16).
Fig. 1.
Epithelial hyperplasia and focal PIN lesions in prostate grafts regenerated from ETS-transduced adult murine prostate cells. (A) Diagram of the ETS lentiviral vector used in the in vivo prostate regeneration assay. Dissociated prostate cells from C57BL/6 mice at 8–12 weeks of age were transduced with the indicated lentivirus and subsequently engrafted into the subrenal capsules of SCID mice and regenerated in vivo for 8 weeks. (B) Representative transilluminated (TI), fluorescent (FL) photographs, and histological sections (H&E staining) of regenerated tissues derived from ETS-transduced prostate cells. ETS-transduced prostate tubules are indicated by arrows. (C) Cellular and nuclear atypia and signet ring cell-like appearance of the epithelial cells in ERG-transduced prostate glands (arrowhead, prominent nucleoli; arrow, intracytoplasmic vacuoles and nuclear displacement). mRFP-tranduced glands are shown as the control.
In contrast to the epithelial glands derived from monomeric red fluorescent protein (mRFP) control vector transduced prostate cells, which formed tubular structures with 2 cell layers and abundant luminal secretions, the grafts derived from ERG transduced prostate cells displayed focal epithelial hyperplasia with cell stratification and loss of cell polarity (Fig. 1B). The proliferative epithelial cells in these ERG-transduced prostate glands demonstrated nuclear pleomorphism and atypia (Fig. 1C), including nuclear enlargement, hyperchromasia, and occasional prominent nucleoli (Fig. 1C, arrowhead). More interestingly, we noted that some ERG-transduced glands displayed micropapillary or cribriform growth patterns, and the epithelial cells exhibited a signet ring cell-like appearance (Fig. 1C) with intracytoplasmic vacuoles and nuclear displacement (Fig. 1C, arrow). These pathological manifestations of ERG-transduced prostate glands resemble the morphological features of signet ring cells that were found in a subset of human PCas harboring the TMPRSS2-ERG gene fusion (20).
Forced Expression of ETV1 in Primary Adult Murine Prostate Cells Induces Epithelial Hyperplasia.
ETV1-associated gene fusions were found to be the second most common chromosomal rearrangement in human PCa (8, 11). To determine whether up-regulation of ETV1 can also transform adult murine prostate cells, we transduced primary prostate cells with a lentiviral vector containing human ETV1 complete coding sequences and performed the in vivo regeneration assay. Similar to ERG-transduced prostate grafts, we found that forced expression of ETV1 in adult prostate cells resulted in hyperplastic lesions (Fig. 1B), characterized by focal hypercellularity and increased epithelial tufting, which is consistent with the observations in ETV1 transgenic mice (8). Taken together, we conclude that high levels of ETS proteins are clearly sufficient to induce neoplastic changes in adult prostate epithelia, but are not sufficient to produce carcinoma in our assay.
ERG-Transduced Prostate Glands Display a Skewed Cell Lineage Composition with Loss of Cytokeratin 5 (CK5)-Positive Basal Cells and Increased CD49f Expression in Luminal Cells.
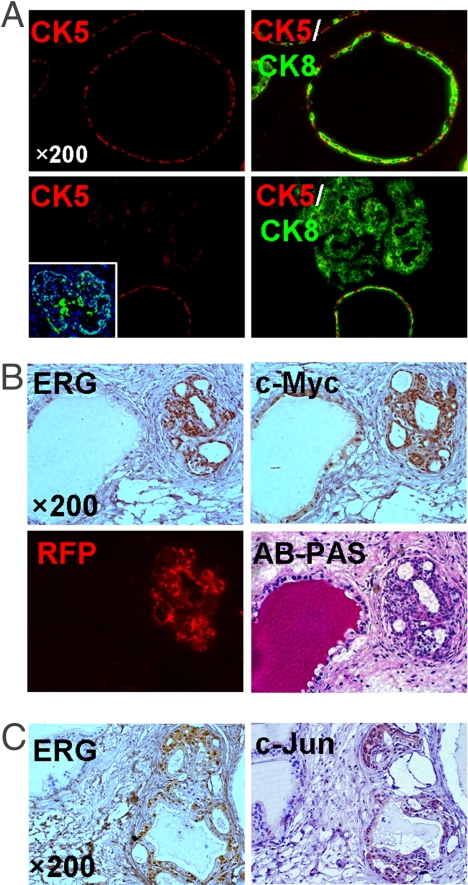
In the wild-type murine prostate tissue and in the normal regenerated prostate tubules, CK5-positive basal cells form an intermittent layer between the luminal cells and the underlying basement membrane (Fig. 2A). In contrast, we found that the frequency of CK5-positive basal cells was significantly diminished in ERG-transduced prostate tubules (Fig. 2A). Previous studies in our lab showed that CD49f (integrin α6), a stem cell marker in multiple epithelial tissues (21, 22), was predominantly expressed on the basal surface of prostate basal cells and colocalized with its extracellular matrix ligand, laminin (22). In contrast to its circumferential expression pattern in mRFP-transduced tubules, immunofluorescent (IF) analysis with a CD49f antibody demonstrated that low, but significant, amounts of CD49f protein were detected on luminal cells in ERG-transduced glands (Fig. S1 B and C). However, the expression pattern of laminin around ERG-transduced prostate tubules was not changed (Fig. S1B). These findings suggest that forced expression of ERG in prostate stem cells may control the lineage choice during differentiation, resulting in the skewed cell lineage composition. Consistently, Klezovitch et al. (13) reported that in addition to the decreased ratio of basal cells to the total prostate epithelial cells, the expression levels of CD104 (integrin β4), a binding partner of CD49f to form a laminin-binding integrin receptor, were elevated in some luminal prostate cells from ERG transgenic mice. Together, these findings indicate that aberrant integrin signaling may be involved in ERG-mediated transformation.
Fig. 2.
ERG overexpression resulted in up-regulation of c-Myc and c-Jun proteins and a skewed cell lineage composition in regenerated prostate tubules. (A) IF analyses of ERG-transduced prostate graft sections (Lower) showing the nuclear staining of ERG in prostate epithelial cells (Inset) and loss of CK5-positive cells in the corresponding tubules compared with the adjunct normal tubules and mRFP-transduced glands (Upper). (B) IHC analysis with anti-ERG and c-Myc antibodies identifies increased levels of c-Myc expression in ERG-positive prostate tubules (Upper). Alcian blue-periodic acid Schiff (AB-PAS) staining and fluorescent (RFP) image of tissue sections containing the same tubules are shown (Lower). (C) IHC staining of regenerated prostate tissues with anti-ERG and c-Jun antibodies reveals the close correlation of ERG overexpression with up-regulation of c-Jun protein.
ERG Overexpression Induces Up-Regulation of c-Myc and c-Jun Protein in Primary Prostate Epithelia.
In a recent study, Sun et al. demonstrated that c-MYC is down-regulated after siRNA-mediated knockdown of ERG in VCaP cells, a human PCa cell line that harbors the TMPRSS2-ERG fusion (23). Using immunohistochemical (IHC) analysis, we found strong nuclear staining of c-Myc in the epithelial cells of ERG-transduced prostate glands (Fig. 2B and Fig. S2A), suggesting that c-MYC could be one of the key downstream effectors that contribute to ERG-mediated prostate oncogenesis. Moreover, we found that the expression levels of c-Jun protein, a known ERG-binding partner (24), were significantly increased in the epithelial cells of ERG-transduced glands compared with adjacent normal glands (Fig. 2C and Fig. S2B). The up-regulation of c-Jun protein on ERG overexpression was further confirmed by Western blot analysis of ERG-transduced PEB-1 cells (Fig. S2C). These findings support the concept that both c-Myc and c-Jun oncogenes may be targets of ERG and may participate in ERG-mediated transformation.
Combined ERG Overexpression and p53 Deletion in Prostate Epithelia Does Not Result in Invasive Adenocarcinoma.
Because ETS proteins alone only have weak transforming effects, we sought to determine whether their role in PCa may be exerted through interaction with other genetic alterations. Therefore, we combined ERG overexpression and other genetic changes frequently involved in PCa and performed the in vivo prostate regeneration assay.
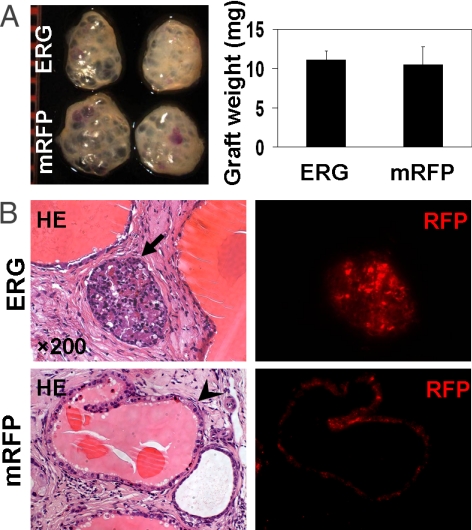
Loss of the tumor suppressor p53 can be identified in approximately one-third of early stage PCas (25). p53 deletion has been shown to synergize with Pten targeted inactivation (26) or Rb deficiency (27) to induce murine PCa, indicating that loss of p53 may play an important role in initiation and progression of PCa. To determine whether loss of p53 can collaborate with ERG overexpression during the development of PCa, prostate cells from adult p53-null mice were transduced with either ERG or RFP lentivirus and implanted in the prostate regeneration assay. Surprisingly, we observed no significant difference in the weights of prostate grafts regenerated from ERG- and mRFP-transduced cells (Fig. 3A). Histological analysis showed that increased levels of ERG protein in prostate epithelial cells from p53-null mice resulted in epithelial hyperplasia and PIN lesions (Fig. 3B), similar to the phenotype caused by ERG overexpression in wild-type prostate epithelial cells (Fig. 1C). We found no evidence to support cooperation between loss of p53 and increased expression of ERG in our 8-week assay, suggesting that 2 oncogenic influences do not always collaborate to advance the disease (28).
Fig. 3.
The absence of synergy between ERG overexpression and p53 deletion for PCa progression. (A) Macroscopic photo and weight of regenerated tissues derived from ERG or mRFP-transduced p53-null murine prostate cells. (B) Representative histological sections of the graft tissues regenerated from p53-null prostate cells, showing that combined ERG overexpression and loss of p53 in murine prostate cells only resulted in PIN lesions, whereas mRFP-transduced p53−/− prostate epithelial cells gave rise to the normal prostate glands. Arrows indicated the lentiviral-transduced prostate tubules. The fluorescent (RFP) images of tissue sections containing the same tubules are also shown.
ERG Collaborates with Aberrant PI3K Pathway to Promote PCa Progression.
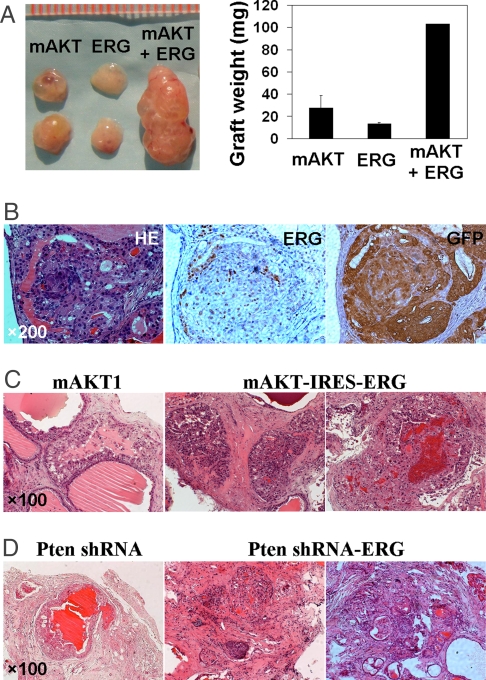
Deletion of the tumor suppressor PTEN occurs in ≈68% of human PCas (29) and results in activation of the PI3K pathway. We demonstrated that increased PI3K signaling via shRNA-mediated PTEN knockdown or overexpression of an activated form of AKT in murine prostate cells causes PIN lesions in the tissue-regeneration model (30, 31). In this study, we combined overexpression of ERG and activated AKT and found that grafts derived from coinfected adult prostate cells weighed 2–3 times more than grafts generated from AKT or ERG overexpression alone (Fig. 4A). In contrast to AKT-induced PIN lesions, the prostate glands that simultaneously overexpressed ERG and AKT/GFP exhibited a cribriform growth pattern with cell crowding and embedded acini (Fig. 4B). The cells in these proliferative foci exhibited nuclear atypia, evidenced by hyperchromatic nuclei, mitotic figures, nuclear contour irregularity, and enlargement. These findings suggest that high levels of ERG protein collaborate with constitutively activated AKT kinase, leading to the development of invasive PCa.
Fig. 4.
Invasive prostate adenocarcinoma induced by ERG overexpression and aberrant PI3K pathway. (A) Photographic overview and weight of regenerated tissues derived from wild-type prostate cells transduced with the indicated lentiviruses. (B) H&E staining and IHC analyses with antibodies against ERG and GFP show prostate adenocarcinoma developed in ERG/mAKT1 coinfected prostate grafts. (C) H&E staining of tissue sections showing invasive adenocarcinomas induced by ERG overexpression and activated AKT kinase using a bicistronic vector. PIN lesions induced by mAKT1 alone are shown as the control. (D) H&E staining of regenerated tissue sections reveals that the combination of ERG overexpression and Pten knockdown resulted in prostate adenocarcinoma, as compared with PIN lesions induced by Pten knockdown.
Because of the relatively low frequency of double infection of ERG and AKT using 2 lentiviral vectors, we prepared a bicistronic construct that contained both AKT- and ERG-coding sequences (Fig. S3). Transduction of prostate epithelial cells with separate lentiviruses (Fig. 4B) or with the single bicistronic construct (Fig. 4C) yielded similar results. The majority of prostate glands in AKT/ERG-transduced regenerated tissues displayed pathological features ranging from well-differentiated to moderately differentiated adenocarcinoma (Fig. 4C), characterized by clusters of fused glands with cribriform pattern, atypical cells and hyperplastic glands infiltrating into the stroma and multiple foci of solid sheets of tumor cells.
Although activation of AKT in human PCa typically occurs through loss of PTEN, the activated AKT and Pten-deficient PCa mouse models demonstrate different degrees of disease progression, because constitutively active AKT only results in PIN lesion, whereas Pten deletion leads to metastatic PCa (32, 33). To determine whether loss of Pten could also cooperate with ERG overexpression, we constructed a single lentiviral vector to express ERG and to knockdown Pten by shRNA (Fig. S3). We consistently found the development of adenocarcinoma in Pten knockdown/ERG overexpressing regenerated glands after 8 weeks of engraftment (Fig. 4D), whereas Pten knockdown alone or ERG overexpression alone only resulted in hyperplasia and PIN lesions. These data suggest that overexpression of ERG protein can collaborate with increased PI3K signaling and may indicate an interaction or cross-talk between these pathways during disease progression, as suggested by Shaffer and Pandolfi (34).
High Levels of ERG Fully Transform Primary Prostate Cells Through Synergy with Enhanced AR Signaling.
AR is commonly mutated or amplified in human PCa (3, 35), and the AR pathway is the most extensively studied pathway in PCa because of its role in late-stage hormone-refractory PCa. Given that up-regulation of ETS transcription factors is mainly driven by the androgen-responsive TMPRSS2 promoter in most samples of human PCa (6, 11), it is reasonable to hypothesize that both ETS overexpression and AR signaling coexist in malignant prostate epithelial cells. However, to date little is known about the potential cross-talk between these 2 transcription factors.
To determine the biological consequences of combined up-regulation of ERG and AR in primary prostate epithelial cells, we used a similar strategy to coexpress these 2 genes by lentiviral vectors. Consistent with the previous findings (31), forced expression of AR single gene impaired the regenerative capacity of the dissociated murine prostate cells, resulting in fewer prostate tubules in AR-transduced prostate grafts (Fig. 5B). Strikingly, we found that the combination of simultaneous up-regulation of ERG and AR in prostate epithelial cells led to the formation of invasive PCa, characterized by poorly formed, tightly packed prostate glands, with the penetration of solid nests of cytologically atypical cells into the surrounding stroma (Fig. 5 B and C). Although graft weights were similar, we noticed that grafts from cells transduced with both ERG and AR were much denser than the translucent ERG-transduced grafts (Fig. 5A). The proliferating cell nuclear antigen (PCNA) staining analysis also indicated a significant increase in cell proliferation in ERG/AR-transduced prostate glands (Fig. S4), accompanied with the absence of smooth muscle actin (SMA) staining (Fig. S4). Interestingly, although the basal layer was maintained in AKT/ERG-expressing glands, IF analysis demonstrated a loss of the CK5-positive basal layer in ERG/AR-expressing glands (Fig. S5), which is a frequent hallmark of human PCa (36). These findings clearly demonstrate that overexpression of ERG protein can strongly synergize with enhanced AR signaling to mediate full transformation of adult murine prostate epithelial cells, resulting in the progression of PIN lesion to invasive adenocarcinoma.
Fig. 5.
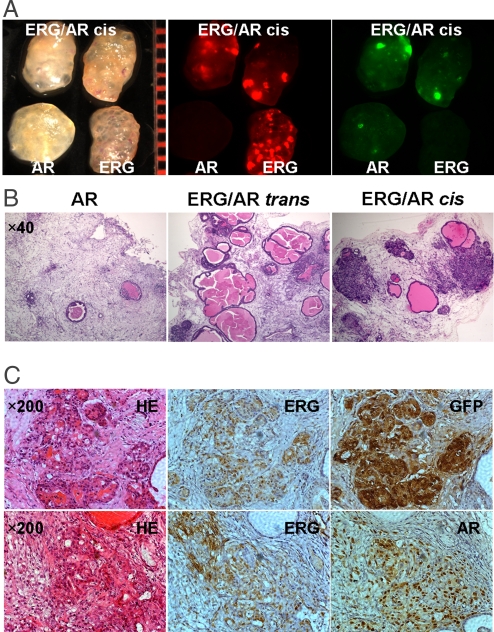
The synergistic effects between ERG overexpression and enhanced AR signaling for PCa progression. (A) Macroscopic transilluminated and fluorescent photos showing the more solid texture in ERG/AR coinfected prostate grafts, compared with the transparent appearance of ERG-transduced grafts. (B) H&E staining of regenerated tissue sections reveals that combined up-regulation of ERG and AR led to the formation of invasive adenocarcinoma in ERG/AR coinfected (ERG/AR cis) prostate grafts, but not in the separately infected/pooled (ERG/AR trans) prostate grafts. Overexpression of AR alone caused an evident decrease in the number of regenerated prostate tubules. (C) H&E staining and IHC analyses with antibodies against ERG, GFP, and AR of regenerated tissue sections showing invasive prostate adenocarcinoma developed in regenerated tissues derived from ERG/AR coinfected prostate cells.
It has been proposed that in addition to an autocrine mechanism (37), AR signaling in PCa cells can act on neighboring malignant cells in a paracrine manner (38). To test whether the observed synergy between ERG and AR occurs in a cell-autonomous manner, we compared the effect of mixing prostate cells infected separately or coinfected with ERG and AR lentiviruses (Fig. 5B, cis and trans). We found that invasive adenocarcinoma was not observed in the regenerated tissues derived from the pooled prostate cells (ERG/AR trans) after separate infection with ERG or AR lentivirus (Fig. 5B), suggesting that in a cell-autonomous manner, both ERG overexpression and enhanced AR signaling are required for the progression to invasive PCa.
Discussion
Despite the high prevalence of ETS-associated gene fusions in human PCa, the role of ETS family transcription factors in prostate oncogenesis is still controversial (17). In this study, we examined the precise function of overexpression of ETS family members in adult murine prostate cells by using a dissociated cell prostate regeneration assay. Given the prepubertal probasin promoter activity that drives expression of ETS proteins in transgenic mouse models, our system can more accurately examine the biological consequences of an acute increase in expression of ETS proteins in adult prostate epithelial cells, because the development of human PCa occurs after sexual maturation. Here, we demonstrate that overexpression of ERG or ETV1 is sufficient to initiate epithelial hyperplasia and focal PIN lesions from adult murine prostate cells, which provide a definitive answer for the causative role of ETS overexpression in prostate tumorigenesis.
However, similar to the intermediate preleukemia state of ERG-transduced hematopoietic cells (39), the introduction of ERG single gene is not sufficient to cause full transformation of prostate epithelial cells. This result is consistent with the conventional concept that at least 3–5 mutations are required to develop a malignant solid tumor in adults (2). Currently, the collaborative effects in oncogenesis are typically investigated by cross-breeding of genetically modified animals to generate compound mouse model. However, the cost and time of cross-breeding and the matching strain genetic background can be prohibitive. In this study, we attempted to tackle these issues by a lentiviral coinfection strategy and demonstrated an in vivo model that represents well the multistep process of PCa progression. We showed that high levels of ERG protein collaborated with alternative signaling pathways to different extents, resulting in the progression of PIN lesions to frank prostate adenocarcinoma. In contrast to the synergistic effects between high levels of ERG and enhanced AR signaling or aberrant PI3K pathway, loss of p53 did not complement ERG overexpression in our tissue regeneration assay for the progression of PCa. These findings are consistent with the current doctrine regarding the evolution of human solid malignant tumors, supporting that multiple but selective genetic changes are required for the progression of individual carcinomas.
Although it is well known that increased AR signaling is associated with PCa progression, transgenic mice with prostate-specific overexpression of wild-type AR did not display any abnormal phenotype (40). However, we found that combined AR and ERG overexpression has strong synergistic effects, resulting in the progression of ERG-induced PIN lesions to invasive adenocarcinoma. These findings suggest an important interaction between ETS transcription factors and AR. Given that the synergy between AR and ERG only occurred in a cell-autonomous manner, we propose that cross-talk may occur at the protein level via the formation of a multicomponent protein complex. This interaction could be mediated by c-Jun, which has been shown to bind to both ERG (24) and AR (41) and found to be up-regulated in ERG expressing regenerated glands. Alternatively, ERG and AR could coregulate a subset of common downstream target genes by binding to their corresponding DNA elements, leading to the full transformation of naïve prostate epithelial cells. Considering the fact that increased ERG expression in most PCas is driven by the androgen-responsive TMPRSS2 promoter, the physical interaction and biological synergy between ERG and AR may represent a positive cascade mechanism for prostate tumorigenesis
Currently there are few treatment options for patients with advanced PCa. Androgen ablation therapy such as finasteride is only effective for a limited duration, and most PCas will usually progress to hormone refractory disease and eventually metastasize (35). Therefore, it is necessary to identify alternative targets for the treatment of advanced PCa in addition to AR. Our findings that ETS family members play an important role in prostate tumorigenesis suggest that ETS transcription factors could be attractive therapeutic targets for PCa harboring ETS-associated gene fusions. Importantly, our findings of the synergistic effects between ERG and enhanced AR signaling or aberrant PI3K pathway provide a rational basis for combination therapy targeting these different signaling pathways, which together may overcome androgen independence and result in long-term remission. With the promising progress in the development of PI3K inhibitors such as SF1126 and NVP-BEZ235 (42, 43) and more potent antiandrogen agents (44), strategies to specifically inhibit ETS transcription factors may be the critical component for combined target therapy in the treatment for ETS overexpressing PCa.
Experimental Procedures
Construction of Lentiviral Vectors and Preparation of Lentiviral Stocks.
Full-length human ERG variant 1 (NM_182918) or ETV1 complete coding sequences were PCR-amplified from the related human-MGC-verified cDNA library clones (Open Biosystems) and subcloned into FUCRW lentiviral vectors (45). The FU-mAKT1-CGW and FU-AR-CGW vectors have been described in ref. 31. The mAKT-IRES-ERG bicistronic lentiviral vector was constructed by sequential subcloning of mAKT1-IRES fragment and ERG cDNA with SpeI-XbaI sites. To generate the Pten shRNA/ERG vector, a Pten shRNA fragment (30), which is transcribed under the control of the H1 promoter, was inserted into FU-ERG-CRW vector. Lentiviral stocks were prepared by using the calcium phosphate precipitation technique as described in ref. 19.
Dissociated Prostate Regeneration Assay.
Lentiviral transduction and prostate regeneration assays were performed as described in ref. 19. The grafts were dissected from the host mice 8 weeks after engraftment, fixed, and sectioned for hematoxylin and eosin (H&E) staining and immunohistological analysis. p53-null mice on the C57BL/6 background were originally obtained from the Jackson Laboratories and raised in our animal research center. All mouse studies and surgical procedures were conducted according to protocols approved by the Division of Laboratory Animal Medicine of the University of California, Los Angeles.
IHC and IF Analysis.
IHC and IF analysis of frozen or paraffin-embedded sections was performed after the previous protocols (19, 22). Briefly, tissue sections were incubated at 4 °C overnight with the following primary antibodies: anti-ERG (sc-353) and AR (Santa Cruz Biotechnology), anti-CD49f (PharMingen), anti-laminin-1 (Sigma), anti-CK5 and CK8 (Covance), anti-c-MYC, c-JUN, and SMA (Abcam), and anti-PCNA (Calbiochem). Mouse anti-GFP monoclonal antibody clone 3C2-G2 was produced in Owen Witte's lab (45). Sections were washed and stained with the corresponding secondary antibodies (19, 22) for 1 h at room temperature, then developed with DAB followed by counterstaining with hematoxylin, or visualized under fluorescent microscopy followed by counterstaining with DAPI.
Supplementary Material
Acknowledgments.
We thank Sanaz Memarzadeh, Rita U. Lukacs, Houjian Cai, Donghui Cheng, and Andrew Tran for technique help and scientific discussion. This work was partly supported by funds from the Prostate Cancer Foundation. O.N.W. is an investigator and Y.Z. is an associate of the Howard Hughes Medical Institute. A.S.G. is supported by an institutional Ruth L. Kirschstein National Research Service Award GM07185. M.A.T. is a scholar of the Leukemia and Lymphoma Society.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905931106/DCSupplemental.
References
- 1.Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 3.Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- 4.Look AT. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278:1059–1064. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- 5.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Evidence of recurrent gene fusions in common epithelial tumors. Trends Mol Med. 2006;12:529–536. doi: 10.1016/j.molmed.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Tomlins SA, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 7.Tomlins SA, et al. TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 2006;66:3396–3400. doi: 10.1158/0008-5472.CAN-06-0168. [DOI] [PubMed] [Google Scholar]
- 8.Tomlins SA, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–599. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- 9.Helgeson BE, et al. Characterization of TMPRSS2:ETV5 and SLC45A3:ETV5 gene fusions in prostate cancer. Cancer Res. 2008;68:73–80. doi: 10.1158/0008-5472.CAN-07-5352. [DOI] [PubMed] [Google Scholar]
- 10.Hermans KG, et al. TMPRSS2:ERG fusion by translocation or interstitial deletion is highly relevant in androgen-dependent prostate cancer, but is bypassed in late-stage androgen receptor-negative prostate cancer. Cancer Res. 2006;66:10658–10663. doi: 10.1158/0008-5472.CAN-06-1871. [DOI] [PubMed] [Google Scholar]
- 11.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8:497–511. doi: 10.1038/nrc2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perner S, et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 2006;66:8337–8341. doi: 10.1158/0008-5472.CAN-06-1482. [DOI] [PubMed] [Google Scholar]
- 13.Klezovitch O, et al. A causal role for ERG in neoplastic transformation of prostate epithelium. Proc Natl Acad Sci USA. 2008;105:2105–2110. doi: 10.1073/pnas.0711711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Thomas TZ, Kasper S, Matusik RJ. A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology. 2000;141:4698–4710. doi: 10.1210/endo.141.12.7837. [DOI] [PubMed] [Google Scholar]
- 15.Wu X, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 16.Tomlins SA, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–188. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carver BS, et al. ETS rearrangements and prostate cancer initiation. Nature. 2009;457:E1. doi: 10.1038/nature07738. discussion E2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King JC, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat Genet. 2009;41:524–526. doi: 10.1038/ng.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xin L, Ide H, Kim Y, Dubey P, Witte ON. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11896–11903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosquera JM, et al. Morphological features of TMPRSS2-ERG gene fusion prostate cancer. J Pathol. 2007;212:91–101. doi: 10.1002/path.2154. [DOI] [PubMed] [Google Scholar]
- 21.Stingl J, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 22.Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun C, et al. TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene. 2008;27:5348–5353. doi: 10.1038/onc.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verger A, et al. Identification of amino acid residues in the ETS transcription factor Erg that mediate Erg-Jun/Fos-DNA ternary complex formation. J Biol Chem. 2001;276:17181–17189. doi: 10.1074/jbc.M010208200. [DOI] [PubMed] [Google Scholar]
- 25.Downing SR, Russell PJ, Jackson P. Alterations of p53 are common in early stage prostate cancer. Can J Urol. 2003;10:1924–1933. [PubMed] [Google Scholar]
- 26.Chen Z, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z, et al. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 2006;66:7889–7898. doi: 10.1158/0008-5472.CAN-06-0486. [DOI] [PubMed] [Google Scholar]
- 28.Portella G, et al. Human papilloma virus 16 E7 oncogene does not cooperate with RET/PTC 3 oncogene in the neoplastic transformation of thyroid cells in transgenic mice. Oncol Res. 2001;12:347–354. doi: 10.3727/096504001108747800. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimoto M, et al. Interphase FISH analysis of PTEN in histologic sections shows genomic deletions in 68% of primary prostate cancer and 23% of high-grade prostatic intra-epithelial neoplasias. Cancer Genet Cytogenet. 2006;169:128–137. doi: 10.1016/j.cancergencyto.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci USA. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xin L, et al. Progression of prostate cancer by synergy of AKT with genotropic and nongenotropic actions of the androgen receptor. Proc Natl Acad Sci USA. 2006;103:7789–7794. doi: 10.1073/pnas.0602567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majumder PK, et al. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: The MPAKT model. Proc Natl Acad Sci USA. 2003;100:7841–7846. doi: 10.1073/pnas.1232229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 34.Shaffer DR, Pandolfi PP. Breaking the rules of cancer. Nat Med. 2006;12:14–15. doi: 10.1038/nm0106-14. [DOI] [PubMed] [Google Scholar]
- 35.Chen CD, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 36.DeMarzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and molecular aspects of prostate cancer. Lancet. 2003;361:955–964. doi: 10.1016/S0140-6736(03)12779-1. [DOI] [PubMed] [Google Scholar]
- 37.Gao J, Arnold JT, Isaacs JT. Conversion from a paracrine to an autocrine mechanism of androgen-stimulated growth during malignant transformation of prostatic epithelial cells. Cancer Res. 2001;61:5038–5044. [PubMed] [Google Scholar]
- 38.Bergerat JP, Ceraline J. Pleiotropic functional properties of androgen receptor mutants in prostate cancer. Hum Mutat. 2009;30:145–157. doi: 10.1002/humu.20848. [DOI] [PubMed] [Google Scholar]
- 39.Pereira DS, et al. Retroviral transduction of TLS-ERG initiates a leukemogenic program in normal human hematopoietic cells. Proc Natl Acad Sci USA. 1998;95:8239–8244. doi: 10.1073/pnas.95.14.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han G, et al. Mutation of the androgen receptor causes oncogenic transformation of the prostate. Proc Natl Acad Sci USA. 2005;102:1151–1156. doi: 10.1073/pnas.0408925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai C, Hsieh CL, Shemshedini L. c-Jun has multiple enhancing activities in the novel cross talk between the androgen receptor and Ets variant gene 1 in prostate cancer. Mol Cancer Res. 2007;5:725–735. doi: 10.1158/1541-7786.MCR-06-0430. [DOI] [PubMed] [Google Scholar]
- 42.Garlich JR, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008;68:206–215. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- 43.Maira SM, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8:440–448. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Memarzadeh S, et al. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell. 2007;12:572–585. doi: 10.1016/j.ccr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.