Abstract
Objective
Bryostatin-1 and related diacylglycerol (DAG) analogues activate RasGRPs in lymphocytes, thereby activating Ras and mimicking some aspects of immune receptor signaling. To define the role of RasGRPs in lymphocyte apoptosis and to identify potential therapeutic uses for DAG analogues in lymphocyte disorders, we characterized the response of lymphoma-derived cell lines to DAG analogues.
Materials and Methods
Human lymphoma-derived B cell lines and mouse primary B cells were treated with bryostatin-1 or its synthetic analogue “pico.” Ras signaling partners and Bcl-2 family members were studied with biochemical assays. Cellular responses were monitored using growth and apoptosis assays.
Results
Stimulation of B cells with DAG analogues results in activation of protein kinase C/RasGRP-Ras-Raf-Mek-Erk signaling and phosphorylation of the proapoptotic BH3-only protein Bim. In vitro, Bim is phosphorylated by Erk on sites previously associated with increased apoptotic activity. In Toledo B cells derived from a non-Hodgkin’s lymphoma (B-NHL), DAG analogue stimulation leads to extensive apoptosis. Apoptosis can be suppressed by either downregulation of Bim or overexpression of Bcl-2. It is associated with the formation of Bak–Bax complexes and increased mitochondrial membrane permeability. Toledo B-NHL cell apoptosis shows a striking dependence on sustained signaling.
Conclusion
In B cells, Erk activation leads directly to phosphorylation of Bim on sites associated with activation of Bim. In Toledo B-NHL cells, the dependence of apoptosis on sustained signaling suggests that Bcl-2 family members could interpret signal duration, an important determinant of B cell receptor–mediated negative selection. Certain cases of B-NHL might respond to DAG analogue treatment by the mechanism outlined here.
Lymphocytes respond differentially depending on the strength and duration of antigen receptor signaling and on concomitant signaling through other receptors [1,2]. Immune receptor stimulation under different conditions results in apparently similar ensuing biochemical events, but these can alternatively promote lymphocyte development, activation, anergy, proliferation, or apoptosis. Control of apoptosis in lymphocytes is particularly important, as cells with strongly self-reactive immune receptors must be culled by this means to avoid autoimmune disorders [3].
The small GTPase Ras plays a key role in transducing immune receptor signals during lymphocyte development and function. Ras in lymphocytes is regulated by RasGRPs, Ras guanyl-releasing proteins [4–6]. These constitute a class of Ras guanyl nucleotide exchange factors (Ras GEFs) that possess regulatory C1 domains functionally similar to the diacylglycerol (DAG)-binding domains of protein kinase C (PKC). Immune receptor signaling results in activation of phospholipase C leading to accumulation of DAG in cellular membranes. By binding DAG through their C1 domains, RasGRPs are recruited to membranes where they interact with substrate Ras and convert it to its activeGTP-bound state. Additionally, membrane DAG recruits and activates PKC, which positively regulates RasGRPs by phosphorylation [5]. Once Ras is activated, it can interact with a variety of down-stream effector systems, the best characterized of which is the Raf-Mek-Erk kinase cascade. The protein kinase Erk phosphorylates many substrates and thereby influences cell proliferation, differentiation, and survival.
In many cell types, including lymphocytes, apoptosis is regulated by theBcl-2 family, which comprises three functionally distinct types of proteins [7]. Proapoptotic Bak and Bax can form multisubunit complexes that compromise the integrity of the outer mitochondrial membrane. This leads to cytochrome-C release, assembly of the proapoptotic molecule APAF-1, and activation of executioner caspases. In healthy cells, according to one popular model, antiapoptotic proteins, such as Bcl-2, Bcl-Xl, and Mcl1 antagonize this process by binding to and neutralizing Bak and Bax. In turn, these antiapoptotic proteins can be titrated by a third class of Bcl-2 protein, the proapoptotic BH3-only proteins [3].
Bim is the key BH3-only regulator of apoptosis in lymphocytes [7–9]. Some evidence supports the idea that Bim gene expression is regulated downstream of immune receptor signaling [10–12]. Additionally, evidence from a number of cell systems supports the proposal that Bim can be positively or negatively regulated by phosphorylation [13–15]. Bim is expressed by alternative splicing as three canonical proteins of decreasing size and abundance: BimEL, BimL, and BimS (Fig. 4F). Erk phosphorylation on Ser69 (Ser65 in rodents) in a BimEL-specific region leads to ubiquitin-dependent proteolysis in a variety of cell types [15,16]. In contrast, the kinase Jnk was shown to phosphorylate BimL on Ser44, Thr56, and Ser58 [17]. Phosphorylation was proposed to facilitate redistribution of BimL from the cytoskeleton to the mitochondria, thereby increasing its apoptotic activity. In T cells, however, Bim is constitutively associated with mitochondria, suggesting that phosphorylation must activate Bim by some other means [18]. Clarifying how Bim function is regulated by phosphorylation in lymphocytes will provide considerable insights into how immune receptor signaling can regulate immune cell apoptosis.
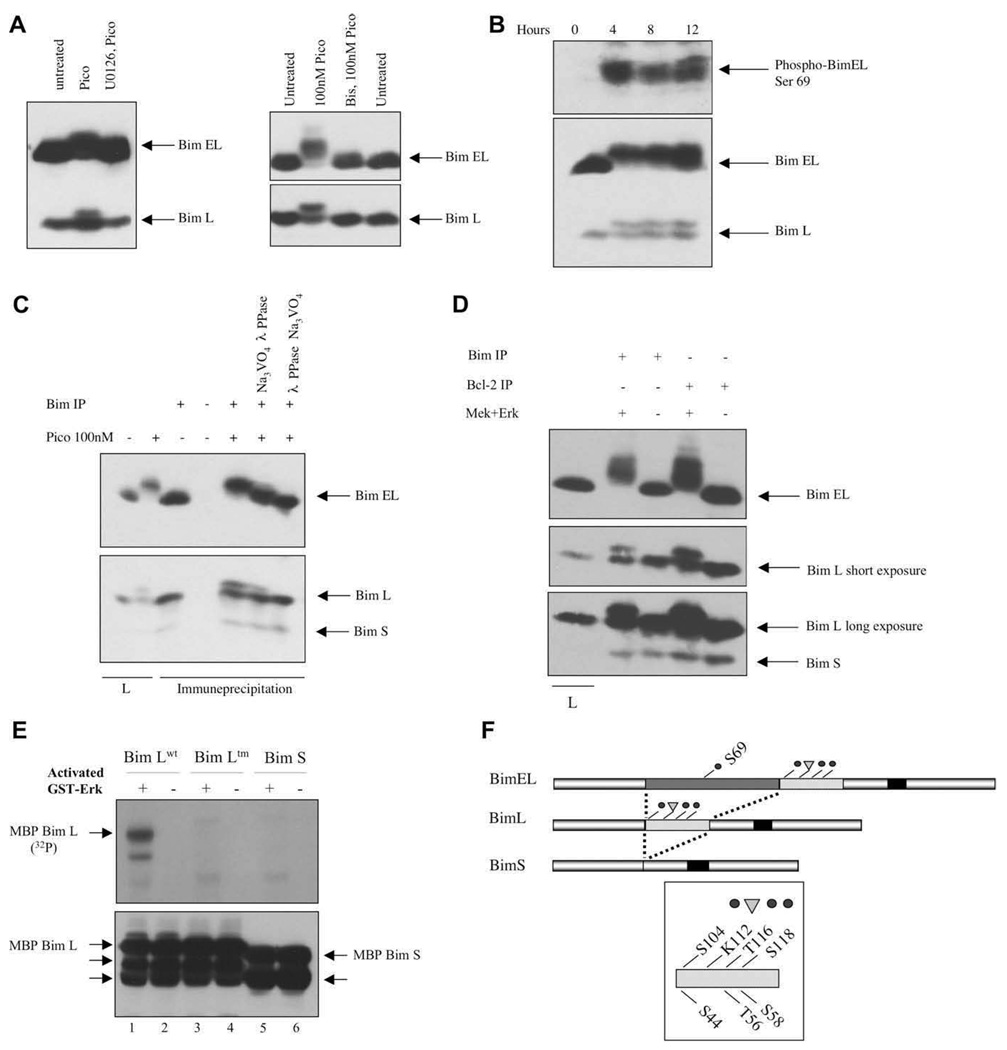
Figure 4.
Phosphorylation of BimEL and BimL by Erk in vivo and in vitro. (A) Cells were treated with 10 nM pico for 28 hours (left panel) or 100 nM for 20 minutes (right panel). Inhibitors were added prior to diacylglycerol (DAG) analogue stimulation. (B) Toledo B-cell non-Hodgkins lymphoma (B-NHL) cells were treated for various periods of time with 10 nM pico and Bim phosphorylation was demonstrated either by probing with an anti-phospho-BimEL (Ser 69) antibody (top panel) or by electrophoretic mobility shifting (bottom panel). (C) The reduced electrophoretic mobility of BimEL and BimL was reversed by in vitro phosphatase treatment. The first two lanes represent lysates (L) analyzed directly. (D) Endogenous Bim proteins, immune-precipitated directly or co-precipitated with anti–Bcl-2 antibodies, were phosphorylated with recombinant Mek plus Erk before electrophoretic mobility shift analysis. (E) Recombinant maltose-binding protein (MBP)-BimL wild-type (wt), MBP-BimL triple mutant (tm), and MBP-BimS were incubated with Mek-activated Erk (odd numbered lanes) in the presence of labeled adenosine triphosphate (ATP). Top panel, radioactive phosphate incorporation was detected by 2 hours autoradiography. Bottom panel, MBP-BimL and MBP-BimS were detected by immunoblotting with an anti-Bim antibody. Unlabeled arrows indicate proteolytic breakdown products of MBP-Bim that preexisted in the protein preparations prior to the kinase assay. Control lanes (even numbers) were from identical paired reactions, but Erk was omitted. (F) Schematic diagram showing the structural relationships between the major Bim splice forms and the positions of the phosphorylation sites and the potential ubiquitination site (K112) discussed in the text.
Here we report that treatment of the Toledo non-Hodgkin’s B cell lymphoma (B-NHL) cell line with DAG analogues leads to the activation of the PKC/RasGRP-Erk signaling axis, proapoptotic Bim phosphorylation and Bim-dependent apoptosis. The properties of this novel apoptosis pathway might shed light on how sustained BCR signaling, in particular, could lead to deletion of strongly self-reactive B cells. Our results may also provide a mechanistic rationale for the treatment of a subset of B-NHL with DAG analogues.
Materials and methods
Reagents and antibodies
The pan-PKC inhibitor Bisindolylmaleimide I was purchased from Calbiochem and was used at 4.6 uM final concentration. The Mek inhibitor U0126 was purchased from Cell Signaling and was used at 1 uM. Tetramethylrhodamine ethyl ester (TMRE) was purchased from Molecular Probes. MG132 (Sigma-Aldrich, St Louis, MO, USA) was used at 10 uM. Lambda phosphatase was purchased from New England BioLabs.
The following antibodies from Cell Signaling were used to detect proteins by immunoblotting: Bim (#4582), Bcl-2 (#2872), Bcl-Xl (#2762), and Mcl1 (#4572). Poly (ADP-ribose) polymerase family, member 1 (PARP-1) antibody was purchased from Santa Cruz Biotechnology (sc-8007). For immune precipitation, we used antibodies to Bax (N20, sc493; Santa Cruz Biotechnology), Bak (NT, 06536 Upstate Biotechnology), and G-Beta (m14, sc-261 Santa Cruz Biotechnology). Antibodies to phospho-BimEL (Ser69 human) were from Chemicon (AB3579). The anti-Bcl-2 antibody 6C8 (BD Biosciences, Mississauga, Ontario, Canada) was used to coprecipitate Bcl-2 and Bim for in vitro Erk phosphorylation studies.
Cells and gene transfer
Toledo B-NHL, Ramos, and RL cell lines were obtained from American Type Culture Collection and were maintained in RPMI supplemented with 10% fetal bovine serum, antibiotics and 5 × 10−5 M β-mercaptoethanol.
The presence of wild-type BimEL, L, and S mRNA sequences in Toledo B-NHL was confirmed by molecular cloning techniques. To effect downregulation of all three Bim isoforms in Toledo B-NHL cells, a small interfering RNA (siRNA) retrovirus vector was employed [19]. Retrovirus was transduced into Toledo B-NHL cells using a helper-free amphotropic packaging cell line and infected cells were selected with 2.5 ug/mL puromycin. Bcl-2 was overexpressed using the pBMGNeo expression plasmid, which carries the inducible metallothionein promoter [20]. After digestion with NotI restriction endonuclease, linear plasmid DNA was introduced into Toledo B-NHL by electroporation, followed by selection of transfected cells in 1.0 mg G418 (active drug)/mL. Expression of Bcl-2 was enhanced by addition of 5 uM CdCl2 overnight and then during pico treatment.
Mouse B cells were isolated from the spleens of 5- to 8-week-old B6 and Rasgrp1−/−; Rasgrp3−/− double-mutant mice (C57Bl/6J background) by magnetic bead technology as described previously [21,22]. Animal studies were done according to protocols approved by The Health Sciences Policy and Welfare Committee at the University of Alberta, in accordance with Canadian Council on Animal Care Guidelines.
Biochemical assays
Detection of RasGRP1 and RasGRP3 by immunoblotting was performed using methods and antibodies we have described previously [23,24]. Ras and Mek and Erk signaling assays were as described previously [24].
To detect Bak–Bax complexes, cells were first lysed in buffer containing, 20 mM Tris pH 7.4, 137 mM NaCl, 2 mM ethylene diamine tetraacetic acid, 10% v/v glycerol, 2% w/v 3[(3-cholamidopropyl) dimethylammonio]-1-propanesulphonate (CHAPS), and protease inhibitors [25]. After addition of antibody and protein A Sepharose, lysates were incubated at 0C for 2 °hours followed by washing three times in the same buffer, except that the CHAPS was reduced to 0.5%. Immune-precipitated samples were resolved by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by immunoblotting with anti-Bak antibodies.
To dephosphorylate Bim, pico-treated Toledo cells were lysed in NP-40 lysis buffer (20 mM Tris pH 8.0, 150 mM NaCl, 10% glycerol, 1% v/v NP-40, 40 mM β-glycerol phosphate and protease inhibitors) and centrifuged to remove nuclei. Bim was immune-precipitated from lysates with protein A Sepharose beads coated with a polyclonal antibody that recognizes the N-terminal region of all three Bim species. After washing in NP-40 lysis buffer three times, beads were washed once in phosphatase reaction buffer consisting of 50 mM Tris (pH 7.5), 100 mM NaCl, 2 mM MnCl2, 2 mM dithiothreitol, 0.1 mM ethylene glycol tetraacetic acid and 0.01% Brij 35. Then 100 uL samples (50% bead volume) were incubated with 1000 units of phage λ phosphatase at 30°C for 30 minutes. In some assays, the phosphatase inhibitor sodium orthovanadate was added at 0.5 mM before addition of enzyme. To control for nonspecific effects of inhibitor or phosphatase on electrophoretic mobility, orthovanadate was also added at the end of the reaction to the experimental samples.
To demonstrate Erk phosphorylation of Bim in vitro, two approaches were used. In the first, Bim was immune-precipitated either directly from NP-40 buffer lysates of RL cells, or coprecipitated with Bcl-2 using an anti–Bcl-2 antibody. We chose RL cells for this experiment because the overexpression of Bcl-2 in these cells likely favors the association of Bim with Bcl-2. The immune-precipitated proteins were incubated at 30°C for 60 minutes in a 50-uL reaction containing recombinant His-tagged-Mek1 (4.0 ug) and GST-Erk2 (4.0 ug), each synthesized in Escherichia coli, 100 uM unlabeled adenosine triphosphate (ATP), 25 mM HEPES (pH 7.4), 10 mM magnesium acetate, and 2 mM dithiothreitol. Phosphorylation was monitored using the electrophoretic mobility shift assay. In the second method, BimL wild-type and BimL triple mutant (Ser44Ala, Thr56Ala, Ser58Ala) cDNAs were expressed in E. coli using the vector pMalC2, which expresses each as a maltose-binding protein (MBP)-BimL fusion protein. GST-Erk was first activated by incubation with recombinant Mek1 and 100 uM unlabeled ATP. Activated Erk was then separated from Mek1 using glutathione beads and used to phosphorylate MBP-BimL in reactions that contained 20 uM unlabeled ATP and 10 uCi of [32Pγ] ATP. Incorporation of label into MBP-BimL was monitored by autoradiography after resolution by sodium dodecyl sulfate polyacrylamide gel electrophoresis.
To quantify Bim and Bcl-2 in downregulation and overexpression studies, immunoblot images were analyzed using ImageQuant TL 2005. Results are representative of duplicate experiments.
Apoptosis and growth assays
To determine the proportion of cells with intact mitochondria, TMRE was added at 100 nM for the final 20 minutes of various culture incubation periods. Flow cytometry analysis clearly discriminated two cell populations that did not overlap in staining properties: apoptotic (unstained) and viable (stained). Toledo B-NHL cells grow with a doubling time of about 22 hours and DAG analogue-induced loss of TMRE staining occurs over a shorter period. Therefore, we expressed viable cells as raw percentages and ignored the possible effects of proliferation of the nonapoptotic fraction. However, the doubling time of Ramos cells is approximately 13 hours and anti–IgM-induced TMRE staining loss was more evident at 48 hours than 24 hours. Therefore, in Figure 7D we have plotted the number of apoptotic cells observed as a percentage of the number of initial cells in each culture (5.4 × 10+5 cells in 2.0 mL). The validity of each method was verified by direct microscopic observation of representative cultures. The dUTP end-labeling TUNEL assay was used according to the manufacturer’s instructions (Roche, Mannheim, Germany).
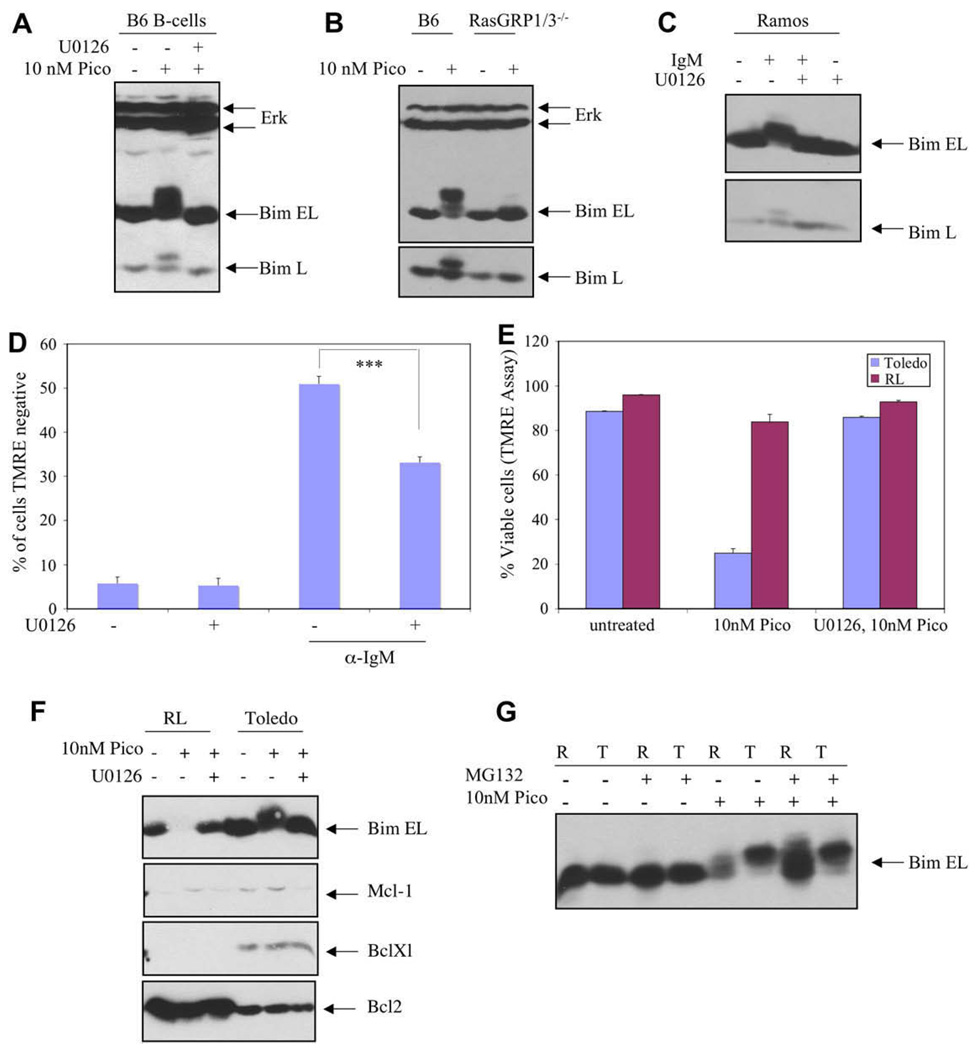
Figure 7.
Similarities and differences between different B-cell populations. (A) Primary splenic B cells isolated from C57Bl/6J mouse (B6) were either incubated without treatment or stimulated with pico for 3.5 hours, with or without prior addition of the Mek inhibitor. BimEL and BimL were analyzed by electrophoretic mobility shift to detect phosphorylation. (B) Similarly, primary B cells from C57Bl/6J (B6) and Rasgrp1 −/−; Rasgrp3 −/− double mutant (RasGRP1/3−/−) mice were compared after 10 nM pico treatment for 4 hours. (C) Ramos B cells were treated with anti-IgM antibodies (10 ug/mL) for 30 minutes alone or with Mek inhibitor pretreatment, followed by analysis of Bim by immunoblotting. (D) Ramos B cells were cultured in medium containing U0126, anti-IgM, or both for 48 hours. Apoptosis was detected using flow cytometry to quantify cells unable to assimilate tetramethylrhodamine ethyl ester (TMRE) and the number of apoptotic cells was plotted as a percent of input cells (***p < 0.0001; see Materials and Methods). Results were duplicated in an independent experiment. (E) Toledo B-cell non-Hodgkins lymphoma (B-NHL) and RL cells were compared in terms of their response to pico treatment using TMRE staining followed by flow cytometry. Incubation with pico was for 24 hours. (F) Toledo B-NHL and RL cells were analyzed for expression of various Bcl-2 family members by immunoblotting. Lysates were prepared from untreated cultures as well as cultures treated for 4 hours with 10 nM pico alone or with the Mek inhibitor. Each gel lane was loaded with 100 ug protein. (G) Toledo B-NHL cells (T) and RL cells (R) were compared in terms of their response to treatment with pico (10 nM) and the proteosome inhibitor MG132 by analyzing BimEL using Bim immunoblotting to detect Bi-mEL mobility and expression level. Treatment was for 5 hours.
Labeled cells were analyzed by flow cytometry using FACScan with an argon laser (480 nM) and Cellquest software (BD Biosciences). Flow cytometry values are averages derived from four separate cultures (±standard deviation of the mean) in two separate experiments. Statistical analysis was performed using the Student’s t-test. Cell proliferation experiments were performed with a model Zf counter (Coulter Electronics).
Results
Stimulation of RasGRPs in Toledo B cells triggers Mek-dependent apoptosis by a mitochondrial pathway
We examined a collection of human B cell–derived cell lines for their responses to DAG analogues such as bryostatin-1 and its synthetic analogue “pico” [26]. We previously demonstrated that these two compounds were indistinguishable in T-cell RasGRP1-based assays [27]. We discovered that treatment of Toledo B-NHL cells with low nanomolar concentration of DAG analogue caused them to assume a granular, vacuolated appearance and to die (Fig. 1A, B).
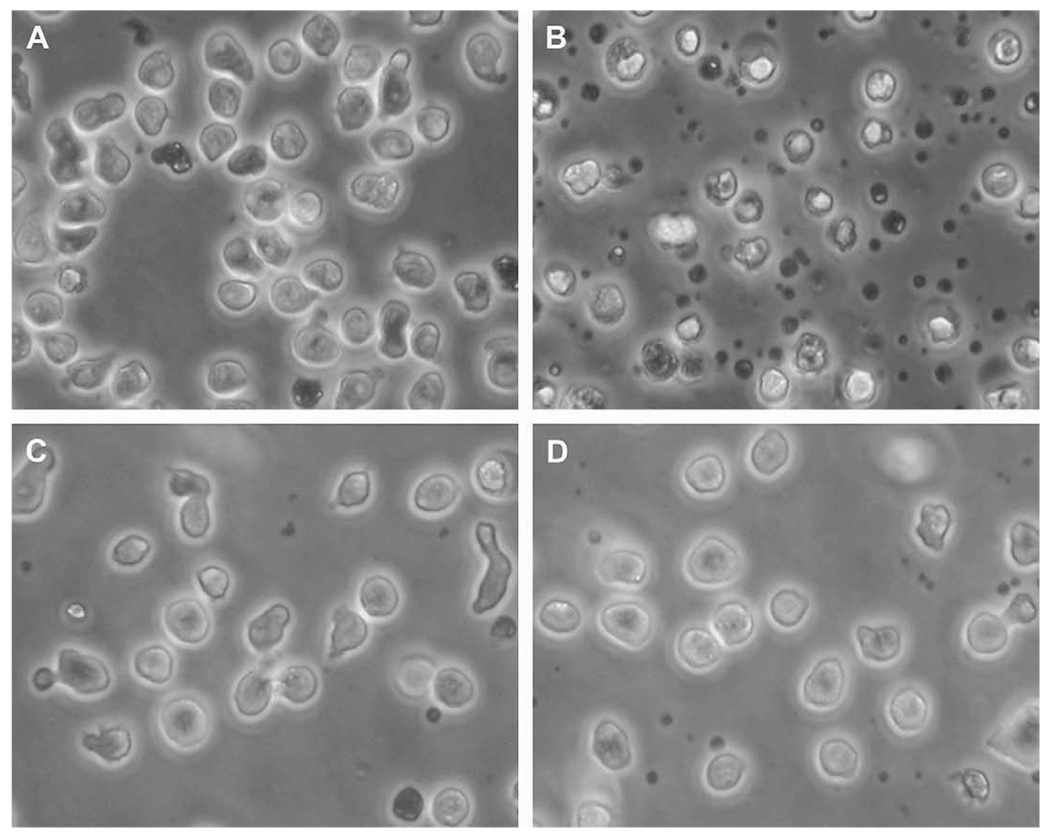
Figure 1.
Diacylglycerol (DAG) analogue induced death in Toledo B-cell non-Hodgkins lymphoma (B-NHL) cells by a protein kinase C (PKC)- and Mek-dependent mechanism. (A–D) Morphology of Toledo B-NHL cells is shown after 48 hours incubation in various conditions: (A) Untreated; (B) 10 nM pico; (C) 10 nM pico plus 4.8 uM Bisindolylmaleimide I; (D) 10 nM pico plus 10 uM U0126.
The PKC inhibitor Bisindolylmaleimide I protected Toledo cells from pico-induced cell death (Fig. 1C). The Mek inhibitor U0126 also blocked the toxic effects of pico (Fig. 1D). Similar results were obtained with the Mek inhibitor PD098059 (data not shown).
Inhibitors of other signaling pathways, such as cyclosporine A (calcineurin), rapamycin (mTOR), Ly294002 (phosphoinositide 3-kinase), JNK inhibitor II (JnkI and Jnk2 mitogen-activated protein [MAP] kinase) and SB 202190 (p38 MAP kinase) did not protect Toledo B-NHL cells from pico-induced death (data not shown).
Like other B cells we have studied, Toledo cells express both RasGRP1 and RasGRP3 (Fig. 2A). Addition of 100 nM pico led to a prompt increase in the level of Ras-GTP in Toledo B cells. This presumably led to activation of Raf, as evidenced by the rapid induction of Mek and Erk phosphorylation (Fig. 2A). After pico treatment, RasGRP3 exhibited a reduced electrophoretic mobility indicative of phosphorylation (Fig. 2A). The pan-PKC inhibitor Bisindo-lylmaleimide I blocked the RasGRP3 electrophoretic mobility shift in pico-stimulated Toledo cells, diminished Ras-GTP accumulation and blocked Mek1 and Erk activation (Fig. 2A). The observation that the PKC inhibitor had a greater effect on Mek1 and Erk than on Ras-GTP may reflect the role of PKC in Raf activation [28]. Authentic bryostatin-1 elicits an indistinguishable signaling response to that seen with pico in Toledo B-NHL cells (Fig. 2B). Thus, compared to other B cells we have studied [6,22], Toledo B-NHL cells have a similar RasGRP signaling system.
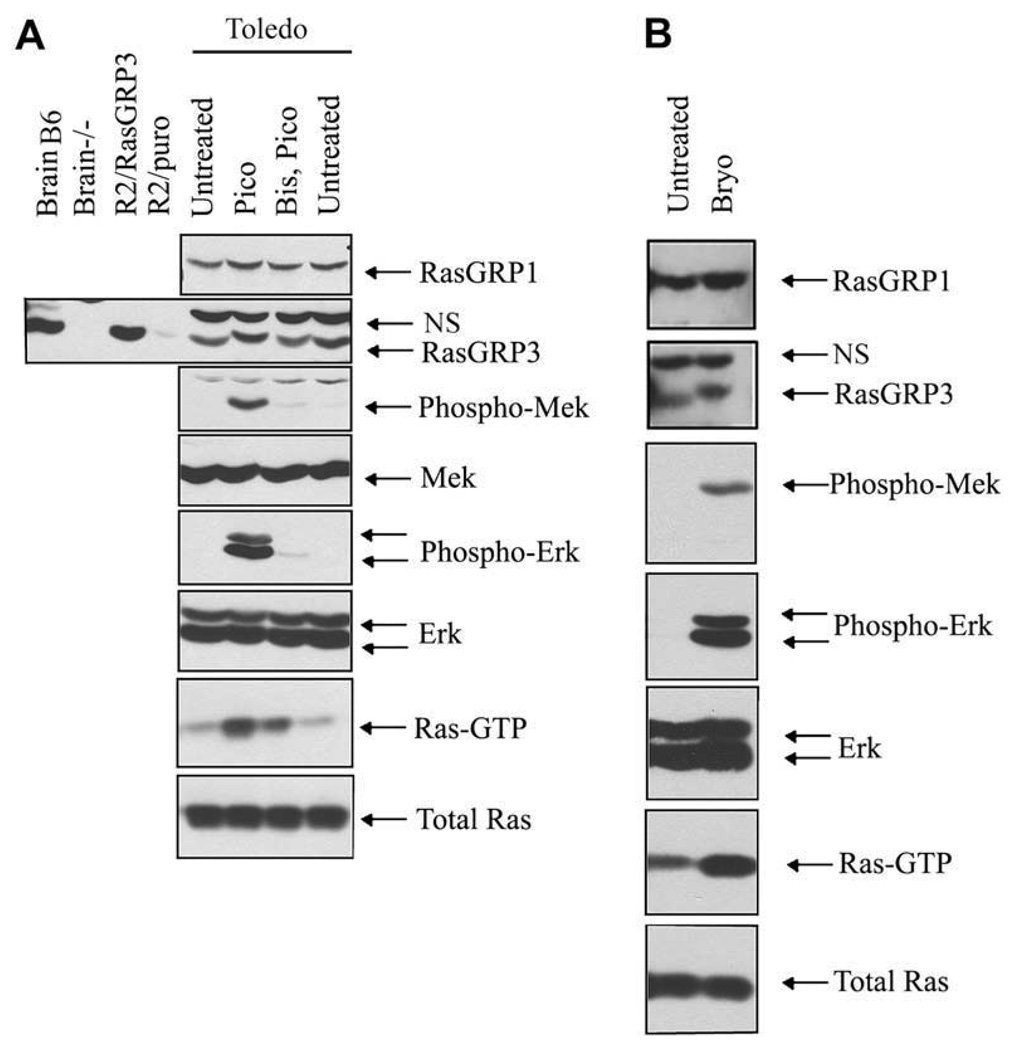
Figure 2.
RasGRP1 and RasGRP3 expression and activation by diacylglycerol (DAG) analogues in Toledo B-cell non-Hodgkins lymphoma (B-NHL). (A) The presence of both RasGRP1 and RasGRP3 was demonstrated by immuno-blotting. Phosphorylation of RasGRP3 is evident from its reduced electrophoretic mobility after pico treatment. The specificity of the RasGRP3 antibody was confirmed by parallel analysis of endogenous RasGRP3 in brain extracts from C57Bl/6J (B6) and RasGRP3 null mutant (−/−) mice, as well as by analysis of RasGRP3 expressed ectopically in rat2 cells with the vector pBabePuro (left four lanes). Pico-induced phosphorylation of Erk and Mek was demonstrated with phospho-antibodies while Ras-GTP was detected with the pull-down assay. The two phospho-Erk species correspond to phospho-Erk1 and phospho-Erk2. Treatment was 100 nM pico for 20 minutes alone or after pretreatment with the pan-PKC inhibitor Bisindolylmaleimide I (Bis). (B) Bryostatin-1 caused a similar activation of the RasGRP signaling system. Cells were treated with 100 nM bryostatin-1 for 10 minutes. NS = nonspecific band.
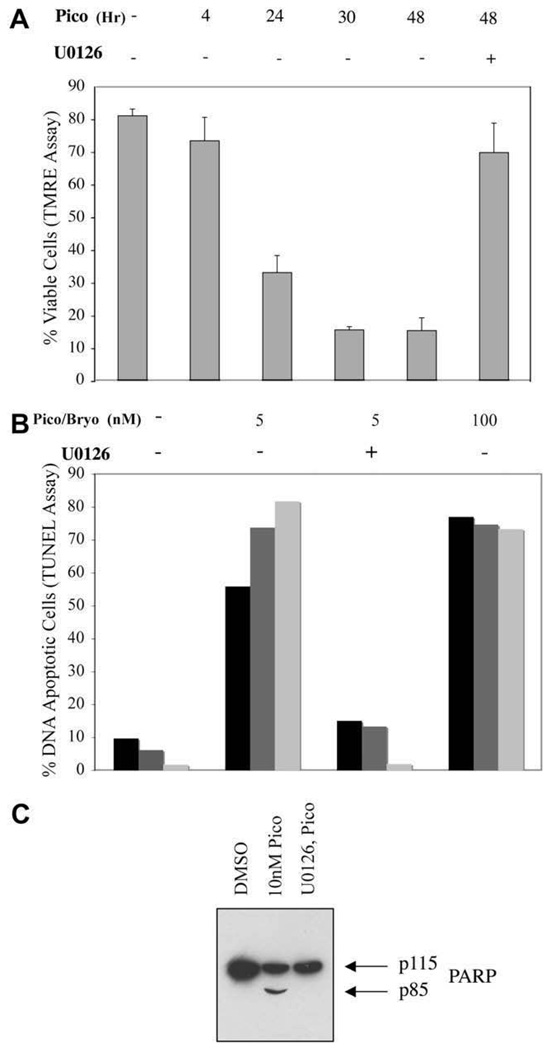
Prolonged treatment of Toledo B-NHL cells with pico led to a progressive loss in their ability to assimilate TMRE into their mitochondria (Fig. 3A), indicating that Toledo B-NHL cells died by apoptosis. Using the TUNEL assay to monitor DNA fragmentation, most cells exhibited nuclear DNA fragmentation by 48 hours (Fig. 3B). The results obtained with pico and bryostatin-1 were very similar. Apoptotic cells also showed extensive nuclear DNA fragmentation as assessed by direct electrophoretic analysis (data not shown). We also observed proteolytic cleavage of PARP-1 implying activation of executioner caspases (Fig. 3C). The Mek inhibitor blocked all of these apoptotic responses. Thus, DAG analogue treatment in Toledo B-NHL cells induces apoptosis by a RasGRP-Ras-Raf-Mek-Erk–mediated mitochondrial pathway.
Figure 3.
Induction of apoptosis by diacylglycerol (DAG) analogue treatment of Toledo B-cell non-Hodgkins lymphoma (B-NHL) cells by a mitochondrial pathway. (A) Cells were treated for various times (in hours) with 10 nM pico then stained with tetramethylrhodamine ethyl ester (TMRE) to detect cells with healthy mitochondria. The Mek-dependence of staining loss was demonstrated by including the Mek inhibitor U0126 throughout the incubation period. (B) Induction of DNA fragmentation after 48 hours 5 or 100 nM pico treatment (black and gray bars represent duplicate cultures) or bryostatin-1 treatment (light bars) was determined using the TUNEL assay. (C) Pico-induced, Mek-dependent cleavage of poly (ADP-ribose) polymerase family, member 1 (PARP-1) after 24 hours treatment with 10 nM pico was demonstrated by immunoblotting. Full-length PARP-1 is indicated by p115 while p85 represents a caspase-cleaved species.
DAG analogue-induced apoptosis involves a Bim-, Bcl-2-, and Bak/Bax-dependent mechanism
We examined the status of various Bcl-2 family members by immunoblotting in order to uncover the connection between Erk activation and increased mitochondrial membrane permeability in Toledo B-NHL cells. Strikingly, the proapoptotic BH3-only protein Bim exhibited an electro-phoretic mobility shift indicative of phosphorylation in DAG analogue treated Toledo B-NHL cells (Fig. 4). Pretreatment with the Mek inhibitor U0126 blocked the pico-induced mobility shift of both BimEL and BimL (Fig. 4A). Similar results were obtained when activation of RasGRP1 and RasGRP3 was blocked by pretreatment with the pan-PKC inhibitor (Fig. 4A).
Phosphorylation of BimEL and BimL was remarkably persistent during pico treatment and was observed as late as 24 hours in one experiment. Modification of BimEL includes phosphorylation on Ser69, as demonstrated with a phosphospecific antibody (Fig. 4B). BimEL and BimL mobility shifts were closely correlated. Contrary to what is seen in other cell types, loss of BimEL, as might be expected from ubiquitination and proteosome-mediated degradation, was modest or not observed. As confirmed in previous studies, the stimulated BimEL and BimL mobility shifts are sensitive to phosphatase treatment (Fig. 4C). BimS was not reproducibly visualized in our immunoblots, but was never found to undergo induced mobility changes (Fig. 4C, D, and Fig.6D). These are potentially important observations because they suggest that Erk can phosphorylate BimL on consensus sites Ser44, Thr56, and Ser58, modifications that have been previously associated with enhanced proapoptotic function.
Figure 6.
Requirement for sustained RasGRP-Erk signaling and Bim phosphorylation in pico-induced apoptosis of Toledo B-cell non-Hodgkins lymphoma (B-NHL) cells. (A) Toledo B-NHL cells were cultured without treatment (diamond), treated for 4 days with 10 nM pico alone (square), or additionally with 4.6 uM bisindolylmaleimide I (circle) or 1.0 uM U0126 (triangle) followed by washing with drug-free medium. Cells were then incubated in regular medium for an additional 4 days and counted. (B) Toledo B-NHL cells were treated with 10 nM pico and assessed for mitochondrial integrity by tetramethylrhod-amine ethyl ester (TMRE) staining at 31 hours. The effects of washing out pico at 9 hours were determined. The effects of adding Bisindolylmaleimide I either immediately before pico (early Bis) or 8 hours later (late Bis) were also compared. (C) Similarly, cells were either untreated (light bars) or treated with 10 nM pico (dark bars) and were examined by TMRE staining at 30 hours without or with U0126 addition at 4, 8 or 12 hours, as indicated. (D) Aliquots of cells from this latter experiment were removed at the indicated times, after a final 30 minutes incubation with or without the Mek inhibitor. Lysates were analyzed for phospho-Erk and Bim by immunoblotting.
To determine whether Erk can phosphorylate BimL directly, endogenous Bim was immune-precipitated and incubated with activated protein kinase. Because Bim physically associated with Bcl-2 might serve as a relatively more effective substrate [13], we also coprecipitated Bim indirectly with an anti–Bcl-2 antibody. In both circum-stances, the samples were assessed using a gel mobility assay to document phosphorylation. Notably, both BimEL and BimL, precipitated in either manner, served as Erk sub-strates, while BimS did not (Fig. 4D). To investigate Erk phosphorylation of BimL in more detail, recombinant full-length BimL was incubated with activated Erk and radiolabeled ATP. As expected, phosphate incorporation into wild-type BimL was readily detected (Fig. 4E). A triple mutant protein with alanine substitutions at positions 44, 56, and 58 was not a substrate, nor was BimS. Collectively, these observations argue that Erk can directly phosphorylate Bim on proapoptotic sites during DAG analogue-induced apoptosis (Fig. 4F).
The critical importance of Bim as an apoptotic effector molecule in this system was confirmed using a retrovirus vector that directs the expression of an siRNA capable of downregulating all three canonical Bim splice forms. Bim-EL protein level was diminished by 44% compared to that seen in cells expressing an irrelevant control vector (Fig. 5A). This reduction of Bim was associated with substantial retention of TMRE staining at 24 hours after pico treatment. It also caused diminished DNA fragmentation at 48 hours (Fig. 5 B, C).
Figure 5.
Regulation of diacylglycerol (DAG) analogue-induced apoptosis in Toledo B-cell non-Hodgkins lymphoma (B-NHL) cells by Bcl-2 family members. (A) Toledo B-NHL cells expressing either the control vector, siLuc, or the siBim vector were examined for Bim expression levels by immunoblotting. Erk was examined as a loading control. Results are representative of two independent experiments. (B) Toledo cells expressing the siLuc and siBim downregulation vectors were compared by tetramethylrhodamine ethyl ester (TMRE) staining after 20 hours (experiment #1) or 26 hours (experiment #2) of 10 nM pico treatment. Results of the two experiments were normalized by expressing the percent viable cells compared to that seen with untreated cells (**p < 0.03). (C) Similarly, siLuc and siBim were compared after 28 hours of 10 nM pico treatment in two separate experiments using the TUNEL assay for nuclear DNA fragmentation (***p < 0.005). (D) Toledo B-NHL cells expressing empty vector (BMG) and overexpressing Bcl-2 were compared, with and without CdCl2 promoter induction, by anti–Bcl-2 immunoblotting. Erk was examined as a loading control. (E) Bcl-2–engineered and empty vector Toledo B-NHL cells were compared in terms of their TMRE staining after 24 hours treatment with 10 nM pico. The results were duplicated in an independent experiment. (F) Similarly, the effects of Bcl-2 overexpression on apoptosis after 48 hours treatment with 10 nM pico was assessed with the TUNEL assay. (G) The pico-induced, Mek-dependent formation of Bak–Bax physical complexes in Toledo B-NHL cells was detected by the presence of Bak in anti-Bax immune- precipitates. Pico treatment was for 24 hours. L = lysate. Similar results were obtained in two additional experiments.
Apoptosis was also potently inhibited by overexpression of Bcl-2 using an inducible vector (Fig. 5D–F). Remarkably, although basal overexpression of plasmid-encoded Bcl-2 only resulted in an approximately twofold increase compared to the endogenous level (Fig. 5D), this was sufficient to prevent loss of TMRE staining in most cells at 24 hours following application of pico (Fig. 5E). When we examined DNA fragmentation at 48 hours after pico treatment, however, more apoptosis inhibition was evident (Fig. 5F) with a higher level of Bcl-2 expression achieved by induced vector expression (approximately threefold compared to the endogenous level).
Finally, we found that Bak and Bax could be immune-precipitated as a complex from lysates prepared from DAG analogue-treated Toledo B cells (Fig. 5G). The induced aggregation of Bak and Bax was Mek-dependent. Furthermore, it was only evident at late time points, increasing in prominence between 24 and 30 hours (data not shown). These studies indicate that DAG analogue-induced RasGRP-Erk signaling leads to Bim activation followed by Bak/Bax activation and apoptosis in a process that can be antagonized by modest overexpression of Bcl-2.
We did not reproducibly observe activation of p38 or Jnk MAP kinases after DAG analogue treatment (Suppl. Fig. S1). Nor did we observe substantial effects of DAG analogue treatment on Bim membrane association using subcellular fractionation methods. Bim was exclusively associated with the particulate fraction in both pico-stimulated and unstimulated cells (Suppl. Fig. S2). Finally, we found that small fractions of Bim and Bcl-2 can be coprecipitated from Toledo B-NHL lysates, but the degree of this physical association was not significantly affected by pico-induced Bim phosphorylation (Suppl. Fig. S3).
DAG analogue-induced apoptosis of Toledo B cells requires prolonged RasGRP-Erk signaling
Stimulated apoptosis of Toledo B cells is a slow process. For example, although Bim was fully phosphorylated by 2 to 4 hours using a dose of 10 nM pico, Bak–Bax complex formation was more evident at 24 to 30 hours. We reasoned that Toledo cells freshly exposed to DAG analogue might not be irretrievably committed to apoptosis and that cell viability might be rescued by halting RasGRP-Erk signaling at intermediate stages. Such signal interruption experiments might provide useful information about the slow execution of the apoptotic program.
In a preliminary experiment, we cultured Toledo cells for 4 days in pico alone, or additionally with either U0126 or Bisindolylmaleimide I. Cells were counted, washed by repeated centrifugation from fresh medium to remove drugs and then recounted (Fig. 6A). After an additional 4-day incubation in drug-free conditions, cells were counted again. Untreated cells increased about fourfold during the first culture period, while pico-treated cells underwent apoptosis, as confirmed by microscopic observation. The presence of Bisindolylmaleimide I during the first 4-day incubation period with pico blocked the appearance of apoptotic cells and such treated cells increased about fourfold during the second culture period. Proliferation of cells after removal of pico plus U0126 was less robust. Nonetheless, U0126 completely suppressed apoptosis, as assessed by microscopic observation. Collectively, these studies show that pico can be washed out of cells and that the inhibitors protect the viability of the cells rather than simply inhibit the execution of the apoptotic program.
Based on the findings of this preliminary experiment, we treated Toledo cells with 10 nM pico alone for 9 hours. Following cell washing and further culturing, TMRE staining was performed at 31 hours to assess cell viability. This experiment revealed that, in contrast with cells cultured continuously in the DAG analogue, most of the cells could be diverted from an apoptotic response after 9 hours exposure to pico (Fig. 6B). Addition of Bisindolylmaleimide I after 8 hours of pico treatment was also very effective at averting loss of TMRE staining and was, in fact, as effective as when added before pico.
In an additional signaling interruption approach, we treated Toledo cells with 10 nM pico and then added the Mek inhibitor at various time points, followed by TMRE staining at 30 hours (Fig. 6C). Again, substantial abrogation of the apoptotic response, as measured by TMRE staining, was achieved when DAG analogue signaling to Erk was interrupted at intermediate stages. In this experiment, we analyzed Erk and Bim phosphorylation after various periods of pico exposure, with or without a final 30 minutes exposure to the Mek inhibitor U0126 (Fig. 6D). These analyses confirmed that Erk, BimEL, and BimL remained phosphorylated at late time points after pico treatment. Importantly, at any time tested, both proteins are rapidly dephosphorylated upon Mek inhibition. Collectively, these experiments show that sustained signaling is required for DAG-analogue induced cell death in Toledo B-NHL cells.
Generality of RasGRP-Erk-Bim signaling in B cells
We examined other B cells to compare their responses to DAG analogues. B cells isolated from mouse spleens showed pico-induced, Mek-dependent phosphorylation of both BimEL and BimL (Fig. 7A). Double-mutant cells lacking RasGRP1 and RasGRP3 did not exhibit BimEL or BimL phosphorylation after pico treatment (Fig. 7B). Primary B cells exhibited substantial spontaneous death during overnight culturing, precluding an investigation of induced apoptosis.
Ramos B cells are well-characterized in terms of BCR and RasGRP signaling [6,24]. We showed that stimulation with anti-IgM antibodies elicited BimEL and BimL phosphorylation (Fig. 7C). Treatment with anti-IgM also resulted in apoptosis as evidenced by the appearance of a substantial population of cells that was unstained by TMRE (Fig. 7D). Importantly, the Mek-inhibitor U0126 substantially blocked this effect. By comparison, RL cells did not exhibit signs of apoptosis (Fig. 7E). We identified two factors that might explain the relative resistance of RL cells to DAG analogues. First, pico stimulation of RL cells led to loss of BimEL (Fig. 7F). This process was reversed by treatment with the Mek inhibitor U0126 (Fig. 7F) or the proteosome inhibitor MG132 (Fig. 7G). Secondly, RL cells expressed relatively high levels of Bcl-2, as expected for a B-NHL cell line harboring a T(14:18) chromosome rearrangement (Fig. 7F). The pico responses of other B-cell lines, such as Pfeiffer, C1R, and RPMI7666 have been studied less extensively. They exhibit pico-induced BimEL and BimL phosphorylation, BimEL degradation and they do not undergo apoptosis (data not shown).
Discussion
Here we show that DAG analogue-induced activation of RasGRPs in B cells leads to Erk-dependent phosphorylation of Bim on both anti- and proapoptotic sites. In Toledo B-NHL cells, RasGRP-mediated activation of Bim leads to extensive apoptosis. Cell lines derived from malignant blood diseases, including B-NHL, are thought to represent clonal cell populations arrested at various stages of blood cell development [29]. From surface markers, BCR gene configuration and sequence analysis of IgG variable regions, Toledo B-NHL cells appear to represent transformed germinal center B cells [30]. Germinal center B cells normally generate novel BCR species through somatic mutation and some of these B cells are deleted in a process thought to require prolonged BCR-antigen encounter [31–34].
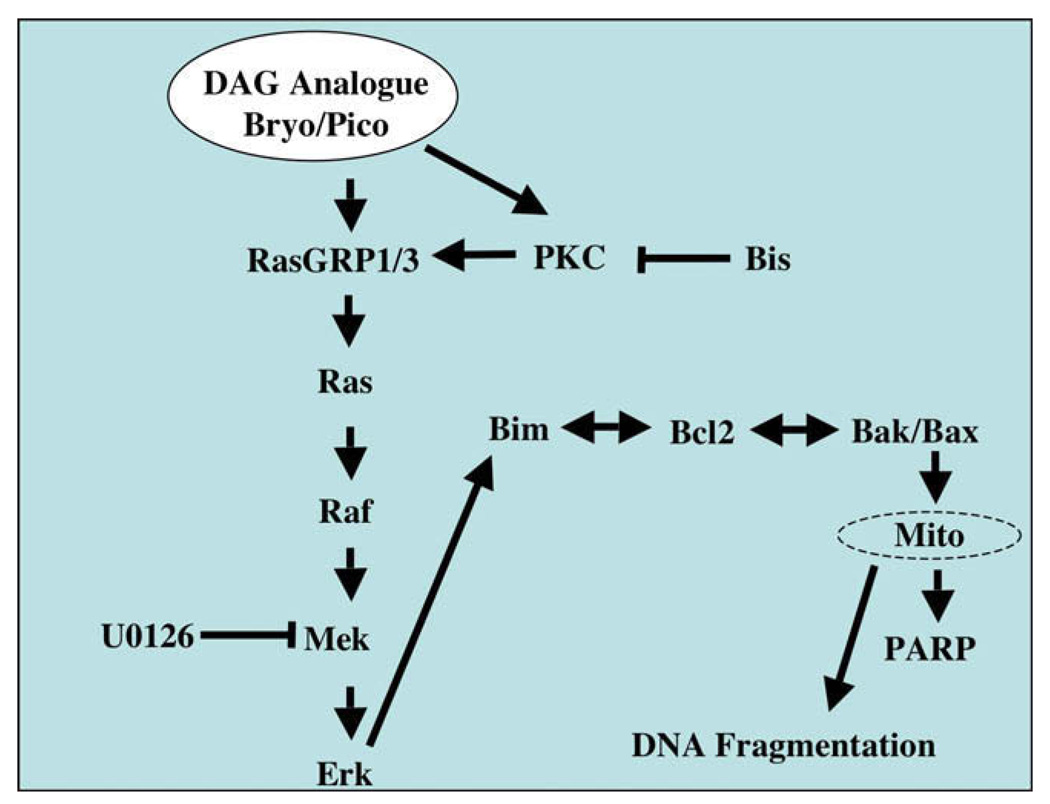
Our studies show that DAG analogue-induced apoptosis in Toledo B-NHL cells requires activation of PKC/RasGRP-mediated Ras-Erk signaling. It also involves Bcl-2 family members previously implicated in control of lymphocyte apoptosis. The Bim downregulation studies and Bcl-2 overexpression studies provide compelling functional data connecting the PKC/RasGRP-Erk and Bcl-2 family regulatory systems. Pharmacological inhibition of RasGRP-Erk signaling blocks Bim phosphorylation, Bak/Bax physical association, loss of TMRE staining, DNA fragmentation, PARP-1 cleavage, and morphological signs of apoptosis. Although we cannot exclude off-target effects of our inhibitors and siRNA reagents, the order of these events after treatment with pico alone is consistent with a more or less linear pathway (Fig. 8). Because RasGRP signaling connects BCR signaling to Ras and important downstream effector systems, we suggest that the DAG analogue response of Toledo B-NHL may provide insights into BCR-mediated negative selection in normal germinal B cells.
Figure 8.
Proposed signaling pathway. Proposed signaling pathway that links protein kinase C (PKC) and RasGRP1/3 to apoptosis in Toledo B-cell non-Hodgkins lymphoma (B-NHL) cells. Single-headed arrows denote positive regulatory influences while double-headed arrows denote reciprocal regulatory effects and physical associations between Bcl-2 family members. Points of action of the Mek inhibitor (U0126) and the pan-PKC inhibitor (Bis) are indicated.
Our previous studies with primary mouse B cells indicated that a primary function of RasGRP1 and RasGRP3 is to promote BCR-induced B cell proliferation [21,22]. However, such proliferation depended substantially on costimulation, which is well-known to affect differentially the proliferative vs apoptotic responses to BCR signaling. Our present studies with mouse B cells and various human B-cell lines indicate that RasGRP- and Mek-dependent proapoptotic Bim phosphorylation may be universal in B cells. Importantly, our experiments with Ramos B cells indicate that Mek-dependent apoptosis is not unique to Toledo B-NHL and that endogenous DAG-generating systems can also promote this pathway. BimL phosphorylation during BCR-stimulated apoptosis in germinal center-derived B-cell lines, including Ramos, has been documented previously [35]. These earlier studies also concluded that Erk, rather than Jnk, positively regulated Bim. Ubiquitin-mediated BimEL degradation, expression of anti-apoptotic proteins and the activity of other biochemical pathways likely prevent the execution of DAG- and DAG analogue-induced apoptosis in most B-cell types.
The mechanisms whereby phosphorylation regulates Bim function remain unclear. There is general agreement that Erk-dependent phosphorylation of the BimEL-specific site Ser69 can lead to ubiquitin-dependent degradation in many cell types and this is clearly an antiapoptotic response. In neurons, however, phosphorylation on this site was proposed to activate the protein by allowing BimEL to recruit Pin1, a peptidyl-prolyl isomerase. Pin-1 was proposed to act as an effector of apoptosis by acting catalytically on other proteins [36]. Additionally, the protein kinase Jnk was shown to phosphorylate Ser44, Thr56, and Ser58 in BimL, sites also present in BimEL (Fig. 4F). Very recently, an elegant genetic analysis of mice bearing Bim nonphosphorylation mutations has confirmed that phosphorylation of Bim can be either anti- or proapoptotic [37]. Mice incapable of ubiquitin-mediated BimEL degradation exhibited increased Bim expression and increased apoptosis. In contrast, Bim mutant mice encoding an alanine substitution in the site equivalent to Thr56 in human BimL (Fig. 4F) exhibited decreased apoptosis and this was attributed to decreased binding of Bim to Bcl-2. Importantly, Erk as well as Jnk contributed to proapoptotic phosphorylation on this site [37].
A key aspect of the current work is the close relationship between Erk activation, BimL phosphorylation, and apoptosis in Toledo B-NHL cells, suggesting that Erk can directly activate Bim in B cells. Previous work with a truncated form of BimL led to the conclusion that Erk was not a BimL kinase [38]. However, our in vitro phosphorylation experiments, which employed both immune-precipitated endogenous Bim proteins and a full-length recombinant BimL, clearly connect Erk activity with proapoptotic Bim phosphorylation sites. However, we were unable to document any consistent effect of Bim phosphorylation on the degree of Bim-Bcl-2 association. This negative result may reflect the technical limitations of our coprecipitation assays. Alternatively, proapoptotic phosphorylation of Bim may increase its activity by some other means, such as recruitment of non–Bcl-2 family proteins [35]. Finally, it should be noted that the three consensus Erk phosphorylation sites common to BimEL and BimL flank Lys112, a potential ubiquitination site in BimEL (Fig. 4F). Thus, proapoptotic phosphorylation might counter BimEL degradation, in addition to activating Bim by a yet to be determined mechanism that can be manifested in BimL.
A noteworthy feature of induced apoptosis in Toledo B-NHL cells is its striking dependence on sustained RasGRP-Erk signaling and Bim phosphorylation. This finding leads to a novel hypothesis concerning the regulation of B-cell deletion. Specifically, the Bcl-2 family of proteins might act as a molecular chronometer that allows the B cells to gauge the duration of BCR signaling. Strongly self-reactive BCR species would engage antigen in a stable manner and elicit both sustained RasGRP-Erk activity and sustained high levels of activated phospho-Bim. While Bim remains in its active, proapoptotic state, dissociation of antiapoptotic proteins such as Bcl-2 from proapoptotic ones such as Bak and Bax would take place slowly, limited by their high mutual affinities. Given sufficient time, active Bim would titrate out and somehow neutralize Bcl-2 and its analogues leaving Bak and Bax to reassort and destroy mitochondrial membrane integrity. Weakly self-reactive, low avidity BCR species would transiently activate RasGRP-Erk signaling to Bim. Counteracted by Bim dephosphorylation activity, such signals would fail to provide the window of opportunity for Bcl-2 family member reassortment.
Previously, BCR-mediated apoptosis in an immature B-cell line, WEHI-231, was enhanced by modest RasGRP1 overexpression [39]. In this system, the effect on apoptosis was found to be independent of signaling through Erk. Rather, effects of RasGRP1 expression on nuclear factor-κB and Bcl-Xl levels were documented. Further studies are required to determine the generality and underlying mechanisms of both Erk-dependent and Erk-independent apoptotic signaling in B cells.
Our results provide further evidence for the equivalence of natural bryostatin-1 and its synthetic analogue pico in RasGRP-based assays. Bryostatin-1 was identified as a compound with anticancer cell effects and it has been evaluated as an anticancer drug in clinical trials. Its action has been interpreted in terms of effects on PKC activation and downregulation. The work presented here outlines a more detailed mechanism of DAG analogue action (Fig. 8) that might be exploited in the treatment of a subset of B-cell tumors. The possibility of combining DAG analogues with Bcl-2 antagonists and proteosome inhibitors should also be considered.
Supplementary Material
Acknowledgments
This work was supported by grants from Canadian Institutes of Health Research, the Alberta Cancer Board and the Alberta Heritage Foundation of Medical Research (J.C.S.), the Intramural Research Program of the National Institutes of Health, National Cancer Center, Center for Cancer Research and a grant from the National Institutes of Health (CA31845). We thank H.M. Hu for the siRNA vectors and C. Bleakley for the Bcl-2 expression vector. We thank Michele Barry, Raymond Lai, Hanne Ostergaard, Ing Swie Goping, Troy Baldwin and Eileen White for useful suggestions.
References
- 1.Kurosaki T. Genetic analysis of B cell antigen receptor signaling. Annu Rev Immunol. 1999;17:555–592. doi: 10.1146/annurev.immunol.17.1.555. [DOI] [PubMed] [Google Scholar]
- 2.Alberola-Ila J, Takaki S, Kerner JD, Perlmutter RM. Differential signaling by lymphocyte antigen receptors. Annu Rev Immunol. 1997;15:125–154. doi: 10.1146/annurev.immunol.15.1.125. [DOI] [PubMed] [Google Scholar]
- 3.Strasser A, Puthalakath H, OReilly LA, Bouillet P. What do we know about the mechanisms of elimination of autoreactive T and B cells and what challenges remain. Immunol Cell Biol. 2008;86:57–66. doi: 10.1038/sj.icb.7100141. [DOI] [PubMed] [Google Scholar]
- 4.Dower NA, Stang SL, Bottorff DA, et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 5.Stone JC. Regulation of Ras in lymphocytes: get a GRP. Biochem Soc Trans. 2006;34:858–861. doi: 10.1042/BST0340858. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y, Liu H, Coughlin J, Zheng J, Li L, Stone JC. Phosphorylation of RasGRP3 on threonine 133 provides a mechanistic link between PKC and Ras signaling systems in B cells. Blood. 2005;105:3648–3654. doi: 10.1182/blood-2004-10-3916. [DOI] [PubMed] [Google Scholar]
- 7.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 8.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes P, Bouillet P, Strasser A. Role of Bim and other Bcl-2 family members in autoimmune and degenerative diseases. Curr Dir Autoimmun. 2006;9:74–94. doi: 10.1159/000090773. [DOI] [PubMed] [Google Scholar]
- 10.Cante-Barrett K, Gallo EM, Winslow MM, Crabtree GR. Thymocyte negative selection is mediated by protein kinase C- and Ca2+dependent transcriptional induction of bim [corrected] J Immunol. 2006;176:2299–2306. doi: 10.4049/jimmunol.176.4.2299. [DOI] [PubMed] [Google Scholar]
- 11.Sandalova E, Wei CH, Masucci MG, Levitsky V. Regulation of expression of Bcl-2 protein family member Bim by T cell receptor triggering. Proc Natl Acad Sci U S A. 2004;101:3011–3016. doi: 10.1073/pnas.0400005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodyear CS, Corr M, Sugiyama F, Boyle DL, Silverman GJ. Cutting edge: Bim is required for superantigen-mediated B cell death. J Immunol. 2007;178:2636–2640. doi: 10.4049/jimmunol.178.5.2636. [DOI] [PubMed] [Google Scholar]
- 13.Ley R, Ewings KE, Hadfield K, Cook SJ. Regulatory phosphorylation of Bim: sorting out the ERK from the JNK. Cell Death Differ. 2005;12:1008–1014. doi: 10.1038/sj.cdd.4401688. [DOI] [PubMed] [Google Scholar]
- 14.Bunin A, Khwaja FW, Kersh GJ. Regulation of Bim by TCR signals in CD4/CD8 double-positive thymocytes. J Immunol. 2005;175:1532–1539. doi: 10.4049/jimmunol.175.3.1532. [DOI] [PubMed] [Google Scholar]
- 15.Luciano F, Jacquel A, Colosetti P, et al. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 16.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein. Bim. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 17.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Swanson BJ, Wang M, et al. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc Natl Acad Sci U S A. 2004;101:7681–7686. doi: 10.1073/pnas.0402293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R, Moudgil T, Ross HJ, Hu HM. Apoptosis of non-small-cell lung cancer cell lines after paclitaxel treatment involves the BH3-only proapoptotic protein Bim. Cell Death Differ. 2005;12:292–303. doi: 10.1038/sj.cdd.4401554. [DOI] [PubMed] [Google Scholar]
- 20.Heibein JA, Goping IS, Barry M, et al. Granzyme B-mediated cytochrome c release is regulated by the Bcl-2 family members bid and Bax. J Exp Med. 2000;192:1391–1402. doi: 10.1084/jem.192.10.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coughlin JJ, Stang SL, Dower NA, Stone JC. The role of RasGRPs in regulation of lymphocyte proliferation. Immunol Lett. 2006;105:77–82. doi: 10.1016/j.imlet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Coughlin JJ, Stang SL, Dower NA, Stone JC. RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J Immunol. 2005;175:7179–7184. doi: 10.4049/jimmunol.175.11.7179. [DOI] [PubMed] [Google Scholar]
- 23.Ebinu JO, Bottorff DA, Chan EY, Stang SL, Dunn RJ, Stone JC. RasGRP, a Ras guanyl nucleotide- releasing protein with calcium-and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 24.Teixeira C, Stang SL, Zheng Y, Beswick NS, Stone JC. Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood. 2003;102:1414–1420. doi: 10.1182/blood-2002-11-3621. [DOI] [PubMed] [Google Scholar]
- 25.Sundararajan R, Cuconati A, Nelson D, White E. Tumor necrosis factor-alpha induces Bax–Bak interaction and apoptosis, which is inhibited by adenovirus E1B 19K. J Biol Chem. 2001;276:45120–45127. doi: 10.1074/jbc.M106386200. [DOI] [PubMed] [Google Scholar]
- 26.Wender PA, Baryza JL, Bennett CE, et al. The practical synthesis of a novel and highly potent analogue of bryostatin. J Am Chem Soc. 2002;124:13648–13649. doi: 10.1021/ja027509+. [DOI] [PubMed] [Google Scholar]
- 27.Stone JC, Stang SL, Zheng Y, et al. Synthetic bryostatin analogues activate the RasGRP1 signaling pathway. J Med Chem. 2004;47:6638–6644. doi: 10.1021/jm0495069. [DOI] [PubMed] [Google Scholar]
- 28.Marais R, Light Y, Mason C, Paterson H, Olson MF, Marshall CJ. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science. 1998;280:109–112. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- 29.Kuppers R, Klein U, Hansmann ML, Rajewsky K. Cellular origin of human B-cell lymphomas. N Engl J Med. 1999;341:1520–1529. doi: 10.1056/NEJM199911113412007. [DOI] [PubMed] [Google Scholar]
- 30.Gabay C, Ben-Bassat H, Schlesinger M, Laskov R. Somatic mutations and intraclonal variations in the rearranged Vkappa genes of B-non-Hodgkin’s lymphoma cell lines. Eur J Haematol. 1999;63:180–191. doi: 10.1111/j.1600-0609.1999.tb01766.x. [DOI] [PubMed] [Google Scholar]
- 31.Carsetti R, Kohler G, Lamers MC. Transitional B cells are the target of negative selection in the B cell compartment. J Exp Med. 1995;181:2129–2140. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan IC. Mechanism of antigen-driven selection in germinal centres. Nature. 1989;342:929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- 33.Shokat KM, Goodnow CC. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375:334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- 34.Pulendran B, Kannourakis G, Nouri S, Smith KG, Nossal GJ. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature. 1995;375:331–334. doi: 10.1038/375331a0. [DOI] [PubMed] [Google Scholar]
- 35.Mouhamad S, Besnault L, Auffredou MT, et al. B cell receptor-mediated apoptosis of human lymphocytes is associated with a new regulatory pathway of Bim isoform expression. J Immunol. 2004;172:2084–2091. doi: 10.4049/jimmunol.172.4.2084. [DOI] [PubMed] [Google Scholar]
- 36.Becker EB, Bonni A. Pin1 mediates neural-specific activation of the mitochondrial apoptotic machinery. Neuron. 2006;49:655–662. doi: 10.1016/j.neuron.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 37.Hubner A, Barrett T, Flavell RA, Davis RJ. Multisite phosphorylation regulates Bim stability and apoptotic activity. Mol Cell. 2008;30:415–425. doi: 10.1016/j.molcel.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ley R, Ewings KE, Hadfield K, Howes E, Balmanno K, Cook SJ. Extracellular signal-regulated kinases 1/2 are serum-stimulated “Bim(EL) kinases” that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. J Biol Chem. 2004;279:8837–8847. doi: 10.1074/jbc.M311578200. [DOI] [PubMed] [Google Scholar]
- 39.Guilbault B, Kay RJ. RasGRP1 sensitizes an immature B cell line to antigen receptor-induced apoptosis. J Biol Chem. 2004;279:19523–19530. doi: 10.1074/jbc.M314273200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.