Abstract
Purpose:
To evaluate whether dexamethasone injected intracamerally at the conclusion of surgery can safely and effectively reduce postoperative inflammation and improve surgical outcomes in eyes with and without glaucoma.
Methods:
Retrospective chart review of 176 consecutive eyes from 146 patients receiving uncomplicated phacoemulsification (PE) (n = 118 total, 82 with glaucoma), glaucoma drainage device (GDD) (n = 35), combined PE/GDD (n = 11) and combined PE/endoscopic cyclophotocoagulation (n = 12). Ninety-one eyes from 76 patients were injected with 0.4 mg dexamethasone intracamerally at the conclusion of surgery. All eyes received standard postoperative prednisolone and ketorolac eyedrops. Outcomes were measured for four to eight weeks by subjective complaints, visual acuity (VA), slit-lamp biomicroscopy, intraocular pressure (IOP) and postoperative complications.
Results:
Dexamethasone significantly reduced the odds of having an increased anterior chamber (AC) cell score after PE (p = 0.0013). Mean AC cell score ± SD in nonglaucomatous eyes was 1.3 ± 0.8 in control and 0.8 ± 0.7 with dexamethasone; scores in glaucomatous eyes were 1.3 ± 0.7 in control and 0.9 ± 0.8 with dexamethasone. Treated nonglaucomatous eyes had significantly fewer subjective complaints after PE (22.2% vs 64.7% in control; p = 0.0083). Dexamethasone had no significant effects on VA, corneal changes, IOP one day and one month after surgery, or long-term complications.
Conclusions:
Intracameral dexamethasone given at the end of cataract surgery significantly reduces postoperative AC cells in eyes with and without glaucoma, and improves subjective reports of recovery in nonglaucomatous eyes. There were no statistically significant risks of IOP elevation or other complications in glaucomatous eyes.
Keywords: cataract surgery, glaucoma, steroid, dexamethasone, inflammation, intraocular pressure
Introduction
Inflammation after intraocular surgery can prolong patient recovery, raise intraocular pressure (IOP), and increase the likelihood of cystoid macular edema (CME), synechial formation, posterior capsule opacification (PCO), and secondary glaucoma. Recent advances in surgical techniques, surgical tools and intraocular lens (IOL) engineering have reduced the amount of inflammation after cataract extraction.1,2 Current peri-operative pharmacologic treatment and prophylaxis consists of topical non-steroidal anti-inflammatory drugs and corticosteroids.
Patient compliance is of concern in the management of postoperative inflammation because multiple eye drops must be taken multiple times per day at regular intervals over the course of weeks.3 Poor compliance compromises the efficacy of topical drugs, which are further limited by corneal absorption and have highly variable intraocular concentrations during the therapeutic course.4,5 Therefore, the opportunity exists to improve management of postoperative inflammation.
Recent reports describe the efficacy of alternate forms of steroid delivery in reducing inflammation after cataract surgery in otherwise healthy eyes. A single posterior sub-Tenon’s capsule injection of triamcinolone acetonide (TA) at the end of phacoemulsification (PE) has been shown to be as effective as steroid eye drops in reducing signs and symptoms of postoperative inflammation.6,7 Additionally, TA injected intracamerally had anti-inflammatory properties equivalent to prednisolone eye drops after cataract surgery.8 Wadwood and colleagues9 described an anterior segment implant that delivers dexamethasone over seven days and reduces post-PE inflammation comparably to dexamethasone eye drops. Intracameral and intravitreal injections of TA given at the end of PE, in conjunction with standard postoperative corticosteroid eye drops, have proven to be beneficial in uveitic eyes.10–12 Betamethasone injected sub-conjunctivally at the end of cataract surgery was also shown to significantly reduce anterior segment inflammation on the first postoperative day, especially in eyes with prior intraocular inflammation.13
Previous studies have largely focused on reducing inflammation after PE in healthy eyes, excluding the glaucomatous population in which inflammation and steroid use are of particular concern. Tight regulation of postoperative IOP is important in these eyes with optic nerve fibers at increased risk for injury. Excessive fibrin and synechial formation can further compromise aqueous outflow and proper functioning of surgical therapies for glaucoma such as tube-shunts. It is therefore important to investigate new methods to safely reduce postoperative inflammation in glaucomatous eyes. Here, we investigated whether an adjunct intracameral injection of 0.4 mg dexamethasone at the conclusion of surgery can safely and effectively reduce postoperative inflammation and improve surgical outcomes in eyes with and without glaucoma.
Patients and methods
We performed a retrospective chart review of 176 consecutive eyes from 146 patients receiving uncomplicated ocular surgery by the Glaucoma Service at the University of Pittsburgh Medical Center (UPMC) Eye Center from August 2006 through April 2008. The surgical procedures comprising this study were (1) PE with IOL implantation, (2) glaucoma drainage device (GDD) implantation, (3) combined PE/GDD, and (4) combined PE and endoscopic cyclophotocoagulation (PE/ECP). All glaucomatous eyes were medically managed until the time of surgery. Thirty-six eyes from 31 patients receiving cataract surgery did not have glaucoma. All remaining eyes were diagnosed with or suspected to have glaucoma. Ninety-one consecutive eyes from 76 patients received intracameral injection of 0.4 mg dexamethasone at the conclusion of surgery. Routine postoperative care was provided and IOP-reducing medications were restarted when judged necessary during follow-up visits.
Surgical technique
All cases were performed under local anesthesia by one of four surgeons (LC, MCH, RJN, JSS). Eyes receiving PE were dilated with two drops each of cyclopentolate hydrochloride 1% and phenylephrine hydrochloride 2.5% given 10 minutes apart. Eyes also received one drop each of ketorolac tromethamine 0.5% or nepafenac 0.1%, and gatifloxacin 0.3% or moxifloxacin 0.5% at the surgeon’s discretion. PE was performed through a clear corneal incision using a stop-and-chop technique and cortex was removed using automated coaxial irrigation/aspiration (I/A) handpieces. A foldable silicone IOL was inserted into the capsular bag except in two cases where the lens was placed in the sulcus. Viscoelastic was aspirated and the incision was hydrated. Following the procedure, all eyes were instilled with one drop each of prednisolone acetate 1%, ketorolac tromethamine 0.5% or nepafenac 0.1%, and gatifloxacin 0.3% or moxifloxacin 0.5%. One hundred of 118 eyes also received one drop of brimonidine tartrate 0.1%. Medications will henceforth be referred to only by active ingredient.
For cases of combined PE/ECP, a second clear corneal incision was made after completion of PE and IOL implantation. An Endo Optiks diode laser (Little Silver, NJ, USA) equipped with a curved endoscopic probe was placed into the ciliary sulcus and the ciliary body was treated with 0.25 W continuous exposure time until ciliary processes whitened. Viscoelastic was removed using I/A. Prednisolone, ketorolac, gatifloxacin, and brimonidine were given postoperatively as described for PE.
Among 46 total GDD and PE/GDD surgeries, Baerveldt tube-shunts were used in 42 cases and Ahmed tube-shunts were used in four cases. PE with IOL implantation was performed immediately preceding GDD placement in the combined surgeries. A limbal bridle suture was then placed, a conjunctival limbal peritomy was performed, Tenon’s capsule was dissected, and recti muscles were isolated. Baerveldt tubes were ligated and the plate was placed under the muscles. The tube tip was pulled through a sclerostomy into the anterior chamber (AC). The tube-shunt was them sutured to sclera and covered with pericardium and the conjunctiva was reapproximated with Tissel fibrin glue. Each eye was given one drop prednisolone, gatifloxacin or moxifloxacin, atropine 1% and tobramycin/dexamethasone 0.3%/0.1% ointment after GDD surgery. Additionally, all PE/GDD cases received one drop ketorolac or nepafenac; nine of 11 cases also received one drop of brimonidine.
Ninety-one consecutive eyes from 76 patients, comprising approximately half of each surgical group, were injected with 0.1 ml of 4 mg/ml dexamethasone through the paracentesis site into the AC at the conclusion of surgery. Dexamethasone was used in approximately the second half of all eyes, reflecting a change in surgical practice.
Postoperative management
All eyes were evaluated by the Glaucoma Service at UPMC Eye Center on the first postoperative day. Subsequent follow-up typically occurred one month after PE, and at one and three weeks after PE/ECP, GDD and PE/GDD unless more frequent visits were required. Anti-glaucoma medications were restarted when IOP reached or exceeded preoperative IOP. Postoperative medication regimen was as follows: (1) PE: ketorolac or nepafenac QID for one week, gatifloxacin or moxifloxacin QID for one week, prednisolone QID for four weeks or with a one drop/week taper over four weeks at the surgeon’s discretion; additionally, 100 of 118 eyes used brimonidine BID for four weeks, (2) GDD: gatifloxacin or moxifloxacin QID for one week, prednisolone QID for four weeks or with a one drop/week taper over four weeks at the surgeon’s discretion, and atropine BID for one week, (3) PE/GDD: ketorolac or nepafenac QID for one week, gatifloxacin or moxifloxacin QID for one week, prednisolone QID for four weeks or with a one drop/week taper over four weeks at the surgeon’s discretion, atropine BID for one week; additionally, nine of 11 eyes used brimonidine BID for four weeks, (4) PE/ECP: ketorolac QID for one week, gatifloxacin QID for one week, prednisolone QID for four weeks and brimonidine BID for four weeks.
Outcome measures
At each clinic visit, outcomes were measured by subjective complaints, visual acuity (VA), slit-lamp biomicroscopy and IOP measurements by Goldmann applanation tonometry. Baseline values were taken from the most recent preoperative clinic visit. Subjective complaints were scored as a 0 for no complaints or 1 for symptoms of pain, blurry vision, redness, foreign body sensation, tearing or photophobia. Postoperative VA was measured by Snellen VA chart and scored as 0 for unchanged or improved VA from baseline or 1 for worsened VA. Corneal changes were scored as 0 or 1 for the presence of new microcystic or stromal edema, Descemet’s membrane folds (DMF) or keratic precipitates (KP). Aqueous cell was graded according to the number of inflammatory cells seen in a 1 × 3 mm high-powered beam at full intensity at a 45°–60° angle as 0 – trace (<5 cells), 1+ (5–10 cells), 2+ (10–20 cells), 3+ (20–30 cells) and 4+ (cells too numerous to count). No eyes included in this study had greater than trace aqueous cell preoperatively. An outcome measure was occasionally incomplete in the medical record; however this did not preclude the use of an otherwise complete case, and the results reflect the missing data accordingly. The development of postoperative complications including elevated IOP defined as greater than 10 mmHg above baseline, pseudophakic CME, choroidal effusions, synechial angle closure, hyphema and AC leaks were followed for four to eight weeks.
Statistical analysis
Data were compiled using Excel 2007 (Microsoft Corporation, Seattle, WA, USA) and Prism 4 for Windows (GraphPad Software Incorporated, La Jolla, CA, USA) and analyzed with R 2008 (R Development Core Team, Vienna, Austria). Logistic regression was used to analyze postoperative complaints, reduced VA, corneal changes and AC cell scores. IOP was analyzed using a generalized estimating equations model for PE and a linear mixed effects model for GDD. P-values < 0.05 were considered significant.
Results
This retrospective chart review of uncomplicated ocular surgery outcomes included 140 eyes from 115 patients with diagnosed or suspected glaucoma and 36 non-glaucomatous eyes from 31 patients. Table 1 shows the number of eyes and glaucoma diagnoses represented, with some eyes having multiple types of glaucoma. Ninety-one consecutive eyes from 76 patients received intracameral injection of dexamethasone at the conclusion of PE, GDD, PE/GDD, and PE/ECP surgeries. No cases with greater than trace aqueous cells preoperatively were included in the study, although seven eyes had a history of uveitis or uveitic glaucoma. Similarly, eyes with greater than trace corneal abnormalities at baseline were excluded from the study; 10 eyes had coexisting corneal disease. Baseline VA and IOP were taken from the most recent preoperative clinic visit, which was a mean ± SD of 32.9 ± 22.3 days prior to surgery. There were no significant differences in mean ages between control and treated eyes in each surgical group (Table 2, p-values not shown). Small sample sizes for combined surgeries precluded meaningful statistical analyses, but the data are included for comparison.
Table 1.
Distribution of glaucoma diagnoses
| Diagnosis | Number of eyes |
|---|---|
| Primary open angle glaucoma | 68 |
| Glaucoma suspect | 16 |
| Chronic angle closure glaucoma | 12 |
| Pseudoexfoliation syndrome | 13 |
| Neovascular glaucoma | 10 |
| Uveitis/uveitic glaucoma | 7 |
| Normotension glaucoma | 7 |
| Congenital/childhood glaucoma | 5 |
| Steroid-induced glaucoma | 5 |
| Pigment dispersion syndrome | 4 |
| Total | 147 |
Table 2.
Measures of ocular inflammation on the first postoperative day
| Surgery | Exp group | GS/Dx glaucoma | n | Mean age ± SD |
Subjective complaints |
Decreased visual acuity |
Corneal changes |
Mean AC cell |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % eyes | p-value | n | % eyes | p-value | n | % eyes | p-value | n | score ± SD | p-value | |||||
| PE | Ctrl | N | 18 | 68.5 ± 10.8 | 17 | 64.7 | 16 | 6.3 | 17 | 47.1 | 17 | 1.3 ± 0.8 | ||||
| PE | Dexa | N | 18 | 64.3 ± 14.8 | 18 | 22.2 | 0.0083** | 18 | 5.6 | 0.61 | 18 | 33.3 | 0.44 | 16 | 0.8 ± 0.7 | 0.0013** |
| PE | Ctrl | Y | 38 | 73.0 ± 12.0 | 36 | 41.7 | 35 | 20.0 | 38 | 47.4 | 38 | 1.3 ± 0.7 | ||||
| PE | Dexa | Y | 44 | 72.2 ± 10.6 | 44 | 36.4 | 0.58 | 31 | 14.6 | 0.61 | 44 | 36.4 | 0.44 | 43 | 0.9 ± 0.8 | 0.0013** |
| GDD | Ctrl | Y | 18 | 65.4 ± 16.1 | 18 | 94.4 | 16 | 68.8 | 18 | 27.8 | 18 | 1.2 ± 1.3 | ||||
| GDD | Dexa | Y | 17 | 61.8 ± 14.6 | 17 | 70.1 | 0.10 | 15 | 66.7 | 0.93 | 17 | 17.7 | 0.48 | 17 | 1.0 ± 0.9 | 0.81 |
| PE/GDD | Ctrl | Y | 4 | 64.2 ± 13.1 | 4 | 100.0 | 4 | 75.0 | 4 | 50.0 | 4 | 1.5 ± 1.0 | ||||
| PE/GDD | Dexa | Y | 7 | 56.0 ± 14.2 | 7 | 71.4 | 7 | 85.7 | 7 | 42.9 | 7 | 1.1 ± 1.5 | ||||
| PE/ECP | Ctrl | Y | 7 | 71.3 ± 8.9 | 7 | 42.9 | 7 | 42.3 | 7 | 28.6 | 7 | 1.6 ± 0.5 | ||||
| PE/ECP | Dexa | Y | 5 | 72.5 ± 5.8 | 5 | 60.0 | 5 | 40.0 | 5 | 40.0 | 5 | 1.2 ± 1.3 | ||||
Abbreviations: AC, anterior chamber; Ctrl, control eye; Dexa, dexamethasone-treated eye; Dx glaucoma, diagnosed glaucoma; ECP, endoscopic cyclophotocoagulation; Exp group, experimental group; GDD, glaucoma drainage device; GS, glaucoma suspect; PE, phacoemulsification; SD, standard deviation.
Measures of postoperative inflammation
Signs and symptoms of ocular inflammation were measured on the first postoperative day (Table 2). Subjective complaints of pain, blurry vision, redness, foreign body sensation, tearing and photophobia were mild and self-limited in nearly all cases, and so were scored in a binary manner. Non-glaucomatous eyes treated with dexamethasone after PE had significantly fewer reports of postoperative complaints, 22.2% compared to 64.7% in untreated eyes (p = 0.0083; Table 2). There was no significant difference in incidence of postoperative complaints in glaucomatous eyes treated with dexamethasone, 36.4% compared to 41.7% in untreated eyes (p = 0.58).
VA was recorded as a binary variable to reflect worsening from preoperative baseline because it was impossible to discern whether VA reduction was secondary to inflammatory changes, glaucoma or coexisting retinal disease. There was no significant difference in postoperative VA between eyes with and without glaucoma (p = 0.85). Glaucomatous eyes treated with dexamethasone after PE had lower incidence of reduced VA, 14.6% compared to 20.0% in controls, but little effect was seen in any other surgical group (Table 2). There was no statistically significant effect of dexamethasone on postoperative VA (PE, p = 0.61; GDD, p = 0.93).
There was no significant difference in corneal changes, including edema, DMF or KP, between eyes with and without glaucoma on the first postoperative day (p = 0.91). Dexamethasone treatment was associated with a lower incidence of inflammatory corneal changes after PE (33.3% in treated nonglaucomatous eyes vs 47.1% in untreated; 36.4% in treated glaucomatous eyes vs 47.4% in untreated) and GDD (17.7% in treated vs. 27.8% in untreated) (Table 2). However, there were insufficient data to conclude a statistically significant effect of dexamethasone on postoperative corneal changes (PE, p = 0.44; GDD, p = 0.48). None of the 10 eyes with coexisting corneal disease developed postoperative corneal changes on exam.
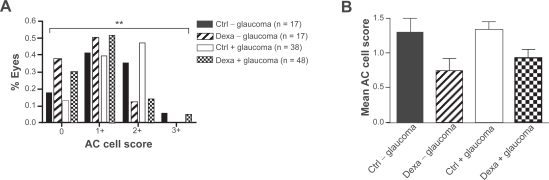
There was no significant difference in the quantity of aqueous inflammatory cells, scored from 0 to 4+, between eyes with and without glaucoma on the first postoperative day (p = 0.81). The frequency distribution of AC cell scores on the first postoperative day after PE was skewed right in the presence of dexamethasone (Figure 1A). Mode AC cell score in glaucomatous eyes was 1+ in 52% treated cases and 2+ in 47% untreated cases. This resulted in a lower mean AC cell score ± SD in nonglaucomatous (control 1.3 ± 0.8, dexamethasone 0.8 ± 0.7) and glaucomatous eyes (control 1.3 ± 0.7, dexamethasone 0.9 ± 0.8) treated with dexamethasone compared to controls (Table 2; Figure 1B). The odds of having an increased AC cell score after PE was significantly reduced in eyes treated with dexamethasone (95% confidence interval: 0.15, 0.63; p = 0.0013).
Figure 1.
(A) Frequency distribution of AC cell scores on the first postoperative day after PE is skewed right in non-glaucomatous and glaucomatous eyes treated with dexamethasone. The odds of having an increased postoperative AC cell score was significantly reduced in eyes treated with dexamethasone (p= 0.0013). (B) Corresponding mean AC cell scores after PE are lower in eyes treated with dexamethasone than in controls.
Abbreviations: AC, anterior chamber; PE, phacoemulsification.
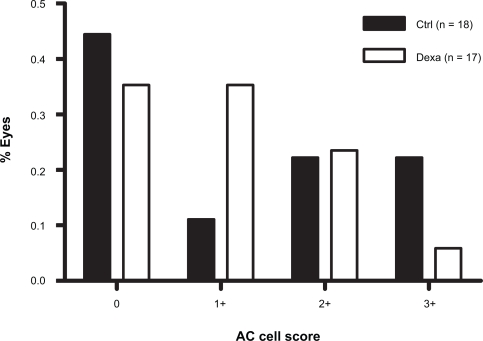
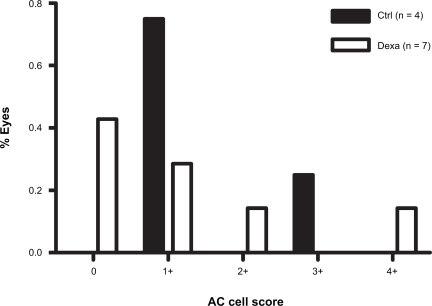
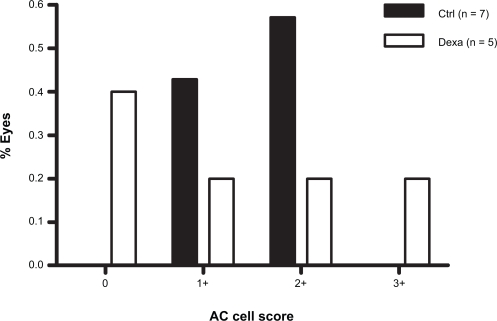
Similar but less pronounced effects of dexamethasone on AC cell score distribution were seen after GDD (Figure 2), PE/GDD (Figure 3) and PE/ECP (Figure 4). A large proportion of eyes receiving GDD surgery had an AC cell score of 0 (44% of control eyes, 35% of treated eyes). However, the frequency distribution of AC cell scores among eyes with a score ≥1+ sloped upward in controls but downward in treated eyes (Figure 2). Mean AC cell scores after GDD were not statistically significant between dexamethasone-treated eyes and control (p = 0.81) (Table 2).
Figure 2.
Frequency distribution of AC cell scores on the first postoperative day after GDD surgery.
Abbreviations: AC, anterior chamber; Ctrl, control; Dexa, dexamethasone; GDD, glaucoma drainage device.
Figure 3.
Frequency distribution of AC cell scores on the first postoperative day after combined PE/GDD surgery.
Abbreviations: AC, anterior chamber; Ctrl, control; Dexa, dexamethasone; GDD, glaucoma drainage device; PE, phacoemulsification.
Figure 4.
Frequency distribution of AC cell scores on the first postoperative day after combined PE/ECP.
Abbreviations: AC, anterior chamber; Ctrl, control; Dexa, dexamethasone; ECP, endoscopic cyclophotocoagulation; PE, phacoemulsification.
Among the seven eyes with a diagnosis of uveitis or uveitic glaucoma, two eyes were untreated and five eyes received dexamethasone. Both untreated eyes had an AC score of 0. Two treated eyes had scores of 1+ cell and three treated eyes had scores of 2+ cell.
IOP and late complications
IOP was measured preoperatively (mean ± SD of 32.9 ± 22.3 days), on the first postoperative day (POD1) for each experimental group, and approximately one month after PE (32.4 ± 8.2 days). There was a significant increase in estimated IOP (preoperative 15.8 ± 0.9 mmHg) of 2.3 ± 0.7 mmHg (p = 0.00065) one day after PE that returned to near-baseline levels after one month (p = 0.56), independent of treatment group (Table 3). IOP after PE was not significantly altered by dexamethasone treatment, regardless of time after surgery or diagnosis of glaucoma (p = 0.12). In fact, dexamethasone was associated with a small decrease of 1.9 ± 1.2 mmHg in IOP rather than a rise in IOP. In glaucomatous eyes, dexamethasone treatment correlated with an overall 0.1 ± 1.6 mmHg increase in estimated IOP compared to untreated nonglaucomatous eyes (p = 0.052).
Table 3.
Statistical analyses of the effects of dexamethasone on postoperative ΔIOP
| Surgery | Experimental group | GS/Dx glaucoma | n | Pre-op mean IOP ± SEM (mmHg) | POD1 mean IOP ± SEM (mmHg) | POMI mean IOP ± SEM (mmHg) |
|---|---|---|---|---|---|---|
| PE | Ctrl | N | 17 | 15.2 ± 0.8 | 19.8 ± 2.0 | 14.4 ± 0.6 |
| PE | Dexa | N | 18 | 14.2 ± 0.8 | 16.4 ± 1.2 | 12.5 ± 0.8 |
| PE | Ctrl | Y | 38 | 15.2 ± 1.1 | 17.1 ± 1.1 | 14.1 ± 0.7 |
| PE | Dexa | Y | 44 | 15.1 ± 0.6 | 17.1 ± 1.1 | 16.3 ± 1.3 |
| GDD | Ctrl | Y | 16 | 27.3 ± 2.2 | 21.2 ± 4.1 | |
| GDD | Dexa | Y | 17 | 28.1 ± 3.3 | 26.9 ± 3.7 | |
| PE/GDD | Ctrl | Y | 4 | 23.0 ± 5.4 | 19.0 ± 9.0 | |
| PE/GDD | Dexa | Y | 7 | 29.9 ± 5.6 | 23.0 ± 9.1 | |
| PE/ECP | Ctrl | Y | 7 | 20.0 ± 3.8 | 20.0 ± 4.7 | |
| PE/ECP | Dexa | Y | 5 | 16.8 ± 0.5 | 28.8 ± 7.7 |
Abbreviations: Ctrl, control eye; Dexa, dexamethasone-treated eye; Dx glaucoma, diagnosed glaucoma; ECP, endoscopic cyclophotocoagulation; GDD, glaucoma drainage device; GS, glaucoma suspect; IOP, intraocular pressure; PE, phacoemulsification; POD1, postoperative day I; POM1, postoperative month I; Pre-op, preoperative; SEM, standard error of the mean.
The incidence of eyes with elevated IOP > 10 mmHg from baseline one day after PE ranged from 4.7%–16.7%, corresponding to one to three eyes, in each experimental group (Table 4). Paracentesis was performed on one glaucomatous control eye and one glaucomatous treated eye on the first postoperative day. Elevated IOP was otherwise self-limited or managed medically. Five treated eyes (11.6%) had POD1 IOP within 10 mmHg of baseline but developed delayed ocular hypertension (at days 12, 22, 29, 33, and 40) and were considered steroid responders; one of these eyes had a known history of prior steroid-induced ocular hypertension. In all five cases, IOP returned to preoperative levels within two to four weeks after discontinuing topical steroids and restarting some or all of the preoperative IOP-lowering medications.
Table 4.
Postoperative complications
| Surgery | Comparison | Estimated ΔIOP ± SE (mmHg) | p-value |
|---|---|---|---|
| PE | POD1 vs preoperative | 2.3 ± 0.7 | 0.00065*** |
| PE | POM1 vs preoperative | −0.4 ± 0.6 | 0.56 |
| PE | Dexa vs ctrl | −1.9 ± 1.2 | 0.12 |
| PE | (Glauco and dexa) vs (nonglauco and ctrl) | 0.1 ± 1.6 | 0.052 |
| GDD | POD1 vs preoperative | −3.6 ± 3.0 | 0.24 |
| GDD | Dexa vs ctrl | 3.0 ± 3.8 | 0.44 |
Abbreviations: Ctrl, control eye; Dexa, dexamethasone-treated eye; Glauco, eye with diagnosed or suspected glaucoma; GDD, glaucoma drainage device; IOP, intraocular pressure; Nonglauco, nonglaucomatous eye; PE, phacoemulsification; POD1, postoperative day 1; POM1, postoperative month 1; SE, standard error.
No significant difference in estimated IOP (preoperative 31.8 ± 8.7 mmHg) was observed one day after GDD (−3.6 ± 3.0 mmHg; p = 0.24) or with dexamethasone treatment (3.0 ± 3.8 mmHg; p = 0.44) compared to controls (Table 3). Three control eyes and two treated eyes had elevated IOP one day after GDD surgery, of which one control eye had retained viscoelastic in the AC (Table 4). Steroid-induced IOP elevation was thought to occur in two control eyes at 34 and 64 days postoperatively, and two treated eyes at 22 and 40 days postoperatively. One of the control eyes had a history of previous steroid-response.
Mean IOPs ± SD one day after PE/GDD were comparable between controls (19.0 ± 15.6 mmHg) and treated eyes (23.0 ± 24.0 mmHg). One treated eye had elevated IOP after PE/GDD resolving after paracentesis; one control eye with a history of steroid-response again had steroid-induced IOP elevation diagnosed 36 days after PE/GDD (Table 4). After PE/ECP, two control eyes (POD1 IOP 30 and 35) and two treated eyes (POD1 IOP 40 and 52) had IOP spikes. One of the control eyes had elevated IOP secondary to retained viscoelastic in the AC. Additionally, one control eye was considered a steroid responder 51 days after PE/ECP.
The surgeries and outcomes of the five eyes with prior history of steroid-induced ocular hypertension were (1) control GDD developed steroid response, (2) control PE/GDD developed steroid response, (3) control PE/ECP with no complications, (4) dexamethasone-treated PE developed steroid response, and (5) dexamethasone-treated GDD developed synechial angle closure but no steroid response.
Late adverse events related to inflammation were uncommon after any surgery (Table 4). Mild CME was seen on fundoscopic exam in one treated eye 33 days after PE, which resolved on exam 10 days later without further intervention. One treated eye developed choroidal effusions after GDD, one control eye developed choroidal effusions after PE/GDD, and one treated eye developed synechial angle closure after GDD. There were 10 postoperative hyphemas and two AC leaks.
Discussion
This study investigated whether dexamethasone injected intracamerally at the conclusion of surgery could safely and effectively reduce postoperative inflammation and improve surgical outcomes in glaucomatous eyes when used in conjunction with standard postoperative medications. We found that dexamethasone treatment significantly reduced the quantity of aqueous inflammatory cells in glaucomatous and control eyes one day after PE (p = 0.0013; Table 2, Figure 1). The 95% confidence interval for the odds ratio (0.15, 0.63) indicates that even the minimum effect of dexamethasone is fairly large in reducing AC cell. Dexamethasone use was also associated with significantly fewer subjective complaints of discomfort, blurry vision, redness, tearing and photophobia in non-glaucomatous eyes (p = 0.0083; Table 2). Postoperative comfort showed moderate improvement when dexamethasone was given after GDD surgery (p = 0.10). Although not statistically significant, there was a trend toward decreased incidence of corneal edema, DMF and KP in treated eyes (Table 2). Since this study involved primarily glaucomatous eyes with varying degrees of visual field defects, VA was scored as postoperative reduction from baseline rather than improvement from baseline. By our methods, no change in postoperative VA reduction was noted in eyes treated with dexamethasone (Table 2).
We also demonstrate that intracameral dexamethasone can safely be given after surgery in eyes with different types of glaucoma with minimal concern for postoperative IOP elevations (Tables 1, 3 and 4). In fact, estimated IOP decreased by 1.9 ± 1.2 mmHg in dexamethasone treated eyes after PE (p = 0.12; Table 3). Paganelli and colleagues7 reported a similar finding of significantly lower IOP up to 28 days after PE in eyes receiving posterior sub-Tenon’s injection of TA compared to prednisolone eyedrops. Dexamethasone in glaucomatous eyes caused almost no deviation (0.1 ± 1.6 mmHg) from estimated IOP in untreated non-glaucomatous eyes. Additionally, there was a low and insignificant incidence of IOP spikes greater than 10 mmHg one day after surgery in dexamethasone-treated and glaucomatous eyes (Table 4). Among eyes with prior history of steroid-induced ocular hypertension, two of the three eyes that were not given dexamethasone subsequently developed a steroid response, and one of the two eyes that were given dexamethasone later had a steroid response. Therefore the risk of recurrent steroid-induced IOP elevation was not increased by intracameral dexamethasone injection. Other postoperative complications associated with inflammation including CME and synechial angle closure were rare in both treated and control eyes (Table 4).
While we show intracameral dexamethasone given in conjunction with a standard course of topical corticosteroids improves postoperative inflammation, it is next important to determine if the use of concurrent topical corticosteroids can be reduced. Unlike topical applications, the intracameral route provides drug delivery directly to the site of interest during the early events of inflammatory cascade activation. Gills and Gills14 were able to replace postoperative steroid drops with increasing concentrations of intracameral TA injected after cataract surgery. We hypothesize that the high relative potency of dexamethasone may confer greater efficacy than TA when given as a single injection. Rapid aqueous volume turnover and short half-life of intraocular dexamethasone, both on the order of several hours, would help minimize the risk of steroid-induced ocular hypertension and corneal and systemic side effects caused by prolonged topical corticosteroid usage.15,16 Injection through the paracentesis site created during surgery also avoids further complications related to sub-Tenon’s and intravitreal injections including globe rupture, central artery occlusion, and choroidal or retinal circulation injections.17–20
The sustained duration of action of sub-Tenon’s and intravitreal injections of TA may preclude safe use in glaucomatous eyes that require strict IOP control.6,7,11,12 TA is detectable for months after intravitreal injection and is reported to increase IOP.21–25 Roth and colleagues demonstrated that IOP spikes >20 mmHg occurred significantly more often after intravitreal TA injection in eyes with prior glaucoma.26 Sub-Tenon’s injection of TA has also been reported to cause ocular hypertension that can be resistant to maximum medical therapy.27–29 In contrast, intracameral injection of low dose TA is reported to have minimal effect on IOP in eyes with no history of glaucoma or steroid-induced ocular hypertension.8 We found no significant rise in postoperative IOP in dexamethasone-treated glaucomatous eyes compared to control. However five (11.6%) treated glaucomatous eyes did develop IOP elevation two to seven weeks after PE, all of which returned to baseline IOP after discontinuation of topical steroids and addition of original antiglaucoma medications. While simultaneous treatment with topical corticosteroids almost certainly contributed to this rise in IOP, adjunct intracameral dexamethasone should be used with some caution in patients known to be steroid responders.
There are several limitations in this study that should be considered. First are the innate pitfalls of a retrospective chart review in which variables are not strictly controlled and occasionally a variable may be missing from the medical record. The academic training environment also introduces bias from variability in clinicians and their preferences in medication regimen, including the course of postoperative topical corticosteroids. We expect minimal selection bias because eyes were treated with dexamethasone in a consecutive manner, reflecting a change in surgical practice. It would have been beneficial if variables were graded more precisely rather than in the binary manner often used in this investigation. Since this study involved primarily glaucomatous eyes with varying degrees of visual field defects, it was impossible to accurately assess VA change in the immediate postoperative period. Furthermore, the experimental groups, especially the combined surgeries were limited by small sample size and insufficient power. Our statistical analyses did not account for the use of fellow eyes as dependent data points, or for ethnic background which may influence the prevalence of open angle glaucoma and tendency toward postoperative inflammation. Lastly, the length of follow-up may have been inadequate to identify all cases of CME and PCO which may present up to three months and five years after cataract surgery, respectively.30,31
Future studies will benefit from a large-scale blinded prospective experimental design, strictly defined grading criteria, pre- and postoperative office visits at regular time intervals, and better control of variables such as postoperative medication regimens. It will be useful to compare the outcomes of intracameral dexamethasone with other routes of delivery such as subconjunctival injection, and with other types of steroids such as TA. It also remains to be determined if a single postoperative injection of intracameral dexamethasone can replace the use of topical steroids altogether. The glaucomatous and uveitic eye populations would be of interest for further investigations because these eyes are particularly prone to a variety of complications from poorly-controlled postoperative inflammation. It will also be useful to study the effect of intracameral dexamethasone after other types of ocular surgery in glaucomatous eyes such as trabeculectomy and canaloplasty. The safety of dexamethasone requires further examination, especially in eyes known to be steroid responders.
In conclusion, this study demonstrates that intracameral dexamethasone given at the end of cataract surgery safely and significantly reduces postoperative inflammatory AC cells in glaucomatous and nonglaucomatous eyes, and improves subjective reports of recovery in nonglaucomatous eyes. We found that dexamethasone did not increase the risk of IOP elevation or other complications in eyes with or without glaucoma, even in those with a prior history of steroid-induced ocular hypertension. EY080908, Bethesda, USA. No author has a financial or proprietary interest in any material or method mentioned.
Table 5.
| Surgerys | Experimental group | GS/Dx glaucoma | n |
POD1 ΔIOP > 10 mmHg |
Steroid response |
Other complications |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # eyes | % | # eyes | % | CME | Choroidal effusion | Synechial angle closure | Hyphema | AC leak | ||||
| PE | Ctrl | N | 18 | 3 | 16.7 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 |
| PE | Dexa | N | 19 | 1 | 5.3 | 0 | 0.0 | 1 | 0 | 0 | 0 | 0 |
| PE | Ctrl | Y | 38 | 3 | 7.9 | 0 | 0.0 | 0 | 0 | 0 | 2 | 0 |
| PE | Dexa | Y | 43 | 2 | 4.7 | 5 | 11.6 | 0 | 0 | 0 | 1 | 0 |
| GDD | Ctrl | Y | 19 | 3 | 15.8 | 2 | 10.5 | 0 | 0 | 0 | 2 | 0 |
| GDD | Dexa | Y | 18 | 2 | 11.1 | 2 | 11.1 | 0 | 1 | 1 | 2 | 1 |
| PE/GDD | Ctrl | Y | 4 | 0 | 0.0 | 1 | 25.0 | 0 | 1 | 0 | 0 | 1 |
| PE/GDD | Dexa | Y | 7 | 1 | 14.3 | 0 | 0.0 | 0 | 0 | 0 | 3 | 0 |
| PE/ECP | Ctrl | Y | 7 | 2 | 28.6 | 1 | 14.3 | 0 | 0 | 0 | 0 | 0 |
| PE/ECP | Dexa | Y | 5 | 2 | 40.0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: AC, anterior chamber; CME, cystoid macular edema; Ctrl, control eye; Dexa, dexamethasone-treated eye; Dx glaucoma, diagnosed glaucoma; ECP, endoscopic cyclophotocoagulation; GDD, glaucoma drainage device; GS, glaucoma suspect; IOP, intraocular pressure; PE, phacoemulsification; POD1, postoperative day 1.
Footnotes
Disclosure
The authors were supported by the (1) Eye and Ear Foundation of Pittsburgh, Research to Prevent Blindness, Pittsburgh, USA, (2) NIH Core Grant for Vision Research EY080908, Bethesda, USA. No author has a financial or proprietary interest in any material or method mentioned.
References
- 1.Dick HB, Schwenn O, Krummenauer F, Krist R, Pfeiffer N. Inflammation after sclerocorneal versus clear corneal tunnel phacoemulsification. Ophthalmology. 1999;107(2):241–247. doi: 10.1016/s0161-6420(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 2.Laurell CG, Zetterström C, Philipson B, Syrén-Nordqvist S. Randomized study of the blood-aqueous barrier reaction after phacoemulsification and extracapsular cataract extraction. Acta Ophthalmol Scand. 1998;76(5):573–578. doi: 10.1034/j.1600-0420.1998.760512.x. [DOI] [PubMed] [Google Scholar]
- 3.Winfield AJ, Jessiman D, Williams A, Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol. 1990;74(8):477–480. doi: 10.1136/bjo.74.8.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ichigashira N, Yamaga N. Intraocular fate of dexamethasone disodium phosphate topically applied to the eyes of rabbits. Steroids. 1978;32(5):615–628. doi: 10.1016/0039-128x(78)90072-7. [DOI] [PubMed] [Google Scholar]
- 5.Krupin T, Waltman SR, Becker B. Ocular penetration in rabbits of topically applied dexamethasone. Arch Ophthalmol. 1974;92(4):312–314. doi: 10.1001/archopht.1974.01010010322012. [DOI] [PubMed] [Google Scholar]
- 6.Negi AK, Browning AC, Vernon SA. Single perioperative triamcinolone injection versus standard postoperative steroid drops after uneventful phacoemulsification surgery: Randomized controlled trial. J Cataract Refract Surg. 2006;32(3):468–474. doi: 10.1016/j.jcrs.2005.12.102. [DOI] [PubMed] [Google Scholar]
- 7.Paganelli F, Cardillo JA, Melo LA, Jr, Oliveira AG, Skaf M, Costa RA. A single intraoperative sub–Tenon’s capsule triamcinolone acetonide injection for the treatment of post–cataract surgery inflammation. Ophthalmology. 2004;111(11):2102–2108. doi: 10.1016/j.ophtha.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Karalezli A, Borazan M, Akova YA. Intracameral triamcinolone acetonide to control postoperative inflammation following cataract surgery with phacoemulsification. Acta Ophthalmol. 2008;86(2):183–187. doi: 10.1111/j.1600-0420.2007.01114.x. [DOI] [PubMed] [Google Scholar]
- 9.Wadood AC, Armbrecht AM, Aspinall PA, Dhillon B. Safety and efficacy of a dexamethasone anterior segment drug delivery system in patients after phacoemulsification. J Cataract Refract Surg. 2004;30(4):761–768. doi: 10.1016/j.jcrs.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Heinz C, Zurek-Imhoff B, Heiligenhaus A. Intraoperative intraocular triamcinolone injection prophylaxis for post-cataract surgery fibrin formation in uveitis associated with juvenile idiopathic arthritis. J Cataract Refract Surg. 2006;32(9):1535–1539. doi: 10.1016/j.jcrs.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Okhravi N, Morris A, Kok HS, et al. Intraoperative use of intravitreal triamcinolone in uveitic eyes having cataract surgery: Pilot study. J Cataract Refract Surg. 2007;33(7):1278–1283. doi: 10.1016/j.jcrs.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Dada T, Dhawan M, Garg S, Nair S. Safety and efficacy of intraoperative intravitreal injection of triamcinolone acetonide injection after phacoemulsification in cases of uveitic cataract. J Cataract Refract Surg. 2007;33(9):1613–1618. doi: 10.1016/j.jcrs.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Corbett MC, Hingorani M, Boulton JE, Shilling JS.Subconjunctival betamethasone is of benefit after cataract surgery Eye 19937 (Pt 6) 744–748. [DOI] [PubMed] [Google Scholar]
- 14.Gills JP, Gills P. Effect of intracameral triamcinolone to control inflammation following cataract surgery. J Cataract Refract Surg. 2005;31(8):1670–1671. doi: 10.1016/j.jcrs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Kwak HW, D’Amico DJ. Evaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. Arch Ophthalmol. 1992;110(2):259–266. doi: 10.1001/archopht.1992.01080140115038. [DOI] [PubMed] [Google Scholar]
- 16.Fraunfelder FT, Fraunfelder FW. Drug-induced Ocular Side Effects. 5th ed. Boston: Butterworth-Heinemann; 2001. [Google Scholar]
- 17.Morgan CM, Schatz H, Vine AK, et al. Ocular complications associated with retrobulbar injections. Ophthalmology. 1988;95(5):660–665. doi: 10.1016/s0161-6420(88)33130-1. [DOI] [PubMed] [Google Scholar]
- 18.Mclean EB. Inadvertent injection of corticosteroid into the choroidal vasculature. Am J Ophthalmol. 1975;80(5):835–837. doi: 10.1016/0002-9394(75)90280-9. [DOI] [PubMed] [Google Scholar]
- 19.Ellis PP. Occlusion of the central retina artery after retrobulbar corticosteroid injection. Am J Ophthalmol. 1978;85(3):352–356. doi: 10.1016/s0002-9394(14)77728-1. [DOI] [PubMed] [Google Scholar]
- 20.Giles CL. Bulbar perforation during periocular injection of corticosteroids. Am J Ophthalmol. 1974;77(4):438–441. doi: 10.1016/0002-9394(74)90449-8. [DOI] [PubMed] [Google Scholar]
- 21.Beer PM, Bakri SJ, Singh RJ, Liu W, Peters GB, Miller M. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110(4):681–686. doi: 10.1016/S0161-6420(02)01969-3. [DOI] [PubMed] [Google Scholar]
- 22.Mason JO, 3rd, Somaiya MD, Singh RJ. Intravitreal concentration and clearance of triamcinolone acetonide in nonvitrectomized human eyes. Retina. 2004;24(6):900–904. doi: 10.1097/00006982-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Kamppeter BA, Cej A, Jonas JB. Intraocular concentration of triamcinolone acetonide after intravitreal injection in the rabbit eye. Ophthalmology. 2008;115(8):1372–1375. doi: 10.1016/j.ophtha.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Bakri JB, Beer PM. The effect of intravitreal triamcinolone acetonide on intraocular pressure. Ophthalmic Surg Lasers Imaging. 2003;34(5):386–390. [PubMed] [Google Scholar]
- 25.Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003;87(1):24–27. doi: 10.1136/bjo.87.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth DB, Realini T, Feuer WJ, et al. Short-term complications of intravitreal injection of triamcinolone acetonide. Retina. 2008;28(1):66–70. doi: 10.1097/IAE.0b013e3181593e38. [DOI] [PubMed] [Google Scholar]
- 27.Inatani M, Iwao K, Kawaji T, et al. Intraocular pressure elevation after injection of triamcinolone acetonide: a multicenter retrospective case-control study. Am J Ophthalmol. 2008;145(4):676–681. doi: 10.1016/j.ajo.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Iwao K, Inatani M, Kawaji T, Koga T, Mawatari Y, Tanihara H. Frequency and risk factors for intraocular pressure elevation after posterior sub-Tenon capsule triamcinolone acetonide injection. J Glaucoma. 2007;16(2):251–256. doi: 10.1097/IJG.0b013e31802d696f. [DOI] [PubMed] [Google Scholar]
- 29.Akduman L, Kolker AE, Black DL, Del Priore LV, Kaplan HJ. Treatment of persistent glaucoma secondary to periocular corticosteroids. Am J Ophthalmol. 1996;122(2):275–277. doi: 10.1016/s0002-9394(14)72027-6. [DOI] [PubMed] [Google Scholar]
- 30.Apple DJ, Solomon KD, Tetz MR, et al. Posterior capsule opacification. Surv Ophthalmol. 1992;37(2):73–116. doi: 10.1016/0039-6257(92)90073-3. [DOI] [PubMed] [Google Scholar]
- 31.Gass JM, Norton ED. Cystoid macular edema and papilledema following cataract extraction: a fluorescein funduscopic and angiographic study. Arch Ophthalmol. 1966;76(5):646–662. doi: 10.1001/archopht.1966.03850010648005. [DOI] [PubMed] [Google Scholar]