Abstract
Objective:
To evaluate the effectiveness of prophylactic administration of nepafenac 0.1% in maintaining mydriasis and in preventing postoperative macular edema following cataract surgery.
Methods:
This was a prospective, randomized, single-masked comparative study in 60 patients undergoing phacoemulsification cataract surgery. Patients were randomized to either the nepafenac or the control group. Nepafenac was administered 3 times daily 1 day before surgery and continued for 6 weeks. The control group received tobramycin-dexamethasone treatment only. Trans-operative mydriasis was measured before surgery, after nuclear emulsification, following cortex aspiration, and at the conclusion of surgery. Macular optical coherence tomography determined central foveal thickness (FT) and total macular volume (TMV) before surgery and at 2 and 6 weeks after surgery. All patients received tobramycin-dexamethasone for 2 weeks after surgery.
Results:
The difference in mean pupil size, at the end of surgery, between the control group (6.84 ± 0.93 mm) and the nepafenac group (7.91 ± 0.74 mm) was statistically significant (p < 0.001). There were no significant differences in FT values between the two groups at any time point; however, TMV at 2 and at 6 weeks was statistically significantly different (p < 0.001), with higher TMV in the control group.
Conclusion:
Prophylactic use of nepafenac was effective in reducing macular edema after cataract surgery and in maintaining trans-operative mydriasis.
Keywords: nepafenac, mydriasis, macular edema, prophylactic, cataract surgery
Introduction
Phacoemulsification with intraocular lens (IOL) implantation is currently the surgical procedure of choice for cataract surgery because it offers the best visual results.1–3 However, despite the advances in surgical techniques (smaller clear corneal incision, and new ultrasound modalities) and improvements in IOL characteristics (acrylic hydrophobic materials), complications related to postoperative inflammation persist and interfere with achieving optimal visual results.4
Eye trauma caused during surgery triggers the inflammatory cascade, releasing a great number of mediators after the inflammatory cells lyse, such as cyclooxygenase-1 (COX-1) and COX-2 enzymes and prostaglandins (PGs). Some of the principal ocular signs and symptoms in which PGs are involved are inflammation, pain, conjunctival hyperemia, miosis, changes in intraocular pressure (IOP), glaucoma, posterior synechiae, posterior capsular opacity, and cystoid macular edema (CME).5
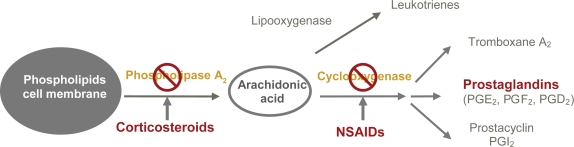
Two important groups of drugs are used to control postoperative inflammation following cataract surgery: non-steroidal anti-inflammatory drugs (NSAIDs), which directly inhibit the COX enzymes, and topical corticosteroids, which act at the level of phospholipase A2, with the resultant inhibition of PG release6–13 (Figure 1). The simultaneous use of NSAIDs and corticosteroids provides a synergistic effect in controlling intraocular inflammation.14
Figure 1.
Mechanism of action of non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids.
Miosis induced by surgical trauma, primarily of the iris, is caused by the release of PGs. When PG release is inhibited with topical NSAIDs, adequate mydriasis is maintained during surgery, thereby decreasing trans-operative complications such as posterior capsule rupture.15 Currently, different non-invasive methods exist that prevent miosis during surgery; however, the use of preoperative topical NSAIDs, in combination with conventional topical mydriatics (anticholinergic and sympathomimetic), is widely practiced.13,16–20
In uncomplicated phacoemulsification surgery, CME continues to be the most common cause of decreased visual acuity (VA) that appears between the 4th and 6th week after surgery. The development of CME is related to the disruption of the hemato-retinal and hemato-aqueous barriers and to the inflammation induced by PGs and other inflammatory mediators.21,22 The incidence of CME ranges from 1% to 6% to 20% to 30%.23,24 These percentages have been diminished with the use of topical NSAIDs.13,25,26
Nepafenac ophthalmic suspension 0.1% (Nevanac®, Alcon Laboratories, Inc., Fort Worth, TX, USA) is a new topical NSAID that is effective in the treatment of pain and postoperative inflammation.27 Nepafenac is a prodrug, which is hydrolyzed in the intraocular tissues to amfenac, a potent inhibitor of COX-1 and COX-2 enzymes.28 The ocular bioavailability and permeability of nepafenac, combined with its rapid bioactivation by ocular tissues, make it a target-specific NSAID for the inhibition of PG formation in the anterior and posterior segments of the eye.29 Its prodrug structure minimizes the risk of toxicity on the corneal surface and enhances its penetration to specific tissues.28,30,31
The purpose of this study was to evaluate the effectiveness of the prophylactic administration of nepafenac 0.1%, in addition to standard antibiotic and corticosteroid treatment, in maintaining trans-operative mydriasis and in preventing postoperative macular edema during the first 6 weeks following cataract surgery by phacoemulsification. To the best of our knowledge, this is the first study assessing the role of nepafenac in maintaining mydriasis during cataract surgery.
Patients and methods
This was a prospective, randomized, single-masked, single-center, longitudinal, experimental and comparative study in patients undergoing phacoemulsification cataract surgery at the Asociación Para Evitar la Ceguera en México, IAP Hospital “Dr Luis Sánchez Bulnes,” Anterior Segment Department. The Ethics and Research Committee of the institution approved the study and patients’ consent.
Sixty (60) patients who met the inclusion/exclusion criteria were included in the study. The inclusion criteria were adult patients 40 years of age or older, regardless of race or gender, who were diagnosed with senile and/or metabolic cataract (according to the Lens Opacities Classification System LOCS III, with classification NO and NC 2–3) and were scheduled for surgery by phacoemulsification and IOL implantation inside the capsular bag, with a normal fundoscopy exam (if observance was possible).
The exclusion criteria included any of the following conditions: pregnancy or breastfeeding; history of ocular inflammatory or infectious eye disease; treatment for eye infection within 30 days prior to inclusion in the study; alterations on the eye surface (including dry eye); history of ocular surgery and/or trauma; and knowledge or suspicion of allergy or hypersensitivity to the preservatives, steroids, topical NSAIDs, or any other component of the study medication. Other exclusion criteria were use of eye medications, including PG analogs; use of topical or systemic steroids within 30 days prior to inclusion in the study; and use of topical or systemic NSAIDs within 14 days prior to inclusion in the study. Patients with non-controlled diabetes mellitus (DM), based on clinical history and blood glucose level (≥126 mg), proliferative diabetic retinopathy, and/or macular edema were excluded from the study. Preoperative mydriasis less than 6 mm prior to the study; synechiae; ocular alteration preventing adequate mydriasis such as iris atrophy; macular alteration documented by optical coherence tomography (OCT), including macular edema of any etiology, macular holes, epiretinal membrane, macular degeneration related to age, and central serous chorioretinopathy; and the use of contact lens in the eye involved during the study were also considered exclusion criteria.
Study protocol
Preoperatively, all patients underwent a thorough ophthalmic examination and review of concurrent medications and medical history. The ophthalmic examination included best-corrected visual acuity (BCVA) (Snellen), slitlamp examination, IOP measurement by Goldmann applanation tonometry, and fundus examination. Macular OCT (Stratus OCT, Carl Zeiss Meditec, Dublin, CA, USA) was performed on all patients prior to surgery. The “Fast Macular Thickness Map” scan was used to determine the foveal thickness (FT, central 1 mm) in microns and total macular volume (TMV) in mm3, and to detect any macular alteration.
The combination of tropicamide 0.8% with phenylephrine 5%, 1 drop every 15 minutes (2 doses) was used as a topical mydriatic in both treatment groups. Additionally, 1 drop of nepafenac was administered every 15 minutes (4 doses) 1 hour prior to surgery in the nepafenac group. The identity of patients receiving preoperative mydriatic or preoperative mydriatic and nepafenac was concealed from the surgeons.
All patients underwent cataract surgery by phacoemulsification and IOL implantation inside the capsular bag (Acrysof IQ, Toric or ReSTOR) under topical (1%) or retrobulbar (99%) anesthesia by 4 experienced surgeons.
Surgeons used the same standardized small-incision phacoemulsification technique on all patients. Briefly, 1.0 mm nasal and 2.8 mm temporal clear corneal incisions were made and a capsulorhexis 5.0 mm in diameter was created. A pre-chopped phacoemulsification technique was used and foldable IOLs were implanted in the capsular bag. The corneal incisions were left sutureless. Phacoemulsification parameters were established prior to all surgeries and were the same in all patients. Cataract surgery was conducted using the Infiniti® Vision System with Ozil technology (Alcon Laboratories, Inc.). The preset parameters used were amplitude of 100%, balanced saline solution without epinephrine for irrigation, with the height of the bottle set at 110 cm, 38 ml/minute aspiration flow rate, 350 mmHg vacuum, and a dynamic rise of 1.
To ensure the standardization of illumination during pupillary measurement, all surgeons used the same microscope (Stativ S-8; Carl Zeiss Optronics, GmbH, Germany) and the illumination was kept constant in all cases. The horizontal and vertical diameters of the pupil were measured in millimeters with a compass under the microscope (directly on the eye) at the following stages: before surgery, after nuclear emulsification, following cortex aspiration, and at the conclusion of surgery. The preset standard magnification of the operating microscope was ensured at each of the 4 time points.
All patients received topical antibiotic-corticosteroid (tobramycin-dexamethasone) treatment 4 times daily for 10 days after surgery. The study group (nepafenac group) received 1 drop of nepafenac 3 times daily 1 day prior to surgery and continued for 6 weeks afterward. The control group did not receive topical nepafenac. All patients were evaluated postoperatively at Day 1 and at Weeks 2 and 6 after surgery. At each visit, BCVA, slitlamp examination to measure cells and flare in the aqueous humor (postoperative anterior chamber inflammation was graded according to the Standardization of Uveitis Nomenclature [SUN]),32 IOP measurements, and fundoscopy were evaluated. Macular OCT was repeated at 2 and 6 weeks by a single observer in order to record FT, TMV, and any other macular alteration.
Statistical analysis
The data were compiled in Microsoft Excel and analyzed using SPSS (version 14, SPSS, Inc. Chicago, IL, USA). Data from patients in the nepafenac and control groups were described as mean values and proportions, and were compared by using the ANOVA or Student’s t test. The Fisher exact test was used to associate the qualitative variables and to determine the relative risk. Statistical significance was established at a p value of <0.05.
The demographic data included age, gender, and evaluated eye, while the clinical data included the presence or absence of diabetes mellitus (without diabetic retinopathy, and the status of nonproliferative diabetic retinopathy), systemic arterial hypertension, and heart disease. The demographic data were compiled and compared between the two groups. The principal variables were mydriasis at the different stages of surgery; and FT and TMV at baseline, 2 weeks, and 6 weeks after surgery.
Results
Sixty patients were included in the study; 30 patients were randomly selected for each group. Table 1 describes the demographic and clinical features of each group. There were no statistically significant differences between the two groups. The average age of patients was 71.9 ± 9.7 years (range, 51 to 88); 22 patients (36.6%) were male. All patients completed the follow-up visits over a 6-week period. There were no serious treatment-related adverse events or toxicity related to the use of nepafenac 0.1%. Additionally, none of the patients presented with inflammatory cells greater than 1 + during the first week of postoperative visits.
Table 1.
Patients’ demographic and pre-operative systemic and ocular pathology
| Parameter |
Group |
*p | |
|---|---|---|---|
| Control (N = 30) | Nepafenac (N = 30) | ||
| Age in years | 0.561 | ||
| Mean ± SD | 71.2 ± 8.8 | 72.6 ± 10.5 | |
| Range | 51 to 85 | 52 to 88 | |
| Gender, N (%) | 0.601 | ||
| Male | 12 (40) | 10 (33.3) | |
| Female | 18 (60) | 20 (66.6) | |
| Eye, N (%) | 0.838 | ||
| Right eye | 14 (46.6) | 15 (50) | |
| Left eye | 16 (53.3) | 15 (50) | |
| Ocular and systemic pathology, N | |||
| DM | 7 | 5 | 0.572 |
| Without DR | 3 | 3 | 1.000 |
| With DR | 4 | 2 | 0.423 |
| Hypertension | 7 | 10 | 0.477 |
| Heart disease | 4 | 3 | 0.662 |
Student’s t test.
Abbreviations: SD, standard deviation; DM, diabetes mellitus; DR, diabetic retinopathy.
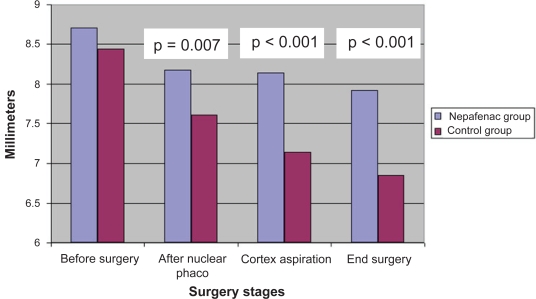
With respect to maintenance of mydriasis during cataract surgery (Table 2), the average preoperative horizontal pupillary diameter was comparable for both groups (8.44 mm and 8.70 mm in the control and the nepafenac groups, respectively; p = 0.143). The difference in pupillary size at the conclusion of surgery was statistically significantly (p < 0.001) different between the two groups (6.84 ± 0.93 mm and 7.91 ± 0.74 mm in the control and the nepafenac groups, respectively). The total reduction in pupil diameter from the beginning to the end of surgery was statistically significantly (p < 0.001) different between the control group (1.59 ± 0.94 mm) and the nepafenac group (0.78 ± 0.56 mm). This change of pupillary size at the conclusion of surgery can be also expressed as a percent total loss of mydriasis (failure to maintain mydriasis), which showed 18.91% loss of mydriasis in the control group compared with 9.03% loss in the nepafenac group (p < 0.001). This indicates that prophylactic use of nepafenac is effective in maintaining mydriasis during cataract extraction and IOL implantation. Figure 2 shows the behavior of the horizontal pupillary size during the different stages of surgery. In the nepafenac group, the greatest failure in maintaining mydriasis occurred after phacoemulsification of the lens nucleus, whereas in the control group, a gradual failure to maintain mydriasis was observed during the entire surgical procedure. Of note, all patients had dark irises. Only 3 patients in the nepafenac group had a reduction in pupil size of ≥1.5 mm at the conclusion of surgery, while 17 patients did so in the control group (p < 0.001) (Table 3), demonstrating that the control group had a relative risk of decreasing pupil size by ≥1.5 mm during surgery 5.7 times greater than the nepafenac group.
Table 2.
Mean horizontal diameter of the pupil during the different stages of cataract surgery
| Surgery stages |
Group |
*p | |
|---|---|---|---|
| Control (mm) | Nepafenac (mm) | ||
| Before surgery | 8.44 ± 0.59 | 8.70 ± 0.75 | 0.143 |
| After nuclear emulsification | 7.60 ± 0.84 | 8.17 ± 0.72 | 0.007 |
| Following cortex aspiration | 7.14 ± 0.87 | 8.13 ± 0.74 | <0.001 |
| Conclusion of surgery | 6.84 ± 0.93 | 7.91 ± 0.74 | <0.001 |
| Total loss of mydriasisa | 1.59 ± 0.94 | 0.78 ± 0.56 | <0.001 |
| Percent total loss | 18.91 | 9.03 | <0.001 |
ANOVA;
Difference between pupil diameter before surgery and pupil diameter at the conclusion of surgery.
Figure 2.
Comparison of horizontal pupil size in the nepafenac and control groups during surgery.
Table 3.
Decrease in pupil diameter (failure to maintain mydriasis) at the end of surgery and its relative risk
|
Group |
*p | ||
|---|---|---|---|
| Control | Nepafenac | ||
| Loss of ≥1.5 mm, N (%) | 17 (85) | 3 (15) | <0.001 |
| Relative risk | 5.7 | 0.18 | |
Fisher’s Exact test.
The average FT by OCT obtained at the different visits is presented in Table 4. There were no statistically significant differences between the control and nepafenac groups in FT (196.03 microns vs. 192.60 microns, p = 0.506) or TMV (6.79 mm3 vs 6.65 mm3, p = 0.056). However, the TMV at 2 weeks and at 6 weeks revealed statistically significant differences (p < 0.001) between both groups, with greater volume in the control group as compared with the nepafenac group (0.35 mm3 at 2 weeks and 0.38 mm3 at 6 weeks). FT at 2 weeks and at 6 weeks between both groups showed no statistically significant differences (p = 0.111 and p = 0.062, respectively), due to the fact that the edema or accumulation of fluids in the macular region was localized outside of the central foveal region. When analyzing the differences between the TMV at baseline and at 2 weeks and at 6 weeks in both groups (Table 5), the differences were statistically significant (p < 0.001) at both time periods, with greater TMV in the control group than in the nepafenac group.
Table 4.
Average foveal thickness (FT) and total macular volume (TMV) in the control and nepafenac groups
| Parameters | Time point |
Group |
*p | |
|---|---|---|---|---|
| Control | Nepafenac | |||
| FT, microns ± SD | Baseline | 196.03 ± 18.32 | 192.60 ± 21.33 | 0.506 |
| 2 weeks | 203.46 ± 19.40 | 195.30 ± 19.66 | 0.111 | |
| 6 weeks | 203.86 ± 17.98 | 194.43 ± 20.26 | 0.062 | |
| TMV, mm3 ± SD | Baseline | 6.79 ± 0.189 | 6.65 ± 0.354 | 0.056 |
| 2 weeks | 7.03 ± 0.212 | 6.68 ± 0.446 | <0.001 | |
| 6 weeks | 7.07 ± 0.242 | 6.69 ± 0.436 | <0.001 | |
ANOVA; p value represents the difference between the control and the nepafenac groups.
Abbreviations: SD, standard deviation; FT, foveal thickness; TMV, total macular volume.
Table 5.
Differences in total macular volume (TMV) from baseline 2 and 6 weeks after surgery
| Time point |
Group |
*p | |
|---|---|---|---|
| Control (mm3 ± SD) | Nepafenac (mm3 ± SD) | ||
| 2 weeks | 0.241 ± 0.115 | 0.031 ± 0.224 | <0.001 |
| 6 weeks | 0.277 ± 0.243 | 0.038 ± 0.242 | <0.001 |
ANOVA.
Abbreviations: TMV, total macular volume; SD, standard deviation.
Additionally, these variables were analyzed in association with DM, and it was evident that in both groups the measurements of the diabetic patients were greater than the measurements of the non-diabetic patients, without having statistically significant differences (Table 6). None of the patients developed clinically significant macular edema associated with vision loss; however, patients in the nepafenac group demonstrated less fluid accumulation in the macular region than those in the control group, suggesting a protective effect with respect to macular edema occurrence.
Table 6.
Differences in total macular volume (TMV) from baseline 2 and 6 weeks after surgery in the presence and absence of diabetes mellitus
| Group | Time point | Diabetes mellitus | *p | |
|---|---|---|---|---|
| Present (N = 7) | Absent (N = 23) | |||
| Control (mm3 ± SD) | 2 weeks | 0.251 ± 0.125 | 0.205 ± 0.064 | 0.181 |
| 6 weeks | 0.294 ± 0.267 | 0.221 ± 0.140 | 0.288 | |
| Present (N = 5) | Absent (N = 25) | |||
| Nepafenac (mm3 ± SD) | 2 weeks | 0.04 ± 0.202 | 0.031 ± 0.232 | 0.464 |
| 6 weeks | 0.096 ± 0.264 | 0.026 ± 0.242 | 0.283 |
Student’s t test.
Abbreviation: TMV, total macular volume.
Discussion
Prostaglandins play an important role in the response to ocular trauma (including surgical trauma), causing inflammation, pain, trans-operative miosis, increased IOP, and pseudophakic CME, among others.5 Nepafenac 0.1% is a new-generation NSAID prodrug, which is hydrolyzed in the intraocular tissues to amfenac, a potent inhibitor of COX-1 and COX-2 enzymes, and thus, an inhibitor of PG synthesis. It has also demonstrated superior penetration of the intraocular tissues with adequate concentrations in the posterior segment to inhibit the synthesis of PGs.27,28,30,31 Multiple studies have demonstrated that NSAIDs are effective drugs for maintaining trans-operative mydriasis13,16–20 and in preventing and treating pseudophakic CME.21–26
The current study demonstrated that topical nepafenac 0.1% is an inhibitor of miosis during cataract surgery. In comparison to the control group, the nepafenac group showed a consistent tendency towards greater pupillary diameter during the different stages of surgery, a decrease in the latter occurring mainly during phacoemulsification of the lens nucleus. In the control group, a gradual failure in maintaining mydriasis was observed during the entire surgical procedure. Intracameral epinephrine was not used in the irrigation solution in this study because it interferes with the actual antimiosis effect of the NSAIDs studied. Shaikh and colleagues analyzed the antimiosis effect of topical prednisolone compared with flurbiprofen. There were no statistically significant differences between the two drugs; however, flurbiprofen produced greater mydriasis.20 It has been reported also that the preoperative use of ketorolac for 3 days is more effective in maintaining mydriasis than the regimen of 1 day and 1 hour preoperatively.13 In this study, nepafenac was administered 1 day preoperatively; however, whether administration 3 days prior to surgery would make any difference in the outcomes is something that should be evaluated in the future.
Optical coherence tomography is a diagnostic tool that objectively quantifies CME by means of changes in the direct measurements in TMV. Therefore, the OCT TMV parameter behaves as an objective indicator of macular swelling that clearly elucidates the amount of postoperative inflammation.33 Compared to fluorescein angiography, OCT is a non-invasive procedure and is useful for evaluations requiring multiple measurements, such as those related to macular edema after cataract surgery. Ozdemir and colleagues reported on the detection of CME after cataract surgery, not by clinical examination of fluorescein angiography, but by OCT.34 OCT has a sensitivity of 96%, specificity of 100%, provides greater evaluation of the axial distribution of accumulated fluids, and has greater reproducibility than angiography.
In the current study, the control group showed greater accumulation of fluids in the macular region compared with the nepafenac group. Clinical macular edema was not seen in any of the patients. Statistical analysis showed that 46 patients per arm (80% power) were needed to show a statistical difference in FT between the two groups. Because no differences were observed in FT between groups at any visit, the authors were able to demonstrate that the accumulation of liquid in the retina occurred outside the central millimeter of the macula, a fact reported previously by Lobo and collaborators. The authors reported that the leakage sites were localized in the vascular regions of the macula and that the edema subsequently reached the central region.35 Furthermore, in a review conducted by Almeida and coworkers, it was found that, despite the absence of clinically significant macular edema, patients treated with ketorolac had a protective effect in comparison with the control group regarding TMV obtained by OCT at 1 week and at 1 month after cataract surgery. The authors reported that the accumulation of fluid in the patients treated with ketorolac was less in the various evaluation periods than in the control group.33 In the current study, patients in the nepafenac group showed a statistically significant difference (p < 0.001) in TMV with respect to baseline at the 2-week and 6-week visits (0.03 mm3 and 0.04 mm3, respectively), whereas in the control group TMV increases were 0.24 mm3 and 0.28 mm3, respectively. Furthermore, when correlating the results with the presence of DM, patients with DM did not have statistically significant differences in TMV in comparison with the non-diabetic patients.
Wolf and colleagues conducted a study similar to this current trial, in which the control group (prednisolone) and the study group (prednisolone-nepafenac) were evaluated and compared with respect to the presence of pseudophakic macular edema, with a follow-up period of 4 weeks. Five patients in the control group (n = 240) had visually significant pseudophakic macular edema compared to zero patients in the study group.36 These results can be related to this study, though a greater sample would be required. Hariprasad and colleagues reported that nepafenac 0.1% was effective in treating CME and diabetic macular edema (DME) in the presence or absence of concomitant steroid therapy. Six patients with CME (3 acute CME and 3 chronic CME) and 1 patient with DME were treated with nepafenac 0.1%. Not only did nepafenac 0.1% reduce the retinal thickness in these patients, but it also restored visual acuity. It is important to note that nepafenac was able to resolve chronic CME, which had failed to respond to conventional steroid/NSAID therapy. Moreover, nepafenac was able to improve DME, a condition that has historically been resistant to pharmacologic treatment.37 Callanan and Williams reported that in a retrospective chart review of 6 eyes of 5 patients, nepafenac was effective in reducing OCT-measured FT in patients with DME.38 In a 15 patient study, Warren and Fox reported significant improvement in visual acuity and retinal thickness after 4 and 12 weeks of 4 times daily treatment with nepafenac 0.1% compared with baseline.39
Until now, the prophylactic use of nepafenac 0.1% for maintaining mydriasis during cataract surgery, as well as the behavior of postoperative macular thickness, has not been reported; however, in this study significant results in these aspects were found. More studies with larger sample sizes comparing nepafenac with other NSAIDs are warranted.
Conclusions
The prophylactic use of nepafenac 0.1% was effective and safe in maintaining mydriasis during cataract surgery as well as in reducing postoperative macular edema. This is the first clinical study evaluating the effect of nepafenac 0.1% on pupil size during cataract surgery.
Acknowledgments
The authors wish to thank Heba Costandy, MD, MS, for medical writing and editing.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Linebarger EJ, Hardten DR, Shah GK, et al. Phacoemulsification and modern cataract surgery. Surv Ophthalmol. 1999;44:123–147. doi: 10.1016/s0039-6257(99)00085-5. [DOI] [PubMed] [Google Scholar]
- 2.Gogate PM, Kulkarni SR, Krishnaiah S, et al. Safety and efficacy of phacoemulsification compared with manual small-incision cataract surgery by a randomized controlled clinical trial: six-week results. Ophthalmology. 2005;112:869–874. doi: 10.1016/j.ophtha.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 3.Riaz Y, Mehta JS, Wormald R, et al. Surgical interventions for age-related cataract. Cochrane Database Syst Rev. 2006;(4):CD001323. doi: 10.1002/14651858.CD001323.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammadpour M, Jafarinasab MR, Javadi MA. Outcomes of acute postoperative inflammation after cataract surgery. Eur J Ophthalmol. 2007;17:20–28. doi: 10.1177/112067210701700104. [DOI] [PubMed] [Google Scholar]
- 5.Podos SM. Prostaglandins, nonsteroidal anti-inflammatory agents and eye disease. Trans Am Ophthalmol Soc. 1976;74:637–660. [PMC free article] [PubMed] [Google Scholar]
- 6.El-Harazi SM, Ruiz RS, Feldman RM, et al. A randomized double-masked trial comparing ketorolac tromethamine 0.5%, diclofenac sodium 0.1%, and prednisolone acetate 1% in reducing post-phacoemulsification flare and cells. Ophthalmic Surg Laser. 1998;29:539–544. [PubMed] [Google Scholar]
- 7.Flach AJ, Dolan BJ, Donahue ME, et al. Comparative effects of ketorolac 0.5% or diclofenac 0.1% ophthalmic solutions on inflammation after cataract surgery. Ophthalmology. 1998;105:1775–1779. doi: 10.1016/S0161-6420(98)99053-4. [DOI] [PubMed] [Google Scholar]
- 8.El-Harazi SM, Ruiz RS, Feldman RM, et al. Efficacy of preoperative versus postoperative ketorolac tromethamine 0.5% in reducing inflammation after cataract surgery. J Cataract Refract Surg. 2000;26:1626–1630. doi: 10.1016/s0886-3350(00)00519-8. [DOI] [PubMed] [Google Scholar]
- 9.Solomon KD, Cheetham JK, DeGryse R, et al. Topical ketorolac tromethamine 0.5% ophthalmic solution in ocular inflammation after cataract surgery. Ophthalmology. 2001a;108:331–337. doi: 10.1016/s0161-6420(00)00543-1. [DOI] [PubMed] [Google Scholar]
- 10.Solomon KD, Vroman DT, Barker D, et al. Comparison of ketorolac tromethamine 0.5% and rimexolone 1% to control inflammation after cataract extraction. Prospective randomized double-masked study. J Cataract Refract Surg. 2001b;27:1232–1237. doi: 10.1016/s0886-3350(00)00832-4. [DOI] [PubMed] [Google Scholar]
- 11.Flach AJ. Topical nonsteroidal antiinflammatory drugs in ophthalmology. Int Ophthalmol Clin. 2002;42:1–11. doi: 10.1097/00004397-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Hirneiss C, Neubauer AS, Kampik A, et al. Comparison of prednisolone 1%, rimexolone 1% and ketorolac tromethamine 0.5% after cataract extraction: a prospective, randomized, double-masked study. Graefes Arch Clin Exp Ophthalmol. 2005;243:768–773. doi: 10.1007/s00417-005-1126-9. [DOI] [PubMed] [Google Scholar]
- 13.Donnenfeld ED, Perry HD, Wittpenn JR, et al. Preoperative ketorolac tromethamine 0.4% in phacoemulsification outcomes: pharmacokinetic-response curve. J Cataract Refract Surg. 2006;32:1474–1482. doi: 10.1016/j.jcrs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Heier JS, Topping TM, Baumann S, et al. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology. 2000;107:2034–2038. doi: 10.1016/s0161-6420(00)00365-1. [DOI] [PubMed] [Google Scholar]
- 15.Muhtaseb M, Kalhoro A, Ionides A. A system for preoperative stratification of cataract patients according to risk of intraoperative complications: a prospective analysis of 1441 cases. Br J Ophthalmol. 2004;88:1242–1246. doi: 10.1136/bjo.2004.046003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon KD, Turkalj JW, Whiteside SB, et al. Topical 0.5% ketorolac vs 0.03% flurbiprofen for inhibition of miosis during cataract surgery. Arch Ophthalmol. 1997;115:1119–1122. doi: 10.1001/archopht.1997.01100160289004. [DOI] [PubMed] [Google Scholar]
- 17.Thaller VT, Kulshrestha MK, Bell K. The effect of pre-operative topical flurbiprofen or diclofenac on pupil dilatation. Eye. 2000;14:642–645. doi: 10.1038/eye.2000.157. [DOI] [PubMed] [Google Scholar]
- 18.Snyder RW, Siekert RW, Schwiegerling J, et al. Acular as a single agent for use as an antimiotic and anti-inflammatory in cataract surgery. J Cataract Refract Surg. 2000;26:1225–1227. doi: 10.1016/s0886-3350(00)00439-9. [DOI] [PubMed] [Google Scholar]
- 19.Srinivasan R, Madhavaranga Topical ketorolac tromethamine 0.5% versus diclofenac sodium 0.1% to inhibit miosis during cataract surgery. J Cataract Refract Surg. 2002;28:517–520. doi: 10.1016/s0886-3350(01)01115-4. [DOI] [PubMed] [Google Scholar]
- 20.Shaikh MY, Mars JS, Heaven CJ. Prednisolone and flurbiprofen drops to maintain mydriasis during phacoemulsification cataract surgery. J Cataract Refract Surg. 2003;29:2372–2377. doi: 10.1016/s0886-3350(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 21.Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc. 1998;96:557–634. [PMC free article] [PubMed] [Google Scholar]
- 22.Miyake K, Ibaraki N. Prostaglandins and cystoid macular edema. Surv Ophthalmol. 2002;47(Suppl 1):S203–S218. doi: 10.1016/s0039-6257(02)00294-1. [DOI] [PubMed] [Google Scholar]
- 23.Ursell PG, Spalton DJ, Whitcup SM, et al. Cystoid macular edema after phacoemulsification: relationship to blood-aqueous barrier damage and visual acuity. J Cataract Refract Surg. 1999;25:1492–1497. doi: 10.1016/s0886-3350(99)00196-0. [DOI] [PubMed] [Google Scholar]
- 24.Mentes J, Erakgun T, Afrashi F, et al. Incidence of cystoid macular edema after uncomplicated phacoemulsification. Ophthalmologica. 2003;217:408–412. doi: 10.1159/000073070. [DOI] [PubMed] [Google Scholar]
- 25.Rho DS. Treatment of acute pseudophakic cystoid macular edema: Diclofenac versus ketorolac. J Cataract Refract Surg. 2003;29:2378–2384. doi: 10.1016/s0886-3350(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 26.Yavas GF, Ozturk F, Kusbeci T. Preoperative topical indomethacin to prevent pseudophakic cystoid macular edema. J Cataract Refract Surg. 2007;33:804–807. doi: 10.1016/j.jcrs.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Lane SS, Modi SS, Lehmann RP. Nepafenac suspension 0.1% for the prevention and treatment of ocular inflammation associated with cataract surgery. J Cataract Refract Surg. 2007;33:53–58. doi: 10.1016/j.jcrs.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 28.Lane SS. Nepafenac: a unique nonsteroidal prodrug. Int Ophthalmol Clin. 2006;46:13–20. doi: 10.1097/01.iio.0000212129.53019.49. [DOI] [PubMed] [Google Scholar]
- 29.Walters T, Raizman M, Ernest P, et al. In vivo pharmacokinetics and in vitro pharmacodynamics of nepafenac, amfenac, ketorolac, and bromfenac. J Cataract Refract Surg. 2007;33:1539–1545. doi: 10.1016/j.jcrs.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Ke TL, Graff G, Spellman JM, et al. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriers. Inflammation. 2000;24:371–384. doi: 10.1023/a:1007001131987. [DOI] [PubMed] [Google Scholar]
- 31.Lindstrom R, Kim T. Ocular permeation and inhibition of retinal inflammation: an examination of data and expert opinion on the clinical utility of nepafenac. Curr Med Res Opin. 2006;22:397–404. doi: 10.1185/030079906X89775. [DOI] [PubMed] [Google Scholar]
- 32.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almeida DR, Johnson D, Hollands H, et al. Effect of prophylactic nonsteroidal antiinflammatory drugs on cystoid macular edema assessed using optical coherence tomography quantification of total macular volume after cataract surgery. J Cataract Refract Surg. 2008;34:64–69. doi: 10.1016/j.jcrs.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 34.Ozdemir H, Karacorlu S, Karacorlu M. Postoperative subretinal fluid associated with cystoid macular edema following cataract surgery. Retina. 2005;25:223–225. doi: 10.1097/00006982-200502000-00022. [DOI] [PubMed] [Google Scholar]
- 35.Lobo CL, Faria PM, Soares MA, et al. Macular alterations after small-incision cataract surgery. J Cataract Refract Surg. 2004;30:752–760. doi: 10.1016/S0886-3350(03)00582-0. [DOI] [PubMed] [Google Scholar]
- 36.Wolf EJ, Braunstein A, Shih C, et al. Incidence of visually significant pseudophakic macular edema after uneventful phacoemulsification in patients treated with nepafenac. J Cataract Refract Surg. 2007;33:1546–1549. doi: 10.1016/j.jcrs.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Hariprasad SM, Callanan D, Gainey S, et al. Cystoid and diabetic macular edema treated with nepafenac 0.1% J Ocul Pharmacol Ther. 2007;23:285–288. doi: 10.1089/jop.2007.0062. [DOI] [PubMed] [Google Scholar]
- 38.Callanan D, Williams P.Topical nepafenac in the treatment of diabetic macular edema Clin OphthalmolIn press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren KA, Fox JE. Topical nepafenac as an alternate treatment for cystoid macular edema in steroid responsive patients. Retina. 2008;28:1427–1434. doi: 10.1097/IAE.0b013e31817e7ead. [DOI] [PubMed] [Google Scholar]