Abstract
Objective:
To evaluate the efficacy and tolerability of Optive™, a new dry eye product containing sodium carboxymethylcellulose (0.5%) and glycerol (0.9%), in patients with keratoconjunctivitis sicca (KCS).
Methods:
This was a non-interventional and observational study including patients with dry eye who required a change of medication or were naïve to dry eye treatment (N = 5,277). Disease severity, tear break-up time (TBUT), tolerability, and change in clinical symptoms were recorded at baseline and at final visit (2 to 4 weeks after first treatment).
Results:
The severity of KCS was mild in 18.6%, moderate in 59.9%, and severe in 21.5% of patients based on physicians’ assessment. TBUT was measured in 4,338 patients before switching to or initiating therapy with Optive and at final visit. Baseline measurement of mean TBUT was 7.7 ± 3.9 seconds. This value increased to 10.0 ± 4.7 seconds at final visit. Most patients (85.4%) reported improvement in local comfort. The majority (75.1%) of patients felt an improvement in symptoms after changing their treatment. Two percent of patients reported adverse events, and 0.4% were treatment-related.
Conclusions:
Optive was well tolerated and improved the symptoms of dry eye after 2 to 4 weeks.
Keywords: keratoconjunctivitis sicca, dry eye, sodium carboxymethylcellulose, glycerol, Optive™
Introduction
Keratoconjunctivitis sicca (KCS), or dry eye, is an eye disease characterized by dryness of the cornea and conjunctiva.1,2 Dry eye is usually a chronic disease, affecting more than 10% of the population worldwide. In particular, it is more common among women and people >65 years old.2,3 Dry eye is a multifactorial disease and is accompanied by increased osmolarity of the tear film and inflammation of ocular surface.1 Symptoms of dry eye vary among patients, and most commonly they include itching, grittiness, burning, sensitivity to bright light, foreign-body sensation, irritation, pain, blurred vision, and contact lens intolerance. In severe cases, dry eye disease can also lead to permanent visual impairment.4,5 Clinical signs of dry eye also vary among patients depending on the specific cause of the disease and include decreased tear film stability as measured by tear break-up time (TBUT).5 Patients with severe KCS may lose the ability to tear in response to neural stimulation,6–8 and are prone to sight-threatening corneal infection and ulceration.2
The symptoms of KCS can be managed and complications can be prevented by the use of artificial tears and lubricating gels that restore normal tear film.9,10 In general, artificial tears moisturize the eye surface by simply increasing the water content of the tear film or by preventing tear evaporation. Osmoprotection, however, is a new approach to treating dry eye by providing protection against hypertonicity below the corneal surface via hydrating the epithelial surface. Evidence suggests that the combination of sodium carboxymethylcellulose (0.5%) and glycerol (0.9%), Optive™ (Allergan Inc., Irvine, CA, USA), not only treats the tear film but also offers osmoprotection.11 The effects of Optive are long-lasting, benefiting patients with dry eye symptoms. The purpose of the present study was to evaluate efficacy and tolerability of Optive in a broad population of patients with KCS in a real life setting.
Methods
Study design
This study was a multicenter, non-interventional, observational, open-label study conducted in Germany. Data collection was initiated in September 2007, and was completed in January 2008. Eight hundred thirty-five participating ophthalmologists provided anonymous patient data using a standardized data-collection instrument. German law does not require the collection of informed consent from patients in non-interventional studies.
Patient selection and symptom measurements
All patients included in the analysis were diagnosed with dry eye symptoms ranging from mild to severe. These patients were either naïve to treatment with any dry eye medication, or were using only 1 artificial tear product at the time of initial assessment. At the initial assessment, patients received or were switched to Optive treatment, depending on if they were naïve to treatment or had been using an artificial tear, respectively. Patient demographics, baseline characteristics (TBUT and severity of dry eye), type of prior treatments, and the reason for changing the KCS treatment were recorded. Adverse events and efficacy outcomes were measured and recorded by physicians at the final exam (2–4 weeks after the initial treatment). Efficacy endpoints were changes in clinical signs and symptoms, local comfort, TBUT, and the assessment of satisfaction with Optive. Local comfort was measured by a 5-point scale ranging from “comfort was markedly improved” to “comfort was worse”. The rating was determined both by physician and patient and was described as the Optive experience versus previous therapy. TBUT was measured using a sterile fluorescein paper diluted with a nonpreserved, balanced salt solution. Statistical analyses of changes in TBUT from baseline to final exam across 3 disease severity categories were done via t-tests for paired samples.
Prior dry eye treatments
This study included analyses of patients who were using the most widely used artificial tears in Germany prior to study entry: Systane® (Alcon Pharma GmbH, Freiburg/Breisgau, Germany), Hylo-Comod® (Ursapharm Arzneimittel GmbH, Saarbrucken, Germany), Lacophtal® (Dr Winzer Pharma GmbH, Berlin, Germany). The major active ingredients in Systane®, Hylo-Comod®, and Lacophtal® were hydroxypropylguar, hyaluronic acid; and povidone, respectively. All other patients using other artificial tears were analyzed together and grouped as “Other”. An additional group included patients who were naïve to dry eye treatment (“Naïve” group).
Results
Patient disposition and baseline characteristics
Of the initial dataset collected (N = 14,012 patients with dry eye) 5,277 patients fulfilled the selection criteria. As shown in Table 1, the majority of the patients were female and over 60 years old. The lack of efficacy of the prior treatments or the expectancy for a higher efficacy with the new treatment in relieving dry eye symptoms was the most commonly reported reason for switching to Optive (68.6%). The severity of KCS was moderate in 3,162 (59.9%) patients (Table 1).
Table 1.
Patient baseline characteristics
| All patients N = 5,277 n (%) | |
|---|---|
| Age | |
| ≤30 years | 209 (4.0) |
| >30 to ≤60 years | 1,953 (37.0) |
| >60 years | 3,007 (57.0) |
| N/Aa | 108 (2.0) |
| Gender | |
| Female | 3,733 (70.7) |
| Male | 1,398 (26.5) |
| N/A | 146 (2.8) |
| Reason to change medicationb | |
| Efficacy Comfort | 3,621 (68.6) |
| Safety | 483 (9.2) |
| Other or Multiple Reasons | 150 (2.8) |
| Severity of dry eye | 1,023 (19.4) |
| Mild | 983 (18.6) |
| Moderate | 3,162 (59.9) |
| Severe | 1,132 (21.5) |
N/A: not applicable due to missing information.
Reasons refer to pre-treatments or, in case of the naïve group, to the expectations for the new treatment.
Prior KCS therapy
Prior to switching to Optive, patients with KCS used a variety of other artificial tear products (n = 66) or were naïve to dry eye treatments. Among the most common treatment choices were Systane® (n = 396; 7.5%), Hylo-Comod® (n = 367; 7.0%), and Lacophtal® (n = 305; 5.8%). Four and a half percent of patients (n = 237) were naïve to treatment. The remaining 75.3% of the patients (n = 3972) used 1 of the other 63 different artificial tears prior to study entry.
At baseline, disease severity varied in patients using different artificial tear products prior to study entry. In the Hylo-comod® group, severe dry eye was more frequent (29.2%), and mild dry eye was less frequent (12.8%) compared with all the other treatment groups, including the Naïve group. On the other hand, in the Lacophtal® treated group, mild dry eye was more frequent (25.9%) and severe dry eye was less frequent (12.5%) compared with other groups that used artificial tears prior to study enrollment. In the Naïve group, mild dry eye was much more frequent (42.6%) and severe dry eye was much less frequent (8.0%) than in any other groups.
Signs and symptoms of dry eye and local comfort with Optive
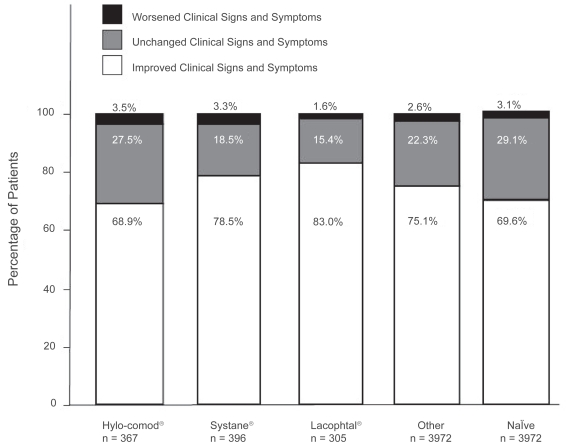
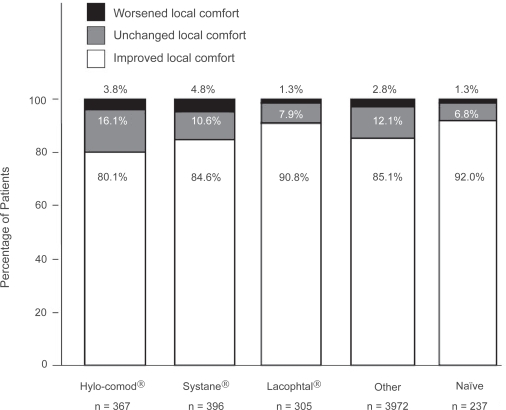
At the final visit, 3963 (75.1%) patients of 5,277 patients reported improvement in clinical signs and symptoms (Figure 1). In particular, 68.9% (n = 253), 78.5% (n = 311), 83.0% (n = 253), 75.1% (n = 2,983), and 69.6% (n = 165) of patients showed improvement in the Hylo-Comod®, Systane®, Lacophtal®, Other, and Naïve group, respectively. Only a few patients (n = 136, 2.6%) reported worsening of the symptoms. Figure 2 illustrates that the majority of all 5,277 patients (85.4%) rated local comfort as an improvement after using Optive for 2 to 4 weeks. Specifically, 80.1% (n = 294), 84.6% (n = 335), 90.8% (n = 277), 85.1% (n = 3,381), and 92.0% (n = 218) of patients reported local comfort improvement in the Hylo-Comod®, Systane®, Lacophtal®, Other, and Naïve group, respectively.
Figure 1.
Change in the clinical signs and symptoms at 2 to 4 weeks after switching to Optive.
Figure 2.
Change in local comfort at 2 to 4 weeks after switching to Optive. The “improvement” category included patients with somewhat to major improvement.
TBUT
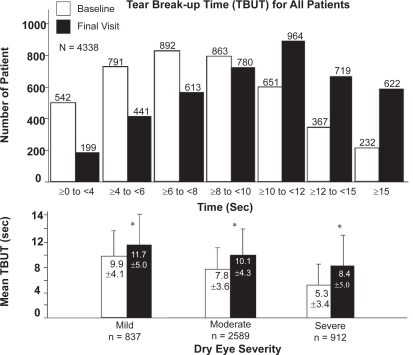
Figure 3 (top panel) illustrates the TBUT across 7 time categories for all patients. At baseline, the highest number of patients had a TBUT of ≥6 to <8 seconds, which increased to >10 to <12 seconds after 2 to 4 weeks of using Optive. In particular, as shown in Figure 3 (bottom panel), TBUT significantly increased from baseline to final assessment in all 3 disease severity categories (p < 0.001). Table 2 summarizes the change in the mean TBUT from baseline to final assessment across all prior treatments used before study entry. Within 2 to 4 weeks, TBUT increased in 29.9% of 4,338 patients, indicating a robust improvement in the clinical sign of KCS.
Figure 3.
Tear break-up time (TBUT) for all patients. Top panel illustrates the change in TBUT from baseline to final visit after switching to Optive across 7 time categories (≥0 to <4, ≥4 to <6, ≥6 to <8, ≥8 to <10, ≥10 to <12, ≥12 to <15, and ≥15 seconds). Bottom panel illustrates the mean TBUT ± standard deviation (seconds) from baseline to final visit across the categories of dry eye disease severity, to which patients were assigned at initial assessment. Asterisk indicates statistical significance (p < 0.001).
Table 2.
Tear break-up time at baseline and at final assessment
| Hylo-Comod® n = 288 | Systane® n = 333 | Lacophtal® n = 245 | Other n = 3,302 | Naïve n = 170 | All patients N = 4,338 | |
|---|---|---|---|---|---|---|
| Baseline TBUT a | ||||||
| Mean ± SDb (Min, Max) | 7.1 ± 3.8 (0.4, 24.0) | 8.1 ± 4.1 (0.3, 30.0) | 8.3 ± 3.5 (0.4, 19.0) | 7.7 ± 4.0 (0.2, 36.0) | 7.8 ± 2.9 (2.0, 20.0) | 7.7 ± 3.9 (0.2, 36.0) |
| Final TBUT | ||||||
| Mean ± SD (Min, Max) | 9.5 ± 4.4 (0.4, 25.5) | 10.1 ± 4.8 (0.3, 45.0) | 10.8 ± 4.0 (0.1, 25.0) | 10.0 ± 4.8 (0.1, 44.0) | 9.5 ± 3.7 (3.0, 22.0) | 10.0 ± 4.7 (0.1, 45.0) |
| Change in Mean TBUT (%) | 2.4 (33.8) | 2.0 (24.7) | 2.5 (30.1) | 2.3 (29.9) | 1.7 (21.8) | 2.3 (29.9) |
Tear break-up time, measured in seconds.
Standard deviation.
Patients satisfaction with Optive
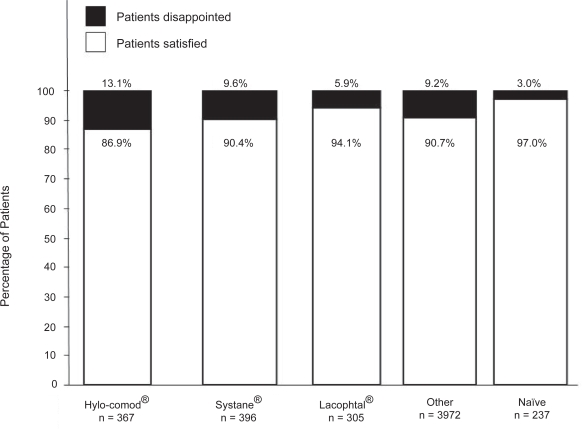
As shown in Figure 4, most patients (n = 4,797, 90.9%) were somewhat to very satisfied with using Optive. In detail, 86.9% (n = 319), 90.4% (n = 358), 94.1% (n = 287), 90.7% (n = 3,603), and 97.0% (n = 230) of patients were satisfied with the new therapy in the Hylo-Comod®, Systane®, Lacophtal®, Other, and Naïve groups, respectively.
Figure 4.
Patient satisfaction with the use of Optive. The results in the “satisfied” category showed here included patients who were somewhat satisfied to very satisfied.
Adverse events
Of 5,277 patients, 107 (2.0%) reported adverse events (AEs), none of which was considered serious. Only 20 of the AEs (0.4% of all patients) were identified as treatment-related. The most commonly reported adverse events were eye irritation (n = 53; 1%), ocular hyperaemia (n = 12; 0.2%), sensation of foreign body (n = 8; 0.2%), blurred vision (n = 7; 0.1%), and drug intolerance (n = 6; 0.1%).
Discussion
In the present study, therapy with Optive greatly improved the signs and symptoms of KCS in the vast majority of patients within 4 weeks. The dual action of Optive is likely the reason that Optive improved signs and symptoms of KCS in our study. Signs and symptoms of dry eye worsened in only a few cases. The vast majority of patients were very satisfied or at least somewhat satisfied with the new product, and the local comfort it provided was rated excellent. Patients valued the comfort of Optive as much better or at least somewhat better compared to their previous dry eye therapy. In accordance with these observations, the clinical sign of KCS (TBUT) was also improved significantly in all stages of the disease (mild, moderate, and severe).
Optive contains sodium carboxymethylcellulose and glycerol, which lubricate the tear film at the eye surface and also promote the growth of epithelial cells to provide osmoprotection.11 The tear film may become damaged under hypertonic stress. Restoring physiologic osmolarity is an important goal in KCS treatment to avoid serious complication, such as sight-threatening corneal infection.12 A number of different products are available for dry eye treatment, including various polymers and numerous products with diverse viscosities and with or without preservative formulations.13 However, these products are not known to possess osmoprotective properties, and therefore likely provide only lubrication and do not protect epithelial cells against hypertonic stress.
The formulations of the artificial tear products play key roles in their efficacy in treating KCS. In particular, in patients who used Hylo-comod®, which is a preservative-free formulation, severe dry eye was more frequent at baseline compared with other treatment groups. In the Lacophtal® group, severe dry eye was less frequent at baseline compared to other groups that had prior artificial tear therapy, which, in part, might be due to the low viscosity of this product. The active ingredients in Optive are sodium carboxymethylcellulose, which is used in several artificial tears and is an effective lubricant, and glycerol, which is a non-blurring and long-lasting moisturizer. Sodium carboxymethylcellulose is an anionic water soluble polymer, which binds to the cell surface and aids in reducing water loss.11 Glycerol restores the lost cellular volume14 and protects cells, but does not increase viscosity that could contribute to blur. Optive also includes a preservative, PURITE®, which is gentle to the ocular surface and has an excellent safety record.15 The safety and tolerability of PURITE® has been also established in a study of 62 patients with mild to moderate dry eye who were treated with 0.5% carboxymethylcellulose that was preserved with PURITE® (Refresh® tears) 4 to 8 times a day for 4 weeks.16 The characteristics of Optive formulation suggest that it would be efficient in restoring physiologic osmolarity of the tear film with minimal side-effects. As anticipated, Optive was well tolerated and efficient in this clinical study. Given the excellent efficacy and tolerability of Optive in the present study, a longer-term treatment with this formulation may result in further improvements.
Conclusion
In this observational study, the use of Optive was effective and well tolerated in patients with dry eye.
Acknowledgments
This study was supported by Allergan, Inc.
Footnotes
Disclosures
The authors have no proprietary interests in Allergan, Inc., or in the product. Patricia Buchholz is an employee of Allergan Inc. Medical writing assistance was provided by Anita Nagypál Schmid, PhD, Pacific Communications, Costa Mesa, CA, USA.
References
- 1.Lemp MA, Baudouin C, Baum J, et al. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Schein OD, Muñoz B, Tielsch JM, et al. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124:723–728. doi: 10.1016/s0002-9394(14)71688-5. [DOI] [PubMed] [Google Scholar]
- 3.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118(9):1264–1268. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 4.Goto E, Yagi Y, Matsumoto Y, Tsubota K. Impaired functional visual acuity of dry eye patients. Am J Ophthalmol. 2002;133:181–186. doi: 10.1016/s0002-9394(01)01365-4. [DOI] [PubMed] [Google Scholar]
- 5.Nichols KK. 2006. Patient-reported symptoms in dry eye disease. Ocul Surf. 2006;4:137–145. doi: 10.1016/s1542-0124(12)70040-x. [DOI] [PubMed] [Google Scholar]
- 6.Heigel TJ, Pflugfelder SC. Aqueous tear production in patients with neurotrophic keratitis. Cornea. 1996;15:106–108. doi: 10.1097/00003226-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Pflugfelder SC, Tseng SCG, Sanabria O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnostic tear-film disorders known to cause ocular irritation. Cornea. 1998;17:38–56. doi: 10.1097/00003226-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Tsubota K. The importance of the Schirmer test with nasal stimulation. Am J Ophthalmol. 1991;111:106–108. doi: 10.1016/s0002-9394(14)76908-9. [DOI] [PubMed] [Google Scholar]
- 9.AAO . Preferred Practice Pattern: Dry Eye Syndrome. San Francisco: American Academy of Ophtalmology (AAO); 2003. [Google Scholar]
- 10.Sheppard JD. Guidelines for the treatment of chronic dry eye disease. Manag Care. 2003;12(12 Suppl):20–25. [PubMed] [Google Scholar]
- 11.Garrett Q, Simmons PA, Xu S, Vehige J, Zhao Z, Ehrmann K, Willcox M. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2007;48:1559–1567. doi: 10.1167/iovs.06-0848. [DOI] [PubMed] [Google Scholar]
- 12.Lemp MA, Chacko B. Diagnosis and treatment of tear deficiencies. In: Tasman W, Jaeger E, editors. Duane’s Clinical Ophthalmology. Philadelphia: Harper and Row; 1997. [Google Scholar]
- 13.Noecker RJ. Comparison of initial treatment response to two enhanced-viscosity artificial tears. Eye Contact Lens. 2006;32:148–152. doi: 10.1097/01.icl.0000181819.63425.a6. [DOI] [PubMed] [Google Scholar]
- 14.Mager WH, Siderius M. Novel insights into the osmotic stress response of yeast. FEMS Yeast Res. 2002;2:251–257. doi: 10.1016/S1567-1356(02)00116-2. [DOI] [PubMed] [Google Scholar]
- 15.Noecker RJ. Effects of common ophthalmic preservatives on ocular health. Adv Ther. 2001;18:205–215. doi: 10.1007/BF02853166. [DOI] [PubMed] [Google Scholar]
- 16.Rozen S, Abelson M, Giovanoni A, Welch D. 1998. Assessment of the comfort and tolerance of 0.5% carboxymethylcellulose preserved with Purite (Refresh tears) in dry eye sufferers. Invest Ophthalmol Vis Sci. 1998;39:S451. [Google Scholar]