Abstract
Irritable bowel syndrome (IBS) is a chronic condition affecting 3%-25% of the general population. As no curative treatment is available, therapy is aimed at reducing symptoms, often with little success. Because alteration of the normal intestinal microflora has been observed in IBS, probiotics (beneficial microbes taken to improve health) may be useful in reducing symptoms. This paper systematically reviews randomized, controlled, blinded trials of probiotics for the treatment of IBS and synthesizes data on efficacy across trials of adequate quality. PubMed, Medline, Google Scholar, NIH registry of clinical trials, metaRegister, and the Cochrane Central Register of Controlled Trials were searched from 1982-2007. We also conducted secondary searches of reference lists, reviews, commentaries, relevant articles on associated diseases, books and meeting abstracts. Twenty trials with 23 probiotic treatment arms and a total of 1404 subjects met inclusion criteria. Probiotic use was associated with improvement in global IBS symptoms compared to placebo [pooled relative risk (RRpooled) 0.77, 95% confidence interval (95% CI) 0.62-0.94]. Probiotics were also associated with less abdominal pain compared to placebo [RRpooled = 0.78 (0.69-0.88)]. Too few studies reported data on other IBS symptoms or on specific probiotic strains to allow estimation of a pooled RR. While our analyses suggest that probiotic use may be associated with improvement in IBS symptoms compared to placebo, these results should be interpreted with caution, given the methodological limitations of contributing studies. Probiotics warrant further study as a potential therapy for IBS.
Keywords: Probiotics, Meta-analysis, Irritable bowel syndrome
INTRODUCTION
Irritable bowel syndrome (IBS) is a chronic condition that severely impacts the quality of life of affected individuals[1,2]. The prevalence of IBS in the general population ranges from 3%-25%[3]. IBS is characterized by intermittent abdominal pain, altered bowel habits (diarrhea and/or constipation) and other gastrointestinal symptoms such as bloating and flatulence in the absence of structural abnormalities in the intestine. The pathophysiology of IBS is multifactorial and may include motor and sensory dysfunction, immune responses, food sensitivities and genetic predisposition[3,4]. Risk factors include female gender (2-3 times more common), acute gastrointestinal infections (e.g. Campylobacter or Salmonella) and psychological factors[3,5,6]. As no curative treatments are available, therapy for IBS is palliative and supportive, targeting specific symptoms, but is notoriously unsatisfactory[7,8]. Although 30% of patients report resolution of symptoms within one year, nearly 70% report that symptoms recur within five years[3].
Studies have observed altered intestinal microflora in IBS patients and an increase in symptoms after enteric infections[9–12], suggesting that restoration of the intestinal microflora may be a useful therapeutic goal. One strategy to restore normal flora is the use of probiotics[13,14]. Probiotics are “beneficial bacteria or yeasts that are ingested to improve health”[15]. Probiotics are also known to modulate the immune response and reduce cytokine production[9,16–18]. Strong evidence for the beneficial role of probiotics exists for the prevention of antibiotic-associated diarrhea, traveler’s diarrhea and pediatric diarrhea[19–22]. There is emerging evidence that probiotics may be useful in preventing or treating Clostridium difficile diarrhea and pouchitis[20,23,24]. Studies of probiotics for IBS have yielded contradictory results, which may be due to a variety of factors: small sample size; variability in trial design; heterogeneity of probiotic strain, dose and treatment duration; and patient characteristics. The wide availability of probiotics as non-prescription products and the lack of a synthesis of data regarding efficacy have prompted us to conduct this meta-analysis.
We conducted a systematic review of randomized, controlled trials published as full articles or meeting abstracts to: (1) assess the characteristics and quality of randomized clinical trials in this area and (2) synthesize data across studies regarding the efficacy of probiotics for IBS.
SEARCH STRATEGY
PubMed, Medline and Google Scholar were searched from 1982-2007 for articles unrestricted by language. Three on-line clinical trial registers were searched: Cochrane Central Register of Controlled Trials (www.cochrane.org), metaRegister of Controlled Trials (www.controlled-trials.com/mrct) and National Institutes of Health (www.clinicaltrials.gov). Secondary and hand searches of reference lists, other studies cross-indexed by authors, reviews, commentaries, books and meeting abstracts also were performed. Search terms included: irritable bowel syndrome, diarrhea, probiotics, risk factors, Rome criteria, Manning criteria, randomized controlled trials, placebo-controlled, bloating and associated author names. Search strategies were broad-based initially, then narrowed to the disease of interest to increase the search network[25]. The procedure for this meta-analysis was designed as suggested by Egger et al with clearly delineated parameters, a priori inclusion and exclusion criteria and standardized data extraction[26,27]. Abstracts of all citations and retrieved studies were reviewed and rated for inclusion. Full articles were retrieved if specific treatments were given for IBS. In some cases, only published abstracts from meetings were available. Published abstracts from meetings were included to lessen the potential for publication bias due to failure to publish negative findings.
INCLUSION AND EXCLUSION CRITERIA
The primary objective of this meta-analysis was to determine the overall efficacy of probiotics for IBS by comparing a common outcome in treated patients with a control group. Inclusion criteria included: randomized, controlled, blinded efficacy trials in humans published as full articles or meeting abstracts in peer-reviewed journals. Exclusion criteria included: pre-clinical studies, safety studies, case reports or case series, phase 1 studies in volunteers, reviews, duplicate reports, trials of unspecified treatments, uncontrolled studies, prebiotic treatments only (no living organisms) or insufficient data in article.
ASSESSMENT OF METHODOLOGICAL QUALITY
Studies that met the inclusion criteria were graded for quality using the Linde Internal Validity Scale (LIVS), which includes the following six items: method of allocation to groups, concealment of allocation, baseline comparability of intervention and placebo groups, blinding of patients, blinding of evaluators, and intention to treat/handling of withdrawals and drop-outs[28,29]. If no information was provided for an item or it was unclear, authors were contacted for more information. If available information was still inadequate, then zero points were given for that item. Total possible scores range from 0 to 6. All trials included in the meta-analysis had a total quality score of 3 or more and those with a score less than 3 were excluded. Two independent reviewers independently assessed inclusion criteria and quality of the trials. Inconsistencies were resolved by discussion.
INTENT-TO-TREAT (ITT) ANALYSIS
Studies were considered to have adhered to intention-to-treat principles if all subjects who were randomized were analyzed with the group to which they were originally assigned and if exclusions were primarily due to patient withdrawal or loss to follow-up. If the investigators excluded patients after randomization due to use of non-study medications or antibiotics, noncompliance with assigned treatment, or non-response to therapy, the analysis was not considered to be ITT.
DATA EXTRACTION
Information on study design, methods, interventions, outcomes, adverse effects and treatments was extracted from each article using a standardized extraction table. When necessary, authors were contacted for data not reported in the original article.
OUTCOMES AND DEFINITIONS
We documented the types of outcomes for trials involving IBS and probiotic in the literature. Outcomes were reported by different studies as either the proportion of subjects reporting improvement or the change in symptom scores from baseline. We did not attempt to synthesize results from studies reporting changes in symptom scores because of numerous challenges including heterogeneity in scales and scoring systems across studies and inconsistent or incomplete reporting of numeric symptom scores. Thus, we selected the proportion of subjects with improvement in global IBS symptoms as the primary outcome for this meta-analysis. Secondary outcomes included the proportion of subjects with improvement in one of three common IBS symptoms: abdominal pain, bloating or flatulence. Documentation of the outcome was based on subject self-report and/or clinician assessment.
META-ANALYSIS METHODS
To estimate pooled relative risks across studies, we first evaluated heterogeneity between and within trials using the χ2 test[30]. The relative risks of responding to probiotic therapy were pooled using a random-effects model if significant heterogeneity was found or a fixed-effects model if the studies were homogenous[31]. The number needed to treat (NNT) was calculated using the reciprocal of the pooled absolute risk reduction. P values less than 0.05 were considered significant. Analyses were performed using Stata software version 9.2 (Stata Corporation, College Station, Texas).
PUBLICATION BIAS
We used a funnel scatterplot to assess the potential for publication bias[32]. Risk ratios were plotted against the standard error of the risk ratio (a surrogate for study size) of each study to detect asymmetry in the distribution of trials. Larger studies usually provide a more precise estimate of the true effect of the treatment and form the narrow spout of the funnel plot. Smaller trials provide less precise estimates, and the increased variability results in a wider cone of the funnel plot. A gap in the funnel plot (commonly, the absence of small studies with negative findings) suggests potential publication bias or methodological problems in smaller studies. Begg’s test was also used to assess potential publication bias[33,34].
STUDY CHARACTERISTICS PREDICTIVE OF POSITIVE FINDINGS
Because there was heterogeneity across studies, we examined study design characteristics that we hypothesized could be associated with results favoring probiotics over placebo. These analyses examined results for the primary outcome variable, reduction in global IBS symptoms. We classified studies as favoring probiotics if the unpooled RR was 0.67 or less. The study by Whorwell et al included 3 different probiotic dose arms but was considered as a single study for the purposes of this analysis[35]. Since one of the 3 arms showed results favoring probiotic, we classified this study as favoring probiotics. Characteristics examined as possible predictors included sample size, LIVS quality score, proportion of female subjects, probiotic dose, treatment duration, attrition > 20%, ITT analysis and use of a proprietary (commercial) vs nonproprietary product. To explore possible predictive variables, we first examined descriptive statistics (median and interquartile range for continuous variables, proportions for categorical variables). To test for statistical significance, we used the Wilcoxon rank-sum test for continuous data and Fisher’s exact test for categorical data.
LITERATURE SCREENING
The literature search yielded 3552 citations on probiotics, of which 789 addressed probiotics and IBS. Based on review of abstracts, 115 were selected for detailed screening.
STUDY SELECTION
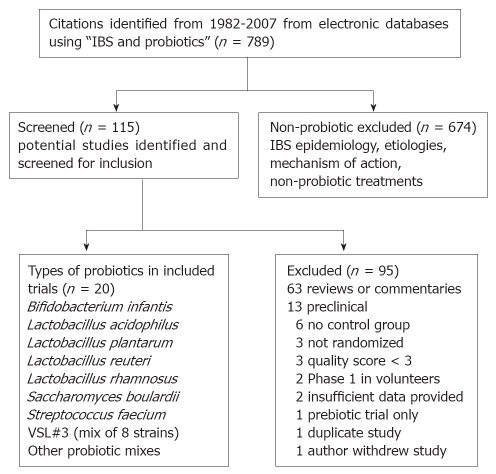
The study selection process is shown in a QUOROM (Quality of Reporting of Meta-analysis) flow diagram (Figure 1)[27]. Overall, 95 studies that were screened failed to meet 1 or more of the inclusion criteria: 63 (66%) were reviews, 13 (14%) were pre-clinical studies, 6 (6%) had no control group[36–41], 3 (3%) were not randomized[42–44], and 10 (10%) were excluded for a variety of reasons. A total of 20 articles met inclusion criteria and provided data on 23 probiotic treatment arms for 1404 patients with IBS (Table 1)[17,35,45–62]. An additional seven trials were excluded after article retrieval and screening for issues related to quality and/or study design (Table 2)[63–68].
Figure 1.
QUOROM flow diagram of included and excluded studies of probiotics for the treatment of Irritable Bowel Syndrome.
Table 1.
Description of 20 randomized, controlled trials of probiotics for IBS included in systematic review
| Reference | Probiotic | Type of control | Number of subjects randomized | Number analyzed | Dose (cfu/d) | Duration of treatment (wk) | % attrition |
| Maupas[45] | Saccharomyces cerevisiae boulardii lyo | Placebo capsules | 34 | 34 | 9 × 109 | 4 | 0 |
| Gade[46] | Streptococcus faecium 40371 | Placebo tablets | 58 | 54 | 1 × 1012 | 4 | 7 |
| Halpern[47] | L. acidophilus (heat killed) “Lacteol Fort” | Placebo capsules | 29 | 18 | 2 × 1010 | 6 | 38 |
| Nobaek[48] | Lactobacillus plantarum DSM9843, in rose hip drink | Placebo plain rose hip drink | 60 | 52 | 5 × 107 | 4 | 13 |
| O'Sullivan[49] | Lactobacillus rhamnosus GG | Placebo tablets | 24 | 19 | 1 × 1010 | 8 | 21 |
| Niedzielin[50] | Lactobacillus plantarum 299v, “ProViva” drink | Placebo drink | 40 | 40 | 2 × 1010 | 4 | 0 |
| Kim[51] | VSL#3 (mix of 8 strains) powder packet1 | Placebo powder | 25 | 25 | 9 × 1011 | 8 | 4 |
| Bausserman[52] | Lactobacillus rhamnosus GG | Placebo capsules | 58 | 50 | 2 × 1010 | 6 | 22 |
| Bittner[53] | Prescript-assist® 29 soil strains and prebiotic “leonardite” | Placebo capsules | 27 | 25 | 2.6 × 108 | 2 | 7 |
| Kajander[54] | L. rhamnosus GG + L. rham. LC705 + Bifido. breve Bb99 +Prop. freudenreichii | Placebo capsules | 103 | 81 | 8-9 × 109 | 24 | 21 |
| Kim[55] | VSL#3 yogurt1 | Placebo yogurt | 48 | 48 | 8 × 109 | 4 | 0 |
| Niv[56] | Lactobacillus reuteri 55730 | Placebo capsules | 54 | 39 | 2 × 108 | 24 | 28 |
| O’Mahony[17] | L. salivaricus UCC4331 | Placebo drink | 54 | 51 | 1 × 1010 | 8 | 16 |
| O’Mahony[17] | Bifido. infantis 35624 | Placebo drink | 53 | 49 | 1 × 1010 | 8 | 16 |
| Kim[57] | B. subtilus + Strept. faecalis | Placebo capsules | 40 | 34 | 3 × 1010 | 4 | 15 |
| Simren[58] | L. plantarum 299v in rose hip drink | plain rose hip drink | 66 | 58 | 2 × 109 | 6 | 12 |
| Whorwell[35] | Bifido. infantis 35624 in 3 doses | Placebo capsules | 362 | 292 | 1 × 106 | 4 | 19 |
| 1 × 108 | |||||||
| 1 × 1010 | |||||||
| Enck[59] | E. coli + Strept faecalis drink | Placebo drink | 297 | 264 | 4.5 × 102 | 8 | 11 |
| Gawronska[60] | L. rhamnosus GG | Placebo capsules | 37 | 37 | 6 × 109 | 4 | 0 |
| Marteau[61] | Bifido. longum, Lact acidophilus, Lactococcus lactis, Strept. thermophilus | Placebo capsules | 106 | 100 | 1 × 1010 | 4 | 6 |
| Simren[62] | Lact. paracasei, Lact acidophilus, Bifido. lactis in yoghurt | Control milk | 74 | 67 | 2 × 1010 | 8 | 9 |
IBS: Irritable bowel syndrome; cfu/d: Colony forming units per day; Bifido.: Bifidobacterium; B.: Bacillus; E.: Escherichia; L.: Lactobacillus; Prop.: Propionibacterium.
VSL#3 is a mixture of 8 probiotic strains (Lactobacillus casei, L. plantarum, L. acidophilus, L. bulgaricus, Bifido. longum, Bifido. breve, Bifido. infantis and Streptococcus thermophilus).
Table 2.
Examples of excluded randomized, controlled trials of probiotics for IBS
| Reference | Probiotic | Number of subjects randomized | Number of subjects analyzed | Dose (cfu/mL) | Duration | Exclusion reason |
| DiBaise[63] | L. plantarum 299v vs placebo | 29 | 20 | 6 × 109 | 4 wk | Withdrawn by author |
| Saggioro[64] | L. plantarum + L. acidophilus | 46 | 39 | 1 × 1011 | 4 wk | Quality score = 2.0 |
| Saggioro[64] | L. plantarum + Bifido. breve | 44 | 37 | 1 × 1010 | 4 wk | Quality score = 2.0 |
| Long[65] | Bifido. (species not given) | 60 | 60 | 6 × 109 | 2 wk | Quality score = 2.5 |
| Kajander[66] | L. rhamnosus GG + L. rham. LC705 + Bifid. breve Bb99 +Prop. freudenreichii | 103 | 83 | 8-9 × 109 | 6 mo | Duplicate study of Kajander K 2005 |
| Bittner[67] | Prescript-assist®, 29 soil strains and prebiotic | 24 | 24 | 2.6 × 108 | Varied | Controls from Bittner 2005 study, phase 4 study |
| Moon[68] | Bacillus subtilis + St. faecium | 34 | 34 | 750 mL/d, cfu/d not given | 4 wk | Outcome data not provided for each group in abstract |
cfu/mL: Colony forming units per milliliter; IBS: Irritable bowel syndrome; L.: Lactobacillus; Bifido.: Bifidobacterium.
STUDY QUALITY
The study quality of 23 treaments was assessed, and 20 trials with LIVS quality scores > 3.0 were included (Table 3). The median quality score was 4 (range 3 to 6). Nine studies did not describe the method of randomization, 8 did not provide baseline comparison of groups, 14 did not specifically state that evaluators were blinded and 20 did not perform intention-to-treat analysis and/or did not fully describe withdrawals. For six studies, the published article or abstract did not contain sufficient information to allow quality scoring, requiring communication with the authors. Only three studies (15%) clearly documented their adherence to intention-to-treat principles[45,50,60].
Table 3.
Quality scoring for 20 randomized, controlled trials of probiotics for IBS (Linde Internal Validity Scale)
| Reference | Total quality score1 | Treatment allocation | Randomization method | Baseline comparison | Patients blinded | Evaluators blinded | Handling and reporting of withdrawals/use of ITT | Data source2 |
| Maupas[45] | 6 | 1 | 1 | 1 | 1 | 1 | 1 | Paper |
| Gade[46] | 4.5 | 1 | 1 | 0 | 1 | 1 | 0.5 | Paper |
| Halpern[47] | 4 | 1 | 1 | 0.5 | 1 | 0.5 | 0 | Paper |
| Nobaek[48] | 3 | 1 | 0 | 0 | 1 | 0.5 | 0.5 | Paper |
| O'Sullivan[49] | 3 | 1 | 0 | 1 | 0.5 | 0.5 | 0 | Author |
| Niedzielin[50] | 4 | 1 | 0 | 1 | 1 | 0 | 1 | Paper |
| Kim[51] | 4.5 | 1 | 0 | 0.5 | 1 | 1 | 1 | Paper |
| Bausserman[52] | 5.5 | 1 | 1 | 1 | 1 | 1 | 0.5 | Paper |
| Bittner[53] | 3 | 1 | 0 | 0 | 0.5 | 0.5 | 1 | Author |
| Kajander[54] | 4.5 | 1 | 1 | 1 | 0.5 | 0.5 | 0 | Paper |
| Kim[55] | 4 | 1 | 0 | 1 | 1 | 0.5 | 0.5 | Paper |
| Niv[56] | 3.5 | 1 | 0 | 0.5 | 1 | 0.5 | 0.5 | Paper |
| O’Mahony[17] | 4.5 | 1 | 1 | 0 | 1 | 1 | 0.5 | Paper |
| Kim[57] | 4 | 1 | 0 | 1 | 0.5 | 0.5 | 1 | Paper |
| Simren[58]3 | 3 | 1 | 0.5 | 0 | 1 | 0.5 | 0 | Author |
| Whorwell[35] | 3.5 | 1 | 0 | 0.5 | 1 | 0.5 | 0.5 | Paper |
| Enck[59]3 | 4 | 1 | 0.5 | 0 | 1 | 0.5 | 1 | Author |
| Gawronska[60] | 4.5 | 1 | 1 | 0 | 1 | 0.5 | 1 | Paper |
| Marteau[61]3 | 4.5 | 1 | 0.5 | 1 | 1 | 0.5 | 0.5 | Author |
| Simren[62]3 | 3.5 | 1 | 0.5 | 0 | 1 | 0 | 1 | Author |
Linde Internal Validity Scale score is based on columns 3-8; range, 0 (poor) to 6 (excellent). (Linde 1996)[29];
Indicates whether additional contact with authors was required to obtain information needed for quality scoring;
Data from published meeting abstract only.
There were a variety of ways in which studies failed to adhere to ITT principles. Seven studies excluded participants who used prohibited/non-study medications, including antibiotics, during the treatment phase[17,46,48,49,51,55,59], while five studies excluded subjects who demonstrated poor compliance with study medications[47,52,54,56,61]. Three studies reported that subjects either dropped out or were excluded due to inadequate response to treatment[49,58,62], while in 4 studies, subjects were excluded for worsening abdominal pain[51,52,54,56]. Often, it was unclear whether subjects with inadequate response or worsening symptoms were excluded at the investigators’ discretion or withdrew from the study of their own accord.
DESCRIPTION OF INCLUDED STUDIES
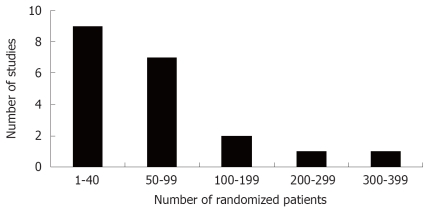
A standardized data extraction table (Table 1) was used to characterize each clinical trial. Twenty randomized controlled trials provided adequate data regarding efficacy in a total of 1404 patients with IBS. In 20 trials, 23 probiotic treatment arms were compared to placebo control arms. Eighteen studies compared a single probiotic treatment arm to placebo, one study compared two probiotic treatments to placebo[17], and one study compared three doses of one probiotic against placebo[35]. The number of patients in each of these studies was generally small, with a median of 54 randomized subjects (range, 25-363; Figure 2). The daily dose of probiotic treatment ranged from 450 to 1 × 1012 colony-forming units (cfu)/day (median = 9 × 109). For the most part, the length of treatment in these studies was brief (median = 4 wk), with 90% of studies having a treatment phase of 8 wk or less.
Figure 2.
Number of randomized patients in 20 randomized, controlled clinical trials of probiotics for the treatment of Irritable Bowel Syndrome.
PROBIOTIC STRAIN
Only two probiotics were tested in multiple trials: Lactobacillus rhamnosus GG in three trials[49,52,60] and Bifidobacterium infantis in two trials[17,35]. None of the L. rhamnosus GG trials provided evaluable data on either the primary or secondary outcomes, which prevented analysis by strain type.
ASSESSMENT AND REPORTING OF OUTCOMES
The outcomes assessed and reported varied widely across the 20 studies. The effect on global IBS symptoms (measured as either proportion with symptom improvement or a reduction in severity scores) was reported in 15/20 (75%) of studies (Table 4) and was the primary outcome for 7 (35%) of studies. Effects on abdominal pain were reported by all studies. But, only 4 (20%) used this as a primary outcome measure[35,50,52,60].
Table 4.
Outcome assessment and reporting for 20 included clinical trials of probiotics for IBS
|
Outcome |
||||||||
| Reference | Global response | Abdominal pain | Bloating/distension | Flatulence | Stool frequency | Mucous | Stool consistency | Dyspepsia |
| Maupas[45] | R | R | R | R | R | R | ||
| Gade[46] | R | R | R | R | R | |||
| Halpern[47] | R | A | A | A | A | A | ||
| Nobaek[48] | R | R | R | A | R | |||
| O’Sullivan[49] | R | R | A | R | A | |||
| Niedzielin[50] | R | R | R | A | A | |||
| Kim[51] | R | R | R | R | R | R | ||
| Bausserman[52] | A | R | R | A | A | |||
| Bittner[53] | A | A | A | |||||
| Kajander[54] | R | R | R | R | R | A | A | |
| Kim[55] | R | R | R | R | R | |||
| Niv[56] | R | R | R | A | ||||
| O’Mahony[17] | R | R | R | A | R | |||
| Kim[57] | R | A | A | A | A | |||
| Simren[58] | R | A | A | A | A | A | A | |
| Whorwell[35] | R | R | R | R | A | |||
| Enck[59] | R | R | A | A | A | |||
| Gawronska[60] | R | |||||||
| Marteau[61] | R | R | A | A | ||||
| Simren[62] | R | A | A | |||||
| Percent reporting | 65% | 80% | 50% | 40% | 25% | 0 | 30% | 5% |
A: Assessed; R: Reported in sufficient detail to allow extraction of data. Bold font indicates that this was the primary outcome identified by the authors for analysis. If author reported no difference between active and placebo groups for a given symptom, but did provide further details, the outcome was classified as assessed only.
Other symptoms were less consistently assessed (e.g. flatulence, 13/20 studies; mucus in stool, 3/20 studies; bloating, 15/20 studies). Only five studies collected some measure of quality of life[17,54,56,61,62]. Seven studies reported data for 3 or more symptoms or outcomes without specifying a primary outcome[17,46,48,49,53,54,56].
Some studies reported the number and proportion of subjects with improvement, while others reported change in numeric symptom scores since baseline. The scales used to measure the severity of IBS symptoms varied widely between studies, making it challenging to compare results across studies. Visual analogue scales were most often used, but still only used by 6 studies[17,46,48,51,55,57]. Likert scales were used by 3 studies[17,49,52], and specific validated scales were used by several studies Gastrointestinal Symptom Rating Scale (GSRS)[52,58] and IBS Severity Scoring System (IBS-SSS)[56,58,62]. Several studies used their own study-specific scale or scoring system[17,35,45,47,50,53,54,59–61]. Often it was unclear whether this scale had been validated.
While many studies assessed a wide range of IBS symptoms, few reported detailed results across the spectrum of symptoms (Table 4), making it more difficult to combine data across studies. For instance, only 8 of 13 studies reporting that they had collected data on flatulence provided this data in their paper and only 5 of 15 reporting they had collected data on stool frequency reported any such data in their paper.
GLOBAL RESPONDERS
The primary outcome selected for this analysis was the proportion of patients in each group with global IBS symptoms by the end of treatment, with ‘responders’ being a dichotomous variable defined by study investigators (Table 5). Of the 23 treatment arms, 14 (61%) had evaluable data for this outcome. Eight treatment arms either did not collect data on global symptom relief[49,52,55,60] or reported change in symptom scores rather than proportion with improvement[17,53,56,57].
Table 5.
Global Improvement in IBS Symptoms in 14 probiotic/placebo treatment arms
| Reference | Probiotic |
Global improvement in IBS symptoms |
Definition of primary outcome1 | |
| Probiotic n/n (%) | Placebo n/n (%) | |||
| Maupas[45] | Saccharomyces cerevisiae boulardii lyo | 13/16 (81) | 13/18 (72) | Improvement of symptoms |
| Gade[46] | Strept faecalis | 26/32 (81) | 9/22 (41) | Improvement of symptoms based on physician assessment |
| Halpern[47] | L. acidophilus | 17/18 (94) | 13/18 (72) | Absence of symptoms |
| Nobaek[48] | L. plantarum | 11/25 (44) | 7/27 (26) | Decrease ≥ 1.5 on VAS symptom scale |
| Niedzielin[50] | L. plantarum | 9/20 (45) | 3/20 (15) | Absence of symptoms |
| Kim[51] | VSL#32 | 4/12 (34) | 5/13 (38) | Satisfactory relief of IBS symptoms |
| Kajander[54] | L. rhamnosus GG + L. rham. LC705 + Bifid. breve Bb99 + Prop. freudenreichii | 31/41 (76) | 17/40 (43) | Symptoms alleviated based on significant reduction of symptom scores |
| Simren[58] | L. plantarum | 10/29 (35) | 11/29 (38) | Reduction ≥ 50% of total symptom score |
| Whorwell[35] | Bifido. infantis (dose, 106 cfu/mL) | 33/74 (44) | 32/76 (42) | Adequate relief of symptoms |
| Whorwell[35] | Bifido. infantis (dose, 108 cfu/mL) | 45/72 (62) | 32/76 (42) | Adequate relief of symptoms |
| Whorwell[35] | Bifido. infantis (dose, 1010 cfu/mL) | 26/71 (37) | 32/76 (42) | Adequate relief of symptoms |
| Enck[59] | E. coli + Strept faecalis | 102/149 (68) | 56/148 (38) | Reduction of ≥ 50% in total symptom score |
| Marteau[61] | Bifido. longum, L. acidophilus, Lactococcus lactis, Strept thermophilus | 20/47 (42.6) | 22/52 (42.3) | Relief of discomfort |
| Simren[62] | L. paracasei, L. acidophilus, Bifido. lactis in yoghurt | 14/33 (42) | 17/34 (50) | Reduction of ≥ 50% in total symptom score |
Unless otherwise stated, all primary outcomes are defined based on patient report.
VSL#3 is a mixture of 8 probiotic strains (Lactobacillus casei, L. plantarum, L. acidophilus, L. bulgaricus, Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis and Streptococcus thermophilus).
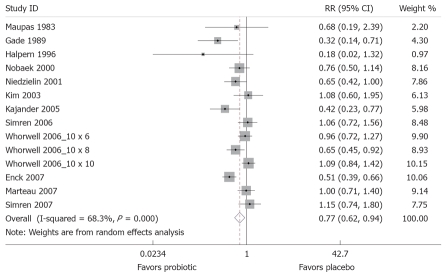
When the meta-analysis model was fitted, the χ2 test for heterogeneity was 41.0 (P <0.001), indicating a high degree of heterogeneity; so a random-effects model was used to pool these results. The forest plot, weighted on sample size, is shown in Figure 3. Compared to placebo, probiotics were significantly protective (less global IBS symptoms compared to placebo at the end of the study) [pooled relative risk (RRpooled) = 0.77; 95% confidence interval (95% CI), 0.62-0.94]. The number needed to treat was 7.3. The funnel plot (Figure 4) is generally symmetrical, showing little evidence of publication bias. Begg’s test did not show statistically significant publication bias (z = 0.93, P = 0.35).
Figure 3.
Forest Plot of randomized controlled trials of 14 treatment arms from 12 studies measuring relative risk of IBS symptoms after probiotic treatment compared to placebo. X-axis is relative risk, with black dot indicating the relative risk, line indicating 95% confidence interval and the size of the grey box proportional to sample size.
Figure 4.
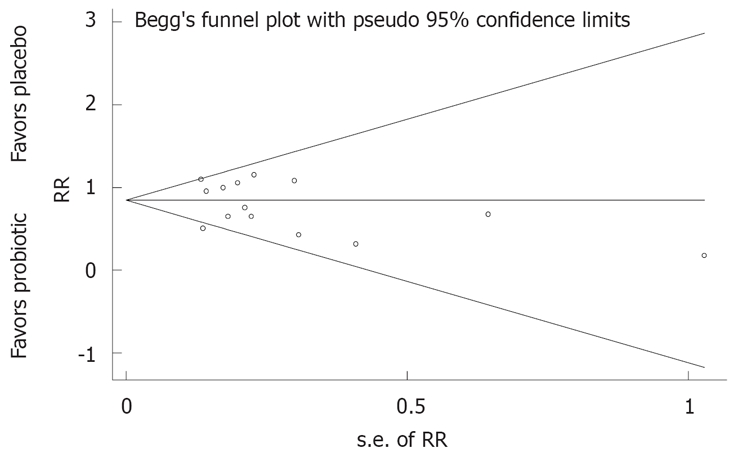
Funnel plots of randomized controlled trials for examining presence of IBS symptoms with probiotic or placebo treatments. RR: Relative risk of global IBS symptoms; s.e. of RR: Standard error of relative risk, an indicator of sample size.
SENSITIVITY ANALYSES
We repeated the meta-analysis weighting by study quality score rather than sample size, with similar results (RRpooled = 0.65; 95% CI, 0.52-0.82). As it appeared that the pooled risk estimate was heavily influenced by one large study[59], we re-ran the analysis excluding the study, but similar results were found (RRpooled = 0.82; 95% CI, 0.67-0.99).
SECONDARY OUTCOMES
A priori secondary outcomes for this study included the proportion of subjects who reported one of three IBS symptoms: abdominal pain, bloating/distension, or flatulence (gas). Of 23 treatment arms, 12 (52%) had evaluable data on at least one of these secondary outcomes. Fourteen treatment arms either did not collect data on these secondary outcomes[45,47,51,58,61,62] or reported symptom scores rather than proportion with symptoms[17,53,56,57]. As only five treatment arms reported proportion of subjects with reduced bloating[46,49,52,54,55] and four reported proportion with improved flatulence[46,48,50,54], further statistical analyses were not performed for these outcomes.
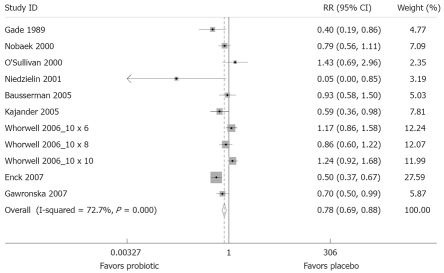
Eight trials (11 probiotic treatment arms) had evaluable data for the proportion of patients reporting abdominal pain at the end of follow-up (Table 6)[35,46,48–50,52,54,59,60]. There was a high degree of heterogeneity (χ2 = 36.6, P < 0.001), and so a random-effects model was used. The forest plot, weighted on sample size, is shown in Figure 5. Compared to placebo, probiotics were associated with less risk of abdominal pain (RRpooled = 0.78; 95% CI, 0.69-0.88). The number needed to treat was 8.9. The funnel plot was generally symmetrical, showing little evidence of publication bias, and Begg’s test did not show statistically significant publication bias (z = -0.70, P = 0.48). The pooled relative risk for abdominal pain was similar when weighted by study quality (RRpooled = 0.61; 95% CI, 0.45-0.81) and after exclusion of the two trials conducted in children (RRpooled = 0.77; 95% CI, 0.68-0.88)[52,60].
Table 6.
Relief of abdominal pain in 11 probiotic/placebo treatment arms
| Reference | Probiotic |
Improvement in abdominal pain |
Definition of secondary outcome1 | |
| Probiotic n/n (%) | Placebo n/n (%) | |||
| Gade[46] | Strept faecalis | 25/32 (78) | 10/22 (45) | Absence or presence of symptom |
| Nobaek[48] | L. plantarum | 9/25 (36) | 5/27 (18) | Decrease ≥ 1.5 on VAS symptom scale |
| O'Sullivan[49] | L. rhamnosus GG | 9/19 (47) | 12/19 (63) | Symptom improved |
| Niedzielin[50] | Lacto plantarum | 20/20 (100) | 11/20 (55) | Absence of symptoms |
| Bausserman[52] | Lacto rhamnosus GG | 11/25 (44) | 10/25 (40) | Decrease of ≥ 1 point symptom score |
| Kajander[54] | L. rhamnosus GG + L. rham. LC705 + Bifid. breve Bb99 +Prop. freudenreichii | 27/41 (66) | 17/40 (43) | Symptoms alleviated |
| Whorwell[35] | Bifido. infantis (106 dose) | 32/74 (43) | 39/76 (52) | Adequate relief of symptoms |
| Whorwell[35] | Bifido. infantis (108 dose) | 42/72 (59) | 39/76 (52) | Adequate relief of symptoms |
| Whorwell[35] | Bifido. infantis (1010 dose) | 28/71 (39) | 39/76 (52) | Adequate relief of symptoms |
| Enck[59] | E. coli + Strept faecalis | 108/149 (72) | 66/148 (45) | ≥ 50% decrease in symptom score |
| Gawronska[60] | Lacto rhamnosus GG | 6/18 (33) | 1/19 (5) | Absence of pain |
All secondary outcomes are defined based on patient report.
Figure 5.
Forest plot of randomized controlled trials of 12 treatment arms from 10 studies measuring relative risk of abdominal pain after treatment with a probiotic compared to placebo. The X-axis depicts relative risk, with black dot indicating the relative risk, line indicating 95% CI and the size of the grey box proportional to sample size.
STUDY CHARACTERISTICS PREDICTING POSITIVE RESULTS
We compared the characteristics of six studies that favored probiotics over placebo (study RR < 0.67 for improvement in global IBS symptoms)[35,46,48,50,54,59] with six studies showing a weak effect or no benefit[45,47,51,58,61,62]. Studies with a stronger probiotic effect were larger than those showing weak or no effect (median 80.5 subjects vs 50 subjects, P = 0.20) and had shorter duration of treatment (median 4 wk vs 6 wk, P = 0.60). but, these differences were not statistically significant. Two-thirds of studies showing strong protective effects used proprietary (commercial) products, compared to 100% of those showing weak or no effect (P = 0.46). In bivariate analyses, no characteristics differed significantly between the two types of studies.
ADVERSE EVENTS
Most studies (17/20, 85%) provided only minimal information about adverse events. Fourteen studies (70%) stated that no serious adverse reactions were noted, but failed to provide any information on how adverse events were ascertained or what types of reactions were considered. Three (15%) of the trials did provide limited data on adverse reactions, including reactions such as “increased intestinal symptoms”, “epistaxis”, “aftertaste”, “anxiety” and “angina”, but did not report rates of adverse reactions by treatment group[17,49,54]. Three trials (15%) did not report any safety data[47,58,59].
We identified 20 clinical trials that met inclusion criteria and provided relevant information about the efficacy of probiotics for IBS symptoms. These trials included 23 probiotic treatment arms and 1404 subjects. Trials were generally small and of short duration and had moderate quality. But, the majority did not follow intention-to-treat principles. Overall, probiotic use was associated with less likelihood of global IBS symptoms compared to placebo (RR = 0.77; 95% CI, 0.62-0.94) and with abdominal pain by the end of follow-up (RR = 0.78; 95% CI, 0.69-0.88). There was not sufficient data to examine other individual IBS symptoms or the efficacy of individual probiotic strains.
STRENGTHS AND LIMITATIONS
We performed a comprehensive review of the literature and made an effort to minimize publication bias by including recent studies as well as those published only as meeting abstracts. Validated quality scoring and data extraction were performed by two reviewers independently, using standardized templates, and differences were resolved by discussion. We excluded studies of poor quality, limiting the impact of serious study design flaws. We selected a primary outcome (global improvement in IBS symptoms) that is clinically relevant and of great concern to IBS patients, as is also true for our secondary outcome (relief of abdominal pain). Communication with study authors was a productive tool for obtaining data not reported in detail in some studies.
Our findings should be interpreted with caution due to important limitations of the existing literature. Two important limitations in the existing trials included the lack of ITT analysis and the presence of heterogeneity in both outcome assessment and study design. A crucial issue is the quality of included studies, with only 3 of 20 studies performing true intention-to-treat analyses. In many studies, participants were excluded from final analyses for reasons such as noncompliance, failure to respond to treatment, or use of prohibited medications. It is difficult to predict how these exclusions may have affected results. But, it is certainly possible that substantial bias could have been introduced, which could account for the apparent beneficial effects observed when data were pooled across studies. Missing values may cause both systematic and unpredictable bias in controlled trial results[69–71]. A recent meta-analysis of chondroitin for osteoarthritis found that small trials and those not analyzed according to ITT principles were more likely to report benefits from chondroitin, while larger studies with greater methodological rigor did not find an effect[72]. Larger studies utilizing ITT have not been performed to examine probiotics as potential therapy for IBS.
Heterogeneity was another important limitation of the published literature, including heterogeneity in the strain and dose of probiotic (which prevented analysis of effects of specific strains); sample size (smaller studies resulted in low power to detect effects in individual studies); duration of treatment and follow-up (short trials do not allow adequate follow-up given the chronic relapsing nature of IBS); and in the assessment and reporting of outcomes. All these sources of heterogeneity made it difficult to combine data from all twenty studies. Another important problem is the lack of systematic data collection and reporting about adverse effects. As a result, it is difficult to be sure that the probiotics studied have been adequately evaluated for safety.
COMPARISON WITH OTHER SYSTEMATIC REVIEWS
To date, no other meta-analysis of probiotics for IBS has been published. Recent published reviews of probiotics for IBS included fewer studies (range, 4 to 12) and focused primarily on evaluating the rationale and potential mechanisms for probiotics as treatment for IBS[9,13,73]. No prior reviews have attempted to calculate a pooled estimate of efficacy, and few reviews provided a detailed summary of individual studies’ outcome data or unpooled risk estimates.
IMPLICATIONS FOR FUTURE RESEARCH
This review highlights important considerations for the design of future studies of probiotics as a potential treatment for IBS (Table 7). There is a need for standardized outcome assessments and larger studies, preferably with longer duration of treatment and follow-up. Future studies should make every effort to minimize loss-to-follow-up and to adhere to ITT principles, analyzing all subjects with the group to which they were originally assigned, notwithstanding potential noncompliance with treatment or the use of other (non-study) medications. Following these methodological principles will provide greater assurance that results are not due to bias. Future studies would benefit from better standardization of outcomes to be studied, including the use of uniform symptom scales. We recommend that future studies examine overall relief of IBS symptoms as an outcome. Although many prior studies primarily reported symptom scores, a statistically significant reduction in symptom score may not be meaningful to an individual patient suffering from IBS. Bijkerk et al examined the validity of 10 methods to assess IBS response and found a single question asking about ‘adequate relief of IBS-related symptoms’ was as valid as more detailed questionnaires on outcome[74]. In order to determine if one probiotic strain is more effective for IBS than others, confirmatory trials with the same probiotic strains are required.
Table 7.
Recommendations for future research studies examining probiotics as a treatment for irritable bowel syndrome
| Recommendations |
| More trials testing the same probiotic strain |
| Larger sample size |
| Longer duration of treatment and follow-up |
| Intention to treat analysis: |
| All participants analyzed with the group to which they were originally assigned, regardless of compliance with treatment, response to treatment, or use of prohibited (non-study) medications. |
| Greater efforts to minimize loss to follow-up |
| Standardized assessment of IBS outcomes |
| Provide some assessment of global relief of IBS symptoms |
| Detailed collection and reporting of data on potential adverse reactions |
Finally, it is important that future studies systematically assess potential adverse effects and provide detailed results, including rates of adverse effects in the treatment and placebo groups.
IMPLICATIONS FOR CLINICAL PRACTICE
While our findings provide preliminary evidence that probiotics may be useful in treating IBS, it is too soon to recommend their use in clinical practice. The pooled relative risks reported here are based on studies with significant methodological limitations, and bias cannot be ruled out as the explanation for these positive findings. Since we did not find any evidence of significant adverse effects from these treatments, and given the lack of available conventional treatments, clinicians should strongly consider discussing the evidence of benefits and risks of probiotics with their patients with IBS. Although the costs of probiotics vary widely, the cost may be similar to other over-the-counter remedies for IBS (such as loperamide).
An important consideration is the lack of regulation of the commercial probiotic products that are currently available. No universal quality assurance programs exist to ensure that commercial products contain the probiotic strain and concentration that are claimed, or to ensure the absence of contamination that could pose risks to consumers. Some resources are available to provide further information about product testing; for example, ConsumerLab is an independent company in the U.S. that tests commercially available health and nutrition products and publishes data about the contents of various commercial products, including the presence of contaminants (http://www.consumerlab.com). They also offer a voluntary certification program. In the summer of 2007, the Food and Drug Administration issued new rules regarding good manufacturing practices for supplement manufacturers, aimed at ensuring more uniform quality of supplements. It remains to be seen whether these new rules will substantially improve the quality and safety of nutritional supplements.
CONCLUSION
In summary, the present meta-analysis suggests that probiotics offer promise for the treatment of IBS. Results should be interpreted cautiously given the methodological limitations of published studies. Future studies are needed, in particular larger studies of longer duration with greater methodological rigor. In addition, more data are needed regarding which specific strains and doses are most likely to be effective. The use of probiotics for IBS warrants further study, particularly given the chronic nature of this condition, its major impact on patients’ quality of life, and the dearth of other effective treatments.
ACKNOWLEDGMENTS
We would like to thank Dr. David Arterburn for his helpful feedback on this manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Supported by Veterans’ Affairs Health Services Research & Development fellowship, TPA 61-029 (Dr. Dublin), National Institute of Aging grant, AG028954-01A1 (Dr. Dublin)
Peer reviewers: Francesco Costa, Dr, Dipartimento di Medicina Interna-U.O. di Gastroenterologia Università di Pisa-Via Roma, 67-56122-Pisa, Italy; Yvan Vandenplas, Professor, Department of Pediatrics, AZ-VUB, Laarbeeklaan 101, Brussels 1090, Belgium
S- Editor Zhong XY L- Editor Alpini G E- Editor Ma WH
References
- 1.Cain KC, Headstrom P, Jarrett ME, Motzer SA, Park H, Burr RL, Surawicz CM, Heitkemper MM. Abdominal pain impacts quality of life in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:124–132. doi: 10.1111/j.1572-0241.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- 2.Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. Initial poor quality of life and new onset of dyspepsia: results from a longitudinal 10-year follow-up study. Gut. 2007;56:321–327. doi: 10.1136/gut.2006.099846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cremonini F, Talley NJ. Irritable bowel syndrome: epidemiology, natural history, health care seeking and emerging risk factors. Gastroenterol Clin North Am. 2005;34:189–204. doi: 10.1016/j.gtc.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Saito YA, Cremonini F, Talley NJ. Association of the 1438G/A and 102T/C polymorphism of the 5-HT2A receptor gene with irritable bowel syndrome 5-HT2A gene polymorphism in irritable bowel syndrome. J Clin Gastroenterol. 2005;39:835; author reply 835–836. doi: 10.1097/01.mcg.0000177239.90005.b5. [DOI] [PubMed] [Google Scholar]
- 5.Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, Jones R, Kumar D, Rubin G, Trudgill N, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770–1798. doi: 10.1136/gut.2007.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruigomez A, Garcia Rodriguez LA, Panes J. Risk of irritable bowel syndrome after an episode of bacterial gastroenteritis in general practice: influence of comorbidities. Clin Gastroenterol Hepatol. 2007;5:465–469. doi: 10.1016/j.cgh.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal A, Whorwell PJ. Irritable bowel syndrome: diagnosis and management. BMJ. 2006;332:280–283. doi: 10.1136/bmj.332.7536.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cremonini F, Talley NJ. Treatments targeting putative mecha-nisms in irritable bowel syndrome. Nat Clin Pract Gastroenterol Hepatol. 2005;2:82–88. doi: 10.1038/ncpgasthep0096. [DOI] [PubMed] [Google Scholar]
- 9.Quigley EM, Flourie B. Probiotics and irritable bowel syndrome: a rationale for their use and an assessment of the evidence to date. Neurogastroenterol Motil. 2007;19:166–172. doi: 10.1111/j.1365-2982.2006.00879.x. [DOI] [PubMed] [Google Scholar]
- 10.Spiller RC. Role of infection in irritable bowel syndrome. J Gastroenterol. 2007;42 Suppl 17:41–47. doi: 10.1007/s00535-006-1925-8. [DOI] [PubMed] [Google Scholar]
- 11.Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852–858. doi: 10.1001/jama.292.7.852. [DOI] [PubMed] [Google Scholar]
- 12.Malinen E, Rinttila T, Kajander K, Matto J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 13.Andresen V, Baumgart DC. Role of probiotics in the treatment of irritable bowel syndrome: potential mechanisms and current clinical evidence. Inter J Probiotics Prebiotics. 2006;1:11–18. [Google Scholar]
- 14.McFarland LV. Normal flora: diversity and functions. Microb Ecol Health Dis. 2000;12:193–207. [Google Scholar]
- 15.Elmer GW, McFarland LV, McFarland M. Introduction. Chapter 1. In: The Power of Probiotics: Improving Your Health with Beneficial Microbes., editor. Binghamton, New York: Haworth Press; 2007. pp. 3–5. [Google Scholar]
- 16.McCarthy J, O’Mahony L, O’Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, Fitzgibbon J, O’Sullivan GC, Kiely B, Collins JK, et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52:975–980. doi: 10.1136/gut.52.7.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 18.Verdu EF, Bercik P, Verma-Gandhu M, Huang XX, Blennerhassett P, Jackson W, Mao Y, Wang L, Rochat F, Collins SM. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55:182–190. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szajewska H, Ruszczynski M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006;149:367–372. doi: 10.1016/j.jpeds.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 20.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 21.McFarland LV, Elmer GW and McFarland M. Meta-analysis of Probiotics for the Prevention and Treatment of Acute Pediatric Diarrhea. Internl J Probiotics Prebiotics. 2006;1:63–76. [Google Scholar]
- 22.McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97–105. doi: 10.1016/j.tmaid.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Kuhbacher T, Ott SJ, Helwig U, Mimura T, Rizzello F, Kleessen B, Gionchetti P, Blaut M, Campieri M, Folsch UR, et al. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. 2006;55:833–841. doi: 10.1136/gut.2005.078303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–114. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw RL, Booth A, Sutton AJ, Miller T, Smith JA, Young B, Jones DR, Dixon-Woods M. Finding qualitative research: an evaluation of search strategies. BMC Med Res Methodol. 2004;4:5. doi: 10.1186/1471-2288-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. QUOROM Group. Br J Surg. 2000;87:1448–1454. doi: 10.1046/j.1365-2168.2000.01610.x. [DOI] [PubMed] [Google Scholar]
- 28.Lim B, Manheimer E, Lao L, Ziea E, Wisniewski J, Liu J, Berman B. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2006;87:CD005111. doi: 10.1002/14651858.CD005111.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Linde K, Ramirez G, Mulrow CD, Pauls A, Weidenhammer W, Melchart D. St John’s wort for depression--an overview and meta-analysis of randomised clinical trials. BMJ. 1996;313:253–258. doi: 10.1136/bmj.313.7052.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke M, Oxman AD, eds Analyzing and Presenting Results: Cochrane Reviewers¡¯ Handbook 4.2 (updated November 2002- Section 8.) In: the Cochrane Library. Oxford: Update Software; 2003, issue 1, cited January 23. 2008 Available from: URL: http://www.cochrane.org/ resources/handbook/index.htm. [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 34.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320:1574–1577. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O’Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 36.Adler SN. The probiotic agent Escherichia coli M-17 has a healing effect in patients with IBS with proximal inflammation of the small bowel. Dig Liver Dis. 2006;38:713. doi: 10.1016/j.dld.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Bazzocchi G, Gionchetti P, Almerigi PF, Amadini C, Campieri M. Intestinal microflora and oral bacteriotherapy in irritable bowel syndrome. Dig Liver Dis. 2002;34 Suppl 2:S48–S53. doi: 10.1016/s1590-8658(02)80164-5. [DOI] [PubMed] [Google Scholar]
- 38.Brigidi P, Vitali B, Swennen E, Bazzocchi G, Matteuzzi D. Effects of probiotic administration upon the composition and enzymatic activity of human fecal microbiota in patients with irritable bowel syndrome or functional diarrhea. Res Microbiol. 2001;152:735–741. doi: 10.1016/s0923-2508(01)01254-2. [DOI] [PubMed] [Google Scholar]
- 39.Colecchia A, Vestito A, La Rocca A, Pasqui F, Nikiforaki A, Festi D. Effect of a symbiotic preparation on the clinical manifestations of irritable bowel syndrome, constipation-variant. Results of an open, uncontrolled multicenter study. Minerva Gastroenterol Dietol. 2006;52:349–358. [PubMed] [Google Scholar]
- 40.Drisko J, Bischoff B, Hall M, McCallum R. Treating irritable bowel syndrome with a food elimination diet followed by food challenge and probiotics. J Am Coll Nutr. 2006;25:514–522. doi: 10.1080/07315724.2006.10719567. [DOI] [PubMed] [Google Scholar]
- 41.Fan YJ, Chen SJ, Yu YC, Si JM, Liu B. A probiotic treatment containing Lactobacillus, Bifidobacterium and Enterococcus improves IBS symptoms in an open label trial. J Zhejiang Univ Sci B. 2006;7:987–991. doi: 10.1631/jzus.2006.B0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Astegiano M, Pellicano R, Terzi E, Simondi D, Rizzetto M. Treatment of irritable bowel syndrome. A case control experience. Minerva Gastroenterol Dietol. 2006;52:359–363. [PubMed] [Google Scholar]
- 43.Tsuchiya J, Barreto R, Okura R, Kawakita S, Fesce E, Marotta F. Single-blind follow-up study on the effectiveness of a symbiotic preparation in irritable bowel syndrome. Chin J Dig Dis. 2004;5:169–174. doi: 10.1111/j.1443-9573.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 44.Sen S, Mullan MM, Parker TJ, Woolner JT, Tarry SA, Hunter JO. Effect of Lactobacillus plantarum 299v on colonic fermentation and symptoms of irritable bowel syndrome. Dig Dis Sci. 2002;47:2615–2620. doi: 10.1023/a:1020597001460. [DOI] [PubMed] [Google Scholar]
- 45.Maupas JL, Champemont P, Delforge M. Treatment of irritable bowel syndrome. Double blind trial of Saccharomyces boulardii. Medecine Chirurgie Digestives. 1983;12:77–79. [Google Scholar]
- 46.Gade J, Thorn P. Paraghurt for patients with irritable bowel syndrome. A controlled clinical investigation from general practice. Scand J Prim Health Care. 1989;7:23–26. doi: 10.3109/02813438909103666. [DOI] [PubMed] [Google Scholar]
- 47.Halpern GM, Prindiville T, Blankenburg M, Hsia T, Gershwin ME. Treatment of irritable bowel syndrome with Lacteol Fort: a randomized, double-blind, cross-over trial. Am J Gastroenterol. 1996;91:1579–1585. [PubMed] [Google Scholar]
- 48.Nobaek S, Johansson ML, Molin G, Ahrne S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231–1238. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 49.O’Sullivan MA, O’Morain CA. Bacterial supplementation in the irritable bowel syndrome. A randomised double-blind placebo-controlled crossover study. Dig Liver Dis. 2000;32:294–301. doi: 10.1016/s1590-8658(00)80021-3. [DOI] [PubMed] [Google Scholar]
- 50.Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001;13:1143–1147. doi: 10.1097/00042737-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, Zinsmeister AR. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 52.Bausserman M, Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J Pediatr. 2005;147:197–201. doi: 10.1016/j.jpeds.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 53.Bittner AC, Croffut RM, Stranahan MC. Prescript-Assist probiotic-prebiotic treatment for irritable bowel syndrome: a methodologically oriented, 2-week, randomized, placebo-controlled, double-blind clinical study. Clin Ther. 2005;27:755–761. doi: 10.1016/j.clinthera.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Kajander K, Hatakka K, Poussa T, Farkkila M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther. 2005;22:387–394. doi: 10.1111/j.1365-2036.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- 55.Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, Thomforde G, Zinsmeister AR. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 56.Niv E, Naftali T, Hallak R, Vaisman N. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome--a double blind, placebo-controlled, randomized study. Clin Nutr. 2005;24:925–931. doi: 10.1016/j.clnu.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 57.Kim YG, Moon JT, Lee KM, Chon NR, Park H. The effects of probiotics on symptoms of irritable bowel syndrome. Korean J Gastroenterol. 2006;47:413–419. [PubMed] [Google Scholar]
- 58.Simren M, Syrous A, Lindh A, Abrahamsson H. Effects of lactobacillus plantarum 299v on symptoms and rectal sensitivity in patients with irritable bowel syndrome (IBS) - A randomized, double-blind controlled trial. Gastroenterology. 2006;130 Suppl 2:A600. [Google Scholar]
- 59.Enck P, Menke G, Zimmermann K, Martens U, Klosterhalfen S. Effective probiotic therapy of the irritable bowel syndrome (IBS): A multi-center clinical trial with primary care physicians. Gastroenterology. 2007;132 Suppl 2:A79. [Google Scholar]
- 60.Gawronska A, Dziechciarz P, Horvath A, Szajewska H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther. 2007;25:177–184. doi: 10.1111/j.1365-2036.2006.03175.x. [DOI] [PubMed] [Google Scholar]
- 61.D'haens GR, Kovacs G, Vergauwe P, Lonovics J, Bouhnik Y, Weiss W, Brunner H, Lavergne-Slove A, Di Stefano AF, Marteau P. A randomized controlled trial of the probiotic combination Lactibiane (R) in irritable bowel syndrome, the Lactibiane (R) study group. Gastroenterology. 2007;132 Suppl 2:A371. [Google Scholar]
- 62.Simren M, Lindh A, Sammelsson L, Olsson J, Posserud I, Strid H, Abrahamsson H. Effect of yoghurt containing three probiotic bacteria in patients with irritable bowel syndrome (IBS) - A randomized, double-blind, controlled trial. Gastroenterology. 2007;132 Suppl 2:A210. [Google Scholar]
- 63.DiBaise JK, Lof J, Taylor K, Quigley EM. Lactobacillus plantarum 299V in the irritable bowel syndrome: A randomized, double-blind, placebo-controlled crossover study. Gastroenterology. 2000;118 Suppl 2:A3163. [Google Scholar]
- 64.Saggioro A. Probiotics in the treatment of irritable bowel syndrome. J Clin Gastroenterol. 2004;38:S104–S106. doi: 10.1097/01.mcg.0000129271.98814.e2. [DOI] [PubMed] [Google Scholar]
- 65.Long ZR, Yu CH, Yang Y, Wang HN, Chi XX. Clinical observation on acupuncture combined with microorganism pharmaceutical preparations for treatment of irritable bowel syndrome of constipation type. Zhongguo Zhen Jiu. 2006;26:403–405. [PubMed] [Google Scholar]
- 66.Kajander K, Korpela R. Clinical studies on alleviating the symptoms of irritable bowel syndrome. Asia Pac J Clin Nutr. 2006;15:576–580. [PubMed] [Google Scholar]
- 67.Bittner AC, Croffut RM, Stranahan MC, Yokelson TN. Prescript-assist probiotic-prebiotic treatment for irritable bowel syndrome: an open-label, partially controlled, 1-year extension of a previously published controlled clinical trial. Clin Ther. 2007;29:1153–1160. doi: 10.1016/j.clinthera.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 68.Moon JT, Kim HS, Park HJ. Effects of probiotics on the intestinal gas volume score and symptoms in patients with irritable bowel syndrome. A randomized double-blind placebo-controlled study. Gastroenterology. 2007;132 Suppl 2:A688. [Google Scholar]
- 69.Porta N, Bonet C, Cobo E. Discordance between reported intention-to-treat and per protocol analyses. J Clin Epidemiol. 2007;60:663–669. doi: 10.1016/j.jclinepi.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 70.Salim A, Mackinnon A, Griffiths K. Sensitivity analysis of intention-to-treat estimates when withdrawals are related to unobserved compliance status. Stat Med. 2008;27:1164–1179. doi: 10.1002/sim.3025. [DOI] [PubMed] [Google Scholar]
- 71.Brittain E, Lin D. A comparison of intent-to-treat and per-protocol results in antibiotic non-inferiority trials. Stat Med. 2005;24:1–10. doi: 10.1002/sim.1934. [DOI] [PubMed] [Google Scholar]
- 72.Reichenbach S, Sterchi R, Scherer M, Trelle S, Burgi E, Burgi U, Dieppe PA, Juni P. Meta-analysis: chondroitin for osteoarthritis of the knee or hip. Ann Intern Med. 2007;146:580–590. doi: 10.7326/0003-4819-146-8-200704170-00009. [DOI] [PubMed] [Google Scholar]
- 73.Camilleri M. Probiotics and irritable bowel syndrome: rationale, putative mechanisms, and evidence of clinical efficacy. J Clin Gastroenterol. 2006;40:264–269. doi: 10.1097/00004836-200603000-00020. [DOI] [PubMed] [Google Scholar]
- 74.Bijkerk CJ, de Wit NJ, Muris JW, Jones RH, Knottnerus JA, Hoes AW. Outcome measures in irritable bowel syndrome: comparison of psychometric and methodological characteristics. Am J Gastroenterol. 2003;98:122–127. doi: 10.1111/j.1572-0241.2003.07158.x. [DOI] [PubMed] [Google Scholar]