Abstract
AIM: To evaluate the protective effect of inactivated hepatitis A vaccine (Healive®) against hepatitis A outbreak in an emergency vaccination campaign.
METHODS: During an outbreak of hepatitis A in Honghe Town, Xiuzhou District, Jiaxing City, Zhejiang Province, two nonrandomized controlled trials were conducted in September 2006. The first trial was to vaccinate 108 anti-HAV negative individuals with close contacts of the patients from September with 1 dose of an inactivated hepatitis A vaccine, Healive®. The control group comprised of 115 individuals with close contacts of the patients before September. The second trial was to vaccinate 3365 primary and secondary school students who volunteered to receive a dose of Healive® and 2572 students who did not receive Healive® serving as its controls. An epidemiological survey was conducted to evaluate the protective efficacy of the vaccine.
RESULTS: A total of 136 hepatitis A cases were reported during an outbreak that started in June, peaked in August and September, and ended after December of 2006. After a massive vaccination of school children in September, the number of cases declined significantly. No hepatitis A was detected in the 108 vaccinated individuals with close contacts of patients, whereas 4 cases of hepatitis A were found in the controls. The infection rate of hepatitis A was not significantly different in the individuals with close contacts of patients whether or not they received the vaccine (P = 0.122). No hepatitis A was detected in the 3365 students who received the vaccine, four cases of hepatitis A were found in the controls. The infection rate of students with or without vaccination was significantly different in the students who received the vaccine (0/3365 vs 4/2572, P = 0.035). The protective efficacy of the vaccine was 100%.
CONCLUSION: Inactivated hepatitis A vaccine demonstrates a good protective effect against an outbreak of hepatitis A.
Keywords: Hepatitis A, Outbreak, Inactivated hepatitis A vaccine, Emergency vaccination, Protective efficacy
INTRODUCTION
Hepatitis A is an acute, usually self-limited disease of the liver caused by hepatitis A virus (HAV). Although it is often a benign infection, up to 15% of the infected persons have a protracted relapsing disease course lasting up to 6 mo[1]. Hepatitis A infection may lead to acute liver failure with a mortality rate of 0.43‰-0.58‰ in 2005 and 2006 according to China Center for Disease Control and Prevention[2]. An estimated 1.5 million clinical cases of hepatitis A occur globally each year. Since HAV is transmitted from person to person, primarily through the faecal-oral route, the incidence of hepatitis A is closely related to the socioeconomic development and hygienic conditions. In China, the incidence of hepatitis A was above 100/100 000 in the 1980s. With the economic growth in the past decade, especially with the usage of live attenuated hepatitis A vaccine and inactivated hepatitis A vaccine[3,4], its incidence has decreased from 20/10 000 in 1996 to 5/10 000 in 2006[5]. Local outbreaks, however, are often reported in small cities and rural areas. In a previous study to investigate the prophylactic use of attenuated hepatitis A vaccine during an outbreak in a village, the live vaccine did not show protective effect, as the infection rate was not significantly different between the vaccinated and control groups[6]. The reason for this might be that the antibody induction period of the attenuated hepatitis A vaccine is relatively long and the seroconversion reaches a peak 2 or 3 mo after injection[7]. In contrast, the antibody induction period of inactivated hepatitis A vaccine is as short as 2 wk[8], and the seroconversion reaches a peak one month after delivery[9]. It is generally recognized that inactivated hepatitis A vaccine could provide good protection against hepatitis A after exposure to HAV[10,11]. Until now, the protective efficacy of inactivated hepatitis A vaccine has not been documented as an emergency vaccination in Chinese population.
Honghe Town, which belongs to the Xiuzhou District, Jiaxing City, Zhejiang Province, is an open rural community in southeastern China. Hepatitis A has been periodically epidemic in this area before vaccines were commonly used, and its incidence has been brought down to the national level with the introduction of hepatitis A vaccination programs. A serological survey in 2002 showed that the anti-HAV positive rate is 67.13% in this area[12]. Great changes have taken place in recent years, as the number of migrants now exceeds that of the local population. Many immigrants live in cramped dwellings and have limited access to appropriate hygienic conditions. Their sewage is drained directly into farmlands and streams without any proper treatment. Moreover, food safety is not guaranteed, as many people get food from unauthorized street stands. Infectious diseases are, thus, very likely to develop and get circulated.
Honghe Town consists of 9 villages with a population of 59 700, including a local population of 27 000 and a migrant population of 32 700. In August and September 2006, a hepatitis A outbreak was detected by the Xiuzhou District Center for Disease Control and Prevention. An emergency vaccination program was implemented for those with close contacts of patients and all school children in Honghe Town to interrupt the outbreak. Here, we report the protective effect of vaccination with Healive®, an inactivated hepatitis A vaccine developed and manufactured in China, on the control of hepatitis A in an open rural community.
MATERIALS AND METHODS
Surveillance of hepatitis A cases
Routine surveillance data on hepatitis A cases were obtained from the reports of physicians, laboratories, and hospitals. The major diagnostic criteria for hepatitis A were nausea, abdominal discomfort, jaundice, dark urine, presence of IgM antibody, and elevated serum alanine aminotransferase (ALT) level.
Considering two weeks needed for antibody induction with inactivated hepatitis A vaccine[6] and a 15-50 d incubation period of hepatitis A, we defined 15 d post-vaccination as the window period. Any case occurring within the window period was excluded from the vaccine protective efficacy analysis.
Composition of the vaccine
The inactivated hepatitis A vaccine, Healive®, developed and manufactured by Sinovac Biotech Co. Ltd. (Beijing, China), was licensed in China in 2002. The vaccine is prepared from the TZ84 strain of HAV. The virus used for vaccine production can grow in human fetal lung diploid fibroblast 2BS cells. Whole viruses were extracted from tissue culture, purified, formalin inactivated, and then adsorbed onto aluminum hydroxide. The pediatric dose contains 250 U/0.5 mL HAV antigen, and the adult dose contains 500 U/1.0 mL HAV antigen. Other ingredients include aluminium hydroxide, sodium dihydrogen phosphate, disodium hydrogen phosphate, sodium chloride, and water for injection.
Study populations and vaccination programs
During the outbreak of hepatitis A in Honghe Town, Xiuzhou District, Jiaxing City, Zhejiang Province, two nonrandomized controlled trials were conducted. Both trials were approved by the Medical Ethics Committee of Xiuzhou District.
Starting in September 2006, anti-HAV tests were performed in individuals with close contacts of hepatitis A patients, of them 108 were negative for HAV. In the first trial, inactivated hepatitis A vaccine, Healive® was emergently delivered to the 108 individuals with close contacts of hepatitis A patients as a post-prophylactic measure after informed consent was obtained. Subjects younger than 16 years old received one injection of a pediatric dose of vaccine containing 250 U/0.5 mL hepatitis A virus antigen and those older than 16 years received one injection of an adult dose of vaccine containing 500 U/1.0 mL hepatitis A virus antigen. These 108 subjects served as the intervention group. Meanwhile, a retrospective investigation and follow-up study were conducted among 44 patients from June to August, who had 115 close contacts and none of them had a previous hepatitis A vaccination. Because the vaccination program was not implemented until September, these 115 close contacts formed a natural non-intervention group. All those with close contacts in the intervention and non-intervention groups were observed for 60 d.
The second trial of the vaccination program was carried out from September 2 to 8 in primary and secondary school students. A written consent form (which provided information about the burden of the disease, the role of vaccine, and the cost of vaccine) was given to each student in the primary and secondary schools in Honghe Town. After the consent forms were signed by the students’ parents, 3365 students who consented to receive the vaccination served as the vaccine group, and 2572 students who did not choose to receive served as the control group. Students having either a history of hepatitis A infection or contraindications for hepatitis A vaccination were excluded from inoculation. One dose of Healive®, containing 250 U/0.5 mL hepatitis A virus antigen, was delivered to all students in the vaccine group.
Data analysis
The protective rate was calculated from the formula: (incidence of non-intervention group-incidence of intervention group)/incidence of non-intervention group. Fisher’s exact test was used to compare the incidence between the intervention and non-intervention groups. Z-test was used to analyze the number of cases. A two-tailed P value less than 0.05 was considered statistically significant. Statistical analysis was carried out using SPSS 11.5 software.
RESULTS
Epidemiology of the hepatitis A outbreak
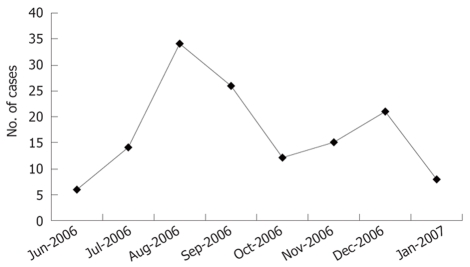
The outbreak of hepatitis A started in June 2006 and ended in January 2007 (Figure 1). A total of 136 cases of hepatitis A were reported. The first wave peaked in August and September in 67.65% (92/136) of the total cases. Forty-four cases of hepatitis A occurred in the second wave of outbreak, accounting for 32.35% (44/136) of the cases. The incidence was 227.86/100 000. Among the 136 reported cases, 64 patients were immigrating population while 72 patients were local population.
Figure 1.
Number of hepatitis A cases by month in Honghe Town during the outbreak of hepatitis A in 2006.
The cases had a different age distribution within the two populations. In the local population, most of the cases [81.9% (59/72)] were found at the age of 20-49 years, while 37.5% (24/64) and 32.8% (21/64) of the cases were found in the migrant population at the ages of 0-10 and 30-39 years, respectively.
Protective efficacy of the vaccine in those with close contacts of the patients
Within the 60-d observation period, 4 subjects in the non-intervention group during the first trial developed clinical symptoms and were diagnosed with hepatitis A. In the intervention group, the earliest and latest emergency injection of the vaccine was given 1 d and 31 d post-contact (the median was 12 d). No hepatitis A clinical symptoms were observed in the intervention group during the 60-d observation period. The statistical test showed the difference in the infection rates between those with close contacts who received vaccination (0/108) and those who did not receive vaccination (4/115) was not significant (P = 0.122). The protective efficacy was 100% in those with close contacts.
Protective efficacy of the vaccine in students
To evaluate the ability of a massive vaccination of students to prevent the outbreak, students were enrolled in the vaccine group or the control group, according to their willingness to receive the vaccine. After the vaccination for 3365 students in early September 2006, one hepatitis A case was reported 3 d after injection. Another 5 cases were detected between September and December 2006 in the control group of 2572 students, on September 16 and 30, October 5, November 1, and December 10. The single case in the vaccine group and September 16 case in the control group were excluded from the incidence analysis. The incidence of hepatitis A in the vaccine and control groups was calculated (Table 1). Fisher’s exact test indicated that the incidence of hepatitis A in the control group was significantly higher than that in the vaccine group (P = 0.035). The protective efficacy was 100% in students who received vaccination.
Table 1.
Hepatitis A incidence in vaccination and control groups
| Group | No. of subjects | No. of cases | Incidence (1/100 000) |
| Vaccine | 3365 | 0 | 0 |
| Control | 2572 | 4 | 155.5 |
Effect of vaccination program on reducing the number of cases
After vaccination, the number of cases was reduced dramatically in the student group (5-19 years old) and in other age groups (Figure 2). Compared with the number of cases before the vaccination program, the number of cases in the student group (5-19 years old) decreased by 70% after the vaccination program (20 cases in July-September vs 6 cases in October- December) (Z = 2.746, P < 0.01). The number of cases decreased by 22.22% (54 cases in July-September vs 42 cases in October-December, (Z = 1.224, P > 0.05) among those outside the student group.
Figure 2.
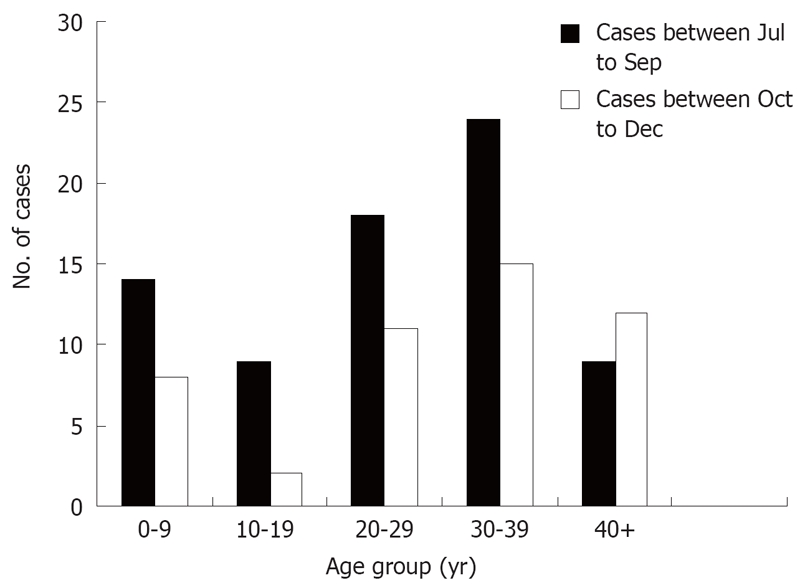
Hepatitis A cases by age group in Honghe Town before and after intervention. The vaccination was implemented in early September 2006.
DISCUSSION
We report a vaccination program in certain populations of an open rural community using an inactivated hepatitis A vaccine as an intervention to a hepatitis A outbreak. This is the first study to demonstrate the protective effect of inactivated hepatitis A vaccine (Healive®) on a Chinese population, and the vaccine was shown to have a 100% protective efficacy, suggesting that the vaccination program reduced the incidence of new hepatitis A cases after a massive vaccination in school children. The precise contribution of the vaccine campaign to the control of the outbreak of hepatitis A is difficult to quantify because the outbreaks are often on the wane over time.
The outbreak of hepatitis A showed a typical epidemic mode: a weak second wave appeared a couple of months after the first wave. One possible reason for the second wave is that 30% of the patients were not hospitalized, and the HAV transmission might have resulted from a delayed quarantine. The appearance of the second wave could also be explained by the fact that the vaccinated population was not large enough to form immunity. In this vaccination campaign, 3365 students and 108 individuals with close contacts of hepatitis A patients were inoculated, leading to a vaccine coverage of 56.7% [3365/(3365 + 2572)] in students and an average vaccine coverage of 5.82% [(3365 + 108)/59700] in the whole community. As vaccination was targeted mainly at students, the vaccination campaign only increased seroconversion in the student group, and hepatitis A virus could still circulate among the susceptible individuals in preschool children and adults. We believe that incomplete interruption of HAV is the consequence of a lack of vaccination coverage in preschool children and adults.
A corroborative survey was conducted to investigate the anti-HAV positive rate when the outbreak occurred in the community (Hai-Tao He, Xiuzhou District Center for Disease Control and Prevention, personal communication, 2006) (Table 2). The anti-HAV positive rate was low in those at the age of 0-4 and 20-39 years, suggesting that a large number of susceptible individuals are the preschool children and adults. These data provide evidence that a low anti-HAV seropositive rate and the lack of a vaccination campaign in preschool children and adults might be the cause of the aforementioned second wave of hepatitis A epidemic.
Table 2.
Anti-HAV sero-positive rate in the community of Honghe Town
|
Age group (yr) |
Total | |||||||
| 0- | 5- | 10- | 20- | 30- | 40- | 50- | ||
| Number of tested persons | 8 | 19 | 97 | 136 | 160 | 71 | 41 | 532 |
| Number of positive results | 4 | 16 | 78 | 103 | 120 | 59 | 34 | 414 |
| Positive rate (%) | 50 | 84.2 | 80.4 | 75.7 | 75 | 83.1 | 82.9 | 77.8 |
It was reported that inactivated hepatitis A vaccine was used in prevention of community-wide outbreaks of hepatitis A[13]. Studies showed that an epidemic of hepatitis A could end eight weeks after 80% of susceptible children and adults received one dose of hepatitis A vaccine[14]; the epidemic may persist for up to 30 wk in another location where less than 50% of the susceptible individuals were vaccinated[15]. It is still unknown what level of vaccination coverage is required to curtail a community-wide outbreak of hepatitis A.
It was difficult to promote vaccination in the local community, as vulnerable individuals besides the students refused to receive the vaccination in spite of our recommendation. Since inactivated hepatitis A vaccine is at expense of the recipients and whole-family vaccination is still unaffordable for many households, school children enjoy the priority in receiving the vaccination. We hope that the government and public health authorities can allocate a larger budget to support emergency vaccination programs to control outbreaks of hepatitis A more rapidly and effectively, especially in areas with a high incidence of HAV infection.
COMMENTS
Background
Hepatitis A is an acute, usually self-limited disease of the liver caused by hepatitis A virus. Although the use of inactivated hepatitis A vaccine as a prophylactic treatment has been reported in Europe and USA, little is known about the application of such a vaccine in control of hepatitis A in China.
Research frontiers
Although hepatitis A is regarded as a benign infection, it may cause serious symptoms. The disease can be prevented by vaccination and hepatitis A vaccine has been proved effective in controlling its outbreak worldwide.
Innovations and breakthroughs
Our study showed that inactivated hepatitis A vaccine demonstrating a good protective effect against outbreak of hepatitis A could be used for emergency vaccination. The study reports the first clinical study evaluating inactivated hepatitis A vaccine in an emergency vaccination campaign during an outbreak of hepatitis A in China.
Applications
Hepatitis A vaccine has several advantages over immune globulin, including long-term protection effect, ease of administration, and widespread availability. Substitution of immunoglobulin by inactivated hepatitis A vaccine and its application in both routine and emergency vaccination during an outbreak of hepatitis are proposed.
Terminology
Hepatitis A: an acute infectious disease of the liver caused by hepatitis A virus. Emergency vaccination: vaccination used during an outbreak of hepatitis A.
Peer review
The present study demonstrated the protective effect of inactivated hepatitis A vaccine against hepatitis A during the outbreak in an open rural community. It provides an interesting insight into the control and prevention of hepatitis in a rural community of China.
Acknowledgments
The authors thank Ye Ning (European Molecular Biology Laboratory, Heidelberg) for helpful suggestions and comments.
Peer reviewers: Jaime Guardia, Professor, Internal Medicine and Liver Unit, Hospital Universitari ‘Vall d’Hebron’, Universitat Autonoma de Barcelona, Barcelona 08035, Spain; Yasuji Arase, Dr, Department of Ggastroenterology, Toranomon Hospital, Tokyo 105-8470, Japan
S- Editor Liu JN L- Editor Wang XL E- Editor Ma WH
References
- 1.Advisory Committee on Immunization Practices (ACIP), Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–23. [PubMed] [Google Scholar]
- 2.Chinese center for disease control and prevention. National incidence and death cases of notifiable class A or class B infectious diseases. Available from: URL: http://www.chinacdc.net.cn/n272442/n272530/n272757/index.html. [Google Scholar]
- 3.Mao JS. Development of live, attenuated hepatitis A vaccine (H2-strain) Vaccine. 1990;8:523–524. doi: 10.1016/0264-410x(90)90001-3. [DOI] [PubMed] [Google Scholar]
- 4.Ren A, Feng F, Ma J, Xu Y, Liu C. Immunogenicity and safety of a new inactivated hepatitis A vaccine in young adults: a comparative study. Chin Med J (Engl) 2002;115:1483–1485. [PubMed] [Google Scholar]
- 5.Sui HT, Liang XF, Yin DP, Cui FQ, Wang HQ. Epidemic characteristics on hepatitis A in China during 1990-2006. Zhongguo Jihua Mianyi. 2007;13:466–469. [Google Scholar]
- 6.Wang X, Ma J, Xu Z, Liu H, Zhang Y, Han C. [Effectiveness of post-exposure prophylaxis using live attenuated hepatitis Alpha vaccine (H(2) strain) among schoolchildren] Zhonghua Yixue Zazhi. 2002;82:955–957. [PubMed] [Google Scholar]
- 7.Ma JC, Han CQ, Ding YX, Liu HB, Zhang Y, Zhang YL, Wang XY, Zhang YW, Xing ZC, Zhao H, et al. Effect of booster immunization of live attenuated hepatitis A vaccine. Zhongguo Shengwu Zhipinxue Zazhi. 2003;16:56–58. [Google Scholar]
- 8.Ren YH, Wu WT, Zhang YC, Xue WH, Kang WX, Ren YF, Han LJ, Li SP, Gao SJ, Cui LY, et al. The study on immunoreaction of low-dose inactivated Chinese hepatitis A vaccine. Zhongguo Jihua Mianyi. 2003;9:114–116. [Google Scholar]
- 9.Andre FE, D’Hondt E, Delem A, Safary A. Clinical assessment of the safety and efficacy of an inactivated hepatitis A vaccine: rationale and summary of findings. Vaccine. 1992;10 Suppl 1:S160–S168. doi: 10.1016/0264-410x(92)90576-6. [DOI] [PubMed] [Google Scholar]
- 10.D’Argenio P, Adamo B, Cirrincione R, Gallo G. The role of vaccine in controlling hepatitis A epidemics. Vaccine. 2003;21:2246–2249. doi: 10.1016/s0264-410x(03)00140-3. [DOI] [PubMed] [Google Scholar]
- 11.Victor JC, Monto AS, Surdina TY, Suleimenova SZ, Vaughan G, Nainan OV, Favorov MO, Margolis HS, Bell BP. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;357:1685–1694. doi: 10.1056/NEJMoa070546. [DOI] [PubMed] [Google Scholar]
- 12.Shen YG, Jiang XL, Gu XJ. Surveillance of Human Immune Level for Hepatitis A in Xiuzhou District of Jiaxin City. Zhejiang Yufang Yixue. 2004;16(10):13. [Google Scholar]
- 13.Werzberger A, Mensch B, Kuter B, Brown L, Lewis J, Sitrin R, Miller W, Shouval D, Wiens B, Calandra G. A controlled trial of a formalin-inactivated hepatitis A vaccine in healthy children. N Engl J Med. 1992;327:453–457. doi: 10.1056/NEJM199208133270702. [DOI] [PubMed] [Google Scholar]
- 14.McMahon BJ, Beller M, Williams J, Schloss M, Tanttila H, Bulkow L. A program to control an outbreak of hepatitis A in Alaska by using an inactivated hepatitis A vaccine. Arch Pediatr Adolesc Med. 1996;150:733–739. doi: 10.1001/archpedi.1996.02170320079014. [DOI] [PubMed] [Google Scholar]
- 15.Craig AS, Sockwell DC, Schaffner W, Moore WL Jr, Skinner JT, Williams IT, Shaw FE, Shapiro CN, Bell BP. Use of hepatitis A vaccine in a community-wide outbreak of hepatitis A. Clin Infect Dis. 1998;27:531–535. doi: 10.1086/514700. [DOI] [PubMed] [Google Scholar]