Abstract
This review provides information on the definition of constipation, normal continence and defecation and a description of the pathophysiologic mechanisms of constipation. In addition, changes in the anatomy and physiology of the lower gastrointestinal tract associated with aging that may contribute to constipation are described. MEDLINE (1966-2007) and CINAHL (1980-2007) were searched. The following MeSH terms were used: constipation/etiology OR constipation/physiology OR constipation/physiopathology) AND (age factors OR aged OR older OR 80 and over OR middle age). Constipation is not well defined in the literature. While self-reported constipation increases with age, findings from a limited number of clinical studies that utilized objective measures do not support this association. Dysmotility and pelvic floor dysfunction are important mechanisms associated with constipation. Changes in GI function associated with aging appear to be relatively subtle based on a limited amount of conflicting data. Additional research is warranted on the effects of aging on GI function, as well as on the timing of these changes.
Keywords: Constipation, Mechanisms, Functional consti-pation, Dysmotility, Older adults, Pelvic floor dysfunction, Gastrointestinal tract
INTRODUCTION
Constipation is a problem that affects all ages. However, it is a common problem in older adults and is often a concern to elders and clinicians. In older people, acute bouts of constipation can occur with acute illness or dietary alterations. In contrast, chronic constipation usually has an insidious onset of many years, often dating to childhood. The symptom experience can range from a mild, acute event that is remedied with a shift in fluid and dietary intake to a chronic condition that requires daily interventions with mixed results. Elders may falsely believe that constipation is a “natural” part of aging[1–4].
The purposes of this review are to: define constipation; provide an overview of normal continence and defecation; and describe the pathophysiologic mechanisms of constipation. In addition, the changes in the anatomy and physiology of the lower GI tract associated with aging that may contribute to constipation are described.
METHODS
To identify the relevant studies on the pathophysiology of constipation in the older adult, a number of strategies were employed. A literature search was conducted that included the following databases and time periods: MEDLINE (1966-2007) and CINAHL (1980-2007). The following MeSH terms were used: (constipation/etiology OR constipation/physiology OR constipation/physiopathology) AND (age factors OR aged OR older OR 80 and over OR middle age).
DEFINITION OF CONSTIPATION
Constipation is not a disease entity, but a general term that is used to describe the difficulties that patients experience with moving their bowels. Clinical and research literature documents that patients and clinicians use different definitions of constipation. Clinicians consider the frequency of defecation episodes, stool weight, colonic transit time, and anorectal manometry as proxy measures for constipation[5,6]. A commonly held belief amongst clinicians is that the problem of constipation is more imagined than real, as “the great majority of those complaining of constipation have a bowel motion (movement) more frequently than three times a week”[7]. The actual problem lies in the definition of constipation. The term itself holds different meanings depending on the individual. Individual perception of bowel function, whether or not the symptoms associated with constipation are endured, is quite distinct from how the medical dictionary defines the problem. Because of the subjective nature of the condition, no consensus exists on the definition of constipation.
Measurement of the frequency of stools has been used to define constipation. “Normal” frequency of stool evacuation comprises a broad or narrow range of time that has large intra- and inter-individual variability. The “usual” range is anywhere from one to three times per day to three times per week[8]. Less than three times per week may be considered normal if this does not represent a change from the usual frequency of baseline defecation events and is not associated with discomfort[9]. This self-report criterion does not describe the entire symptom experience of constipation.
Patients define constipation quite differently from clinicians. A study of 531 general practice patients found that 50% of them gave a different definition of constipation than their physicians[10]. Most patients define it by one or more of the following symptoms: hard stools, infrequent stools, the need for excessive straining, a sense of incomplete evacuation, and/or an excessive amount of time spent on the toilet or in unsuccessful evacuation[11]. Patients may perceive any or all of the following as constipation: straining to expel hard, dry stools; difficulty with initiating a bowel movement or an inability to defecate when desired; feelings of incomplete evacuation; and/or abdominal cramping and bloating. Subjective reports among middle aged and older individuals have identified the most common definition of constipation as being difficulty with defecation[12].
Most patients with a complaint of constipation have a functional disorder that affects the colon and/or anorectum. “Functional” is used to describe symptoms or problems that have no underlying anatomic abnormalities. However, the normal function of an organ has changed. Functional bowel disorders are functional gastrointestinal disorders with symptoms that arise in the middle or lower gastrointestinal tract[4]. Functional constipation is defined as the reduced frequency of bowel movements and/or an altered act of evacuation[13].
Functional bowel disorders, including functional constipation, are diagnosed primarily through patients’ reports of symptoms. As a result, a symptom based classification is needed for clinical diagnosis, evidence based management, and research. Since 1989, an international panel of experts has met four times and issued recommendations on the diagnosis and management of Irritable Bowel Syndrome (IBS), as well as diagnostic criteria for other functional bowel disorders. Known as the RomeI, Rome II, and Rome III (Table 1) criteria, these recommendations have evolved to include functional anorectal disorders. All disorders are defined by specific symptoms. However, functional disorders of defecation include the results of diagnostic tests as part of the definition[14].
Table 1.
Rome III criteria for defining functional constipation
| Loose stools rarely present with laxative use and insufficient criteria for IBS and |
| Two or more of the following (fulfilled for the last 3 mo with symptom onset at least 6 mo prior to diagnosis): |
| Fewer than three bowel movements per week |
| Straining1 |
| Lumpy or hard stools1 |
| Sensation of incomplete evacuation1 |
| Sensation of anorectal obstruction or blockade1 |
| Manual maneuvers (e.g., digital evacuation, support of the pelvic floor) |
| To facilitate a bowel movement1 |
≥ 25% of defecations. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders and functional abdominal pain. Gastroenterology 2006; 130: 1486.
Rome III as a functional bowel disorder that presents as persistently difficult, infrequent, or seemingly incomplete defecation, which does not meet IBS criteria, defines functional constipation. Patients are diagnosed with functional constipation if they are devoid of organic alterations and present with at least two or more of the symptoms listed in Table 1, fulfilled for at least three months with symptom onset at least six months prior to diagnosis, and insufficient criteria for IBS[4].
Several changes occurred in the development of the diagnostic criteria between RomeIand Rome III, in particular between Rome II and III. Studies using the Rome II criteria yielded lower prevalence rates for constipation than those using RomeIcriteria. A factor that contributed to this decrease was a change in the Rome II criteria that did not allow for laxative-induced loose stools[15]. Rome III includes this criterion. In addition, the frequency of occurrence of the various criteria was modified from > 25% to ≥ 25%. This approach was taken to be consistent with the criteria for other functional bowel disorders[4]. Since Rome II, more consistent criteria for the diagnosis and management of constipation have appeared in the literature.
With this definition of constipation as background information, the next section of this paper reviews normal continence and defecation prior to a discussion of the pathophysiology of constipation and constipation in the older adult.
NORMAL CONTINENCE AND DEFECATION
Normal anatomy and physiology of the gastrointestinal tract are well documented in the literature. Normal continence and defecation are complex processes that are altered in someone with constipation. Continence is the ability to retain feces until an acceptable time for defecation. Defecation is the evacuation of fecal material from the colon. Both functions involve complex physiologic processes that are not completely understood. Voluntary regulation through the central nervous system (CNS) and involuntary intrinsic reflex mechanisms are involved in both of these functions. Fecal continence is maintained by anatomic factors, anorectal sensation, and rectal compliance[16]. Problems with continence and defecation can arise from an extrinsic disorder involving the central or peripheral nervous systems; from an intrinsic disorder of the colon, rectum, or anal sphincters; or from a combination of these mechanisms.
Normal stool output is about 200 mg daily. Activity in the proximal colon determines the consistency and volume of delivery of contents to the rectum. The rectum is a reservoir. As rectal filling gradually proceeds, anorectal sampling permits subconscious perception of the consistency of the content. An intact internal anal sphincter (IAS) ensures continence.
Autonomic neurons relay the anorectal sensation of the rectal contents. Activation of these afferents results in both conscious perception and activation of local reflexes, such as the rectoanal inhibitory reflex to begin the relaxation of the IAS. Reflex voluntary contraction of the external anal sphincter (EAS) maintains continence until voluntary defecation is possible. A similar reflex contraction occurs to maintain continence when a rise in abdominal pressure occurs, such as during a cough or with positional changes. Partial external contraction is also observed during the passage of flatus, and coupled with an intact anorectal sensation this is the mechanism through which fecal continence is maintained during the passage of gas. Preservation of continence depends on the normal functioning of anorectal sensation, the appropriate perception of that sensory information, the integrity of lower and higher reflex arcs, and the action of the internal and external anal sphincters.
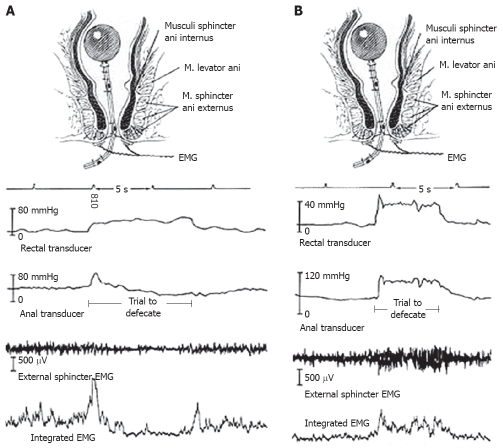
Defecation (Figure 1A) begins with rectal sensory awareness of a critical level of filling, which is relayed to the cerebral cortex as the perception of the need to evacuate the rectum. The actual volume that triggers the perception is dependent on the condition of the rectum (e.g. mucosal inflammation, rectal wall compliance) and the character of the contents (e.g. consistency, volume). When appropriate, the individual adopts a sitting or squatting position. This position results in a straightening of the anorectal angle that allows more effective propulsion of the rectal contents. The EAS and puborectalis muscles relax. The rectal contents provoke reflex relaxation of the IAS and the individual can then bear down. Abdominal pressure rises, abdominal wall muscles tense and relaxation of the pelvic floor allows some stool to enter the lower rectum. This movement of stool initiates a spontaneous rectosigmoid contraction, which pushes the stool through the relaxed anal canal[16].
Figure 1.
Manometric and electromyographic recordings seen with normal (A) and abnormal (B) defecation. EMG: Electromyography; Footnote: Wald A. Manometry. In: Schuster MM, Crowel MD, Koch KL, eds. Atlas of Gastrointestinal Motility in Health and Disease 2nd ed, Hamilton, and Ontario: BC Decker; 2002: 289-303.
Large propulsive contractions of the rectum occur until the rectum is empty. Sensory input from the anus maintains the propulsive activity until the rectum is fully voided. This reflex appears to be mediated at the level of the spinal cord, as even spinally injured patients can evacuate a complete stool from the rectum, once initiated[17]. Such patients tend to use digital rectal stimulation to initiate the propulsive contractions of the recto-sigmoid colon. As stool passes through the anal canal, it stretches the EAS and creates a traction force on it. After the last bolus of stool has passed, the closing reflex of the EAS is stimulated by the release of traction. Therefore, anal continence is maintained following defecation.
CHANGES IN THE LOWER GASTROINTE-STINAL TRACT ASSOCIATED WITH AGING
Age-related anatomic changes within the lower gastrointestinal tract may contribute to delayed transit time and decreased stool water content[18]. These changes can include intestinal wall atrophy, reduced blood supply, and intrinsic neuronal changes. However, no significant functional changes are readily apparent in the aging gastrointestinal tract. Secretion and absorption remain relatively constant. This constancy is thought to be due to the redundancy in each segment of the intestinal tract.
Gut transit time and colonic motility are similar in healthy older adults compared to younger participants[19]. In contrast, elderly people with chronic illness who report constipation have a prolonged total gut transit time of 4 to 9 d (normal < 3 d), with evacuation delayed through the lowest part of the large bowel and rectum. Nursing home residents have even more prolonged transit times of up to three weeks in those least mobile which makes them highly susceptible to slow transit constipation (STC) and overflow fecal incontinence[20]. Colonic function appears to be more influenced by factors associated with aging (e.g. chronic disease, immobility, and medications) than aging itself.
Age-related neurodegenerative changes in the enteric nervous system (ENS) may be key to functional changes observed with advanced age. In colons of people older than age 65, a 37% loss of enteric neurons was found when compared with younger people[21]. The number of nerve cells present in the myenteric plexus of the human large intestine was examined using laminar preparations of the muscularis externa in two groups of participants aged 20 to 35 and over 65 years. In addition, the collagen and elastic system related fibers in the myenteric ganglia were qualitatively evaluated. The total number of neurons was decreased in older individuals. Both collagen and elastic system fibers were more numerous in the ganglia from the older participants. The authors concluded that the decrease in neuron density with age is accompanied by an apparent increase in the fibrous components of the myenteric ganglia. These findings suggest that neurodegenerative changes may contribute to the disturbed colonic motility seen in the aging population.
Older people have age-related reductions in IAS pressure and pelvic muscle strength[22,23], as well as changes in rectal sensitivity[24] and anal function. Women in particular, experience a larger decrease in squeeze pressures with aging especially after menopause, and due to injuries sustained during vaginal delivery[25,26]. These changes increase both the risk and the potential for constipation.
The interrelationship between aging and parity in the anorectal squeeze pressure in women is difficult to determine from currently available data. However, the authors suggested that menopausal effects might be relevant[3]. These age-related changes are not in and of them pathologic, but may contribute to the development of constipation in the elderly.
PATHOPHYSIOLOGIC MECHANISMS OF CONSTIPATION
Two mechanisms explain the pathophysiology of constipation[27,28]. Colonic motility dysfunction, or dysmotility, is failure of coordinated motor activity to move stool through the colon. It is sometimes associated with: dietary factors, medications that can alter motility; or systemic disease (e.g. neurologic, metabolic, or endocrine disorders). Others exhibit abnormalities of the enteric nerves, such as decreased volume of interstitial cells of Cajal (ICC) and other neural elements[29]. The second mechanism involves pelvic floor dysfunction, or disorders of the anorectum and pelvic floor, which result in outlet dysfunction and an inability to adequately evacuate rectal contents. Functional constipation may occur as a result of disordered movement through the sigmoid colon and/or anorectum. Both mechanisms coexist in some patients[28], making it difficult to determine the exact underlying mechanisms for constipation.
Physiology of dysmotility
Dysmotility results in colonic delay (i.e. abnormally prolonged colonic transit time). Three types of colonic delay have been identified: right colonic (colonic inertia), left colonic, and rectosigmoid. Additionally, delay can occur in patients with no colonic dysmotility[30]. Mechanisms of delay include: dysfunction of the autonomic nervous system, disruption in the ENS[31], disruptions in the neuroendocrine system[32,33], and/or colonic myopathy[34,35].
Impaired colonic propulsive activity may represent a major mechanism for colonic dysmotility. In patients with constipation (n = 45), there were fewer mass movements segmental contractions[36]. No differences in post awakening values were found in patients with chronic constipation, which suggests that the brain-gut control of fundamental mechanisms governing colonic motility is preserved[37].
A disorder of the ICC may have a role in the development of diminished or absent colonic motor activity[38]. In patients with STC, the number of ICC was significantly decreased in all layers of the colonic wall[29], including the external muscle layer[39]. Thus, constipation in patients with colonic inertia is attributable to weak or absent electric activity.
When compared with healthy controls, patients with STC exhibit reduced daytime colonic pressure waves and a higher frequency of periodic rectal motor activity (PRMA) that were unrelated to proximal colonic activity. Their findings suggest that excessive and uncoordinated phasic rectal activity may further impede stool transport and contribute to STC[40].
The gastrocolic response to ingestion of a meal in individuals with constipation is characterized by a shorter contractile activity in all three-colon segments and significantly fewer high amplitude propagated contractions (HAPCs)[41,42]. Changes in rectal wall contractility in response to feeding, as well as with the administration of a cholinergic agonist, and a smooth muscle relaxant, are decreased in constipated patients. This finding suggests an abnormality in rectal muscular wall contractility[43].
The gastrocolic response after ingestion of a standardized liquid meal and the response to a local chemical stimulus were investigated in 10 healthy controls and 10 patients with STC. Increases in motility after a meal and bisacodyl were seen in healthy participants, but not in patients with STC. Timing of the high amplitude propagating contractions was prolonged and decreased in number in the patients with STC. In addition, symptom reports of a cramp felt at the time of a HAPC were significantly lower than in controls (P < 0.05)[44].
The nerve fibers in the colonic circular muscle may be abnormal in patients with STC. A reduction in the density of excitatory nerve fibers with tachykinin and enkephalin immunoreactivity was found in the colonic circular muscle of patients with STC, whereas innervation of all the other layers was normal[45]. Small sensory fiber dysfunction has also been suggested in patients with STC[46].
Abnormalities in neurotransmitters may contribute to dysmotility and the subsequent development of constipation. Changes in excitatory and inhibitory neurotransmitters have been evaluated with conflicting results. Levels of vasoactive intestinal peptide (VIP) were found to be unchanged[47] or increased[33] in patients with STC. The release of acetylcholine was found to be depressed in colonic tissue specimens from constipated patients[48]. Excessive nitric oxide was found in ICC preparations from the distal colon of patients with STC[49]. A decrease in serotonin immunoreactivity in the muscular mucosa and circular muscle was identified in patients who underwent subtotal colectomy for colonic inertia[50]. How changes in the release of various neurotransmitters contribute to the pathogenesis of STC has not been described in detail.
A number of gut hormones (i.e. cholecystokinin, peptide YY, somatostatin, enteroglucagon, pancreatic peptides) are thought to have potent effects on gastrointestinal motility. Plasma cholecystokinin and peptide YY have not been found to be altered in patients with STC[51]. However, specific abnormalities in circulating gut hormones have been identified in patients with STC including: higher levels of circulating somatostatin, lower levels of somatostatin integrated with an incremental meal response, and decreased levels of enteroglucagon 30-60 min after a meal[52]. Significantly fewer enteroglucagon and serotonin immunoreactive cells were found in patients with STC[33]. However, how changes in these hormones contribute to the pathogenesis of STC has not been described.
Changes in physiology associated with disease states
Disease states that alter slowly wave patterns or spike responses will alter contraction and motility[53]. Abnormalities in colonic motility seen in diabetic patients with constipation are due in part to altered autonomic neural control manifested as an abnormal gastrocolonic response. Slow wave patterns appear unaltered in healthy participants compared to patients with constipation and diabetes. Minimal spike potential activity is seen in both healthy and diabetic patients during fasting. Following a meal, spike potential activity quickly increases during the first 10 min and is sustained for 30 min in healthy participants. This activity is inhibited by the pre-administration of an anticholinergic drug, which suggests that the postprandial response is mediated through the cholinergic nervous system. In diabetic patients without constipation, the response to a meal is the same as in controls. In chronic insulin dependent diabetic patients with constipation, the normal postprandial increase in spike potential is not present. The lack of spike potential leads to abnormal postprandial motor activity in the colon, which results in constipation[54,55].
Pelvic floor dysfunction
The second major mechanism for constipation is pelvic floor dysfunction, which results in disordered defecation. It is most commonly due to dysfunction of the pelvic floor muscles or anal sphincters[15]. Different terms that are used to describe these disorders include anismus, pelvic-floor dyssynergia, paradoxical pelvic floor contraction, obstructed defecation, functional rectosigmoid obstruction, and functional fecal retention in childhood[56]. The pathophysiology of these disorders is not completely understood.
Physiology of pelvic floor dysfunction
When constipation is accompanied by an immobile perineum, patients have impaired balloon expulsion, impaired and delayed artificial stool expulsion, decreased straightening of the anorectal angle, decreased descent of the pelvic floor with defecation, and prolonged rectosigmoid transit times. All are thought to be signs of pelvic floor dysfunction rather than delayed transit time[57].
When compared to healthy controls, patients with obstructed defecation demonstrate lower intrarectal pressure and defecation indices and higher anal residual pressures on anorectal manometry recordings during straining (Figure 1B). Impaired rectal contraction, paradoxical anal contraction, or inadequate anal relaxation seen in patients with obstructed defecation suggests that rectoanal coordination is impaired[58].
The overall frequency of propagating sequences in the colon does not differ between patients with obstructed defecation and healthy controls. In fact, patients with obstructed defecation were found to have a significant increase in the frequency of retrograde and antegrade propagating sequences (P < 0.05) in the left colon and a significant reduction in the amplitude of propagating pressure waves throughout the entire colon (P < 0.03). In the 15 min before defecation, controls showed a highly significant increase in frequency (P = 0.001) and amplitude (P = 0.01) of propagating sequences. In contrast, patients did not demonstrate this or the typical spatiotemporal organization of propagating sequences normally observed before expulsion of stool[59].
Neural influences on pelvic floor dysfunction
Parasympathetic afferent nerves are stimulated by both slow or cumulative and fast or intermittent distention of the rectum, whereas sympathetic afferent nerves are only stimulated by fast distention. In a study that examined the role of sympathetic afferent nerves in the mediation of rectal filling sensations, women with obstructed defecation were found to have either blunted or absent rectal sensory perception[60]. Participants experienced a nonspecific sensation in the pelvis or lower abdomen with fast distention, which suggested that sympathetic efferents were deficient. In spite of this, rectal wall compliance was normal in the patients with obstructed defecation[61].
The gastrocolic reflex has been evaluated in patients with obstructed defecation. It was found to be absent or prolonged in patients with obstructive defecation in whom transit time is prolonged. The gastrocolic reflex was found to be intact if slow transit was absent[62].
Patients with STC can also have anorectal motility disturbances. The minimum relaxation volume, the rectal defecatory threshold, the rectal maximal tolerable volume, and the rectal compliance are significantly higher in patients with STC than in healthy controls (P < 0.01 or P < 0.05)[63]. The anorectal reflex is active in puborectalis paradoxical syndrome, but the rectoanal reflex is not, indicating a possible myogenic defect in the puborectalis muscle[64].
How people develop defecation disorders is unclear. In two-thirds of patients, dyssynergic or obstructed defecation appears to be an acquired behavioral disorder of defecation and in the rest the process of defecation may not have been learned since childhood[65].
PATHOPHYSIOLOGY OF CONSTIPATION IN THE OLDER ADULT
Constipation is often considered a natural part of aging but it is a disorder that is not caused by aging itself. Although changes in the gastrointestinal tract associated with aging may predispose one to develop constipation, the disorder usually has a multifactoral etiology and may be a lifetime disorder.
As shown in Table 2, numerous factors may contribute to the development of constipation[6–9,66,67]. Though bowel transit time and frequency of bowel movements do not change with aging, a number of comorbid conditions may contribute to the development of constipation[66]. Some data suggest that older adults perceive constipation as straining during defecation rather than decreased bowel frequency[68,69]. Another study of elderly individuals who reported constipation demonstrated that straining and hard bowel movements were the most frequent complaints[69]. A determination of the most likely etiology for constipation requires identification of the primary complaint[66].
Table 2.
Etiology of constipation in the older adult
| Endocrine and metabolic disease |
| Diabetes mellitus |
| Hypothyroidism |
| Neurologic disease |
| Autonomic neuropathy |
| Cerebrovascular disease |
| Multiple sclerosis |
| Parkinson’s disease |
| Spinal cord injury |
| Psychological conditions |
| Anxiety |
| Depression |
| Structural abnormalities |
| Anorectal conditions: fissures, hemorrhoids, rectal prolapse or rectocele |
| Obstructive colonic lesions |
| Lifestyle |
| Dehydration |
| Low calorie diet |
| Low fiber diet |
| Immobility |
| Iatrogenic |
| Medications |
Aging is associated with changes in the structure and function of the colon and defecatory mechanisms. Regional differences in colonic properties and in neurotransmitter functions have implications for normal function and dysfunction[70].
Rectal sensation plays a critical role in normal defecation and may change with aging. In one study elderly patients with constipation and a history of fecal impaction had impaired rectal and perineal sensation and required significantly larger volumes of rectal distention to stimulate the normal urge to defecate[24]. A second report described impaired rectal perception of stool in elderly patients with constipation[71], while sensation appeared to remain intact in those patients without constipation.
Disordered defecation can occur as a result of injury to the pudendal nerve. The incidence of increased pudendal nerve terminal motor latency, an indicator of pudendal nerve dysfunction, is increased in elderly females[3]. Injury to the pudendal nerves can lead to abnormal perineal descent, which can impact rectal emptying by causing partial prolapse of the anal canal by the anterior rectal mucosa[72].
Several types of anorectal abnormalities occur in older people with constipation including dyschezia and pelvic dyssynergia. Dyschezia is characterized by reduced tone, increased compliance, and impaired sensation such that a greater degree of rectal distention is required to induce the defecatory mechanism[24]. Seen most commonly in frail elders, these individuals have recurrent rectal impactions, a frequent consequence of which is fecal soiling. Fecal soiling affects 28% of older people. However, it is a problem that is not assessed by doctors or nurses[73]. Pelvic dyssynergia, also termed anismus, involves a failure to relax the pelvic floor and external anal sphincter muscles during defecation.
IMPLICATIONS FOR FUTURE RESEARCH
Constipation is not a straightforward problem. However, recent research on the mechanisms and effects of constipation on the elderly is extremely sparse. Until the mechanisms of constipation are completely understood, it is likely that treatment will be at best, minimally successful. Dysmotility and pelvic floor dysfunction are clearly important mechanisms associated with constipation, but further work is needed to understand the anatomic, physiologic, and lifestyle factors that affect these mechanisms. In addition, longitudinal data on gastrointestinal motility are needed to determine the effects of aging on normal lower GI and pelvic floor anatomy and function. Studies are needed that evaluate changes in the physiology of the pelvic floor in women over the menopause transition and whether these changes contribute to functional outcomes seen at that time and on into old age.
Changes in GI function associated with aging appear to be relatively subtle based on a limited amount of conflicting data. Additional research is warranted on the effects of aging on GI function, as well as on the timing of these changes. A deeper understanding of the basic mechanisms of dysfunction, changes in the colonic wall, and pelvic floor dysfunction in the older adult could provide new directions for the assessment and management of constipation in this vulnerable group.
While significant progress has occurred in the development of consensus criteria for the assessment of constipation the definition is not standardized. The Rome criteria constitute a self-reported, complaint-based diagnostic system with a significant overlap between the criteria for dysmotility and pelvic dysfunction disorders. Studies are needed to clarify the discrete differences between colonic motor dysfunction and functional defecation disorders.
Supported by The Betty Irene Moore Fellowship
Peer reviewers: Stefan Wirth, Professor, Dr, Children’s Hospital, Heusnerstt. 40, Wuppertal 42349, Germany; Diego Garcia-Compean, MD, Professor, Faculty of Medicine, University Hospital, Department of Gastroenterology, Autonomous University of Nuevo Leon, Ave Madero y Gonzalitos, 64700 Monterrey, NL, Mexico
S- Editor Zhong XY L- Editor Alpini GD E- Editor Ma WH
References
- 1.Chan AO, Cheng C, Hui WM, Hu WH, Wong NY, Lam KF, Wong WM, Lai KC, Lam SK, Wong BC. Differing coping mechanisms, stress level and anorectal physiology in patients with functional constipation. World J Gastroenterol. 2005;11:5362–5366. doi: 10.3748/wjg.v11.i34.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang L, Toner BB, Fukudo S, Guthrie E, Locke GR, Norton NJ, Sperber AD. Gender, age, society, culture, and the patient’s perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130:1435–1446. doi: 10.1053/j.gastro.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 3.Laurberg S, Swash M. Effects of aging on the anorectal sphincters and their innervation. Dis Colon Rectum. 1989;32:737–742. doi: 10.1007/BF02562120. [DOI] [PubMed] [Google Scholar]
- 4.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 5.Ashraf W, Park F, Lof J, Quigley EM. An examination of the reliability of reported stool frequency in the diagnosis of idiopathic constipation. Am J Gastroenterol. 1996;91:26–32. [PubMed] [Google Scholar]
- 6.Koch T, Hudson S. Older people and laxative use: literature review and pilot study report. J Clin Nurs. 2000;9:516–525. doi: 10.1046/j.1365-2702.2000.00357.x. [DOI] [PubMed] [Google Scholar]
- 7.Campbell AJ, Busby WJ, Horwath CC. Factors associated with constipation in a community based sample of people aged 70 years and over. J Epidemiol Community Health. 1993;47:23–26. doi: 10.1136/jech.47.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaefer DC, Cheskin LJ. Constipation in the elderly. Am Fam Physician. 1998;58:907–914. [PubMed] [Google Scholar]
- 9.Abyad A, Mourad F. Constipation: common-sense care of the older patient. Geriatrics. 1996;51:28–34, 36. [PubMed] [Google Scholar]
- 10.Herz MJ, Kahan E, Zalevski S, Aframian R, Kuznitz D, Reichman S. Constipation: a different entity for patients and doctors. Fam Pract. 1996;13:156–159. doi: 10.1093/fampra/13.2.156. [DOI] [PubMed] [Google Scholar]
- 11.Koch A, Voderholzer WA, Klauser AG, Muller-Lissner S. Symptoms in chronic constipation. Dis Colon Rectum. 1997;40:902–906. doi: 10.1007/BF02051196. [DOI] [PubMed] [Google Scholar]
- 12.Ross DG. Subjective data related to altered bowel elimination patterns among hospitalized elder and middle-aged persons. Orthop Nurs. 1993;12:25–32. doi: 10.1097/00006416-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Drossman DA. The functional gastrointestinal disorders and the Rome II process. Gut. 1999;45 Suppl 2:II1–II5. doi: 10.1136/gut.45.2008.ii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130:1510–1518. doi: 10.1053/j.gastro.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 15.Thompson WG, Irvine EJ, Pare P, Ferrazzi S, Rance L. Functional gastrointestinal disorders in Canada: first population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig Dis Sci. 2002;47:225–235. doi: 10.1023/a:1013208713670. [DOI] [PubMed] [Google Scholar]
- 16.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil. 2006;18:507–519. doi: 10.1111/j.1365-2982.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 17.Read NW. Feedback regulation and sensation. Dig Dis Sci. 1994;39:37S–40S. [PubMed] [Google Scholar]
- 18.Lynch AC, Anthony A, Dobbs BR, Frizelle FA. Anorectal physiology following spinal cord injury. Spinal Cord. 2000;38:573–580. doi: 10.1038/sj.sc.3101076. [DOI] [PubMed] [Google Scholar]
- 19.Hanani M, Fellig Y, Udassin R, Freund HR. Age-related changes in the morphology of the myenteric plexus of the human colon. Auton Neurosci. 2004;113:71–78. doi: 10.1016/j.autneu.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Loening-Baucke V, Anuras S. Sigmoidal and rectal motility in healthy elderly. J Am Geriatr Soc. 1984;32:887–891. doi: 10.1111/j.1532-5415.1984.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 21.Brocklehurst JC, Kirkland JL, Martin J, Ashford J. Constipation in long-stay elderly patients: its treatment and prevention by lactulose, poloxalkol-dihydroxyanthroquinolone and phosphate enemas. Gerontology. 1983;29:181–184. doi: 10.1159/000213112. [DOI] [PubMed] [Google Scholar]
- 22.Gomes OA, de Souza RR, Liberti EA. A preliminary investigation of the effects of aging on the nerve cell number in the myenteric ganglia of the human colon. Gerontology. 1997;43:210–217. doi: 10.1159/000213852. [DOI] [PubMed] [Google Scholar]
- 23.McHugh SM, Diamant NE. Anal canal pressure profile: a reappraisal as determined by rapid pullthrough technique. Gut. 1987;28:1234–1241. doi: 10.1136/gut.28.10.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McH ugh SM, Diamant NE. Effect of age, gender, and parity on anal canal pressures. Contribution of impaired anal sphincter function to fecal incontinence. Dig Dis Sci. 1987;32:726–736. doi: 10.1007/BF01296139. [DOI] [PubMed] [Google Scholar]
- 25.Read NW, Abouzekry L, Read MG, Howell P, Ottewell D, Donnelly TC. Anorectal function in elderly patients with fecal impaction. Gastroenterology. 1985;89:959–966. doi: 10.1016/0016-5085(85)90194-5. [DOI] [PubMed] [Google Scholar]
- 26.Ryhammer AM, Laurberg S, Sorensen FH. Effects of age on anal function in normal women. Int J Colorectal Dis. 1997;12:225–229. doi: 10.1007/s003840050094. [DOI] [PubMed] [Google Scholar]
- 27.Sultan AH, Kamm MA, Hudson CN. Pudendal nerve damage during labour: prospective study before and after childbirth. Br J Obstet Gynaecol. 1994;101:22–28. doi: 10.1111/j.1471-0528.1994.tb13005.x. [DOI] [PubMed] [Google Scholar]
- 28.Cheung O, Wald A. Review article: the management of pelvic floor disorders. Aliment Pharmacol Ther. 2004;19:481–495. doi: 10.1111/j.1365-2036.2004.01886.x. [DOI] [PubMed] [Google Scholar]
- 29.Sagar PM, Pemberton JH. Anorectal and pelvic floor function. Relevance of continence, incontinence, and constipation. Gastroenterol Clin North Am. 1996;25:163–182. doi: 10.1016/s0889-8553(05)70370-8. [DOI] [PubMed] [Google Scholar]
- 30.Wedel T, Spiegler J, Soellner S, Roblick UJ, Schiedeck TH, Bruch HP, Krammer HJ. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology. 2002;123:1459–1467. doi: 10.1053/gast.2002.36600. [DOI] [PubMed] [Google Scholar]
- 31.Mertz H, Naliboff B, Mayer EA. Symptoms and physiology in severe chronic constipation. Am J Gastroenterol. 1999;94:131–138. doi: 10.1111/j.1572-0241.1999.00783.x. [DOI] [PubMed] [Google Scholar]
- 32.Bassotti G, Villanacci V. Slow transit constipation: a functional disorder becomes an enteric neuropathy. World J Gastroenterol. 2006;12:4609–4613. doi: 10.3748/wjg.v12.i29.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Salhy M, Norrgard O, Spinnell S. Abnormal colonic endocrine cells in patients with chronic idiopathic slow-transit constipation. Scand J Gastroenterol. 1999;34:1007–1011. doi: 10.1080/003655299750025110. [DOI] [PubMed] [Google Scholar]
- 34.Sjolund K, Fasth S, Ekman R, Hulten L, Jiborn H, Nordgren S, Sundler F. Neuropeptides in idiopathic chronic constipation (slow transit constipation) Neurogastroenterol Motil. 1997;9:143–150. doi: 10.1046/j.1365-2982.1997.d01-46.x. [DOI] [PubMed] [Google Scholar]
- 35.Knowles CH, Nickols CD, Scott SM, Bennett NI, de Oliveira RB, Chimelli L, Feakins R, Williams NS, Martin JE. Smooth muscle inclusion bodies in slow transit constipation. J Pathol. 2001;193:390–397. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH797>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 36.Knowles CH, Scott SM, Lunniss PJ. Slow transit constipation: a disorder of pelvic autonomic nerves? Dig Dis Sci. 2001;46:389–401. doi: 10.1023/a:1005665218647. [DOI] [PubMed] [Google Scholar]
- 37.Bassotti G, Chistolini F, Nzepa FS, Morelli A. Colonic propulsive impairment in intractable slow-transit constipation. Arch Surg. 2003;138:1302–1304. doi: 10.1001/archsurg.138.12.1302. [DOI] [PubMed] [Google Scholar]
- 38.Bassotti G, Germani U, Fiorella S, Roselli P, Brunori P, Whitehead WE. Intact colonic motor response to sudden awakening from sleep in patients with chronic idiopathic (slow-transit) constipation. Dis Colon Rectum. 1998;41:1550–1555; discussion 1555-1556. doi: 10.1007/BF02237305. [DOI] [PubMed] [Google Scholar]
- 39.Shafik A, Shafik AA, El-Sibai O, Mostafa RM. Electric activity of the colon in subjects with constipation due to total colonic inertia: an electrophysiologic study. Arch Surg. 2003;138:1007–1011; discussion 1011. doi: 10.1001/archsurg.138.9.1007. [DOI] [PubMed] [Google Scholar]
- 40.Tong WD, Liu BH, Zhang LY, Zhang SB, Lei Y. Decreased interstitial cells of Cajal in the sigmoid colon of patients with slow transit constipation. Int J Colorectal Dis. 2004;19:467–473. doi: 10.1007/s00384-003-0577-x. [DOI] [PubMed] [Google Scholar]
- 41.Rao SS, Sadeghi P, Batterson K, Beaty J. Altered periodic rectal motor activity: a mechanism for slow transit constipation. Neurogastroenterol Motil. 2001;13:591–598. doi: 10.1046/j.1365-2982.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- 42.Bassotti G, Imbimbo BP, Betti C, Dozzini G, Morelli A. Impaired colonic motor response to eating in patients with slow-transit constipation. Am J Gastroenterol. 1992;87:504–508. [PubMed] [Google Scholar]
- 43.Grotz RL, Pemberton JH, Levin KE, Bell AM, Hanson RB. Rectal wall contractility in healthy subjects and in patients with chronic severe constipation. Ann Surg. 1993;218:761–768. doi: 10.1097/00000658-199312000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Schryver AM, Samsom M, Smout AI. Effects of a meal and bisacodyl on colonic motility in healthy volunteers and patients with slow-transit constipation. Dig Dis Sci. 2003;48:1206–1212. doi: 10.1023/a:1024178303076. [DOI] [PubMed] [Google Scholar]
- 45.Porter AJ, Wattchow DA, Hunter A, Costa M. Abnormalities of nerve fibers in the circular muscle of patients with slow transit constipation. Int J Colorectal Dis. 1998;13:208–216. doi: 10.1007/s003840050163. [DOI] [PubMed] [Google Scholar]
- 46.Knowles CH, Scott SM, Wellmer A, Misra VP, Pilot MA, Williams NS, Anand P. Sensory and autonomic neuropathy in patients with idiopathic slow-transit constipation. Br J Surg. 1999;86:54–60. doi: 10.1046/j.1365-2168.1999.00994.x. [DOI] [PubMed] [Google Scholar]
- 47.Tzavella K, Riepl RL, Klauser AG, Voderholzer WA, Schindlbeck NE, Muller-Lissner SA. Decreased substance P levels in rectal biopsies from patients with slow transit constipation. Eur J Gastroenterol Hepatol. 1996;8:1207–1211. doi: 10.1097/00042737-199612000-00014. [DOI] [PubMed] [Google Scholar]
- 48.Mitolo-Chieppa D, Mansi G, Rinaldi R, Montagnani M, Potenza MA, Genualdo M, Serio M, Mitolo CI, Rinaldi M, Altomare DF, et al. Cholinergic stimulation and nonadrenergic, noncholinergic relaxation of human colonic circular muscle in idiopathic chronic constipation. Dig Dis Sci. 1998;43:2719–2726. doi: 10.1023/a:1026615730533. [DOI] [PubMed] [Google Scholar]
- 49.Zhao RH, Baig MK, Thaler KJ, Mack J, Abramson S, Woodhouse S, Tamir H, Wexner SD. Reduced expression of serotonin receptor(s) in the left colon of patients with colonic inertia. Dis Colon Rectum. 2003;46:81–86. doi: 10.1007/s10350-004-6500-x. [DOI] [PubMed] [Google Scholar]
- 50.Mollen RM, Hopman WP, Kuijpers HH, Jansen JB. Plasma cholecystokinin, plasma peptide YY and gallbladder motility in patients with slow transit constipation: effect of intestinal stimulation. Digestion. 2000;62:185–193. doi: 10.1159/000007812. [DOI] [PubMed] [Google Scholar]
- 51.van der Sijp JR, Kamm MA, Nightingale JM, Akkermans LM, Ghatei MA, Bloom SR, Jansen JB, Lennard-Jones JE. Circulating gastrointestinal hormone abnormalities in patients with severe idiopathic constipation. Am J Gastroenterol. 1998;93:1351–1356. doi: 10.1111/j.1572-0241.1998.00345.x. [DOI] [PubMed] [Google Scholar]
- 52.Battle WM, Snape WJ Jr, Alavi A, Cohen S, Braunstein S. Colonic dysfunction in diabetes mellitus. Gastroenterology. 1980;79:1217–1221. [PubMed] [Google Scholar]
- 53.Meunier P, Rochas A, Lambert R. Motor activity of the sigmoid colon in chronic constipation: comparative study with normal subjects. Gut. 1979;20:1095–1101. doi: 10.1136/gut.20.12.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schang JC, Devroede G. Fasting and postprandial myoelectric spiking activity in the human sigmoid colon. Gastroenterology. 1983;85:1048–1053. [PubMed] [Google Scholar]
- 55.Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360–1368. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 56.Pezim ME, Pemberton JH, Levin KE, Litchy WJ, Phillips SF. Parameters of anorectal and colonic motility in health and in severe constipation. Dis Colon Rectum. 1993;36:484–491. doi: 10.1007/BF02050015. [DOI] [PubMed] [Google Scholar]
- 57.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. Am J Gastroenterol. 1998;93:1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 58.Dinning PG, Bampton PA, Andre J, Kennedy ML, Lubowski DZ, King DW, Cook IJ. Abnormal predefecatory colonic motor patterns define constipation in obstructed defecation. Gastroenterology. 2004;127:49–56. doi: 10.1053/j.gastro.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 59.Gosselink MJ, Schouten WR. Rectal sensory perception in females with obstructed defecation. Dis Colon Rectum. 2001;44:1337–1344. doi: 10.1007/BF02234795. [DOI] [PubMed] [Google Scholar]
- 60.Gosselink MJ, Hop WC, Schouten WR. Rectal compliance in females with obstructed defecation. Dis Colon Rectum. 2001;44:971–977. doi: 10.1007/BF02235485. [DOI] [PubMed] [Google Scholar]
- 61.Gosselink MJ, Schouten WR. The gastrorectal reflex in women with obstructed defecation. Int J Colorectal Dis. 2001;16:112–118. doi: 10.1007/s003840000270. [DOI] [PubMed] [Google Scholar]
- 62.Liu S, Zou K, Song J. A study of anorectal manometry in patients with chronic idiopathic constipation. J Tongji Med Univ. 2000;20:351–352. doi: 10.1007/BF02888204. [DOI] [PubMed] [Google Scholar]
- 63.Shafik A, Shafik AA, El-Sibai O, Ahmed I. Study of the role of the second defecation reflex: anorectal excitatory reflex in the pathogenesis of constipation. J Am Coll Surg. 2003;196:729–734. doi: 10.1016/S1072-7515(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 64.Rao SS, Tuteja AK, Vellema T, Kempf J, Stessman M. Dyssynergic defecation: demographics, symptoms, stool patterns, and quality of life. J Clin Gastroenterol. 2004;38:680–685. doi: 10.1097/01.mcg.0000135929.78074.8c. [DOI] [PubMed] [Google Scholar]
- 65.De Lillo AR, Rose S. Functional bowel disorders in the geriatric patient: constipation, fecal impaction, and fecal incontinence. Am J Gastroenterol. 2000;95:901–905. doi: 10.1111/j.1572-0241.2000.01926.x. [DOI] [PubMed] [Google Scholar]
- 66.Donald IP, Smith RG, Cruikshank JG, Elton RA, Stoddart ME. A study of constipation in the elderly living at home. Gerontology. 1985;31:112–118. doi: 10.1159/000212689. [DOI] [PubMed] [Google Scholar]
- 67.Whitehead WE, Drinkwater D, Cheskin LJ, Heller BR, Schuster MM. Constipation in the elderly living at home. Definition, prevalence, and relationship to lifestyle and health status. J Am Geriatr Soc. 1989;37:423–429. doi: 10.1111/j.1532-5415.1989.tb02638.x. [DOI] [PubMed] [Google Scholar]
- 68.Harari D, Gurwitz JH, Avorn J, Bohn R, Minaker KL. How do older persons define constipation? Implications for therapeutic management. J Gen Intern Med. 1997;12:63–66. doi: 10.1046/j.1525-1497.1997.12110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Talley NJ, O'Keefe EA, Zinsmeister AR, Melton LJ 3rd. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895–901. doi: 10.1016/0016-5085(92)90175-x. [DOI] [PubMed] [Google Scholar]
- 70.Camilleri M, Lee JS, Viramontes B, Bharucha AE, Tangalos EG. Insights into the pathophysiology and mechanisms of constipation, irritable bowel syndrome, and diverticulosis in older people. J Am Geriatr Soc. 2000;48:1142–1150. doi: 10.1111/j.1532-5415.2000.tb04793.x. [DOI] [PubMed] [Google Scholar]
- 71.Bannister JJ, Abouzekry L, Read NW. Effect of aging on anorectal function. Gut. 1987;28:353–357. doi: 10.1136/gut.28.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Engel AF, Kamm MA. The acute effect of straining on pelvic floor neurological function. Int J Colorectal Dis. 1994;9:8–12. doi: 10.1007/BF00304293. [DOI] [PubMed] [Google Scholar]
- 73.O’Keefe EA, Talley NJ, Zinsmeister AR, Jacobsen SJ. Bowel disorders impair functional status and quality of life in the elderly: a population-based study. J Gerontol A Biol Sci Med Sci. 1995;50:M184–M189. doi: 10.1093/gerona/50a.4.m184. [DOI] [PubMed] [Google Scholar]