Summary
The retinal pigment epithelium (RPE) consists of a monolayer of cuboidal, pigmented cells that is located between the retina and the choroid. The RPE is vital for growth and function of the vertebrate eye and improper development results in congenital defects, such as microphthalmia or anophthalmia, or a change of cell fate into neural retina called transdifferentiation. The transcription factors microphthalmia-associated transcription factor (Mitf) and orthodenticle homolog 2 (Otx2) are crucial for RPE development and function; however, very little is known about their regulation. Here, by using a Wnt-responsive reporter, we show that the Wnt/β-catenin pathway is activated in the differentiating mouse RPE. Cre-mediated, RPE-specific disruption of β-catenin after the onset of RPE specification causes severe defects, resulting in microphthalmia with coloboma, disturbed lamination, and mislocalization of adherens junction proteins. Upon β-catenin deletion, the RPE transforms into a multilayered tissue in which the expression of Mitf and Otx2 is downregulated, while retina-specific gene expression is induced, which results in the transdifferentiation of RPE into retina. Chromatin immunoprecipitation (ChIP) and luciferase assays indicate that β-catenin binds near to and activates potential TCF/LEF sites in the Mitf and Otx2 enhancers. We conclude that Wnt/β-catenin signaling is required for differentiation of the RPE by directly regulating the expression of Mitf and Otx2. Our study is the first to show that an extracellular signaling pathway directly regulates the expression of RPE-specific genes such as Mitf and Otx2, and elucidates a new role for the Wnt/β-catenin pathway in organ formation and development.
Keywords: RPE, β-catenin, Eye, Mouse, Development
INTRODUCTION
The RPE originates from the optic neuroepithelium of the ventral forebrain, which undergoes morphogenetic movements leading to formation of the optic cup. The resulting inner layer of the optic cup develops into the neural retina and the outer layer differentiates into RPE. Both retina and RPE are specified early, prior to optic cup formation. Subsequent to RPE specification, a period of differentiation and maturation follows, resulting in dramatic morphological, structural and functional changes (Rizzolo, 2007; Strauss, 2005). Interestingly, the RPE fate is reversible for several days following the initial activation of differentiation, as evidenced by a propensity to downregulate RPE-specific genes, to hyperproliferate and to differentiate into retina, a process considered to be transdifferentiation (Stroeva, 1960; Zhao et al., 1995). Thus, it is crucial that mechanisms exist to maintain RPE differentiation in the optic cup.
RPE specification and differentiation are regulated by two key regulatory transcription factors, Mitf and Otx2. Disruption of either gene, similar to genetic ablation of the RPE, results in microphthalmia and coloboma during murine eye development (Martinez-Morales et al., 2001; Raymond and Jackson, 1995; Scholtz and Chan, 1987). Mitf isoforms and Otx2 transactivate essential genes for terminal pigment differentiation in the RPE and neural crest (e.g. tyrosinase-related protein 1; Tyrp1) and for RPE-specific functions (Bharti et al., 2006; Martinez-Morales et al., 2004). Initiation and maintenance of Mitf and Otx2 expression is controlled by interaction with surrounding extraocular tissues, including the extraocular mesenchyme (Fuhrmann et al., 2000; Gage et al., 1999; West-Mays et al., 1999). A few candidate regulators have been identified (Fuhrmann et al., 2000; Muller et al., 2007; Perron et al., 2003; Zhang and Yang, 2001); however, the exact mechanisms controlling the expression of Mitf and Otx2 are not known. The Wnt/β-catenin pathway (http://www.stanford.edu/~rnusse/wntwindow.html) is an excellent candidate because it is active in the developing RPE; activation results in cytoplasmic stabilization of β-catenin, which then translocates into the nucleus and associates with TCF/LEF transcription factors. Interestingly, Wnt/β-catenin signaling promotes differentiation of neural crest-derived pigmented cells by direct transactivation of the Mitf-M promoter (Schmidt and Patel, 2005). Although melanocytes and RPE cells originate from different tissues, some aspects of the mechanisms regulating pigment cell differentiation in different lineages could be similar.
MATERIALS AND METHODS
Mouse lines
Tyrp1-Cre mice were provided by P. Chambon and M. Mark (IGBMC, France) (Mori et al., 2002). B6.129-Ctnnb1tm2Kem/KnwJ (β-cateninFL) and Gt(ROSA)26Sor (ROSA26R) mice are available at Jackson Laboratories (Brault et al., 2001; Soriano, 1999). BATgal and β-cateninfloxdel/+ mice were generated as previously described (Brault et al., 2001; Maretto et al., 2003). Tyrp1-Cre;β-cateninfloxdel/+ mice were mated with β-cateninFL/FL animals to generate Tyrp1-Cretg/0;β-cateninfloxdel/FL embryos (referred to generally as mutant embryos). Littermate β-cateninfloxdel/FL mice served as controls. For timed pregnancies, counting started on the day a vaginal plug was detected as E0.5. We observed subtle pigment irregularities in Tyrp1-Cretg/0;β-catenin+/+ RPE at P0, which were not accompanied with any of the defects observed in Tyrp1-Cretg/0;β-cateninfloxdel/FL RPE, such as transdifferentiation (not shown). For genotyping, the primers and cycling parameters have been described elsewhere (Brault et al., 2001; Soriano, 1999). For detecting lacZ, the following primers were used: 5′-GCAGACCGTTTTCGCTCGG-3′; 5′-CGACCGCATGGTCAGAAGC-3′.
Histology, in situ hybridization, immunohistochemistry and cell counts
For X-Gal labeling, tissue was fixed for 20 minutes and then postfixed in 4% paraformaldehyde. For histological analysis, eponate resin-embedded embryos were sectioned (500 nm) and stained with Toluidine Blue. Whole-mount in situ hybridization was performed as previously described (Fuhrmann et al., 2009). For immunohistochemistry on coronal sections, the following primary antibodies were used: Mitf (Exalpha), Vsx2 (N-terminal, Exalpha), Otx2 (Chemicon), Tuj1/acTubb3 (Covance), Neurod (1:300, Santa Cruz), Pou4f/Brn3b (Santa Cruz), β-catenin (Sigma), Neurofilament M (Chemicon), Conductin (Santa Cruz), β-galactosidase (Cappel, or gift from Tom Glaser, University of Michigan, Ann Arbor, MI, USA), ZO-1 (Zymed). Antigen retrieval with citrate buffer or 1% Triton X-100 was performed when necessary. Alexa 488/568/647-conjugated secondary antibodies (Molecular Probes), donkey-anti-goat TRITC (Jackson ImmunoResearch), Alexa Fluor 488/568 phalloidin (Molecular Probes), VECTASTAIN Elite ABC and VECTOR Peroxidase Substrate kits (Vector Laboratories) were used. Epifluorescence images were taken with an upright Olympus BX51 microscope and a Microfire CCD camera (Optronics) and, if necessary, background subtraction was applied. Confocal images were taken with an Olympus FV1000 and processed using ImageJ (NIH) and Photoshop CS2 (Adobe). β-galactosidase-labeled cells were counted from alternating sections in the dorsal and ventral E11 RPE based on their position from a line drawn from the center of the lens through the optic nerve. The total number was combined, averaged by the number of sections per eye and subjected to Student's t-test.
Chromatin immunoprecipitation (ChIP)
ChIP on RPE from C57BL/6 embryos was carried out as described (Clark et al., 2008). Pre-cleared supernatant was incubated overnight at 4°C with 12 μg of anti-β-catenin antibody (BD Laboratories), 12 μg of mouse IgG (Jackson Laboratories), or no antibody (input). Immunopurified DNA encompassing the six potential TCF/LEF binding sites in the Mitf-D enhancer (-1393, -1389, -389, -364, -321 and -132) was PCR-amplified with the following primers: 1 (-1224 to -1434), 5′-CCCTGTGTTTGTTCCGTTCT-3′ and 5′-AAGGAGCTGTGGCATAATCG-3′; 2 (-359 to -563), 5′-TGGTGAGCCAGGCTAAGAAT-3′ and 5′-CAAAGCTCAGCTAATTGACAGC-3′; and 3 (+18 to -202), 5′-TGAAGCCTTAGTGAGCTTGC-3′ and 5′GATCTCGAGAGGTCCCAACA-3′. A region of the Mitf-D open-reading frame was amplified using the primers 5′-AGCTCAGAGGCACCAGGTAA-3′ and 5′-TGGAGTTAAGAGTGAGCATAGCC-3′. The following primers were used on the Otx2 T0 enhancer to amplify the region flanking the putative TCF/LEF binding site: (-4 to -200), 5′-AGAAAACGTGAGCTCCCAAA-3′ and 5′-CGAGTTTCGGCCTCTGAGTA-3′. 5′-GTGTTGGTGTGACCACGTTC-3′ and 5′-CTCCCACCTTTTCCAAACAA-3′ were used to amplify a region in the Otx2 open-reading frame.
Luciferase assays
A 2248bp-fragment of the RPE-specific Mitf-D enhancer (Bharti et al., 2008) was cloned from mouse BAC DNA (RP23-9A13) and inserted into pGL3B. HEK293T cells were transfected with 1 ng pRL-TK and 50 ng reporter construct (MitfD>luc, MitfDMS>luc, Otx2T0>luc, Otx2T0MS>luc), 50 ng constitutively active β-catenin (Yost et al., 1996), 50 ng dominant-negative TCF3 (ΔTCF3) (Molenaar et al., 1996) or empty vector (pCMS-EGFP), using lipofectamine/lipofectaminePLUS according to the manufacturer's instructions (Invitrogen). Firefly and renilla luciferase activities were measured 24 hours post-transfection using a Modulus Microplate Multimode plate reader (Turner Biosystems) after injecting either 100 μl D-luciferin or coelenterazine (Biotum). Data are presented as mean±s.e.m. from four separate experiments.
RESULTS AND DISCUSSION
TCF/LEF activation in the embryonic RPE is dependent on β-catenin expression
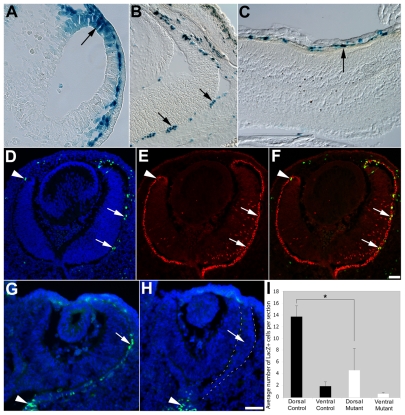
To investigate the temporal pattern of Wnt/β-catenin activity during mouse RPE development, we analyzed mice transgenic for multimerized TCF/LEF consensus sites driving a lacZ reporter gene (BATgal; Maretto et al., 2003). TCF/LEF activation is detectable in the dorsal optic vesicle at embryonic day (E) 9.5 (Fig. 1A), in the peripheral and dorsal RPE in the optic cup at E12.5 (Fig. 1D-F), and in scattered cells at embryonic and postnatal ages (Fig. 1B,C); however, activation ceases after postnatal day 30 (P30; not shown). RPE-specific reporter activation was confirmed up to P16 by colocalization of β-galactosidase and Otx2 expression (Fig. 1D-F; see also Fig. S1 in the supplementary material; data not shown). Although this is consistent with chick and zebrafish transgenic Wnt pathway reporters (Cho and Cepko, 2006; Dorsky et al., 2002), particular mouse TCF/LEF reporters show differences in activity in the developing RPE (this study) (Fuhrmann et al., 2009; Liu et al., 2006). These differences might be due to positional effects of transgene insertion sites, caused by β-catenin-independent activation, or due to context-specific usage of the distinct minimal promoters of the various Wnt reporters (Fuhrmann et al., 2009; Hsu et al., 1998; Labbe et al., 2000). To confirm that β-catenin is required for BATgal activity, we disrupted β-catenin expression in the embryonic RPE by using Tyrp1-Cre, which is active in the dorsal optic vesicle by E9.5, earlier than previously reported (see Fig. S1A in the supplementary material) (Mori et al., 2002). In mutant mice (Tyrp1-Cretg/0;β-cateninfloxdel/FL), loss of β-catenin expression is first observed in the dorsal RPE at E10.75, whereas the ventral RPE has not yet undergone Cre-mediated recombination (see Fig. S2 in the supplementary material). Importantly, in Tyrp1-Cretg/0;β-cateninfloxdel/FL RPE, a significant reduction of reporter expression is observed in the dorsal optic cup (nearly 3-fold; P=0.037; Fig. 1G-I), suggesting that β-catenin operates through activating TCF/LEF factors. To further confirm Wnt/β-catenin activity in the embryonic RPE, we observed that expression of the universal target gene Axin2 starts in the periphery and extends into central regions (see Fig. S1G,I in the supplementary material) (Burns et al., 2008; Fuhrmann et al., 2009; Jho et al., 2002).
Fig. 1.
TCF/LEF activity in the developing RPE in mouse is dependent on β-catenin. (A-C) X-gal staining marks TCF/LEF-responsive cells (arrows) in BATgal RPE at E9.5 (26 somites; A), E15.5 (B) and P16 (C). (D-F) Colocalization of β-galactosidase (D; green; DAPI in blue) and Otx2 (E, red) in the dorsal (arrows) and ventral (arrowheads) RPE at E12.5. (F) Merge of D and E. (G,H) β-galactosidase-labeled cells in E11 BATgal control (G, green, arrow) and Tyrp1-Cretg/0;β-cateninfloxdel/FL RPE (H, between dashed lines, arrow). Arrowheads indicate the optic nerve. (I) Quantification of β-galactosidase-labeled cells in dorsal and ventral RPE of control and mutant embryos. Note the statistically significant reduction in TCF/LEF-responsive cells in dorsal RPE (P=0.039), but not in ventral, non-transdifferentiated RPE (P=0.089), of mutant embryos. Scale bars: 50 μm.
Loss of β-catenin results in severe ocular defects and mislocalization of adherens junction proteins in mutant RPE
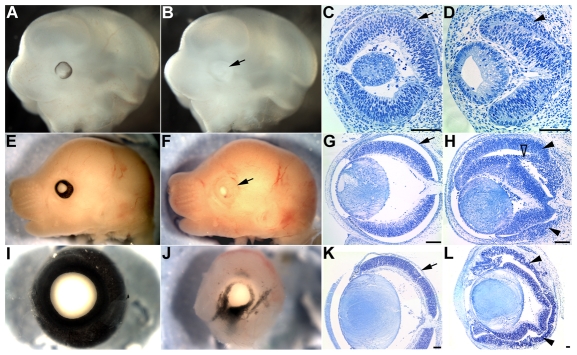
Conditional disruption of β-catenin in the RPE results in severe ocular defects (Fig. 2). Pigmentation is absent or dramatically reduced in Tyrp1-Cretg/0;β-cateninfloxdel/FL RPE, indicating that β-catenin is required for pigment synthesis (Fig. 2B,F,J). The mutant RPE becomes hypercellular, starting dorsally and later extending ventrally (Fig. 2D,H,L). This is likely to be due to the delayed expression of Cre in the ventral optic vesicle, although we cannot exclude that cell non-autonomous mechanisms contribute to the propagation of RPE defects in the ventral optic cup. In Tyrp1-Cretg/0;β-cateninfloxdel/FL eyes, the optic fissure fails to close, resulting in a coloboma (see Fig. S3B in the supplementary material). Postnatal mutant eyes are microphthalmic (n=18; not shown), show abnormal folding in the RPE (Fig. 2L) and exhibit defective optic nerve formation, and ganglion cells fail to exit the retina in a normal manner (compare Fig. S3C and S3D in the supplementary material). We also observed retinal defects that include disorganization, and abnormal folding and bridge formation between the retinal surface and the mutated RPE (Fig. 2H,L). These defects are similar to those described in mice with a mutation in Mitf, with ablation of the RPE or with retina-specific inactivation of β-catenin (Fu et al., 2006; Raymond and Jackson, 1995; Scholtz and Chan, 1987), and are consistent with a requirement of the RPE for proper morphogenesis and lamination of the retina.
Fig. 2.
Severe eye defects induced by RPE-specific deletion of β-catenin. Lateral views of control (A,E,I) and Tyrp1-Cretg/0;β-cateninfloxdel/FL mutant (B,F,J) eyes at E11.5 (A,B), E15.5 (E,F) and P0 (I,J). (C,D,G,H,K,L) Toluidine Blue staining of coronal sections of control (C,G,K) and mutant (D,H,L) eyes at E11.5 (C,D), E15.5 (G,H) and P0 (K,L). Note the absence of pigment (B,F,J; arrows) and hypercellularity (arrowheads in D,H,L) of the RPE. The disorganized retina forms bridges to the mutant RPE (open arrowhead in H). Arrows in C,G,K indicate control RPE. (L) At P0, the RPE and retinal layers in mutant eyes are folded extensively. Scale bars: 50 μm.
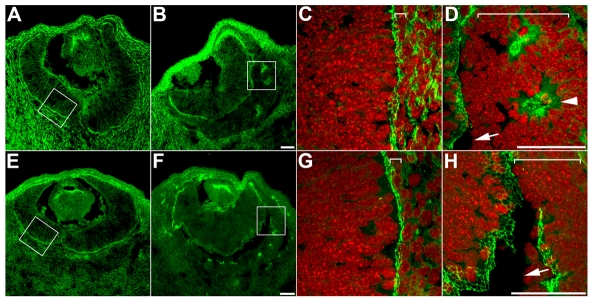
Analysis of Tyrp1-Cretg/0;β-cateninfloxdel/FL eyes harboring the ROSA26R reporter show that β-galactosidase-positive cells (inactivated for β-catenin) become progressively excluded and are replaced by cells expressing β-catenin, which appear to originate from the endogenous retina (see Fig. S4 in the supplementary material). As β-catenin localizes in adherens junctions, we examined whether the expression patterns of F-actin and ZO-1 (Tjp1 - Mouse Genome Informatics), which are initially associated with adherens junctions in the RPE (Rizzolo, 2007), are altered. In mutant RPE, subtle changes start to appear at E11 (not shown), and significant mislocalization was obvious at E12.5 (Fig. 3). The highest concentration of F-actin and ZO-1 is normally observed at the apical surface of the retina and the RPE in a uniform line, which is composed of a honeycomb-like network (Fig. 3A,C,E,G). In Tyrp1-Cretg/0;β-cateninfloxdel/FL RPE, however, expression of F-actin and ZO-1 is discontinuous along the apical surface and mislocalized to rosette structures (Fig. 3B,D,F,H) indicating that β-catenin is crucial for the proper localization of adherens junction proteins along the apical border of the RPE.
Fig. 3.
Cell adhesion is perturbed in Tyrp1-Cretg/0;β-cateninfloxdel/FL RPE. At E12.5, F-actin (A-D) and ZO-1 (E-H) are enriched in apical adherens junctions in the retina and RPE in control (A,C,E,G) and in Tyrp1-Cretg/0;β-cateninfloxdel/FL (B,D,F,H) retinas. In mutant RPE, F-actin and ZO-1 are mislocalized to rosette structures (D, arrowhead) and are absent at the apical border (D,H, arrows). Boxes in A,B,E,F mark areas magnified in C,D,G,H, counter-labeled with DAPI (red). Brackets delineate the RPE. Scale bars: 50 μm.
Upon β-catenin deletion, the RPE transdifferentiates into neural retina
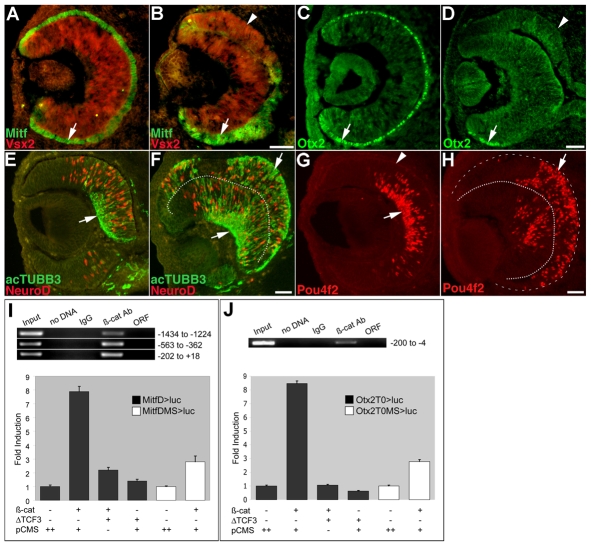
Tyrp1-Cretg/0;β-cateninfloxdel/FL eyes show phenotypical changes that strikingly resemble those in mice homozygous for a mutation in Mitf (Mi/Mi) or with compound mutations of the Otx1 and Otx2 genes; the RPE transdifferentiates into retina, accompanied with a loss of RPE-specific morphology and gene expression, hyperproliferation, and an upregulation of retina-specific genes expressed in an inverse orientation (Bumsted and Barnstable, 2000; Nguyen and Arnheiter, 2000; Martinez-Morales et al., 2001). To determine whether transdifferentiation occurs in Tyrp1-Cretg/0;β-cateninfloxdel/FL RPE, we assessed changes in RPE and retina-specific gene expression. At E11, Mitf is normally expressed throughout the RPE, and expression of the earliest marker of retinal progenitor cells, Vsx2 (formerly known as Chx10) (Burmeister et al., 1996; Clark et al., 2008), is restricted to the retina (Fig. 4A). In mutant RPE, expression of Mitf and Otx2 is downregulated and replaced by that of Vsx2, starting at 29 somites (Fig. 4B,D; not shown). The dorsal portion comprises almost exclusively cells that have undergone recombination and do not express β-catenin or Mitf (see Fig. S2 in the supplementary material), suggesting that depletion of β-catenin results in a loss of the RPE cell fate. To analyze the extent of transdifferentiation, double labeling with retinal cell type-specific markers at E12.5 was performed. Acetylated class III β-tubulin (acTubb3) is normally restricted to the presumptive ganglion cell layer; however, in Tyrp1-Cretg/0;β-cateninfloxdel/FL RPE, acTubb3 expression is upregulated in an inverse orientation (Fig. 4E,F). Ectopic expression of the amacrine and photoreceptor precursor marker Neurod is as widespread in the RPE as it is in the retina, suggesting that the time-course of neurogenesis occurs similarly in both tissues (Fig. 4E,F). Furthermore, the differentiation of ganglion cells is induced, as shown by ectopic expression of Pou4f2/Brn3b (Fig. 4H). Together, our results demonstrate that RPE-specific disruption of β-catenin results in transdifferentiation into retina. Because cell adhesion defects are prominent after downregulation of Mitf, they are less likely to underlie the change in cell fate we observed in the Tyrp1-Cretg/0;β-cateninfloxdel/FL RPE.
Fig. 4.
RPE-specific loss of β-catenin induces transdifferentiation into retina. (A,B) Mitf (green) is present throughout the RPE in E11 controls (A, arrow) and in the ventral Tyrp1-Cretg/0;β-cateninfloxdel/FL RPE (B, arrow). The dorsal mutant RPE is devoid of Mitf (B, arrowhead). Vsx2 is normally detected in the retina (A, red) and ectopically expressed in mutant RPE (B, arrowhead). (C,D) At E11.5, Otx2 is expressed throughout the RPE in controls (C, arrow) and in a few cells in the ventral RPE of mutant embryos (D, arrow), but is undetectable in the dorsal portion (D, arrowhead) of the RPE in mutant embryos. (E-H) Retinal neurogenesis occurs in E12.5 Tyrp1-Cretg/0;β-cateninfloxdel/FL RPE. (E) Normally, acTubb3 (green, arrow) is expressed in ganglion cell precursors, and Neurod (red) in amacrine and photoreceptor precursors. (F) Both markers are ectopically expressed in an inverse orientation in the transdifferentiated RPE (arrow). (G,H) Pou4f2 labels ganglion cells at the basal surface (arrows). Arrowhead in G marks the RPE. Dotted lines in F and H indicate the retina and RPE boundary; dashed line indicates the RPE and choroid boundary. (I, top) ChIP was performed on primary E12.5 mouse RPE using a β-catenin antibody (β-cat Ab). β-catenin associates with regions of the Mitf-D enhancer containing putative TCF/LEF sites. Locations of the amplicons are indicated to the right. (Bottom) An Mitf-D pGL3B reporter construct (MitfD>luc) is activated by constitutively active β-catenin (β-cat) in HEK293T cells. Cotransfection with ΔTCF3 or mutation of TCF/LEF sites (MitfDMS>luc) diminishes reporter activation. (J, top) β-catenin associates with the Otx2 T0 enhancer in vivo as shown by ChIP. (Bottom) β-catenin activates an Otx2 pGL3B reporter construct (Otx2T0>luc), whereas ΔTCF3 or mutation of TCF/LEF sites (Otx2T0MS>luc) reduces reporter activation. Input, amplification from non-immunoprecipitated chromatin. Negative controls used were mouse IgG, H2O (no DNA) or open-reading frame primers (ORF). The ChIP assays were independently repeated four times (twice using whole E9.5 embryos and twice using E12.5 RPE). The transactivation assays were repeated a minimum of four times and representative examples are shown. pCMS is an empty vector. Scale bars: 50 μm.
β-catenin binds near and activates putative TCF/LEF sites in the Mitf and Otx2 enhancers
Expression of both Otx2 and Mitf proteins is rapidly downregulated in Tyrp1-Cretg/0;β-cateninfloxdel/FL RPE; therefore, we asked whether β-catenin associates with their enhancers in vivo. We identified six putative TCF/LEF binding sites (Hallikas et al., 2006) in a 2248bp-fragment of the RPE-specific Mitf-D enhancer, positioned at -1393, -1389, -389, -364, -321 and -132 relative to the transcriptional start site. β-catenin binds at or near these sites, as determined by ChIP using native RPE lysate from E12.5 embryos (Fig. 4I). To examine whether β-catenin can transcriptionally activate Mitf, the Mitf-D enhancer was cloned into the pGL3B luciferase reporter. β-catenin produced an 8-fold increase in luciferase activity, and this activation was reduced by co-transfection with ΔTCF3 or by mutation of all potential TCF/LEF binding sites (Fig. 4I). Furthermore, one putative TCF/LEF binding site was identified in the T0 enhancer of Otx2 (Martinez-Morales et al., 2003), within in a region that is amplified by PCR after immunoprecipitation with a β-catenin antibody (Fig. 4J). β-catenin also produced an 8.5-fold increase in Otx2-pGL3B luciferase reporter activity, which was repressed by co-transfection with ΔTCF3 or by mutating the putative TCF/LEF binding site (Fig. 4J). These results support our model that β-catenin, in association with TCF/LEF factors, mediates the transcriptional activation of Mitf-D and Otx2 in the RPE.
A few other factors have been shown to promote RPE development; for example, TGFβ/activin and sonic hedgehog signaling (Fuhrmann et al., 2000; Huh et al., 1999; Sakami et al., 2008; Zhang and Yang, 2001). RPE-promoting signals could exert different functions depending on location and timing; an activin-like factor and sonic hedgehog might specify the RPE fate in the dorsal and ventral optic vesicle, respectively, while Wnt/β-catenin ensures proper RPE differentiation in the optic cup. The source of the actual Wnt ligand could be the RPE itself, the adjacent retina or the extraocular mesenchyme (e.g. Wnt2b, Wnt3, Wnt5a, Wnt7b) (Liu et al., 2003). However, targeted inactivation of the RPE-specific factors Tcf1 (Tcf7 - Mouse Genome Informatics) and/or Lef1 leads either to early embryonic lethality or does not appear to cause obvious eye defects, which might be due to functional redundancy (Galceran et al., 1999; Galceran et al., 2000; van Genderen et al., 1994; Verbeek et al., 1995). Interestingly, ectopic activation of Wnt/β-catenin in the presumptive retina is not sufficient to promote a change into RPE-like tissue, suggesting that additional factors are required (Cho and Cepko, 2006; Fu et al., 2006).
In conclusion, our report is the first to show a direct role for an extracellular signaling pathway in controlling development of the mammalian RPE. Although we cannot rule out that cell adhesion defects can independently interfere with RPE differentiation, our results strongly suggest that β-catenin, via TCF/LEF activation, is essential for maintaining cell fate in the developing RPE by the direct regulation of Mitf and Otx2 expression. Thus, this mechanism of Mitf regulation appears to be evolutionary conserved between the RPE and neural crest-derived melanocytes. It remains to be determined what the source(s) of the actual ligand(s) is and how Wnt/β-catenin signaling integrates with other putative regulatory pathways to control RPE development.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/15/2505/DC1
Supplementary Material
We are grateful to Pierre Chambon, Manuel Mark and Peter Klein for providing Tyrp1-Cre mice and BATgal mice, respectively. We thank Amber Mathiesen, Andrew Loudon and the Levine and Marc labs for technical help; ChangJiang Zou for providing the Mitf-D enhancer fragment; and Rich Dorsky, Wolfgang Baehr and Ed Levine for helpful comments. This project was supported by the NIH (EY14954, EY014800) and by an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology, University of Utah. Deposited in PMC for release after 12 months.
References
- Bharti, K., Nguyen, M. T., Skuntz, S., Bertuzzi, S. and Arnheiter, H. (2006). The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye. Pigment Cell Re.s 19, 380-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti, K., Liu, W., Csermely, T., Bertuzzi, S. and Arnheiter, H. (2008). Alternative promoter use in eye development: the complex role and regulation of the transcription factor MITF. Development 135, 1169-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault, V., Moore, R., Kutsch, S., Ishibashi, M., Rowitch, D. H., McMahon, A. P., Sommer, L., Boussadia, O. and Kemler, R. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253-1264. [DOI] [PubMed] [Google Scholar]
- Bumsted, K. M. and Barnstable, C. J. (2000). Dorsal retinal pigment epithelium differentiates as neural retina in the microphthalmia (mi/mi) mouse. Invest. Ophthalmol. Vis. Sci. 41, 903-908. [PubMed] [Google Scholar]
- Burmeister, M., Novak, J., Liang, M. Y., Basu, S., Ploder, L., Hawes, N. L., Vidgen, D., Hoover, F., Goldman, D., Kalnins, V. I. et al. (1996). Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat. Genet. 12, 376-384. [DOI] [PubMed] [Google Scholar]
- Burns, C. J., Zhang, J., Brown, E. C., Van Bibber, A. M., Van Es, J., Clevers, H., Ishikawa, T. O., Taketo, M. M., Vetter, M. L. and Fuhrmann, S. (2008). Investigation of Frizzled-5 during embryonic neural development in mouse. Dev. Dyn. 237, 1614-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, S. H. and Cepko, C. L. (2006). Wnt2b/beta-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development 133, 3167-3177. [DOI] [PubMed] [Google Scholar]
- Clark, A. M., Yun, S., Veien, E. S., Wu, Y. Y., Chow, R. L., Dorsky, R. I. and Levine, E. M. (2008). Negative regulation of Vsx1 by its paralog Chx10/Vsx2 is conserved in the vertebrate retina. Brain Res. 1192, 99-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsky, R. I., Sheldahl, L. C. and Moon, R. T. (2002). A transgenic Lef1/beta-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish development. Dev. Biol. 241, 229-237. [DOI] [PubMed] [Google Scholar]
- Fu, X., Sun, H., Klein, W. H. and Mu, X. (2006). beta-catenin is essential for lamination but not neurogenesis in mouse retinal development. Dev. Biol. 299, 424-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann, S., Levine, E. M. and Reh, T. A. (2000). Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development 127, 4599-4609. [DOI] [PubMed] [Google Scholar]
- Fuhrmann, S., Riesenberg, A. N., Mathiesen, A. M., Brown, E. C., Vetter, M. L. and Brown, N. L. (2009). Characterization of a transient TCF/LEF-responsive progenitor population in the embryonic mouse retina. Invest. Ophthalmol. Vis. Sci. 50, 432-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage, P. J., Suh, H. and Camper, S. A. (1999). Dosage requirement of Pitx2 for development of multiple organs. Development 126, 4643-4651. [DOI] [PubMed] [Google Scholar]
- Galceran, J., Farinas, I., Depew, M. J., Clevers, H. and Grosschedl, R. (1999). Wnt3a-/-like phenotype and limb deficiency in Lef1-/-Tcf1-/- mice. Genes Dev. 13, 709-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galceran, J., Miyashita-Lin, E. M., Devaney, E., Rubenstein, J. L. and Grosschedl, R. (2000). Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development 127, 469-482. [DOI] [PubMed] [Google Scholar]
- Hallikas, O., Palin, K., Sinjushina, N., Rautiainen, R., Partanen, J., Ukkonen, E. and Taipale, J. (2006). Genome-wide prediction of mammalian enhancers based on analysis of transcription-factor binding affinity. Cell 124, 47-59. [DOI] [PubMed] [Google Scholar]
- Hsu, S. C., Galceran, J. and Grosschedl, R. (1998). Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with beta-catenin. Mol. Cell. Biol. 18, 4807-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh, S., Hatini, V., Marcus, R. C., Li, S. C. and Lai, E. (1999). Dorsal-ventral patterning defects in the eye of BF-1-deficient mice associated with a restricted loss of shh expression. Dev. Biol. 211, 53-63. [DOI] [PubMed] [Google Scholar]
- Jho, E. H., Zhang, T., Domon, C., Joo, C. K., Freund, J. N. and Costantini, F. (2002). Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 22, 1172-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe, E., Letamendia, A. and Attisano, L. (2000). Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc. Natl. Acad. Sci. USA 97, 8358-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H., Mohamed, O., Dufort, D. and Wallace, V. A. (2003). Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev. Dyn. 227, 323-334. [DOI] [PubMed] [Google Scholar]
- Liu, H., Thurig, S., Mohamed, O., Dufort, D. and Wallace, V. A. (2006). Mapping canonical Wnt signaling in the developing and adult retina. Invest. Ophthalmol. Vis. Sci. 47, 5088-5097. [DOI] [PubMed] [Google Scholar]
- Maretto, S., Cordenonsi, M., Dupont, S., Braghetta, P., Broccoli, V., Hassan, A. B., Volpin, D., Bressan, G. M. and Piccolo, S. (2003). Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA 100, 3299-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Morales, J. R., Signore, M., Acampora, D., Simeone, A. and Bovolenta, P. (2001). Otx genes are required for tissue specification in the developing eye. Development 128, 2019-2030. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales, J. R., Dolez, V., Rodrigo, I., Zaccarini, R., Leconte, L., Bovolenta, P. and Saule, S. (2003). OTX2 activates the molecular network underlying retina pigment epithelium differentiation. J. Biol. Chem. 278, 21721-21731. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales, J. R., Rodrigo, I. and Bovolenta, P. (2004). Eye development: a view from the retina pigmented epithelium. BioEssays 26, 766-777. [DOI] [PubMed] [Google Scholar]
- Molenaar, M., van de Wetering, M., Oosterwegel, M., Peterson-Maduro, J., Godsave, S., Korinek, V., Roose, J., Destree, O. and Clevers, H. (1996). XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86, 391-399. [DOI] [PubMed] [Google Scholar]
- Mori, M., Metzger, D., Garnier, J. M., Chambon, P. and Mark, M. (2002). Site-specific somatic mutagenesis in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 43, 1384-1388. [PubMed] [Google Scholar]
- Muller, F., Rohrer, H. and Vogel-Hopker, A. (2007). Bone morphogenetic proteins specify the retinal pigment epithelium in the chick embryo. Development 134, 3483-3493. [DOI] [PubMed] [Google Scholar]
- Nguyen, M. and Arnheiter, H. (2000). Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development 127, 3581-3591. [DOI] [PubMed] [Google Scholar]
- Perron, M., Boy, S., Amato, M. A., Viczian, A., Koebernick, K., Pieler, T. and Harris, W. A. (2003). A novel function for Hedgehog signalling in retinal pigment epithelium differentiation. Development 130, 1565-1577. [DOI] [PubMed] [Google Scholar]
- Raymond, S. M. and Jackson, I. J. (1995). The retinal pigmented epithelium is required for development and maintenance of the mouse neural retina. Curr. Biol. 5, 1286-1295. [DOI] [PubMed] [Google Scholar]
- Rizzolo, L. J. (2007). Development and role of tight junctions in the retinal pigment epithelium. Int. Rev. Cytol. 258, 195-234. [DOI] [PubMed] [Google Scholar]
- Sakami, S., Etter, P. and Reh, T. A. (2008). Activin signaling limits the competence for retinal regeneration from the pigmented epithelium. Mech. Dev. 125, 106-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, C. and Patel, K. (2005). Wnts and the neural crest. Anat. Embryol. 209, 349-355. [DOI] [PubMed] [Google Scholar]
- Scholtz, C. L. and Chan, K. K. (1987). Complicated colobomatous microphthalmia in the microphthalmic (mi/mi) mouse. Development 99, 501-508. [DOI] [PubMed] [Google Scholar]
- Soriano, P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71. [DOI] [PubMed] [Google Scholar]
- Strauss, O. (2005). The retinal pigment epithelium in visual function. Physiol. Rev. 85, 845-881. [DOI] [PubMed] [Google Scholar]
- Stroeva, O. G. (1960). Experimental analysis of the eye morphogenesis in mammals. J. Embryol. 8, 349-368. [Google Scholar]
- van Genderen, C., Okamura, R. M., Farinas, I., Quo, R. G., Parslow, T. G., Bruhn, L. and Grosschedl, R. (1994). Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 8, 2691-2703. [DOI] [PubMed] [Google Scholar]
- Verbeek, S., Izon, D., Hofhuis, F., Robanus-Maandag, E., te Riele, H., van de Wetering, M., Oosterwegel, M., Wilson, A., MacDonald, H. R. and Clevers, H. (1995). An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature 374, 70-74. [DOI] [PubMed] [Google Scholar]
- West-Mays, J. A., Zhang, J., Nottoli, T., Hagopian-Donaldson, S., Libby, D., Strissel, K. J. and Williams, T. (1999). AP-2alpha transcription factor is required for early morphogenesis of the lens vesicle. Dev. Biol. 206, 46-62. [DOI] [PubMed] [Google Scholar]
- Yost, C., Torres, M., Miller, J. R., Huang, E., Kimelman, D. and Moon, R. T. (1996). The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 10, 1443-1454. [DOI] [PubMed] [Google Scholar]
- Zhang, X.-M. and Yang, X.-J. (2001). Temporal and spatial effects of Sonic Hedgehog signaling in chick eye morphogenesis. Dev. Biol. 233, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, S., Thornquist, S. C. and Barnstable, C. J. (1995). In vitro transdifferentiation of embryonic rat retinal pigment epithelium to neural retina. Brain. Res. 677, 300-310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.