Summary
The developing ocular lens provides an excellent model system with which to study the intrinsic and extrinsic cues governing cell differentiation. Although the transcription factors Pax6 and Sox2 have been shown to be essential for lens induction, their later roles during lens fiber differentiation remain largely unknown. Using Cre/loxP mutagenesis, we somatically inactivated Pax6 and Sox2 in the developing mouse lens during differentiation of the secondary lens fibers and explored the regulatory interactions of these two intrinsic factors with the canonical Wnt pathway. Analysis of the Pax6-deficient lenses revealed a requirement for Pax6 in cell cycle exit and differentiation into lens fiber cells. In addition, Pax6 disruption led to apoptosis of lens epithelial cells. We show that Pax6 regulates the Wnt antagonist Sfrp2 in the lens, and that Sox2 expression is upregulated in the Pax6-deficient lenses. However, our study demonstrates that the failure of differentiation following loss of Pax6 is independent of β-catenin signaling or Sox2 activity. This study reveals that Pax6 is pivotal for initiation of the lens fiber differentiation program in the mammalian eye.
Keywords: Pax6, Sox2, Lens, Crystallin, Wnt, β-catenin, Mouse
INTRODUCTION
Lens development is a complex process in which a single epithelial layer undergoes several stages of competence, induction and differentiation, ultimately forming a highly specialized organ (Grainger et al., 1997; Lovicu and Robinson, 2004; Ogino and Yasuda, 2000). The vertebrate lens comprises only two types of cells: an anterior lens epithelium (LE) and the derived lens fiber cells (LFCs). This, along with its morphological isolation from surrounding tissues, makes the lens an ideal model for the study of tissue growth and differentiation (Bhat, 2001; Lovicu and Robinson, 2004).
The transcription factor (TF) Pax6 is essential for eye development in vertebrates and invertebrates (Ashery-Padan and Gruss, 2001; Gehring, 1996; Grindley et al., 1995; Hogan et al., 1988; Walther et al., 1991). Interestingly, normal development of the mammalian eye is dependent on normal Pax6 dosage, as heterozygotes suffer from pan-ocular disorders such as aniridia in humans and Small eye (Sey) in mice (Glaser et al., 1994; Glaser et al., 1990).
Pax6 is expressed throughout all stages of lens development, except in terminally differentiated LFCs. During early organogenesis, Pax6 is detected in both the lens-inducing optic vesicles, and in the lens-forming surface ectoderm (SE) (Walther et al., 1991). Expression of Pax6 in the SE is required to render it competent for induction into a lens (Fujiwara et al., 1994; Quinn et al., 1996). Inductive signals from the optic vesicle (OV) trigger a thickening of the SE known as the lens placode (LP), which is intrinsically dependent on Pax6 expression (Ashery-Padan et al., 2000). Between embryonic day (E) 10 and E11, the LP invaginates and detaches from the SE to form the lens vesicle. The anterior cells of the lens vesicle remain as the undifferentiated LE and retain high expression of Pax6. By contrast, the posterior cells elongate and differentiate into primary LFCs and lose Pax6 expression. In the fetal and postnatal stages of development, at the transitional zone between LE and LFCs, the equatorial epithelial cells undergo proliferation, cell cycle exit, migration and elongation, and finally mature into secondary fiber cells deposited around a nucleus of primary LFCs, all this in a distinct spatial order (Bassnett and Beebe, 1992; Beebe et al., 1982; Rafferty and Rafferty, 1981). Although Pax6 expression is maintained in the LE and in the equatorial transitional zone, its role in maintaining an epithelial phenotype or in LFC differentiation remains largely unknown.
LFCs express high levels of lens-specific crystallins (Bassnett and Beebe, 1992; Beebe et al., 1982; Beebe and Piatigorsky, 1976; Rafferty and Rafferty, 1981). Crystallins have distinct expression patterns, making them the definitive markers of lens differentiation. The crystallins of the βγ supergroup are highly expressed in developing LFCs and are used as markers for LFC differentiation, whereas α-crystallins are differentially expressed between LE and LFCs (Robinson and Overbeek, 1996). Pax6 activation and its binding to promoters of crystallin genes have been studied extensively (Cvekl et al., 2004; Duncan et al., 1996; Muta et al., 2002; Wawrousek et al., 1990; Yang et al., 2006). Based on its expression pattern and regulation of crystallins, it has been suggested that Pax6 plays a dual role, acting as both an activator and a repressor of crystallin expression (Duncan et al., 1998). Furthermore, in vitro and chromatin-binding assays indicate that Pax6 co-operates with the Sox2 TF on specific crystallin enhancers during early stages of lens formation (Kamachi et al., 2001; Kondoh et al., 2004). However, the roles of Pax6 and Sox2 in the control of crystallin gene expression during LFC differentiation have not been studied in vivo.
Along with TFs, many growth factors and signaling pathways have been reported to be involved in LFC differentiation (Lovicu and McAvoy, 2005). Most notably, FGF signaling is differentially activated along the anterior-posterior axis of the lens, with increased activity at the posterior side of the lens equator (de Iongh et al., 1997; Garcia et al., 2005; Robinson, 2006). By contrast, Wnt signaling is believed to be antagonistic to LFC differentiation. Wnt receptors, co-receptors and downstream proteins are expressed in the LE (Ang et al., 2004; Chen et al., 2004; Chen et al., 2006). When the Wnt co-receptor Lrp6 was deleted in mice, aberrant LFCs appeared in the anterior pole of the lens (Stump et al., 2003). Upon inactivation of the canonical Wnt effector β-catenin, LE markers, proliferation and differentiation were disrupted (Cain et al., 2008). These findings suggest a role for Wnt signaling in LE cell fate.
Owing to the severe ocular phenotype of Pax6 mutants, the later developmental roles of Pax6 could only be extrapolated from in vitro research. Herein, we introduce the first in vivo loss-of-function model of Pax6 and its presumed transcriptional partner Sox2. We show that the loss of Pax6 prevents LFC differentiation and results in cell death and in an increase in Sox2. However, conditional deletion of Sox2 reveals that it is dispensable for LFC differentiation. Furthermore, overexpression of β-catenin results in a differentiation failure that is similar to, but independent of, that observed following Pax6 loss. These findings place Pax6 upstream in the cascade of events leading to the differentiation of LE into lens fibers in mammals.
MATERIALS AND METHODS
Mouse lines
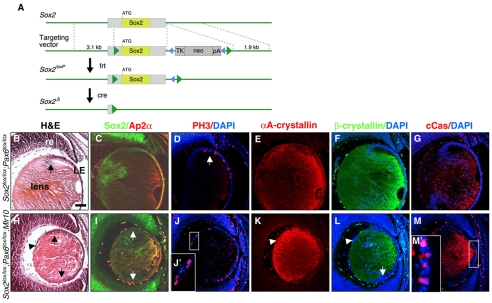
Mouse lines employed in this study were: Pax6lox (Ashery-Padan et al., 2000), Mlr10 (Zhao et al., 2004), Catnblox(ex3) (Harada et al., 1999) and BATlacZ (Nakaya et al., 2005) and are described in Fig. S1 in the supplementary material. The Sox2loxP line (see Fig. 7A) contains two loxP sites inserted around the single exon of the murine Sox2 gene using conventional gene-targeting methods (Joyner, 1995). In the gene-targeting vector, loxP-frt-pMC1neopA-frt, the neo gene is flanked by frt sites. Flp recombinase activity within the B6.SJL-Tg(ACTFLPe)9205Dym/J mouse line (Rodriguez et al., 2000) was used to delete the neo selection cassette.
Fig. 7.
Arrest of LFC differentiation following Pax6 loss is not mediated by upregulation of Sox2. (A) Sox2lox targeting vector and somatic deletion allele. The neo selection cassette is flanked by frt sites (blue triangles). The single Sox2 exon is flanked by loxP sites (green triangles). (B-M′) Cre-mediated deletion results in the Sox2Δ allele. Sox2lox/lox;Pax6lox/lox control (B-G) and Sox2lox/lox;Pax6lox/lox;Mlr10 E14.5 double somatic mutant (H-M′) mouse lenses analyzed by H&E staining (B,H) and antibody labeling for Sox2 and Ap2α (C,I, green and red, respectively), phosphohistone H3 (PH3, red in D,J,J′), αA-crystallin (E,K), β-crystallin (F,L) and cleaved caspase 3 (cCas, red in G,M,M′). Counterstaining was with DAPI (D,F,G,J,L,M). Arrows indicate the lens equator, arrowheads to the aberrant cells in the lens posterior. LE, lens epithelium; re, retina. Scale bar: 100 μm.
Histology, immunofluorescence analysis, BrdU, TUNEL and X-Gal assays
Paraffin sections (10 μm) were stained with Hematoxylin and Eosin (H&E) using standard procedures. Immunofluorescence analysis was performed on paraffin sections as previously described (Ashery-Padan et al., 2000) using the following primary antibodies: rabbit anti-Pax6 (1:1000, Chemicon), mouse anti-Ap2α (1:50, Santa Cruz), rabbit anti-cleaved caspase 3 (1:100, Cell Signaling), goat anti-αA-crystallin (1:1000, Santa Cruz), goat anti-αB-crystallin (1:100, Santa Cruz), rabbit anti-βB1-crystallin (1:250, Santa Cruz), rabbit anti-γF-crystallin (1:50, Santa Cruz), rabbit anti-cyclin D1 (1:250, Thermo Scientific), rat anti-Ki67 (1:100, Dako), goat anti-p57Kip2 (1:100, Santa Cruz), rabbit anti-Prox1 (1:50, Acris) and rabbit anti-Sox2 (1:500, Chemicon). Secondary antibodies were conjugated to RRX or Cy2 (Jackson ImmunoResearch). Nuclei were visualized with DAPI (0.1 μg/ml, Sigma). For cell cycle quantification, BrdU (10 μl/g of 14 mg/ml) was injected 1.5 hours before sacrifice. Slides were stained with anti-phosphohistone H3 (1:500, Santa Cruz), fixed for 10 minutes in 4% paraformaldehyde, then stained with mouse anti-BrdU (1:100, Chemicon) as described (Marquardt et al., 2001). Five eyes were used from each genotype, and the percentage of marker-positive nuclei was calculated from total DAPI-positive nuclei. A two-tailed Student's t-test was used for statistical analysis. X-Gal staining on cryosections was performed as described (Liu et al., 2003).
Confocal quantification of Sox2 expression
Images of E14.5 lenses were taken using a confocal microscope CLSM410 (Zeiss) and the signal was measured within the linear range using the Range Indicator application (Zeiss LSM Imager). Five nuclei each from the extreme anterior, equator and posterior of lens sections were measured for intensity (pixel values 0-255) and divided by the retinal nucleus intensity for the same section, using ImageJ software (NIH).
In situ hybridization (ISH)
ISH was performed using DIG-labeled RNA probes (Yaron et al., 2006). The Prox1 probe was produced from a 947 bp PCR fragment (forward, 5′-CAGATGCCTAGTTCCACAGACC-3′; reverse, 5′-AGAGCGTTGCAATCTCTACTCG-3′). Other ISH probes used were: Cryaa, Cryab and cMaf (Robinson and Overbeek, 1996), Sox1, Sfrp2 (Leimeister et al., 1998) and Six3 (Oliver et al., 1995). All analyses presented in this study were conducted on at least five eyes of each genotype, from at least two different litters.
RESULTS
Somatic mutation of Pax6 in the lens results in small eyes due to lens defects
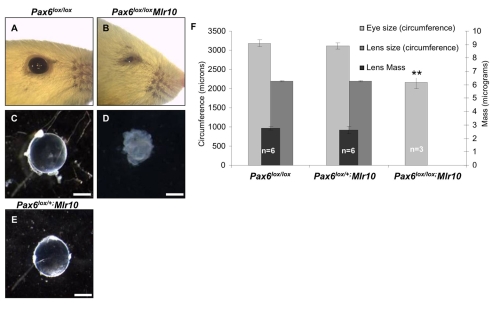
To study the role of Pax6 in the lens after the lens vesicle stage, we employed the Mlr10 transgene (Zhao et al., 2004) and the Pax6lox allele (Ashery-Padan et al., 2000) and established Pax6lox/lox;Mlr10 somatic mutants. Pax6lox/lox littermates were used as controls. Pax6lox/lox;Mlr10 eyes were significantly smaller than those of controls (Fig. 1B,D,F; 65% of circumference, P<0.001). This reduction in size was attributed to the decrease in lens tissue, which appeared opaque and shapeless (Fig. 1D). In a previous study, Pax6 was shown to have lens-autonomous dosage requirements at the LP stage (Davis-Silberman et al., 2005). To determine whether there is haploinsufficiency at later stages of lens development, we investigated the phenotype of the heterozygous Pax6lox/+;Mlr10 littermates. We did not identify any differences in lens weight, size or opacity between the Pax6lox/+;Mlr10 animals and controls (Fig. 1E,F). This result implies that a diploid dose of Pax6 is not required after the lens structure has formed.
Fig. 1.
Microopthalmia in Pax6lox/lox;Mlr10 mice. (A-E) Eyes (A,B) and isolated lenses (C-E) from P30 control (A,C), Pax6lox/lox;Mlr10 (B,D) and Pax6lox/+;Mlr10 (E) mice. (F) Quantification of eye circumference (light-gray bars), lens circumference (dark-gray bars) and dry mass (black bars) of controls, Pax6lox/+;Mlr10 and Pax6lox/lox;Mlr10 mice. Lens size and mass could not be measured in Pax6lox/lox;Mlr10 mice (D,F). **P=0.004. Scale bars: 500 μm.
Somatic inactivation of Pax6 by E14.5 leads to failure in LFC differentiation
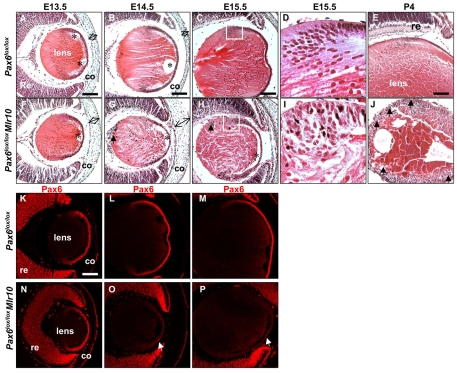
To characterize the morphological defects of Pax6lox/lox;Mlr10 mice and to determine the onset of Pax6 inactivation, we conducted a histological analysis of eyes from embryonic and postnatal stages and monitored Pax6 loss by immunostaining (Fig. 2) and by activity of human alkaline phosphatase (hAP) from the Z/AP reporter (see Fig. S2 in the supplementary material) (Lobe et al., 1999). In controls, Pax6 expression was high in the anterior LE and equator of the lens, and diminished as the cells underwent differentiation (Fig. 2K-M). At E13.5, prior to the loss of Pax6 protein from the LE (Fig. 2K,N), the Pax6lox/lox;Mlr10 and control lenses appeared similar in size (Fig. 2A,F). After Pax6 loss at E14.5 (Fig. 2O), Pax6lox/lox;Mlr10 lenses were slightly more elongated, and a few small nucleated cells were detected in the posterior part of the lens (Fig. 2G, arrow). The cornea of E14.5 mutants (Fig. 2G, double arrow) was thicker than in the controls (Fig. 2B), which suggests failure of the lens to induce mesenchymal condensation (Sevel and Isaacs, 1988). By E15.5, the mutant lenses appeared significantly smaller than the controls (Fig. 2H; 76% of circumference, n=8, P<0.002). In E15.5 controls, the nuclei of the equatorial transitional zone, in which LE cells undergo differentiation, were organized in a characteristic bow pattern (Fig. 2C,D). By contrast, in Pax6lox/lox;Mlr10 lenses, transitional zone cells were disorganized (Fig. 2H,I). At later stages, the Pax6lox/lox;Mlr10 lenses remained significantly smaller than controls, as lens fiber formation seemed to be arrested from ∼E14.5 (Fig. 2; see Fig. S3 in the supplementary material). By P4, the Pax6lox/lox;Mlr10 lens seemed to be mostly composed of epithelial cells surrounding a few fiber cells (Fig. 2J). In the adult [postnatal day (P) 30], only remnants of lens tissue were detected in the mutant (Fig. 1D). These morphological defects suggest that Pax6-deficient LE cells fail to differentiate and instead accumulate at the lens equator and in the posterior lens, and that the LFCs detected in the Pax6lox/lox;Mlr10 lenses probably originate prior to Pax6 loss.
Fig. 2.
Pax6 ablation in the lens epithelium coincides with failure of lens fiber cell differentiation and morphological defects in the lens. (A-J) Hematoxylin and Eosin (H&E) staining of control (A-E) and Pax6lox/lox;Mlr10 (F-J) eyes in E13.5 (A,F), E14.5 (B,G), E15.5 (C,D,H,I) and P4 (E,J) mice. D and I are higher magnifications of the boxed regions from C and H, respectively. Arrows mark posterior nucleated cells. Double arrows demonstrate the thickness of presumptive cornea of controls (A,B) and mutants (F,G). Asterisks mark fixation artifacts. (K-P) Pax6 protein in Pax6lox/lox controls (K-M) and Pax6lox/lox;Mlr10 mutants (N-P) at E13.5 (K,N), E14.5 (L,O) and E15.5 (M,P). White arrows indicate a few cells that retain some Pax6 activity owing to the mosaic nature of Cre activity. co, cornea; re, retina. Scale bars: 100 μm.
Pax6-deficient LE cells fail to exit the cell cycle at the lens equator
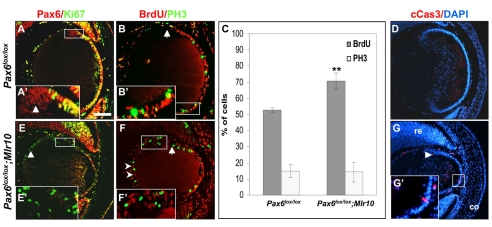
The first step in LFC differentiation is cell cycle exit (Rafferty and Rafferty, 1981). Pax6 is expressed in proliferating LE cells, as it co-localized with Ki67, a marker of actively proliferating cells (Fig. 3A) (Endl et al., 1997). However, Pax6 was also expressed after the cells had undergone cell cycle exit (Fig. 3A′, arrow). This pattern of expression suggests that either Pax6 is involved in maintaining the proliferation capacity of the anterior LE, or that it is required for cell cycle exit in the LE of the equatorial zone. To distinguish between these possibilities, we characterized the distribution of Ki67 in Pax6lox/lox;Mlr10 embryos. The loss of Pax6 was accompanied by a change in the distribution of Ki67. In Pax6lox/lox;Mlr10 lenses, Ki67+ cells were detected posterior to the lens equator, a region which is normally devoid of proliferating cells (Fig. 3A,E). To further determine the cell cycle stage of Pax6-deficient cells at E14.5, we quantified the percentage of cells in the S and M phases using BrdU incorporation and phosphorylated histone H3 (PH3) immunostaining, respectively. Both markers were only detected anterior to the equator in control lenses (Fig. 3B), whereas in Pax6lox/lox;Mlr10 mutants, proliferating cells were abundant in the transitional zone and in the posterior lens (Fig. 3F′ and arrowheads). In these regions, BrdU was detected in 46.2±3.5% (±s.d.) of the nuclei, and PH3 was detected in 32.8±6.2% of the nuclei. We therefore concluded that Pax6 is required for the cell cycle exit of LE cells at the lens equator.
Fig. 3.
Pax6-deficient lens epithelium fails to exit the cell cycle at the lens equator and undergoes apoptosis. (A-B′,E-F′) Antibody labeling of control (A,B) and Pax6lox/lox;Mlr10 (E,F) lenses from E14.5 mouse embryos. (A,E) Expression of Pax6 (red) and Ki67 (green) with co-expression in the lens epithelium (LE) of Ki67 and Pax6 (A, yellow). Some Ki67- cells express Pax6 (A′, arrowhead). In Pax6lox/lox;Mlr10, Pax6- Ki67+ cells are detected in the transitional zone (E′) and in the posterior lens (E, arrowhead). (B,F) Phosphohistone H3 (PH3, green) and BrdU (B,F, red) were detected in the Pax6lox/lox LE up to the lens equator (arrow, B) but also posterior to the lens equator in the Pax6lox/lox;Mlr10 lenses (F,F′) and in the posterior lens (F, arrowheads). (C) Quantitative analysis reveals a significant increase in the percentage of BrdU+ cells in Pax6lox/lox;Mlr10 lenses (70.7±5.1% s.d.) as compared with the control LE (52.8±1.4%, **P<0.001). The percentage of PH3+ cells is not altered in the Pax6- LE (control 14.3±6.2%; mutant 14.7±4.1%). (D,G,G′) Cleaved caspase 3 (cCas3)-positive cells detected in the LE of Pax6lox/lox;Mlr10 lenses (G,G′, red, arrowhead) but not in the control E14.5 mouse embryo (D). Counterstaining is with DAPI (blue). co, cornea; re, retina. Scale bar: 100 μm.
To determine whether Pax6 loss alters the cell cycle dynamics in the anterior LE itself, we quantitatively analyzed BrdU+ and PH3+ cells in the LE of control and Pax6-deficient lenses (Fig. 3C). A significant increase in the BrdU incorporation index was observed in the LE following Pax6 loss (70.7±5.15%), as compared with the controls (52.8±1.4%, P<0.001). The proportion of PH3+ cells was similar between the genotypes (14.3±6.2% in Pax6lox/lox;Mlr10 and 14.7±4.0% in Pax6lox/lox). This suggests a prolonged S phase in the Pax6-deficient LE, which is reminiscent of the phenotype reported in Pax6-deficient cerebral cortex (Estivill-Torrus et al., 2002).
Apoptosis in the Pax6-deficient lens
Although cells in Pax6lox/lox;Mlr10 lenses continued to proliferate, the overall size of the lens was reduced (Fig. 2). To test whether this tissue loss was due to apoptosis, we performed TUNEL analysis, which demonstrated an increase in apoptotic cells in the Pax6lox/lox;Mlr10 lenses (not shown). To perform a quantitative analysis, we detected the cleaved form of caspase 3 (cCas3, Fig. 3D,G). However, the number of cCas3+ cells in the Pax6lox/lox;Mlr10 lenses was low (2.6±2.0 per section, n=8), suggesting that apoptosis is only partially responsible for the significant reduction in lens size, which is instead primarily due to the arrest in LFC differentiation. Interestingly, cCas3+ cells were never BrdU+ (not shown). Thus, the longer S phase observed in the Pax6- LE is probably not an immediate trigger of apoptosis.
Pax6 is not required for the regulation of crystallin genes at late stages of lens development
The αA-crystallin (Cryaa) promoter has been shown to bind and to be activated by Pax6 in vitro (Cvekl et al., 1995; Yang and Cvekl, 2005; Yang et al., 2006). Accordingly, Pax6 was found to be required in vivo for the onset of Cryaa expression during early stages of lens development (Ashery-Padan et al., 2000; Cvekl et al., 1995). To examine whether Pax6 regulates crystallin expression during secondary LFC differentiation, we characterized the distribution of crystallin transcripts and proteins in the Pax6-deficient lenses. Cryaa protein was detected in both the LE and LFCs of control lenses, with elevated expression in the latter (Robinson and Overbeek, 1996) (Fig. 4A). Intriguingly, in Pax6lox/lox;Mlr10 lenses, Cryaa protein was maintained in the LE and in the posterior aberrant cells, similar to its expression in controls (Fig. 4F; see also Fig. S4 in the supplementary material). As crystallins are ultra-stable proteins (Jaenicke, 1996), Cryaa might be detected because of its low turnover, rather than continued expression. We therefore examined Cryaa transcripts by in situ hybridization (ISH). Cryaa transcripts were detected in the LE of E14.5, E15.5 and E18.5 Pax6lox/lox;Mlr10 lenses in a similar distribution to that of Cryaa protein (Fig. 4B,G), confirming that Pax6 is not required for the low-level Cryaa expression in the LE at these stages (Fig. 4G and data not shown).
Fig. 4.
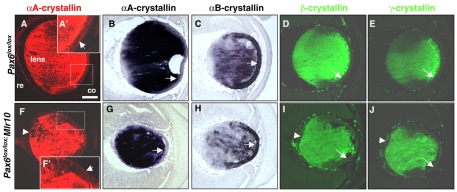
Pax6 is not essential for maintaining the expression of α-crystallins or for the downregulation of βγ-crystallins during LFC differentiation. Control (A-E) and Pax6lox/lox;Mlr10 mutant (F-J) E14.5 mouse lenses labeled with antibodies against αA-crystallin (Cryaa, A,F, red), β-crystallins (D,I, green) and γ-crystallins (E,J, green), or subjected to ISH with probes against αA-crystallin (B,G) and αB-crystallin (C,H). Arrows indicate the LE, arrowheads to Pax6- cells accumulating in the posterior lens. co, cornea; re, retina. Scale bar: 100 μm.
Unlike Cryaa, αB-crystallin (Cryab) is strongly expressed in the LE of the E12.5-15.5 developing lens and is reduced in LFCs, overlapping with Pax6 expression (Robinson and Overbeek, 1996) (Fig. 4C). This, together with the results of extensive in vitro research (Gopal-Srivastava et al., 1996; Yang et al., 2004), suggest that Pax6 is an important regulator of Cryab expression. However, Cryab expression was maintained in both control and Pax6lox/lox;Mlr10 lenses, with high expression in the LE (Fig. 4C,H). This suggests that Pax6 is not required for Cryab expression during the later stages of lens development.
β- and γ-crystallins are expressed throughout lens development exclusively in LFCs. Specifically, βB1-crystallin (Crybb1) expression is initiated precisely when lens fibers begin to elongate (Brahma, 1988; Duncan et al., 1996), making it an ideal marker for LFC differentiation. To examine LFC differentiation in Pax6lox/lox;Mlr10 mutants, an antibody against Crybb1 that identifies most β-crystallin (Cryb) proteins was employed. Cryb was detected in the LFCs of control and Pax6lox/lox;Mlr10 lenses in a similar pattern (Fig. 4D,I). Cryb was not detected in the LE, transitional zone and aberrant posterior cells of the Pax6-deficient lenses (Fig. 4I), confirming the undifferentiated state of these cells. Previous in vitro studies suggested that the high level of Pax6 in the LE suppresses Crybb1 (Duncan et al., 1996). However, removal of Pax6 from the Pax6lox/lox;Mlr10 lenses was not sufficient to induce upregulation of Cryb in the LE in vivo (Fig. 4I).
Similar to Cryb, γ-crystallins (Cryg) are expressed in mature LFCs and are possible targets for Pax6 regulation based on in vitro studies (Kralova et al., 2002; Yang et al., 2004). Cryg was not detected in the LE, transitional zone or aberrant posterior cells of Pax6lox/lox;Mlr10 lenses (Fig. 4J).
Taken together, these results demonstrate that Pax6 is not required for the expression of α-crystallins or for the maintenance of an undifferentiated fate in the LE by inhibiting LFC-specific crystallins. Importantly, cells at the equator and on the posterior side of Pax6lox/lox;Mlr10 lenses do not express any crystallin LFC marker (Fig. 4I,J, arrowheads). Therefore, Pax6 is primarily required for the normal differentiation of LFCs and this activity does not depend on its regulation of crystallin expression.
Pax6 requirement for LFC differentiation is not mediated through Prox1, Sox1 or cMaf
Several TFs have been shown to be essential for LFC differentiation in vivo, namely Prox1, Sox1 and cMaf (Maf - Mouse Genome Informatics). To determine whether the lack of LFC differentiation observed in the Pax6lox/lox;Mlr10 mice is mediated through one of these TFs, we characterized their expression in Pax6-deficient Pax6lox/lox;Mlr10 lenses.
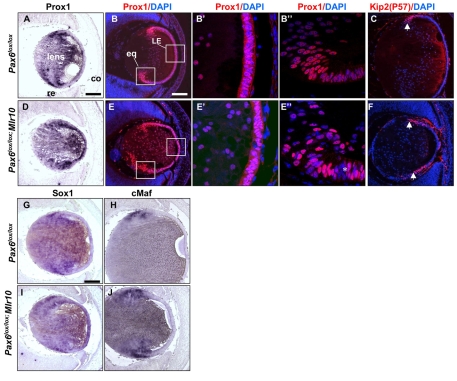
Prox1 is essential for the elongation of primary LFCs, exit from the cell cycle and the expression of several γ-crystallins (Wigle et al., 1999). Prox1 expression in the LP is dependent on Pax6 activity (Ashery-Padan et al., 2000). At E14.5, Prox1 transcripts were detected in both control and Pax6lox/lox;Mlr10 lenses (Fig. 5A,D). As Prox1 protein is differentially localized during lens development (Duncan et al., 2002), we examined its spatial distribution at E14.5 by immunolabeling. In both control and Pax6lox/lox;Mlr10 lenses, Prox1 protein was detected in the nuclei and cytoplasm of LE cells, whereas in the equator and in differentiating LFCs it was mainly nuclear (Fig. 5B,E). The level of Prox1 expression varied among Pax6lox/lox;Mlr10 cells (Fig. 5E,E″, asterisk). However, most nuclei maintained Prox1 expression at E14.5 and during later stages (E15.5; data not shown). In accordance with the maintenance of Prox1 in Pax6lox/lox;Mlr10 lenses, expression of its downstream targets - cell cycle inhibitory genes p57Kip2 (Cdkn1c) and p27Kip1 (Cdkn1b) (Wigle et al., 1999) - was detected in the equatorial region of both control and Pax6-deficient lenses (Fig. 5C,F and data not shown). These results show that during secondary LFC differentiation, Pax6 does not regulate the expression of Prox1 or of its cell cycle inhibiting targets, but is still essential for cell cycle arrest.
Fig. 5.
Intrinsic requirement for Pax6 in LFC differentiation is not mediated through Prox1, Sox1 or cMaf. (A-J) Expression patterns in E14.5 (A,B,D,E,G,I) or E15.5 (C,F,H,J), control (A-C,G,H) and Pax6lox/lox;Mlr10 (D-F,I,J) mouse eyes, showing Prox1 transcript (A,D) and protein (B,E, boxed regions magnified in B′,B″,E′,E″, red), p57Kip2 protein (C,F, red), and Sox1 (G,I) and cMaf (H,J) transcripts. Asterisk in E″ marks a patch of low-level Prox1 expression. Counterstaining is with DAPI (B-C,E-F, blue). co, cornea; eq, lens equator; LE, lens epithelium; re, retina. Scale bars: 100 μm.
TFs of the Sox family are expressed during, and are involved in, lens development (Kamachi et al., 1995; Kamachi et al., 1998). One of these, Sox1, has been shown to be essential for complete elongation of LFCs and for expression of γ-crystallins (Nishiguchi et al., 1998). Sox1 was expressed in all lens cells at E14.5-15.5, with a marked increase in differentiating LFCs (Nishiguchi et al., 1998) (Fig. 5G). The same expression pattern was observed in Pax6lox/lox;Mlr10 lenses, indicating that Pax6 is not crucial for Sox1 expression (Fig. 5I).
Finally, cMaf is a lens-specific member of the large Maf gene family. cMaf has been shown to be essential for LFC elongation and γ-crystallin expression (Kawauchi et al., 1999; Ring et al., 2000; Yoshida et al., 2001; Yoshida and Yasuda, 2002). In Pax6lox/lox;Mlr10 lenses, the expression of cMaf was similar to in controls. cMaf transcripts were detected throughout the lens, with elevated expression at the lens equator (Sakai et al., 1997) (Fig. 5H,J).
Taken together, the apparently normal upregulation of Sox1 and cMaf at the lens equator, as well as the normal distribution of Prox1 protein, demonstrate that despite Pax6 loss, the cells in the transitional zone are able to respond to extracellular signals and activate some differentiation markers. However, even with the activation of these factors, execution of the lens fiber differentiation program requires Pax6.
Pax6 negatively regulates Sox2 in the equatorial zone of the embryonic lens
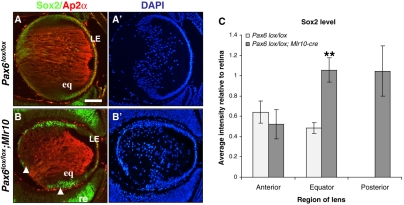
The Sox2 TF is expressed in the developing lens and has been implicated to function with Pax6 in initiating crystallin expression (Kamachi et al., 1998; Kamachi et al., 2001; Kondoh et al., 2004; Stevanovic et al., 1994; Yang et al., 2004). Furthermore, direct regulation of Sox2 by Pax6 has been demonstrated in neural progenitor cells (Wen et al., 2008). The role of Sox2 and whether it interacts with Pax6 during later stages of lens development are unknown. We therefore characterized the expression of Sox2 following Pax6 loss. We utilized Ap2α (Tcfap2a), a TF that is expressed in the anterior LE and is essential for early lens development, as a marker for the anterior LE (Pontoriero et al., 2008; West-Mays et al., 1999). Double immunolabeling for Ap2α and Sox2 revealed that in the control lens at E14.5, Ap2α is co-expressed with Sox2 in the anterior LE, whereas in the transitional zone only Sox2 was detected (Fig. 6A). In the conditional mutant, Ap2α was restricted to a small population of the most anterior cells of the LE. By contrast, Sox2+ cells were detected in a much wider population of cells at the Pax6lox/lox;Mlr10 equator and at a high level of expression, similar to that in the retina. Ectopic cells at the lens posterior were also intensely Sox2 positive (Fig. 6B). Sox2 expression was quantified by confocal microscopy. In controls, only a low level of Sox2 was observed in the anterior LE, the same as in the lens equator and about half of that in the retina (Fig. 6A). Anterior LE cells of the Pax6lox/lox;Mlr10 had expression levels comparable to those of controls, but equatorial and posterior cells showed a 2.2-fold increase in expression (P=0.0001), attaining levels greater than in the retina (Fig. 6B,C). Therefore, following Pax6 ablation, cells of the lens equator fail to differentiate into LFCs, increase at the expense of anterior Ap2α+ LE, express high levels of Sox2 and expand into the posterior lens.
Fig. 6.
Pax6 downregulates Sox2 in the lens equator. (A,B) Immunofluorescent detection of Sox2 (green) and Ap2α (red) in E14.5 control (A) and Pax6lox/lox;Mlr10 (B) mouse lenses. Arrowheads in B indicate elevated expression of Sox2 at the lens equator and in the posterior lens. (A′,B′) Counterstaining of A,B with DAPI. (C) Quantification of Sox2 protein by confocal image analysis (n=6, **P<0.001). eq, lens equator; LE, lens epithelium; re, retina. Scale bar: 100 μm.
The differentiation failure and proliferation of aberrant LE are not mediated through Sox2
Sox2 is known to be involved in the determination of stem cell fate and in the proliferation of neural stem cells (Episkopou, 2005). To examine the role of Sox2 in the lens and to determine whether the significant increase in Sox2 expression in Pax6lox/lox;Mlr10 lenses mediates the observed differentiation failure, we established the Sox2lox allele, which includes two loxP sequences flanking the single exon of the murine Sox2 gene (Fig. 7A). This allele was employed in combination with Mlr10 to inactivate either Sox2 alone (Sox2lox/lox;Mlr10) or Sox2 together with Pax6 (Sox2lox/lox;Pax6lox/lox;Mlr10). The Sox2lox/lox;Mlr10 embryos and adult mice did not exhibit any abnormal ocular phenotypes (not shown). This is in agreement with an apparent reduction in Sox2 expression at E12.5-15.5 (Nishiguchi et al., 1998), suggesting that the low-level expression of Sox2 in E14.5 lenses is not essential for lens development.
In the Sox2lox/lox;Pax6lox/lox;Mlr10 double somatic mutants, both Pax6 and Sox2 are deleted exclusively in the lens (Fig. 7). Accordingly, Sox2 protein was not detected in the Sox2lox/lox;Pax6lox/lox;Mlr10 lens, but was preserved in the adjacent optic cup, where Cre is not active (Fig. 7I). Despite the obvious loss of Sox2, the ocular phenotype of the double somatic mutant was strikingly similar to that of Pax6lox/lox;Mlr10 mutants. The Sox2lox/lox;Pax6lox/lox;Mlr10 lenses were smaller than controls and epithelial cells accumulated posterior to the lens equator (Fig. 7H). The anterior LE, as identified by Ap2α expression, was reduced in size (Fig. 7I). Moreover, similar to the phenotype of Pax6lox/lox;Mlr10, in Sox2lox/lox;Pax6lox/lox;Mlr10 lenses αA-crystallin protein and transcripts were strongly expressed in the LFCs and weakly in the LE and in the aberrant posterior cells (Fig. 7K and data not shown), whereas β-crystallin was absent from cells of the lens equator and from the aberrant posterior cells (Fig. 7L). Failure of cell cycle exit was also evident in Sox2lox/lox;Pax6lox/lox;Mlr10 lenses (Fig. 7J,J′). Finally, some Sox2lox/lox;Pax6lox/lox;Mlr10 LE cells underwent apoptosis, as demonstrated by cCas3 immunostaining (Fig. 7M,M′). Therefore, when Sox2 overexpression is prevented, Pax6-null LE cells undergo the same LFC differentiation failure and cell death as observed in lenses that overexpress Sox2. Thus, the LFC differentiation failure observed in Pax6lox/lox;Mlr10 mutants is independent of Sox2.
Ectopic Wnt/β-catenin activity inhibits LFC differentiation
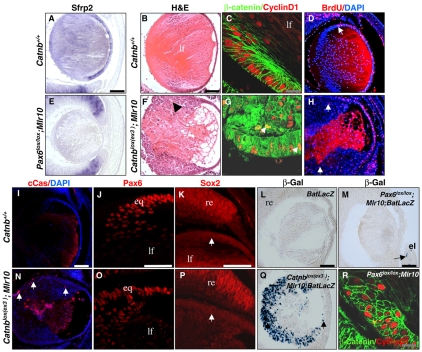
Sox2 is a known target of Wnt signaling in the retina (Van Raay et al., 2005), and members of the Sox family modulate β-catenin activity (Sinner et al., 2007; Sinner et al., 2004). Therefore, we examined a possible connection between loss of Pax6 and canonical Wnt signaling. We first characterized the expression of Sfrp2, a secreted inhibitor of Wnt signaling and a target of Pax6 (Kim et al., 2001). In control E14.5 lenses, Sfrp2 was detected anterior to the lens equator (Fig. 8A) (Chen et al., 2004). By contrast, Sfrp2 was not detected in Pax6lox/lox;Mlr10 lenses (Fig. 8E). Thus, Pax6 regulates Sfrp2 in the LE, which might play a role in the attenuation of Wnt signaling during LFC differentiation.
Fig. 8.
β-catenin overexpression leads to LFC differentiation failure independently of Pax6. (A,E) Sfrp2 transcripts in control (A) and Pax6lox/lox;Mlr10 (E) E14.5 eyes. (B-D,F-K,N-P) E15.5 control (B-D,I-K) and Catnblox(ex3);Mlr10 (F-H,N-P) lenses. (B,F) H&E staining reveals aberrant accumulation of cells at the lens equator of Catnblox(ex3);Mlr10 mice (F, arrowhead). (C,G) β-catenin and cyclin D1 (green and red, respectively) detected by antibody labeling. (D,H) BrdU+ cells (red) found anterior to the lens equator in the control (D, arrow) accumulate posterior to the lens equator of Catnblox(ex3);Mlr10 lenses (H, arrows). (I,N) cCas3 (red) is not detected in controls (I) but is detected in Catnblox(ex3);Mlr10 lenses (N, arrows). (J,O) Pax6 protein is detected in the Catnblox(ex3);Mlr10 lenses (O), as in the control (J). (K,P) Sox2 is weakly expressed in control LE and in the lens equator of Catnblox(ex3);Mlr10 (arrows). (L,M,Q) β-galactosidase activity (β-Gal, blue) in E14.5 lenses of BATlacZ (L), Catnblox(ex3);Mlr10;BATlacZ (Q) and Pax6lox/lox;Mlr10;BATlacZ (M). β-galactosidase activity is detected in the developing eyelid (el, arrow) but not in lenses of Pax6lox/lox;Mlr10;BATlacZ (M). (R) Antibody labeling against β-catenin (green) and cyclin D1 (red). Counterstaining is with DAPI (blue, D,H,I,N). el, eyelid; eq, lens equator; re, retina; lf, lens fiber. Scale bars: white/black, 100 μm; gray, 400 μm.
Taking this into consideration, we hypothesized that overexpression of β-catenin (Catnb; Ctnnb1) would result in LFC differentiation failure. To test this hypothesis, we established Catnblox(ex3);Mlr10-Cre gain-of-function mutants. In the Catnblox(ex3) allele, Cre-mediated deletion of exon 3 results in accumulation of β-catenin in the nucleus, enabling expression of its target genes (Harada et al., 1999).
Catnblox(ex3);Mlr10 adult lenses were significantly smaller than controls (not shown). At E15.5, the morphology of Catnblox(ex3);Mlr10 lenses was abnormal, with epithelial cells accumulating at the lens equator and in the posterior lens (Fig. 8F), similar to the Pax6lox/lox;Mlr10 phenotype (Fig. 2H-J).
The transcriptional control function of β-catenin, as opposed to its structural role, depends on its cellular localization. In the control, β-catenin was detected primarily in the cell membranes (Fig. 8C), whereas in the Catnblox(ex3);Mlr10 lenses it was detected in the cytoplasm and nuclei (Fig. 8G). Nuclear localization was detected by co-immunostaining with an antibody against cyclin D1 (Fig. 8C,G), a plausible target of the canonical Wnt pathway (Shtutman et al., 1999; Tetsu and McCormick, 1999). Similar to in Pax6lox/lox;Mlr10, proliferation, as detected by BrdU, was detected in the large mass of small nucleated cells of the equator and posterior lens (Fig. 8H). Apoptotic cells were detected in the Catnblox(ex3);Mlr10 lens, but not in controls (Fig. 8I,N).
Pax6 was apparently unaffected by the activation of the Wnt pathway in Catnblox(ex3);Mlr10 lenses, as it showed strong expression in the anterior LE and weak expression in the equator and in the aberrant cells of the posterior lens (Fig. 8J,O). In contrast to in Pax6lox/lox;Mlr10 lenses, Sox2 was not upregulated at the equator of Catnblox(ex3);Mlr10 lenses (Fig. 8P), suggesting Pax6-dependent repression of Sox2 in lens cells. Moreover, it seems that Wnt/β-catenin does not activate Sox2 in the mammalian lens.
The canonical Wnt pathway is inactive during secondary LFC differentiation and is not regulated by Pax6
To directly examine whether Wnt/β-catenin signaling is active in Pax6lox/lox;Mlr10 lenses, we employed the BATlacZ transgene (Nakaya et al., 2005). In this reporter line, lacZ is expressed under control of the Tcf/Lef promoter, which is activated by β-catenin. As expected, in Catnblox(ex3);Mlr10;BATlacZ embryos, β-galactosidase activity was detected in most lens cells, especially in the nucleated, undifferentiated cells at the equator and posterior of the lens (Fig. 8Q). In Pax6lox/lox;Mlr10;BATlacZ animals, β-galactosidase activity was identical to that of control littermates and was not detected in the lens at E14.5 (Fig. 8L,M). Furthermore, β-catenin remained confined to the cellular membrane and did not enter the nucleus of Pax6lox/lox;Mlr10 lenses (Fig. 8R). This indicates that the failure of Pax6-negative cells to differentiate into LFCs is unlikely to be mediated through Wnt/β-catenin transcriptional activity.
DISCUSSION
In this study, we established the first in vivo model in which Pax6 is abolished from a formed embryonic lens, constituting a direct tool for the study of the role of Pax6 during secondary lens fiber differentiation. The findings presented reveal that Pax6 is essential for lens fiber differentiation but is dispensable for maintaining a lens epithelial identity. This role of Pax6 is not mediated by changes in canonical Wnt pathway activity, or by the upregulation of Sox2 observed in Pax6-deficient lenses. Known transcriptional regulators of LFC differentiation - Sox1, cMaf and Prox1 - are not dependent on Pax6 activity, but are, however, insufficient to enable lens fiber differentiation without Pax6. Therefore, Pax6 activity within the lens is crucial for cell cycle exit and for initiation of the lens fiber differentiation program in the mammalian eye.
Robustness of fetal stage LE to haploinsufficiency of Pax6
The vertebrate eye is sensitive to changes in Pax6 dosage: both reduction and elevation result in severe ocular phenotypes (Duncan et al., 2004; Glaser et al., 1994; Glaser et al., 1990; Hogan et al., 1988; Sanyal and Hawkins, 1979; Schedl et al., 1996). We have previously shown that the lens is intrinsically sensitive to Pax6 dosage reduction, as somatic inactivation of one copy of Pax6 in the SE mimics the lens phenotype of Pax6 heterozygotes (Davis-Silberman et al., 2005).
In contrast to the phenotype observed in the SE following Pax6 reduction, we observed no phenotypic difference between Pax6lox/+;Mlr10 lenses and controls, even in adult mice (1 year old, not shown). Therefore, a diploid dose of Pax6 is not necessary during the late stages of lens development, in contrast to the sensitivity to Pax6 reduction during formation of the LP. This confirms previous hypotheses, which attributed the Pax6 dosage requirement to lens placode formation, based on the analysis of lens development in Pax6+/- mutants (van Raamsdonk and Tilghman, 2000) or deletion of the Pax6 ectoderm enhancer (Dimanlig et al., 2001).
Pax6 is required for cell cycle exit, cell survival and lens fiber differentiation
Pax6 is expressed in both the proliferating anterior LE and in the transitional zone, including non-proliferating cells (Ki67- BrdU-; Fig. 3). Pax6 loss from the whole lens alters cell proliferation in both regions, increasing the proportion of cells in the S phase in the LE and preventing cell cycle exit in the transitional zone (Fig. 3). Pax6 involvement in cell cycle regulation has been reported in the developing retina (Marquardt et al., 2001; Oron-Karni et al., 2008). During brain development, Pax6 loss results in a shortened cell cycle during early corticogenesis but a prolonged S phase during later stages (Estivill-Torrus et al., 2002).
Pax6 involvement in cell cycle regulation might be through its direct interactions with cell cycle components, including the retinoblastoma protein (pRb; Rb1), which has been found to be associated with Pax6 in vitro and in lens extracts (Cvekl et al., 1999). Accordingly, the phenotype of pRb loss-of-function includes cell differentiation arrest, persistent proliferation and reduced survival - a phenotype reminiscent of Pax6lox/lox;Mlr10 lenses (Morgenbesser et al., 1994; Pan and Griep, 1994). Other proposed mechanisms include direct association of Pax6 with the centrosomes or mitotic chromosomes in proliferating cortical progenitors and cultured cells, respectively (Tamai et al., 2007; Zaccarini et al., 2007). The relevance of the above findings to Pax6 function in cell cycle regulation in the lens remains to be investigated.
Pax6 is known to bind, activate and repress crystallin gene expression in vitro and in vivo during early stages of development (Cvekl and Duncan, 2007; Cvekl et al., 2004). During the late stages of newt lens regeneration, which emulates normal lens development, Pax6 has been shown to be needed for LFC differentiation but not for crystallin maintenance (Madhavan et al., 2006). In accordance with this, our results show that removal of Pax6 does not alter the expression of α-crystallins in the LE, but at the same time precludes the upregulation of crystallin expression observed in differentiating LFCs (Fig. 4). The requirement for Pax6 for the onset of LFC differentiation can be explained by the recently proposed chromatin remodeling model (Yang et al., 2006), according to which TFs operate in a temporal order on enhancer sequences of the Cryaa gene, each TF enabling chromatin remodeling and activity of further TFs. In Pax6lox/lox;Mlr10 mutants, Pax6 might enable basal expression of Cryaa and Cryab by `opening' chromatin to transcription prior to mutation onset in LE cells. After the initiation of α-crystallin expression, Pax6 is dispensable for its maintenance in the LE. In the transitional zone, upregulation of Cryaa and the initiation of β- and γ-crystallin activation do require Pax6.
Pax6 seems to govern some, but not all, of the processes associated with LFC differentiation. In the Pax6lox/lox;Mlr10 lenses, expression of differentiation regulators (Sox1, cMaf and Prox1) and cell cycle inhibitors (p27Kip1 and p57Kip2) is not lost in the transitional zone (Fig. 5). In fact, the transitional zone seems to be expanded, probably due to the continued proliferation of Pax6-deficient cells. This expansion might also be occurring at the expense of the anterior LE, as can be seen by the large population of Sox2+ cells at the equator and the relatively small population of anterior Ap2α+ cells (Fig. 6). It appears that cells at the Pax6-deficient lens equator are competent to respond to some external cues that trigger the expression of transitional zone markers. However, without Pax6, these factors are insufficient to bring about cell cycle exit, or to activate crystallin expression and cellular elongation. Knockout models of Sox1, cMaf and Prox1 show that these TFs are directly essential for crystallin accumulation and elongation of LFCs (Kawauchi et al., 1999; Nishiguchi et al., 1998; Ring et al., 2000; Wigle et al., 1999; Yoshida and Yasuda, 2002). In the transitional zone, Pax6 is co-expressed with these factors and has been found to co-operate with cMaf (Sakai et al., 2001; Yoshida et al., 2001). Thus, although Pax6 is not required for the onset of expression of Sox1, cMaf and Prox1, it might function with them to regulate LFC differentiation.
Lens inversion experiments have demonstrated that lens polarity is dependent on the cellular environment (Coulombre and Coulombre, 1963). Since then, numerous growth factor families have been reported to influence LFC differentiation (reviewed by Lovicu and McAvoy, 2005). Most notably, FGFs were shown to initiate LFC differentiation in a concentration-dependent manner (Robinson, 2006). Mlr10-Cre-mediated inactivation of three FGF receptors resulted in complete arrest of LFC differentiation at the lens vesicle stage and reduced expression of Prox1, cMaf, p27Kip1 and p57Kip2 (Zhao et al., 2008). This phenotype was more severe than that of the Pax6 mutant presented here, which suggests that Pax6 is not absolutely essential for the capacity of cells to respond to FGF signaling, although it might regulate some components of this pathway.
A complex relationship between Pax6 and Sox2: Pax6 inhibits the expression of Sox2 at the lens equator
Pax6 and Sox2 have been shown to form a functional complex that is required for the activation of crystallin genes at the placodal stage (Cvekl et al., 2004; Kamachi et al., 2001; Kondoh et al., 2004; Smith et al., 2005). In addition, Pax6 has been shown to bind enhancer sequences of Sox2 and to activate Sox2 expression in lens cells (Inoue et al., 2007; Lengler et al., 2005) and in neuronal progenitors (Wen et al., 2008), suggesting a positive effect of Pax6 on Sox2 expression.
We show that during late stages of development, Pax6 ablation results in a dramatic increase in Sox2 expression in the transitional zone but not in the anterior LE (Fig. 6C). Sox2 is associated with maintenance of a progenitor phenotype and stem cell characteristics (Graham et al., 2003; Loh et al., 2008; Pan and Thomson, 2007). Therefore, the observed upregulation of Sox2 might be the result of reversion to a more primal state that lacks the capacity to differentiate. However, by deleting Sox2 in Pax6-deficient lenses, we demonstrated that the increase in Sox2 is not the cause of the observed phenotype. The analysis of Sox2-deficient lenses suggests that Sox2 is not required at later stages of lens development (Fig. 7 and not shown). Moreover, when LE cells fail to differentiate because of β-catenin activation, Sox2 expression does not increase (Fig. 8P), contradicting the notion that Sox2 upregulation is the default result of differentiation failure in the LE.
The Wnt pathway and LFC differentiation
During lens induction, Wnt signaling in the SE is essential for preventing ectopic lens formation in the surrounding head ectoderm, and overexpression of β-catenin in the SE prevents lens induction and inhibits expression of both Pax6 and Sox2 (Miller et al., 2006; Smith et al., 2005; Stump et al., 2003). The involvement of the canonical Wnt pathway in LFC differentiation is still under debate. β-catenin loss-of-function phenotypes have been largely attributed to its structural, rather than transcriptional, role (Kreslova et al., 2007; Smith et al., 2005). Nevertheless, many components of the Wnt signaling pathway are expressed in distinct temporal and spatial patterns throughout lens development (Ang et al., 2004; Chen et al., 2004; Lovicu and McAvoy, 2005). In addition, the Wnt co-receptor Lrp6 has been shown to delay LFC differentiation (Stump et al., 2003). These findings suggest that canonical Wnt/β-catenin signaling does play an antagonistic role in LFC differentiation.
Recently, lens-specific β-catenin loss-of-function mutants were established (Catnblox/lox;Mlr10). Analysis of these mutants revealed that β-catenin is required for proliferation and differentiation of the LE (Cain et al., 2008). In accordance with this, the constitutive stabilization of β-catenin conducted in the current study resulted in the prevention of cell cycle exit and of LFC differentiation (Fig. 8). The seemingly similar phenotypes of β-catenin gain-of-function and Pax6-deficient lenses, together with the downregulation of Sfrp2 in the latter, led to the hypothesis that the phenotype of the Pax6lox/lox;Mlr10 lens is mediated by alterations in the canonical Wnt/β-catenin signaling pathway. This hypothesis was tested in this study through the use of the BATlacZ transgene (Nakaya et al., 2005). The lacZ reporter was activated in the lens of E14.5 β-catenin gain-of-function mutants, enabling detection of canonical Wnt pathway activity in the lens. lacZ was not active in control or Pax6lox/lox;Mlr10 lenses. From this, we infer that β-catenin transcriptional control activity does not play a major role in the LE at E14.5. Moreover, it seems that the phenotype of Pax6lox/lox;Mlr10 lenses is not mediated by Wnt/β-catenin signaling, although Pax6 involvement in LFC differentiation through the non-canonical Wnt pathways remains to be investigated.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/15/2567/DC1
Supplementary Material
We thank Joachim Graw and Lena Remizova for helpful comments on the manuscript; Corinne Lobe and Andreas Nagy for the Z/AP reporter line; Terry P. Yamaguchi for the BATlacZ reporter line; Peter Gruss for the mouse lines established in his laboratory; and Robin Lovell-Badge, Guillermo Oliver, Cornelia Leimeister, Amir Rattner and Leif Lundh for providing constructs for ISH probes. Research in R.A.-P.'s laboratory is supported by the Israel Science Foundation, the Binational Science Foundation, the AMN foundation, the Glaucoma Research Foundation, the Israeli Ministry of Health and the E. Matilda Ziegler Foundation. The research of M.L.R. is supported by NEI R01EY12995.
References
- Ang, S. J., Stump, R. J., Lovicu, F. J. and McAvoy, J. W. (2004). Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr. Patterns 4, 289-295. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan, R. and Gruss, P. (2001). Pax6 lights-up the way for eye development. Curr. Opin. Cell Biol. 13, 706-714. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan, R., Marquardt, T., Zhou, X. and Gruss, P. (2000). Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 14, 2701-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett, S. and Beebe, D. C. (1992). Coincident loss of mitochondria and nuclei during lens fiber cell differentiation. Dev. Dyn. 194, 85-93. [DOI] [PubMed] [Google Scholar]
- Beebe, D. C. and Piatigorsky, J. (1976). Differential synthesis of crystallin and noncrystallin polypeptides during lens fiber cell differentiation in vitro. Exp. Eye Res. 22, 237-249. [DOI] [PubMed] [Google Scholar]
- Beebe, D. C., Compart, P. J., Johnson, M. C., Feagans, D. E. and Feinberg, R. N. (1982). The mechanism of cell elongation during lens fiber cell differentiation. Dev. Biol. 92, 54-59. [DOI] [PubMed] [Google Scholar]
- Bhat, S. P. (2001). The ocular lens epithelium. Biosci. Rep. 21, 537-563. [DOI] [PubMed] [Google Scholar]
- Brahma, S. K. (1988). Ontogeny of beta B1-crystallin polypeptide during chicken lens development. Exp. Eye Res. 47, 507-510. [DOI] [PubMed] [Google Scholar]
- Cain, S., Martinez, G., Kokkinos, M. I., Turner, K., Richardson, R. J., Abud, H. E., Huelsken, J., Robinson, M. L. and de Iongh, R. U. (2008). Differential requirement for beta-catenin in epithelial and fiber cells during lens development. Dev. Biol. 321, 420-433. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Stump, R. J., Lovicu, F. J. and McAvoy, J. W. (2004). Expression of Frizzleds and secreted frizzled-related proteins (Sfrps) during mammalian lens development. Int. J. Dev. Biol. 48, 867-877. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Stump, R. J., Lovicu, F. J. and McAvoy, J. W. (2006). A role for Wnt/planar cell polarity signaling during lens fiber cell differentiation? Semin. Cell Dev. Biol. 17, 712-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombre, J. L. and Coulombre, A. J. (1963). Lens Development: Fiber Elongation and Lens Orientation. Science 142, 1489-1490. [DOI] [PubMed] [Google Scholar]
- Cvekl, A. and Duncan, M. K. (2007). Genetic and epigenetic mechanisms of gene regulation during lens development. Prog. Retin. Eye Res. 26, 555-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl, A., Kashanchi, F., Sax, C. M., Brady, J. N. and Piatigorsky, J. (1995). Transcriptional regulation of the mouse alpha A-crystallin gene: activation dependent on a cyclic AMP-responsive element (DE1/CRE) and a Pax-6-binding site. Mol. Cell. Biol. 15, 653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl, A., Kashanchi, F., Brady, J. N. and Piatigorsky, J. (1999). Pax-6 interactions with TATA-box-binding protein and retinoblastoma protein. Invest. Ophthalmol. Vis. Sci. 40, 1343-1350. [PubMed] [Google Scholar]
- Cvekl, A., Yang, Y., Chauhan, B. K. and Cveklova, K. (2004). Regulation of gene expression by Pax6 in ocular cells: a case of tissue-preferred expression of crystallins in lens. Int. J. Dev. Biol. 48, 829-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Silberman, N., Kalich, T., Oron-Karni, V., Marquardt, T., Kroeber, M., Tamm, E. R. and Ashery-Padan, R. (2005). Genetic dissection of Pax6 dosage requirements in the developing mouse eye. Hum. Mol. Genet. 14, 2265-2276. [DOI] [PubMed] [Google Scholar]
- de Iongh, R. U., Lovicu, F. J., Chamberlain, C. G. and McAvoy, J. W. (1997). Differential expression of fibroblast growth factor receptors during rat lens morphogenesis and growth. Invest. Ophthalmol. Vis. Sci. 38, 1688-1699. [PubMed] [Google Scholar]
- Dimanlig, P. V., Faber, S. C., Auerbach, W., Makarenkova, H. P. and Lang, R. A. (2001). The upstream ectoderm enhancer in Pax6 has an important role in lens induction. Development 128, 4415-4424. [DOI] [PubMed] [Google Scholar]
- Duncan, M. K., Li, X., Ogino, H., Yasuda, K. and Piatigorsky, J. (1996). Developmental regulation of the chicken beta B1-crystallin promoter in transgenic mice. Mech. Dev. 57, 79-89. [DOI] [PubMed] [Google Scholar]
- Duncan, M. K., Haynes, J. I., 2nd, Cvekl, A. and Piatigorsky, J. (1998). Dual roles for Pax-6: a transcriptional repressor of lens fiber cell-specific beta-crystallin genes. Mol. Cell. Biol. 18, 5579-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, M. K., Cui, W., Oh, D. J. and Tomarev, S. I. (2002). Prox1 is differentially localized during lens development. Mech. Dev. 112, 195-198. [DOI] [PubMed] [Google Scholar]
- Duncan, M. K., Xie, L., David, L. L., Robinson, M. L., Taube, J. R., Cui, W. and Reneker, L. W. (2004). Ectopic Pax6 expression disturbs lens fiber cell differentiation. Invest. Ophthalmol. Vis. Sci. 45, 3589-3598. [DOI] [PubMed] [Google Scholar]
- Endl, E., Steinbach, P., Knuchel, R. and Hofstadter, F. (1997). Analysis of cell cycle-related Ki-67 and p120 expression by flow cytometric BrdUrd-Hoechst/7AAD and immunolabeling technique. Cytometry 29, 233-241. [PubMed] [Google Scholar]
- Episkopou, V. (2005). SOX2 functions in adult neural stem cells. Trends Neurosci. 28, 219-221. [DOI] [PubMed] [Google Scholar]
- Estivill-Torrus, G., Pearson, H., van Heyningen, V., Price, D. J. and Rashbass, P. (2002). Pax6 is required to regulate the cell cycle and the rate of progression from symmetrical to asymmetrical division in mammalian cortical progenitors. Development 129, 455-466. [DOI] [PubMed] [Google Scholar]
- Fujiwara, M., Uchida, T., Osumi-Yamashita, N. and Eto, K. (1994). Uchida rat (rSey): a new mutant rat with craniofacial abnormalities resembling those of the mouse Sey mutant. Differentiation 57, 31-38. [DOI] [PubMed] [Google Scholar]
- Garcia, C. M., Yu, K., Zhao, H., Ashery-Padan, R., Ornitz, D. M., Robinson, M. L. and Beebe, D. C. (2005). Signaling through FGF receptor-2 is required for lens cell survival and for withdrawal from the cell cycle during lens fiber cell differentiation. Dev. Dyn. 233, 516-527. [DOI] [PubMed] [Google Scholar]
- Gehring, W. J. (1996). The master control gene for morphogenesis and evolution of the eye. Genes Cells 1, 11-15. [DOI] [PubMed] [Google Scholar]
- Glaser, T., Lane, J. and Housman, D. (1990). A mouse model of the aniridia-Wilms tumor deletion syndrome. Science 250, 823-827. [DOI] [PubMed] [Google Scholar]
- Glaser, T., Jepeal, L., Edwards, J. G., Young, S. R., Favor, J. and Maas, R. L. (1994). PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat. Genet. 7, 463-471 [erratum appears in Nat. Genet. (1994) 8, 203]. [DOI] [PubMed] [Google Scholar]
- Gopal-Srivastava, R., Cvekl, A. and Piatigorsky, J. (1996). Pax-6 and alphaB-crystallin/small heat shock protein gene regulation in the murine lens. Interaction with the lens-specific regions, LSR1 and LSR2. J. Biol. Chem. 271, 23029-23036. [DOI] [PubMed] [Google Scholar]
- Graham, V., Khudyakov, J., Ellis, P. and Pevny, L. (2003). SOX2 functions to maintain neural progenitor identity. Neuron 39, 749-765. [DOI] [PubMed] [Google Scholar]
- Grainger, R. M., Mannion, J. E., Cook, T. L., Jr and Zygar, C. A. (1997). Defining intermediate stages in cell determination: acquisition of a lens-forming bias in head ectoderm during lens determination. Dev. Genet. 20, 246-257. [DOI] [PubMed] [Google Scholar]
- Grindley, J. C., Davidson, D. R. and Hill, R. E. (1995). The role of Pax-6 in eye and nasal development. Development 121, 1433-1442. [DOI] [PubMed] [Google Scholar]
- Harada, N., Tamai, Y., Ishikawa, T., Sauer, B., Takaku, K., Oshima, M. and Taketo, M. M. (1999). Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 18, 5931-5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan, B. L., Hirst, E. M., Horsburgh, G. and Hetherington, C. M. (1988). Small eye (Sey): a mouse model for the genetic analysis of craniofacial abnormalities. Development 103Suppl, 115-119. [DOI] [PubMed] [Google Scholar]
- Inoue, M., Kamachi, Y., Matsunami, H., Imada, K., Uchikawa, M. and Kondoh, H. (2007). PAX6 and SOX2-dependent regulation of the Sox2 enhancer N-3 involved in embryonic visual system development. Genes Cells 12, 1049-1061. [DOI] [PubMed] [Google Scholar]
- Jaenicke, R. (1996). Stability and folding of ultrastable proteins: eye lens crystallins and enzymes from thermophiles. FASEB J. 10, 84-92. [DOI] [PubMed] [Google Scholar]
- Joyner, A. L. I. (1995). Gene targeting: a practical approach. In The Practical Approach Series (ed. B. D. H. D. Rickwood), pp. 234. New York: IRL Press at Oxford University Press.
- Kamachi, Y., Sockanathan, S., Liu, Q., Breitman, M., Lovell-Badge, R. and Kondoh, H. (1995). Involvement of SOX proteins in lens-specific activation of crystallin genes. EMBO J. 14, 3510-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi, Y., Uchikawa, M., Collignon, J., Lovell-Badge, R. and Kondoh, H. (1998). Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development 125, 2521-2532. [DOI] [PubMed] [Google Scholar]
- Kamachi, Y., Uchikawa, M., Tanouchi, A., Sekido, R. and Kondoh, H. (2001). Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 15, 1272-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi, S., Takahashi, S., Nakajima, O., Ogino, H., Morita, M., Nishizawa, M., Yasuda, K. and Yamamoto, M. (1999). Regulation of lens fiber cell differentiation by transcription factor c-Maf. J. Biol. Chem. 274, 19254-19260. [DOI] [PubMed] [Google Scholar]
- Kim, A. S., Anderson, S. A., Rubenstein, J. L., Lowenstein, D. H. and Pleasure, S. J. (2001). Pax-6 regulates expression of SFRP-2 and Wnt-7b in the developing CNS. J. Neurosci. 21, RC132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh, H., Uchikawa, M. and Kamachi, Y. (2004). Interplay of Pax6 and SOX2 in lens development as a paradigm of genetic switch mechanisms for cell differentiation. Int. J. Dev. Biol. 48, 819-827. [DOI] [PubMed] [Google Scholar]
- Kralova, J., Czerny, T., Spanielova, H., Ratajova, V. and Kozmik, Z. (2002). Complex regulatory element within the gammaE- and gammaF-crystallin enhancers mediates Pax6 regulation and is required for induction by retinoic acid. Gene 286, 271-282. [DOI] [PubMed] [Google Scholar]
- Kreslova, J., Machon, O., Ruzickova, J., Lachova, J., Wawrousek, E. F., Kemler, R., Krauss, S., Piatigorsky, J. and Kozmik, Z. (2007). Abnormal lens morphogenesis and ectopic lens formation in the absence of beta-catenin function. Genesis 45, 157-168. [DOI] [PubMed] [Google Scholar]
- Leimeister, C., Bach, A. and Gessler, M. (1998). Developmental expression patterns of mouse sFRP genes encoding members of the secreted frizzled related protein family. Mech. Dev. 75, 29-42. [DOI] [PubMed] [Google Scholar]
- Lengler, J., Bittner, T., Munster, D., Gawad Ael, D. and Graw, J. (2005). Agonistic and antagonistic action of AP2, Msx2, Pax6, Prox1 AND Six3 in the regulation of Sox2 expression. Ophthalmic Res. 37, 301-309. [DOI] [PubMed] [Google Scholar]
- Liu, H., Mohamed, O., Dufort, D. and Wallace, V. A. (2003). Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev. Dyn. 227, 323-334. [DOI] [PubMed] [Google Scholar]
- Lobe, C. G., Koop, K. E., Kreppner, W., Lomeli, H., Gertsenstein, M. and Nagy, A. (1999). Z/AP, a double reporter for cre-mediated recombination. Dev. Biol. 208, 281-292. [DOI] [PubMed] [Google Scholar]
- Loh, Y. H., Ng, J. H. and Ng, H. H. (2008). Molecular framework underlying pluripotency. Cell Cycle 7, 885-891. [DOI] [PubMed] [Google Scholar]
- Lovicu, F. J. and Robinson, M. L. (2004). Development of the Ocular Lens. Cambridge: Cambridge University Press.
- Lovicu, F. J. and McAvoy, J. W. (2005). Growth factor regulation of lens development. Dev. Biol. 280, 1-14. [DOI] [PubMed] [Google Scholar]
- Madhavan, M., Haynes, T. L., Frisch, N. C., Call, M. K., Minich, C. M., Tsonis, P. A. and Del Rio-Tsonis, K. (2006). The role of Pax-6 in lens regeneration. Proc. Natl. Acad. Sci. USA 103, 14848-14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt, T., Ashery-Padan, R., Andrejewski, N., Scardigli, R., Guillemot, F. and Gruss, P. (2001). Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105, 43-55. [DOI] [PubMed] [Google Scholar]
- Miller, L. A., Smith, A. N., Taketo, M. M. and Lang, R. A. (2006). Optic cup and facial patterning defects in ocular ectoderm beta-catenin gain-of-function mice. BMC Dev. Biol. 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenbesser, S. D., Williams, B. O., Jacks, T. and DePinho, R. A. (1994). p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature 371, 72-74. [DOI] [PubMed] [Google Scholar]
- Muta, M., Kamachi, Y., Yoshimoto, A., Higashi, Y. and Kondoh, H. (2002). Distinct roles of SOX2, Pax6 and Maf transcription factors in the regulation of lens-specific delta1-crystallin enhancer. Genes Cells 7, 791-805. [DOI] [PubMed] [Google Scholar]
- Nakaya, M. A., Biris, K., Tsukiyama, T., Jaime, S., Rawls, J. A. and Yamaguchi, T. P. (2005). Wnt3a links left-right determination with segmentation and anteroposterior axis elongation. Development 132, 5425-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi, S., Wood, H., Kondoh, H., Lovell-Badge, R. and Episkopou, V. (1998). Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 12, 776-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino, H. and Yasuda, K. (2000). Sequential activation of transcription factors in lens induction. Dev. Growth Differ. 42, 437-448. [DOI] [PubMed] [Google Scholar]
- Oliver, G., Mailhos, A., Wehr, R., Copeland, N. G., Jenkins, N. A. and Gruss, P. (1995). Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development 121, 4045-4055. [DOI] [PubMed] [Google Scholar]
- Oron-Karni, V., Farhy, C., Elgart, M., Marquardt, T., Remizova, L., Yaron, O., Xie, Q., Cvekl, A. and Ashery-Padan, R. (2008). Dual requirement for Pax6 in retinal progenitor cells. Development 135, 4037-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, G. and Thomson, J. A. (2007). Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 17, 42-49. [DOI] [PubMed] [Google Scholar]
- Pan, H. and Griep, A. E. (1994). Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 8, 1285-1299. [DOI] [PubMed] [Google Scholar]
- Pontoriero, G. F., Deschamps, P., Ashery-Padan, R., Wong, R., Yang, Y., Zavadil, J., Cvekl, A., Sullivan, S., Williams, T. and West-Mays, J. A. (2008). Cell autonomous roles for AP-2alpha in lens vesicle separation and maintenance of the lens epithelial cell phenotype. Dev. Dyn. 237, 602-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn, J. C., West, J. D. and Hill, R. E. (1996). Multiple functions for Pax6 in mouse eye and nasal development. Genes Dev. 10, 435-446. [DOI] [PubMed] [Google Scholar]
- Rafferty, N. S. and Rafferty, K. A., Jr (1981). Cell population kinetics of the mouse lens epithelium. J. Cell. Physiol. 107, 309-315. [DOI] [PubMed] [Google Scholar]
- Ring, B. Z., Cordes, S. P., Overbeek, P. A. and Barsh, G. S. (2000). Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development 127, 307-317. [DOI] [PubMed] [Google Scholar]
- Robinson, M. L. (2006). An essential role for FGF receptor signaling in lens development. Semin. Cell Dev. Biol. 17, 726-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, M. L. and Overbeek, P. A. (1996). Differential expression of alpha A- and alpha B-crystallin during murine ocular development. Invest. Ophthalmol. Vis. Sci. 37, 2276-2284. [PubMed] [Google Scholar]
- Rodriguez, C. I., Buchholz, F., Galloway, J., Sequerra, R., Kasper, J., Ayala, R., Stewart, A. F. and Dymecki, S. M. (2000). High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat. Genet. 25, 139-140. [DOI] [PubMed] [Google Scholar]
- Sakai, M., Imaki, J., Yoshida, K., Ogata, A., Matsushima-Hibaya, Y., Kuboki, Y., Nishizawa, M. and Nishi, S. (1997). Rat maf related genes: specific expression in chondrocytes, lens and spinal cord. Oncogene 14, 745-750. [DOI] [PubMed] [Google Scholar]
- Sakai, M., Serria, M. S., Ikeda, H., Yoshida, K., Imaki, J. and Nishi, S. (2001). Regulation of c-maf gene expression by Pax6 in cultured cells. Nucleic Acids Res. 29, 1228-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal, S. and Hawkins, R. K. (1979). Dysgenetic lens (dyl)-a new gene in the mouse. Invest. Ophthalmol. Vis. Sci. 18, 642-645. [PubMed] [Google Scholar]
- Schedl, A., Ross, A., Lee, M., Engelkamp, D., Rashbass, P., van Heyningen, V. and Hastie, N. D. (1996). Influence of PAX6 gene dosage on development: overexpression causes severe eye abnormalities. Cell 86, 71-82. [DOI] [PubMed] [Google Scholar]
- Sevel, D. and Isaacs, R. (1988). A re-evaluation of corneal development. Trans. Am. Ophthalmol. Soc. 86, 178-207. [PMC free article] [PubMed] [Google Scholar]
- Shtutman, M., Zhurinsky, J., Simcha, I., Albanese, C., D'Amico, M., Pestell, R. and Ben-Ze'ev, A. (1999). The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 96, 5522-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinner, D., Rankin, S., Lee, M. and Zorn, A. M. (2004). Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development 131, 3069-3080. [DOI] [PubMed] [Google Scholar]
- Sinner, D., Kordich, J. J., Spence, J. R., Opoka, R., Rankin, S., Lin, S. C., Jonatan, D., Zorn, A. M. and Wells, J. M. (2007). Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol. Cell. Biol. 27, 7802-7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A. N., Miller, L. A., Song, N., Taketo, M. M. and Lang, R. A. (2005). The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev. Biol. 285, 477-489. [DOI] [PubMed] [Google Scholar]
- Stevanovic, M., Zuffardi, O., Collignon, J., Lovell-Badge, R. and Goodfellow, P. (1994). The cDNA sequence and chromosomal location of the human SOX2 gene. Mamm. Genome 5, 640-642. [DOI] [PubMed] [Google Scholar]
- Stump, R. J., Ang, S., Chen, Y., von Bahr, T., Lovicu, F. J., Pinson, K., de Iongh, R. U., Yamaguchi, T. P., Sassoon, D. A. and McAvoy, J. W. (2003). A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev. Biol. 259, 48-61. [DOI] [PubMed] [Google Scholar]
- Tamai, H., Shinohara, H., Miyata, T., Saito, K., Nishizawa, Y., Nomura, T. and Osumi, N. (2007). Pax6 transcription factor is required for the interkinetic nuclear movement of neuroepithelial cells. Genes Cells 12, 983-996. [DOI] [PubMed] [Google Scholar]
- Tetsu, O. and McCormick, F. (1999). Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398, 422-426. [DOI] [PubMed] [Google Scholar]
- van Raamsdonk, C. D. and Tilghman, S. M. (2000). Dosage requirement and allelic expression of PAX6 during lens placode formation. Development 127, 5439-5448. [DOI] [PubMed] [Google Scholar]
- Van Raay, T. J., Moore, K. B., Iordanova, I., Steele, M., Jamrich, M., Harris, W. A. and Vetter, M. L. (2005). Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron 46, 23-36. [DOI] [PubMed] [Google Scholar]
- Walther, C., Guenet, J. L., Simon, D., Deutsch, U., Jostes, B., Goulding, M. D., Plachov, D., Balling, R. and Gruss, P. (1991). Pax: a murine multigene family of paired box-containing genes. Genomics 11, 424-434. [DOI] [PubMed] [Google Scholar]
- Wawrousek, E. F., Chepelinsky, A. B., McDermott, J. B. and Piatigorsky, J. (1990). Regulation of the murine alpha A-crystallin promoter in transgenic mice. Dev. Biol. 137, 68-76. [DOI] [PubMed] [Google Scholar]
- Wen, J., Hu, Q., Li, M., Wang, S., Zhang, L., Chen, Y. and Li, L. (2008). Pax6 directly modulate Sox2 expression in the neural progenitor cells. NeuroReport 19, 413-417. [DOI] [PubMed] [Google Scholar]
- West-Mays, J. A., Zhang, J., Nottoli, T., Hagopian-Donaldson, S., Libby, D., Strissel, K. J. and Williams, T. (1999). AP-2alpha transcription factor is required for early morphogenesis of the lens vesicle. Dev. Biol. 206, 46-62. [DOI] [PubMed] [Google Scholar]
- Wigle, J. T., Chowdhury, K., Gruss, P. and Oliver, G. (1999). Prox1 function is crucial for mouse lens-fibre elongation. Nat. Genet. 21, 318-322. [DOI] [PubMed] [Google Scholar]
- Yang, Y. and Cvekl, A. (2005). Tissue-specific regulation of the mouse alphaA-crystallin gene in lens via recruitment of Pax6 and c-Maf to its promoter. J. Mol. Biol. 351, 453-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Chauhan, B. K., Cveklova, K. and Cvekl, A. (2004). Transcriptional regulation of mouse alphaB- and gammaF-crystallin genes in lens: opposite promoter-specific interactions between Pax6 and large Maf transcription factors. J. Mol. Biol. 344, 351-368. [DOI] [PubMed] [Google Scholar]
- Yang, Y., Stopka, T., Golestaneh, N., Wang, Y., Wu, K., Li, A., Chauhan, B. K., Gao, C. Y., Cveklova, K., Duncan, M. K. et al. (2006). Regulation of alphaA-crystallin via Pax6, c-Maf, CREB and a broad domain of lens-specific chromatin. EMBO J. 25, 2107-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron, O., Farhy, C., Marquardt, T., Applebury, M. and Ashery-Padan, R. (2006). Notch1 functions to suppress cone-photoreceptor fate specification in the developing mouse retina. Development 133, 1367-1378. [DOI] [PubMed] [Google Scholar]
- Yoshida, K., Kim, J. I., Imaki, J., Hiromi, I., Nishi, S., Matsuda, H., Harada, T., Harada, C., Ohno, S. and Sakai, M. (2001). Proliferation in the posterior region of the lens of c-maf-/- mice. Curr. Eye Res. 23, 116-119. [DOI] [PubMed] [Google Scholar]
- Yoshida, T. and Yasuda, K. (2002). Characterization of the chicken L-Maf, MafB and c-Maf in crystallin gene regulation and lens differentiation. Genes Cells 7, 693-706. [DOI] [PubMed] [Google Scholar]
- Zaccarini, R., Cordelieres, F. P., Martin, P. and Saule, S. (2007). Pax6p46 binds chromosomes in the pericentromeric region and induces a mitosis defect when overexpressed. Invest. Ophthalmol. Vis. Sci. 48, 5408-5419. [DOI] [PubMed] [Google Scholar]
- Zhao, H., Yang, Y., Rizo, C. M., Overbeek, P. A. and Robinson, M. L. (2004). Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Invest. Ophthalmol. Vis. Sci. 45, 1930-1939. [DOI] [PubMed] [Google Scholar]
- Zhao, H., Yang, T., Madakashira, B. P., Thiels, C. A., Bechtle, C. A., Garcia, C. M., Zhang, H., Yu, K., Ornitz, D. M., Beebe, D. C. et al. (2008). Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 318, 276-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.