Summary
Several different populations of interneurons in the murine cortex, including somatostatin (SST)- or parvalbumin (PV)-expressing cells, are born in the ventral ganglionic eminences during mid-gestation and then migrate tangentially to the cortex. SST is expressed by some interneuron progenitors in the cerebral cortex and in migrating populations in the ventrolateral cortex at birth. However, PV (also known as PVALB) is not expressed by interneurons until the second postnatal week after reaching the cortex, suggesting that molecular cues in the cerebral cortex might be involved in the differentiation process. BMP4 is expressed at high levels in the somatosensory cortex at the time when the PV+ interneurons differentiate. Treatment of cortical cultures containing interneuron precursors is sufficient to generate PV+ interneurons prematurely and inhibit SST differentiation. Furthermore, overexpression of BMP4 in vivo increases the number of interneurons expressing PV, with a reduction in the number of SST+ interneurons. PV+ interneurons in the cortex express BMP type I receptors and a subpopulation displays activated BMP signaling, assessed by downstream molecules including phosphorylated SMAD1/5/8. Conditional mutation of BMP type I receptors in interneuron precursors significantly reduces the number of cortical PV+ interneurons in the adult brain. Thus, BMP4 signaling through type I receptors regulates the differentiation of two major medial ganglionic eminence-derived interneuron populations and defines their relative numbers in the cortex.
Keywords: Bone morphogenetic protein 4, Interneuron, BMP type I receptor, Ganglionic eminence, GABA, Mouse
INTRODUCTION
Interneurons differentiate in the developing cerebral cortex from precursors generated in the ventral ganglionic eminences (GEs), which migrate tangentially to the cerebral cortex (Anderson et al., 1997; Lavdas et al., 1999; Walsh and Cepko, 1992; Wichterle et al., 1999; Wichterle et al., 2001). Calretinin-containing interneurons preferentially arise from the caudal ganglionic eminence (CGE), whereas parvalbumin (PV; PVALB - Mouse Genome Informatics)- and somatostatin (SST)-containing interneurons are generated from the medial ganglionic eminence (MGE) (Butt et al., 2005; Gonchar and Burkhalter, 1997; Xu et al., 2004). The MGE-derived PV+ interneurons include chandelier and large basket cells, which exhibit fast spiking firing patterns, and the SST+ interneurons include small basket cells and Martinotti cells, which exhibit regular spiking and burst spiking, respectively (Butt et al., 2005; DeFelipe, 1997; Wang et al., 2004).
Differential expression of genes in the MGE may be partially responsible for the fate specification of progenitors (Flames et al., 2007; Fogarty et al., 2007; Wonders et al., 2008), but less is known about the molecular signaling mechanisms that regulate the differentiation of the precursors of PV+ and SST+ interneurons once they reach the cortex. Interneuron development may be influenced by the bone morphogenetic proteins (BMPs) that signal through a heterodimeric complex of type I (BMPR1A, BMPR1B and ALK2, also known as ACVR1) and type II (BMPR2) receptors (Gulacsi and Lillien, 2003; Koenig et al., 1994; Liu et al., 1995; ten Dijke et al., 1994; Yung et al., 2002), and loss of BMP receptor signaling alters interneuron subtype specification in the cortex and spinal cord (Samanta et al., 2007; Wine-Lee et al., 2004). SMAD4, a downstream mediator of BMP signaling, has been implicated in PV expression in the cerebellum, and BMP2 can generate PV+ cells in vitro from a fate-restricted progenitor isolated from the MGE (Chojnacki and Weiss, 2004; Zhang et al., 1996; Zhang et al., 1997; Zhou et al., 2003). However, it is unclear if BMP signaling plays a role in vivo in the differentiation of cortical PV+ and SST+ interneurons.
Here we show that BMP4 is a signaling cue in the cortex that can cause differentiation of a subset of PV+ interneurons, with a reduction in SST+ interneurons. We find that PV+ interneurons express BMP type I receptors and that null mutations of these receptors in interneuron progenitors have significantly fewer PV+ interneurons in the somatosensory cortex. Hence, we identify dorsal BMP4 signaling through BMP type I receptors as an important signal that selectively enhances PV+ interneuron differentiation and limits the number of SST+ interneurons.
MATERIALS AND METHODS
Generation and maintenance of mouse lines
Neuron specific enolase (Nse)-Bmp4 transgenic mice were maintained in an FVB background; their generation has been reported earlier (Gomes et al., 2003). Olig1-cre (Olig1cre/cre) mice were maintained in a C57BL/6 background, and Bmpr1a floxed (Bmpr1afx/fx), Bmpr1a+/- and Bmpr1b+/- mice were maintained in a 129Sv:C57BL/6 mixed background. The generation of Olig1-cre, Bmpr1afx/fx, Bmpr1a+/- and Bmpr1b+/- mice has already been reported (Lu et al., 2002; Mishina et al., 2002; Yi et al., 2000). Olig1cre/cre mice were bred with Bmpr1a+/- to get Olig1cre/+; Bmpr1a+/-, which were then bred with Bmpr1b+/- to generate Olig1cre/cre; Bmpr1a+/-; Bmpr1b+/- mice. The Bmpr1afx/fx animals were bred with Bmpr1b+/- to generate Bmpr1afx/fx; Bmpr1b+/- mice. In the final mating Olig1cre/cre; Bmpr1a+/-; Bmpr1b+/- animals were bred to Bmpr1afx/fx; Bmpr1b+/- to get Olig1cre/+; Bmpr1afx/+; Bmpr1b+/- (control) and Olig1cre/+; Bmpr1afx/-; Bmpr1b-/- (Bmpr1a/b double null) animals. The excision of the Bmpr1a floxed allele by Olig1-cre expression has already been reported (Samanta et al., 2007). The Olig1cre/cre mice were bred with ROSAGFP/GFP (Jackson Labs) (Mao et al., 2001) mice to get Olig1cre/+; ROSAGFP/+ progeny.
Neurosphere culture
The MGE was isolated from the E12 mouse telencephalon (CD1 mice, Charles River) and cells were dissociated using 0.25% trypsin (Invitrogen). Dissociated cells (5×104 cells/ml) were grown as neurospheres in DMEM/F12 (Invitrogen) supplemented with N2 and B27 (Invitrogen) nutrient additives and bFGF (20 ng/ml; R&D Systems) for 5 days. For phenotypic analysis cells were plated on poly-D-lysine (PDL)-coated coverslips at clonal density (104 cells/ml) in low bFGF (0.5 ng/ml) alone or with BMP4 (20 ng/ml; R&D Systems) or noggin (250 ng/ml; R&D Systems) for 7-9 days. In some samples sonic hedgehog (SHH; 10 nM; R&D Systems) was added for the first 2 days followed by low bFGF (0.5 ng/ml) alone or with BMP4 (20 ng/ml) for the next 7 days.
Cortical neuron culture
The cerebral cortex was isolated from P0 mouse (CD1 or Olig1cre/+; ROSAGFP/+ mice) brains after removing the meninges and dissociated in 0.25% trypsin. Dissociated cells (105 cells/ml) were filtered through a wire mesh to eliminate all debris and plated on PDL-coated coverslips in Neurobasal Medium (Invitrogen) supplemented with B27 and 5% FBS. One hour after plating the media was changed to Neurobasal Medium and B27 with or without BMP4 (20 ng/ml) for 4 days.
Immunocytochemistry and immunohistochemistry
Cultured cells were fixed in 4% formaldehyde for 15 minutes before performing immunocytochemistry. Mice were transcardially perfused with cold HBSS (Invitrogen) and the brains were fixed in 4% paraformaldehyde for 2 hours. The fixed brains were cryoprotected in 30% sucrose solution overnight and frozen in Tissue-Tek OCT before being cryosectioned for immunohistochemistry. The fixed cells and tissue sections were incubated with primary antibody overnight at 4°C in phosphate buffered saline (PBS) containing 1% BSA, 0.25% Triton X-100 and 2% goat serum. After washes the cell and the tissue sections were incubated with fluorophore-conjugated Alexa Fluor goat secondary antibodies (1:500; Invitrogen) in a solution containing 1% BSA, 0.25% Triton X-100 and DAPI nuclear stain (Invitrogen) for 1 hour before a second set of washes and were then mounted in ProLong Gold antifade reagent (Invitrogen). The primary antibodies used were rabbit anti-PV (1: 1000; Swant), mouse anti-PV (1:1000; Swant), rat anti-SST (1:500; Chemicon), mouse anti-βIII-tubulin (1:1000; Sigma), rabbit anti-GABA (1:1000; Sigma), mouse anti-GABA (1:1000; Sigma), guinea pig anti-VGLUT1 (1:1000; Synaptic Systems); rabbit anti-BMPR1A (1:100; Abgent), rabbit anti-BMPR1B (1:100; Orbigen); rabbit anti-pSMAD1/5/8 (1:100; Cell Signaling).
Cell counts and statistical analysis
In cell culture experiments, images were acquired from three immunostained coverslips from independent culture wells for each treatment condition using a Zeiss Axiovert epifluorescence microscope. Cells were counted manually using NIH ImageJ software and counts were averaged. For cortical cultures from Olig1cre/+; ROSAGFP/+ mice ∼800 GFP+ cells were counted and examined for PV expression per sample. Each experiment was repeated three times for statistical analysis. Coronal mouse brain sections (10 μm) close to Bregma -0.1 mm and Bregma +1.7 mm, based on landmarks defined by the Paxinos Atlas of the Mouse Brain, were used for the analysis of primary somatosensory cortex and the hippocampal dentate gyrus, respectively. Images of the entire primary somatosensory cortex or dentate gyrus were obtained from four brain sections separated by 40 μm using a Zeiss Axiovert epifluorescence microscope and counted manually using NIH ImageJ software. Since the PV and SST cells are limited to the cortical layers II-VI, cells were counted only from these layers and at least three brains were analyzed for each genotype. ImageJ software was also used to measure the area of the cortex (∼1 mm2/section) or dentate gyrus from which the cells were counted. To study colocalization, images were acquired using a Zeiss UV LSM510 confocal scanning microscope. All counts and image acquisitions were performed blinded. In experiments with only two conditions, Student's t-test assuming unequal variance was used to determine statistical significance, and for multicondition experiments, one-way ANOVA followed by Tukey's post hoc test was used. All data are presented as mean±s.e.m.
Quantitative real-time polymerase chain reaction (QRT-PCR)
QRT-PCR was performed using the Mastercycler ep realplex system (Eppendorf). Total RNA from tissue and cells was isolated using the RNAqueous-4PCR Kit (Ambion) following the manufacturer's instructions. The isolated RNA was treated with TURBO DNase (Ambion) to eliminate all residual DNA contamination. cDNA was prepared using the Thermoscript RT-PCR Kit (Invitrogen). QRT-PCR was performed with an initial denaturation of 10 minutes at 95°C, followed by 40 cycles of 15-second denaturation at 95°C and 1 minute of annealing and elongation at 60°C. The DNA amplification was detected from the fluorescent signal produced by the SYBR Green 1 dye in the SYBR Green PCR Mastermix (Applied Biosystems). All samples were run in duplicates for each set of PCR primers and GAPDH was used as the internal control. The specificity of the PCR primers was confirmed in advance by sequencing the PCR products amplified from brain cDNA. For further confirmation of primer specificity, the QRT-PCR of the experimental samples was followed with a melting curve program whereby the melting kinetics of the amplicon was examined by raising the temperature gradually from 60°C to 95°C over a 20-minute period on the realplex system.
In situ hybridization
Cryosections (20 μm) from desired brain regions were washed three times with PBS containing 0.1% Tween 20 (PBST) and treated with proteinase K (1 μg/ml, Invitrogen) for 5 minutes. Sections were then fixed in 4% paraformaldehyde and 0.1% glutaraldehyde for 30 minutes. Following three PBST washes, digoxigenin (DIG)-labeled antisense mRNA probes were added and the sections were incubated in a humidified hybridization chamber at 68°C overnight. On the following day, slides were washed at 68°C with high stringency wash buffer twice for 1 hour each, and with low stringency wash buffer three times for 1.5 hours each. Slides were then washed three times with Tris-buffered saline with 0.1% Tween 20 (TBST) followed by overnight incubation in anti-DIG alkaline phosphatase antibody (Roche, 1:2000) in 5% goat serum. Slides were subsequently washed three times in TBST containing 2 mM levamisole and transferred into developing solution containing NBT-BCIP (Roche) for 6 hours to overnight. Finally, sections were dehydrated through an ethanol gradient, cleared in xylene and mounted with Histomount (Zymed) for analysis and storage.
RESULTS
Interneurons in the cortex express parvalbumin during the second postnatal week
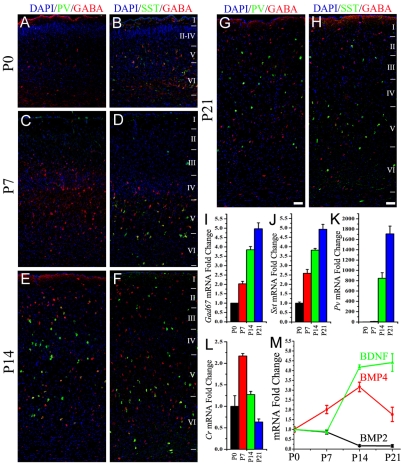
To analyze the timeline of PV+ interneuron differentiation in the cortex, P0, P7, P14 and P21 brain sections were immunostained for PV, SST and GABA. GABA- or SST-expressing cells were present in the cerebral cortex at P0 (Fig. 1A) and P7 (Fig. 1B), but PV-expressing cells were absent at these times. This was also true for the ventral areas of the brain, where SST and GABA immunoreactive cells, but not PV+ cells, were present (see Fig. S1A-C in the supplementary material). However, at both P14 and P21 a large number of PV+ cells were widely distributed across layers II-VI (Fig. 1E,G) suggesting that PV+ interneurons differentiate some time between P7 and P14. SST- and GABA-expressing cells also increased in number from P14 through to P21 and were widely distributed throughout layers I-VI (Fig. 1F,H). mRNA transcript levels for the different interneuron subtype markers were quantified in tissue samples of the somatosensory cortex (Fig. 1I-L). Similar to the observations with immunohistochemistry, the expression of both Gad67 (Gad1 - Mouse Genome Informatics; expressed by GABA-expressing cells) and Sst increased progressively from birth through to P21 in the cortex (Fig. 1I,J) suggesting a gradual increase in cell numbers. The expression pattern of calretinin (referred to here as Cr) mRNA was more complex and reached a peak at P7 (Fig. 1L). The expression of Pv mRNA, however, increased by ∼800-fold at P14 from levels at P0 and doubled to ∼1700-fold by P21 (Fig. 1K), consistent with the immunohistochemistry demonstrating very low expression at birth and at P7.
Fig. 1.
Parvalbumin (PV) is not expressed by interneurons until after they migrate to the cortex in postnatal mouse brain. (A-D) At postnatal day 0 (P0) and P7, somatostatin (SST, green in B,D) and GABA (red) immunoreactive cells can be observed in the cortex but PV expression (green in A,C) is absent. DAPI-labeled nuclei are in blue. (E-H) At P14 PV+ cells (green in E,G) appear in large numbers in the cortex (E) and PV expression is maintained at P21 (G). SST (green in F,H) and GABA (red) continue to be expressed through to P14 (F) and P21 (H). (I-L) mRNA expression levels of Gad67 (I), Sst (J), Pv (K) and calretinin (Cr, L) in the somatosensory cortex at the indicated postnatal time points. (M) Comparison of mRNA expression of Bmp2, Bmp4 and Bdnf postnatally. Scale bars: 50 μm.
Levels of mRNA for BMP family members were examined to determine whether they are expressed in the cortex at times consistent with a role in interneuron differentiation. Bmp4 expression increased at P7 and reached its peak at P14, coinciding with the appearance of PV+ interneurons in the cortex (Fig. 1M). Brain derived neurotrophic factor (Bdnf; Fig. 1M), which has also been implicated in PV+ interneuron differentiation (Huang et al., 1999), did not increase at P7 but increased by about ∼4-fold at P14. Although BMP2 influences PV expression in vitro (Chojnacki and Weiss, 2004), Bmp2 expression is lower at P14 (Fig. 1M) relative to levels found in the P0 cortex, suggesting it does not play a significant role in PV+ interneuron differentiation in vivo. Thus Bmp4 is expressed in the somatosensory cortex in a pattern consistent with role for BMP4 in differentiation of PV+ interneurons in vivo.
Effects of BMP4 signaling on MGE progenitors in vitro
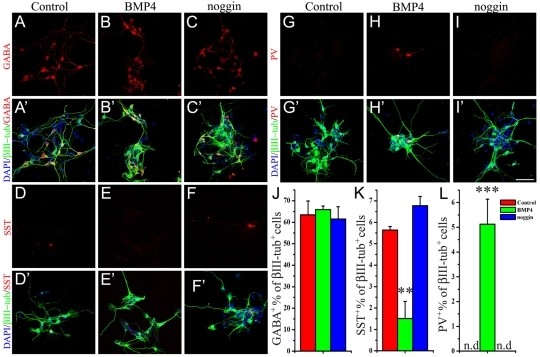
To examine the effects of BMP4 signaling on interneuron differentiation, MGE progenitors were cultured as neurospheres, dissociated and plated for differentiation in the presence of low bFGF alone (control), or with BMP4 or the BMP antagonist noggin for 7 days in vitro (DIV). BMP4 treatment significantly increased the numbers of neurons (βIII-tubulin+) and GABAergic interneurons (GABA+/βIII-tubulin+) generated from the MGE-derived progenitors compared with the control or noggin conditions (see Fig. S2A-D in the supplementary material). However, GABA+ cells constituted ∼65% of neurons under all three conditions (Fig. 2A-C′,J) and thus BMP4 treatment promoted neuronal differentiation but did not actively instruct a GABAergic fate. However, BMP4 treatment altered the phenotype of the GABAergic neurons. PV+ interneurons were completely absent in the control and noggin groups but constituted 5.1±1.0% (Fig. 2D-F′,K) of neurons in the BMP4-treated group. Conversely, BMP4 treatment significantly reduced the percentage of SST+ neurons (1.5±0.8%) compared with control (5.6±0.2%) or noggin (6.8±0.4%) treatment (Fig. 2G-I′,L). Taken together, these observations suggest that BMP4 signaling inhibits SST+ interneuron differentiation from MGE progenitors and enhances differentiation of PV+ interneurons.
Fig. 2.
BMP4 is required for the generation of PV+ interneurons from medial ganglionic eminence (MGE) progenitors in vitro. (A-I′) bFGF (20 ng/ml)-responsive neurospheres were grown from E12 MGE, dissociated and plated for 7 days in low bFGF (0.5 ng/ml) alone (Control) or in the presence of BMP4 (20 ng/ml) or noggin (250 ng/ml). Differentiated cells immunostained for GABA (red, A-C′), SST (red, D-F′) or PV (red, G-I′), βIII-tubulin (green), with DAPI-labeled nuclei in blue. Note the absence of PV+ cells in the control (G) and noggin-treated (I) groups. A′-I′ are merged images. (J-L) Quantification of the average percentages of GABA+, SST+ and PV+ interneurons under each condition (see key). **P<0.01, ***P<0.001 by ANOVA; n.d., not detected. Scale bar: 50 μm.
Timeline of the effects of BMP4 on PV differentiation
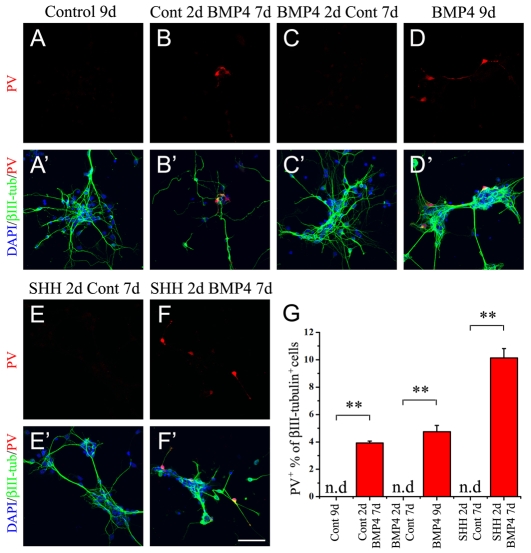
BMPs can affect patterning, exit from cell cycle, lineage commitment and the differentiation of neural progenitors (Gomes et al., 2003; Gross et al., 1996; Lim et al., 2000; Ohyama et al., 2005; Sasai et al., 1995). To further define the effects of BMP4 on PV fate specification, progenitors from the MGE were plated in low mitogen (bFGF) alone or with BMP4 for 2 DIV. Under these low mitogen conditions, the MGE cells rapidly begin to express immature markers for the neuronal lineage, such as βIII-tubulin. For the next 7 DIV, the media conditions were left unchanged for some samples but for others it was changed to BMP4 from control or vice versa. No cells expressing PV were detected when progenitors were allowed to differentiate in control conditions for 9 DIV (Fig. 3A,A′,G), similar to what was observed at 7 DIV (Fig. 2K). If BMP4 acted on early MGE progenitors to promote PV+ interneuron differentiation, we hypothesized that a short 2 DIV treatment with BMP4 might be sufficient to induce PV expression. However, when BMP4 was removed after the initial 2 days of treatment, no PV+ cells were detected even after 7 DIV (Fig. 3C,C′,G). This suggested that BMP4 did not act on an early MGE progenitor to promote a PV fate. By contrast, when BMP4 was introduced into the media for 7 days after 2 DIV in low mitogen, during which time the progenitors partially differentiate, 3.9±0.1% of the neurons expressed PV (Fig. 3B,B′,G), which was comparable to MGE progenitors left for 9 days in BMP4 (4.8±0.5%) (Fig. 3D,D′,G). This further suggested that BMP4 acted to promote a PV+ fate only after progenitors had already committed to the neuronal lineage.
Fig. 3.
BMP4 causes differentiation of already committed precursors but is not sufficient to induce PV lineage commitment from progenitors in vitro. (A-F′) bFGF (20 ng/ml)-responsive neurospheres from E12 MGE were dissociated and plated for 2 days in low bFGF (0.5 ng/ml) alone (Control) or in the presence of BMP4 (20 ng/ml) or SHH (10 nM). After 2 days the media was changed to either low bFGF or BMP4 for the following 7 days. Cells were immunostained for PV (red) and βIII-tubulin (green), with DAPI-labeled nuclei in blue. Note the absence of PV+ interneurons in each condition lacking BMP4 during the final 7 days (A,A′,C,C′,E,E′). BMP4 treatment for the first 2 days after plating was not sufficient to induce PV+ interneuron differentiation (C,C′). SHH treatment during the first 2 days expanded the progenitor pool but was not sufficient to cause PV expression (E,-F′). A=′-F′ are merged images. (G) Quantification of the average percentages of PV+ interneurons under different conditions. **P<0.01, Student's t-test; n.d., not detected. Scale bar: 50 μm.
Another crucial signaling molecule regulating interneuron development is SHH, which regulates the expression of transcription factors such as NKX2.1, DLX1 and DLX2 that maintain the ventral identity of interneuron progenitors (Ericson et al., 1995; Kohtz et al., 1998; Xu et al., 2005). Loss of SHH signaling causes a significant reduction in GABAergic interneurons in the cortex, including interneurons containing PV and SST (Xu et al., 2005). To determine whether SHH could induce PV expression from MGE progenitors, cells plated in low mitogen were given a 2-day pulse of SHH, which was then removed from the media for the next 7 DIV to allow differentiation. Although there was an increase in the number of GABAergic neurons, no PV+ cells could be detected at the end of the 9 DIV (Fig. 3E,E′,G). However, when BMP4 was introduced into the media after the 2-day pulse of SHH, the fraction of neurons that expressed PV doubled to 10.1±0.7% (Fig. 3F,F′,G). This presumably reflects expansion of the ventral progenitors by SHH, which then differentiate in the presence of BMP4 (Zhu et al., 1999b). Overall, these observations suggest that BMP4 is required for PV expression by fate-committed MGE progenitors but is not sufficient for interneuron lineage specification in vitro.
Interneuron precursors that have migrated to the cortex can respond to BMP4
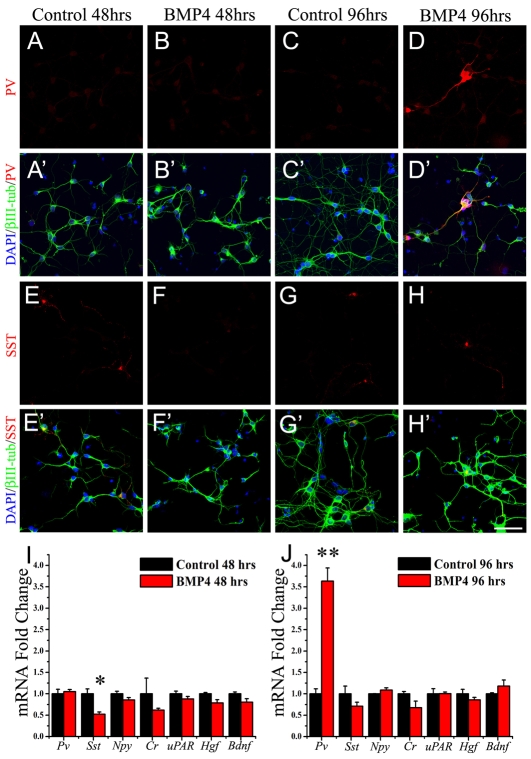
Precursors that give rise to PV+ and SST+ interneurons are present in the cerebral cortex around birth (Cobos et al., 2005; Liodis et al., 2007; Zhao et al., 2008). To determine whether BMP4 can prematurely differentiate interneuron precursors present in the postnatal cortex to a PV+ phenotype, dissociated cells from P0 cortex were treated with BMP4 for 4 DIV. After 2 DIV no PV immunoreactive cells were present in either untreated or BMP-treated cultures (Fig. 4A,B), and there were no differences in the levels of Pv mRNA measured using quantitative real-time PCR (Fig. 4I). However, fewer cells expressing SST were found after 2 days of BMP4 treatment (Fig. 4E-F′), and there was a significant ∼50% reduction in Sst mRNA (Fig. 4I). After 4 DIV PV+ cells could be detected in the BMP4-treated group (Fig. 4D) but were completely absent in untreated controls (Fig. 4C). This remarkable premature expression of PV is almost 10 days earlier than what is seen in vivo (Fig. 1C) and over 3 weeks earlier than what has been reported in studies using cortical neuron cultures (Patz et al., 2004; Xu et al., 2004). There was a corresponding ∼4-fold increase in Pv mRNA expression in the BMP4-treated cultures at 4 days (Fig. 4J). Interestingly, the reduction in Sst mRNA did not persist at 4 DIV (Fig. 4G-H′,J) suggesting that there is a subpopulation of SST+ precursors that do not respond to BMP4 and can thus differentiate. mRNA levels for other interneuron markers, including Cr and neuropeptide Y (Npy), did not differ between BMP4-treated and the untreated conditions on either day (Fig. 4I,J) suggesting that the effects of BMP4 were specific to the PV+ subtype. Overall GABAergic differentiation, assessed by the expression of Gad67 mRNA, was also not affected by BMP4 treatment (data not shown). Loss of the urokinase plasminogen activator receptor (uPAR, Plaur - Mouse Genome Informatics) that is required for hepatocyte growth factor (HGF) activation and function can affect the number of PV+ cells in vivo (Powell et al., 2003). However, we did not observe any change in the mRNA levels of Hgf or uPAR, suggesting that they are not downstream of BMP4-mediated PV expression (Fig. 4I,J). BDNF signaling has also been implicated in the expression of PV in the visual cortex (Huang et al., 1999; Jones et al., 1994), but Bdnf mRNA levels also did not differ between the two conditions (Fig. 4I,J).
Fig. 4.
BMP4 can induce PV expression in cortical neuron cultures. (A-H′) Cortical neuron cultures were prepared from P0 mouse brains and grown in the presence or absence (Control) of BMP4 (20 ng/ml). Cells were immunostained for PV (red, A-D′) or SST (red, E-H′) and βIII-tubulin (green), with DAPI-labeled nuclei in blue. PV immunoreactivity is absent under both conditions at 48 hours after treatment (A-B′). At 96 hours PV immunoreactive cells could be detected only in the BMP4-treated cultures (C-D′). Fewer SST+ interneurons were observed at 48 hours in the BMP-treated (F) compared with the untreated condition (E) but the numbers were similar at 96 hours (G,H). A′-H′ are merged images. (I,J) mRNA levels of Pv, Sst, neuropeptide Y (Npy), calretinin (Cr), urokinase plasminogen activator receptor (uPAR), hepatocyte growth factor (Hgf) and brain-derived neurotrophic factor (Bdnf) were compared in the cortical neuron cultures at 48 hours and 96 hours with or without BMP4 treatment. Note the remarkable increase in Pv mRNA expression 96 hours after BMP4 treatment. *P<0.01, Student's t-test. Scale bar: 50 μm.
To exclude the possibility that BMP4 might be ectopically inducing PV expression in some glutamatergic neurons in cortical cultures, we immunostained cortical cultures after 4 days of BMP4 treatment with GABA and the glutamate transporter VGLUT1 (SLC17A7 - Mouse Genome Informatics). Of the PV+ neurons in culture, the majority colabeled with GABA (82.2±2.9%; see Fig. S3A-A‴ in the supplementary material) and almost none expressed VGLUT1 (2.8±1.4%; see Fig. S3B-B‴ in the supplementary material). To investigate the origin of the precursors in the cortex that can differentiate into PV+ interneurons in the presence of BMP4, we prepared cortical cultures from P0 Olig1cre/+; ROSAGFP/+ mice (Lu et al., 2002; Mao et al., 2001). In these mice, all cells derived from an OLIG1+ lineage are labeled with GFP. OLIG1 and OLIG2 are expressed strongly in the MGE progenitors during development and can give rise to different populations of cortical interneurons (Miyoshi et al., 2007; Samanta et al., 2007). When cortical cultures from P0 Olig1cre/+; ROSAGFP/+ mice were treated with BMP4 for 4 days, almost all (91.9±1.7%) of the PV+ interneurons observed were also positive for GFP (see Fig. S4B-C in the supplementary material), suggesting that they were derived from an OLIG1+ lineage, and constituted 7.3±0.7% of the total GFP+ cells (see Fig. S4D in the supplementary material). Similar to our previous observation, no PV+ cells were observed in the absence of BMP4 treatment (see Fig. S4A-A‴,C,D in the supplementary material).
Hence, BMP4 can prematurely differentiate PV+ interneurons from OLIG1 lineage precursors present in the cerebral cortex at birth through mechanisms independent of changes in BDNF or HGF signaling.
Role of BMP4 signaling in the generation of interneuron subtypes in vivo
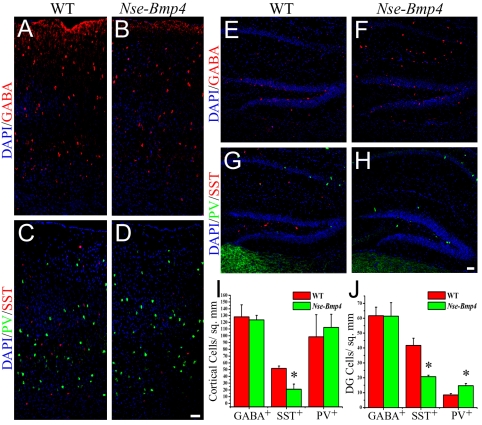
To determine whether BMP4 signaling regulates PV+ interneurons in vivo, we examined interneuron differentiation in mice that overexpress Bmp4 under the control of a neuron specific enolase (Nse, Eno2 - Mouse Genome Informatics) promoter (Nse-Bmp4). These animals overexpress BMP4 abundantly in the hippocampus and at lower levels in the cortex (see Fig. S5 in the supplementary material) (Gomes et al., 2003). Since interneurons in the hippocampus share the same origins as cortical interneurons (Pleasure et al., 2000), we examined interneuron differentiation in the hippocampus. The numbers of GABA-positive interneurons in the dentate gyrus of Nse-Bmp4 animals and wild-type (WT) brains did not differ (Fig. 5E,F,J). However, there was an extraordinary ∼70% increase (WT 8.5±0.8 cells/mm2 versus Nse-Bmp4 14.7±1.5 cells/mm2) in the number of PV-expressing interneurons and ∼50% reduction (WT 41.7±4.9 cells/mm2 versus Nse-Bmp4 20.8±1.0 cells/mm2) in the number of SST-expressing interneurons in the dentate gyrus of the Nse-Bmp4 animals (Fig. 5G,H,J). Thus, BMP4 overexpression in the hippocampus in vivo augments the generation of PV-expressing interneurons at the expense of SST+ interneuron differentiation.
Fig. 5.
Overexpression of BMP4 in vivo affects PV+ and SS+ interneuron differentiation. (A-D) Primary somatosensory cortex of P21 wild-type (WT) and Nse-Bmp4 mice immunostained for GABA (red in A,B) or SST (red in C,D) and PV (green in C,D), with DAPI-labeled nuclei in blue. Note the reduction in SST+ interneurons in the transgenic cortex (D) compared with the WT brain (C). (E-H) Dentate gyrus of the hippocampus of wild-type (WT) and Nse-Bmp4 mice immunostained for GABA (red in E,F) or SST (red in G,H) and PV (green in G,H), with DAPI-labeled nuclei in blue. Note the reduction in SST+ and increase in PV+ interneurons in the transgenic dentate gyrus (H) compared with WT (G). (I,J) Quantification of the immunopositive cells per unit area of the cortex (I) and dentate gyrus (J). *P<0.05, Student's t-test. Scale bars: 50 μm.
We also examined interneuron differentiation in the somatosensory cortex of Nse-Bmp4 mice, where levels of BMP4 overexpression are lower. The total number of GABA-expressing interneurons did not differ between Nse-Bmp4 and WT animals (Fig. 5A,B,I). However, there was a ∼60% reduction (WT 51.8±3.6 cells/mm2 versus Nse-Bmp4 21.1±7.6 cells/mm2) in the number of SST-expressing interneurons in the somatosensory cortex of Nse-Bmp4 compared with WT mice. There was no significant change in the number of PV+ interneurons (Fig. 5C,D,I), suggesting that the suppression of SST+ interneuron differentiation requires lower doses of BMP4 than for the stimulation of PV expression. Alternatively, the cortical expression of the Nse transgene might coincide with the earlier onset of SST expression in the cortex, thus preferentially affecting the number of SST interneurons.
PV+ and SST+ interneurons express BMP type I receptors
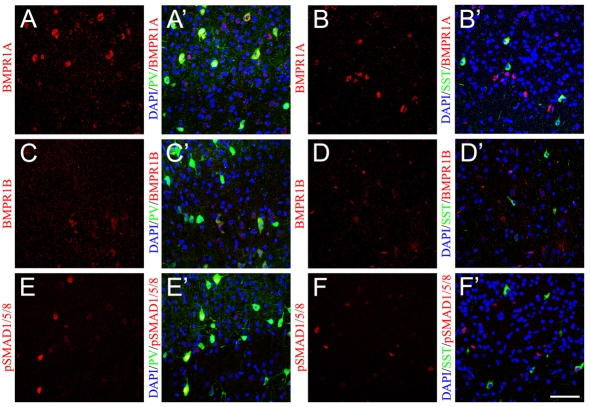
BMPs signal by binding to the high affinity type I receptors (BMPR1A, BMPR1B, ALK2), which then bind to the BMP type II receptor (BMPR2). BMPR2 phosphorylates and activates BMPR1, which phosphorylates downstream molecules like the R-Smads (SMAD1/5/8) that can bind Co-Smads (SMAD4) that then translocate to the nucleus and bind to target genes. BMPR1A is expressed widely in the postnatal mouse brain, whereas BMPR1B has a more limited expression (Zhang et al., 1998). Interestingly, immunostaining for BMPR1A demonstrated that the almost all PV+ (Fig. 6A,A′) and SST+ (Fig. 6B,B′) interneurons expressed BMPR1A at P14. BMPR1B was expressed at lower levels and was restricted to subpopulations of PV+ (Fig. 6C,C′) and SST+ (Fig. 6D,D′) interneurons. Similar limited expression of BMPR1B and a broader expression of BMPR1A in SST+ interneurons was observed at P0 and P7 (see Fig. S6A-D′ in the supplementary material). Thus the expression patterns of the BMP type I receptors did not explain the differential actions of BMP4 on their respective precursors in the cortex.
Fig. 6.
A subpopulation of PV+ interneurons is exposed to strong BMP4 signaling in vivo. (A-B′) BMPR1A (red) is expressed at high levels in PV+ (green, A′) and SST+ (green, B′) interneurons at P14. (C-D′) BMPR1B (red) is expressed at lower levels and is restricted to subpopulations of PV+ (C′) and SST+ (D′) interneurons at P14. (E-F′) Strong nuclear pSMAD1/5/8 (red) staining is present in a subpopulation of PV+ (E′) but is rarely seen in SST+ (F′) interneurons. A′-F′ are merged images. Scale bar: 50 μm.
Recently, lineage tracing using different regulatory elements of the Dlx genes has shown that two distinct precursor populations from the MGE take different migratory routes to the cortex (Ghanem et al., 2007). Although both precursor populations generate PV+ interneurons, only one of them gives rise to majority of the SST+ interneurons in the cortex. This suggests that two subpopulations of PV+ interneuron precursors take different migratory routes to the cortex and might be exposed to different signals. Indeed, when we coimmunostained for pSMAD1/5/8 at P14, only a subpopulation of PV+ interneurons showed intense nuclear staining (Fig. 6E,E′). Conversely, SST+ cells rarely had nuclear pSMAD1/5/8 and showed a more diffuse cytoplasmic staining (Fig. 6F,F′). Since SST+ interneurons appear earlier in the cortex, we also examined the SST+ interneurons at P0 and P7 for nuclear pSMAD1/5/8. At both P0 and P7 nuclear pSMAD1/5/8 was rarely observed in SST+ interneurons (see Fig. S6E-F′ in the supplementary material). This suggests that SST+ cells in vivo are not exposed to significant levels of BMPs, whereas a subset of PV+ cells receive strong BMP signals.
BMPR1 signaling is required for the differentiation of a subpopulation of PV+ interneurons
Since both the PV+ and SST+ interneurons expressed BMP type I receptors, we hypothesized that BMP type I receptors might be involved in mediating the effects of BMP4. We have shown previously that loss of BMPR1A alone does not affect the number of PV interneurons in the cortex (Samanta et al., 2007). Since Bmpr1a null animals have functional BMPR1B receptors, BMPR1B might compensate for the loss of BMPR1A. To investigate whether the combined loss of BMPR1A and BMPR1B can affect the maturation of PV interneurons, we generated Bmpr1a/b double null (Olig1cre/+; Bmpr1afx/-; Bmpr1b-/-) animals that have both Bmpr1a and Bmpr1b ablated in OLIG1 lineage cells (Lu et al., 2002; Yi et al., 2000). As Olig1/2-expressing cells can give rise to cortical interneurons, including those expressing PV (Miyoshi et al., 2007; Samanta et al., 2007), and the PV+ cells observed in vitro were from an OLIG1+ lineage (see Fig. S4 in the supplementary material), the loss of BMP type I receptors in the OLIG1 lineage would affect the subsequent differentiation of PV+ interneurons in the somatosensory cortex.
The Bmpr1a/b mutants were compared with littermate controls (Olig1cre/+; Bmpr1afx/+; Bmpr1b+/-), which were heterozygous for Bmpr1a and Bmpr1b in the OLIG1 lineage, for changes in the number of interneuron subtypes in the cortex. At P0, when some SST+ interneurons can be observed in the cortex, the Bmpr1a/b mutants had a strong trend towards an increase in SST+ interneurons in the somatosensory cortex (see Fig. S7C in the supplementary material). Regions of the ventrolateral cortex that include the piriform area contain a large population of SST+ interneurons at P0 (see Fig. S1 in the supplementary material). When comparing Bmpr1a/b null brains with control brains, we found a significant ∼35% increase (control 223.1±16.5 cells/mm2 versus Bmpr1a/b mutant 300.5±18.6 cells/mm2) in the number of SST+ interneurons in the piriform area. At a later time point, P21, we found that the increased number of SST+ interneurons seen at P0 did not persist in the Bmpr1a/b double mutant brain (Fig. 7A-C). In the absence of increased cell death in the mutant cortex at P21 (data not shown), this suggests a premature expression of SST in interneurons at P0 in the absence of BMP type I receptor signaling. However, the Bmpr1a/b animals had a ∼17% reduction (control 93.5±2.9 cells/mm2 versus Bmpr1a/b mutant 77.8±2.7 cells/mm2) in PV+ interneurons in the somatosensory cortex compared with the control animals (Fig. 7A,B,E). There were no significant differences in the total numbers of GABA+ interneurons in the somatosensory cortex (see Fig. S8A,B in the supplementary material) between controls and Bmpr1a/b double null animals. This confirms the limited role for BMP4 signaling in overall GABAergic interneuron differentiation. Thus, BMPR1A/B signaling mediates the effects of BMP4 on differentiation of a subset of PV+ interneurons, and the absence of BMPR1A/B signaling can cause premature differentiation of SST+ interneurons.
Fig. 7.
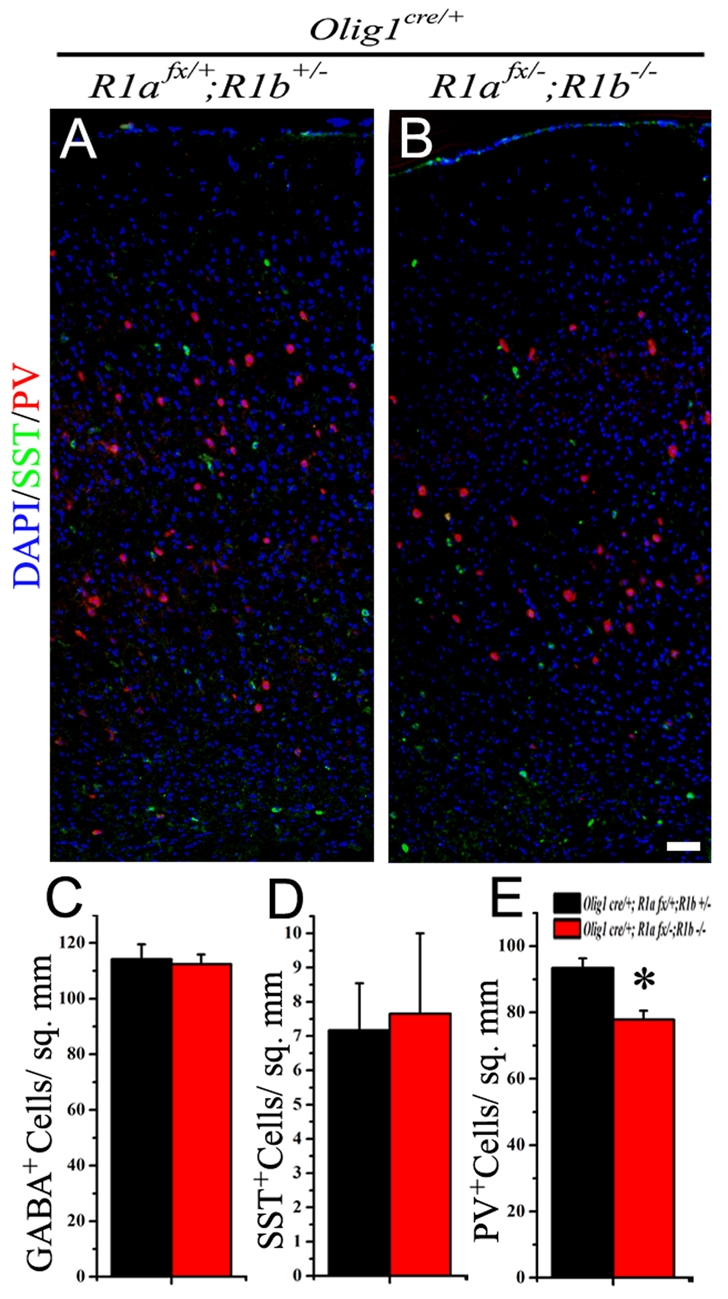
Loss of BMP type I receptors reduces the number of PV+ interneurons in the cortex. (A,B) The number of PV+ interneurons (red) in the somatosensory cortex of Bmpr1a/b double null mice (B, R1afx/-; R1b-/-) are reduced compared with the control brains (A, R1afx/+; R1b+/-) but the number of SST+ interneurons (green) is unchanged (A,B). (C-E) Quantification of GABA+, SST+ and PV+ cells for the control (black) and Bmpr1a/b null (red) mice. *P<0.05, Student's t-test. Scale bar: 50 μm.
DISCUSSION
Although we find that BMP signaling regulates interneuron subtype specification, we do not find any role for it in the primary specification of the GABAergic lineage in vitro or in vivo. The numbers of GABA-expressing cells in the cortex of both BMP4-overexpressing mice and Bmpr1a/b double null mutants are comparable to controls. Although BMPs have been reported to increase GABAergic lineage commitment from MGE progenitors (Yung et al., 2002), our studies indicate that the effect of BMPs on progenitor cells is to promote neuronal lineage commitment and hence more GABAergic neurons. Thus we found that BMP4 treatment significantly increased the numbers both of neurons (βIII-tubulin+) and GABAergic interneurons (GABA+/βIII-tubulin+) generated from the E12.5 MGE-derived progenitors, but did not change the overall percentage of GABAergic interneurons. This is consistent with prior studies that have shown the proneuronal effect of BMP signaling (Li et al., 1998; Mabie et al., 1999; Zhu et al., 1999a).
Although BMP4 does not affect GABAergic lineage commitment, it has differential effects on PV+ and SST+ interneuron differentiation. Our studies in vitro suggest that BMP4 acts on interneuron precursors that are derived from progenitors that can be expanded by SHH signaling and have already been specified to induce PV expression. Treatment of P0 cortical cultures with BMP4 caused premature PV expression, suggesting that some OLIG1 lineage precursors present are responsive to BMP signaling even at that time. In turn, this suggests that it is the appearance of the BMP4 ligand in the cortex that is responsible for the induction of PV and this is very dose-dependent. This is supported by the observation that Nse-Bmp4 mice had significantly more PV+ interneurons in the hippocampus. In the WT brain, BMP4 expression is lower in the hippocampus than in the cortex, and hence ectopic overexpression of BMP4 in the Nse-Bmp4 transgenic animals had a greater effect on PV+ interneuron differentiation in the hippocampus.
Null mutation of Bmpr1a/b reduced the overall number of PV+ interneurons, but many PV+ cells remained in these brains. There are a number of possible reasons for why PV+ interneurons still developed in these animals. First, a third type 1 BMP receptor, ALK2, is expressed in the brain, albeit at low abundance, and it is possible that this receptor compensated for the loss of BMPR1A/B. Second, it is possible that there is a second population of PV+ interneurons that is not dependent upon BMP signaling and that partially compensates for the loss of the BMP-responsive population. Although the Bmpr1a/b double mutants had a reduced number of PV+ interneurons, there was no reduction in the overall size of the GABA+ population. The similar numbers of GABA+ interneurons and the lack of increased cell death (data not shown) suggest that the cells that do not differentiate to PV+ interneurons survive and express GABA. From our studies it does not appear that these cells switch their subtype to SST+ interneurons in the absence of BMPR1 signaling and might instead be converted to some other subtype. The Bmpr1a/b double mutants die between P17 and P24, which prevents us from analyzing the number of PV+ interneurons in the adult animal to examine if compensatory mechanisms would eventually restore the numbers of PV+ interneurons to normal levels.
PV expression in cortical interneurons has been linked to synaptic activity and the resulting increase in levels of BDNF in the cortex (Huang et al., 1999; Itami et al., 2007), and Bdnf mutants have reduced levels of PV+ interneurons but normal numbers of GABA+ interneurons (Jones et al., 1994). This is similar to what we observe in the Bmpr1a/b double mutants. There were no changes in BDNF expression after BMP4 treatment of cultured cells, suggesting that BMP does not act by regulating BDNF expression. However, BMP4 expression peaks in the cortex around the same time as BDNF, suggesting that BMP4 and BDNF might cooperate in the differentiation of the PV+ interneurons in the cortex. Similar cooperative effects between BMPs and BDNF have been reported for other cell populations in the brain, and some of the effects of BMPs may have been mediated through BDNF (Galter et al., 1999; Gratacos et al., 2001). Unlike BDNF, little is known about the effects of BMP4 signaling on synaptic plasticity, or whether BMP4 expression is regulated by synaptic activity, although recently BMP signaling was implicated in enhancing synaptic plasticity in the hippocampus (Sun et al., 2007). Very little PV expression can be seen in organotypic cultures deprived of activity even after 28 DIV, and BDNF was unable to upregulate PV expression in such cultures (Patz et al., 2004; Xu et al., 2004). We observe PV expression in vitro 4 days after BMP4 treatment. As PV expression is thought to be activity-dependent, BMP4 possibly increases the spontaneous neuronal activity in culture, thus accelerating the expression of PV. Indeed, increased BMP signaling resulting from mutation of the BMP antagonist chordin in the hippocampal neurons or the application of BMP ligands enhances presynaptic transmitter release (Sun et al., 2007).
In contrast to the effects on PV expression, BMP signaling suppressed SST interneuron differentiation after BMP4 treatment of cultured MGE progenitor cells and cortical cultures, and after overexpression of BMP4 in vivo. A simple interpretation would be that BMP signaling converts interneurons from a SST+ to a PV+ phenotype, but our results do not support this conclusion. First, in the MGE progenitor cultures, the number of PV+ cells induced by BMP4 treatment did not precisely match the decrease in the number of SST+ cells, and in the cortical cultures the timing of the effects on SST and PV expression differed. Second, the decrease in SST+ interneurons in the cortex of Nse-Bmp4 mice was not accompanied by an increase in PV+ interneurons. Further, there was no increase in SST+ cells in the cortex of the Bmpr1a/b double mutant at P21 when we observed a reduction in PV+ interneurons. The dosage of BMP4 required to suppress SST expression also appears to be lower than that required to induce PV expression. In the P0 cortex, when levels of BMP4 are lower relative to the P14 cortex, mutation of Bmpr1a/b causes a premature expression of SST from the precursors, suggesting that even at the relatively lower levels BMP4 can suppress SST expression. Together these observations suggest that the effects of BMP4 on SST+ interneuron differentiation are by its action on a different population of precursors committed to become SST+ interneurons. This is consistent with previous studies that have shown that PV+ and SST+ cells differentiate from separate pools of committed precursors (Flames et al., 2007; Fogarty et al., 2007; Wonders et al., 2008).
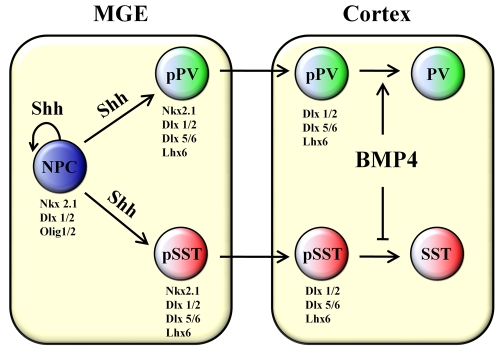
Our observations propose a novel role for BMP4 whereby it regulates the interneuron subtype differentiation of committed precursors in the cerebral cortex (Fig. 8). By contrast, BMP signaling appears to regulate the numbers of calbindin-containing interneurons by acting directly on the proliferation of subventricular zone progenitor cells (Samanta et al., 2007). Our findings are similar to the reported effects of BMPs on hypothalamic dopaminergic neuron differentiation of fate-committed subpallial progenitors (Ohyama et al., 2005). Interestingly, like the PV+ and SST+ interneurons in the cortex, the hypothalamic dopaminergic neurons are also derived from an NKX2.1 lineage. In our model (Fig. 8) increased expression of BMP4 in the cortex during the second postnatal week is required for the differentiation of a subpopulation of PV+ interneurons from the postmigratory PV precursor pool. In the PV+ population of interneurons, morphologically distinct subsets like the chandelier and large basket cells are present that exhibit fast spiking firing patterns, and the SST+ positive population includes small basket cells and Martinotti cells that exhibit regular spiking and burst spiking, respectively (Butt et al., 2005; DeFelipe et al., 1989; Kubota and Kawaguchi, 1994). Many PV+ interneurons also express the potassium channel KV3.1 (KCNC1 - Mouse Genome Informatics) and SST+ interneurons coexpress CR or NPY (Kawaguchi and Kondo, 2002; Wonders and Anderson, 2006). Although BMP4 can influence the expression of PV and SST, it remains to be investigated whether other molecular and physiological properties of these interneuron subtypes are also differentially affected by BMP signaling. Future studies addressing these questions will elucidate the broader role of BMP signaling in the maintenance of cortical interneuron diversity.
Fig. 8.
Model for the effects of BMP4 on two populations of MGE-derived interneuron precursors. The PV+ and SST+ interneuron precursors (pPV and pSST, respectively) derived from the neural progenitor cells (NPC), migrate tangentially from the MGE to the cortex after downregulation of Olig1/2 and Nkx2.1 expression. BMP4 enhances the differentiation of PV+ interneurons (solid arrow) and inhibits the differentiation of SST+ interneurons (inhibitory bar) from their precursors. The genes expressed in each cell type are indicated.
Competing interests statement
There are no competing interests or other relevant issues to disclose.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/15/2633/DC1
Supplementary Material
We thank Yuji Mishina for providing the Bmpr1a mice; David Rowitch for the Olig1-cre, and Karen Lyons and Henry Kronenberg for the Bmpr1b mouse lines. This project was supported by NIH grants NS 20013 and NS 20778. Deposited in PMC for release after 12 months.
References
- Anderson, S. A., Eisenstat, D. D., Shi, L. and Rubenstein, J. L. (1997). Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278, 474-476. [DOI] [PubMed] [Google Scholar]
- Butt, S. J., Fuccillo, M., Nery, S., Noctor, S., Kriegstein, A., Corbin, J. G. and Fishell, G. (2005). The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron 48, 591-604. [DOI] [PubMed] [Google Scholar]
- Chojnacki, A. and Weiss, S. (2004). Isolation of a novel platelet-derived growth factor-responsive precursor from the embryonic ventral forebrain. J. Neurosci. 24, 10888-10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos, I., Calcagnotto, M. E., Vilaythong, A. J., Thwin, M. T., Noebels, J. L., Baraban, S. C. and Rubenstein, J. L. (2005). Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat. Neurosci 8, 1059-1068. [DOI] [PubMed] [Google Scholar]
- DeFelipe, J. (1997). Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J. Chem. Neuroanat. 14, 1-19. [DOI] [PubMed] [Google Scholar]
- DeFelipe, J., Hendry, S. H. and Jones, E. G. (1989). Visualization of chandelier cell axons by parvalbumin immunoreactivity in monkey cerebral cortex. Proc. Natl. Acad. Sci. USA 86, 2093-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson, J., Muhr, J., Placzek, M., Lints, T., Jessell, T. M. and Edlund, T. (1995). Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell 81, 747-756. [DOI] [PubMed] [Google Scholar]
- Flames, N., Pla, R., Gelman, D. M., Rubenstein, J. L., Puelles, L. and Marin, O. (2007). Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J. Neurosci. 27, 9682-9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty, M., Grist, M., Gelman, D., Marin, O., Pachnis, V. and Kessaris, N. (2007). Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J. Neurosci. 27, 10935-10946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galter, D., Bottner, M., Krieglstein, K., Schomig, E. and Unsicker, K. (1999). Differential regulation of distinct phenotypic features of serotonergic neurons by bone morphogenetic proteins. Eur. J. Neurosci. 11, 2444-2452. [DOI] [PubMed] [Google Scholar]
- Ghanem, N., Yu, M., Long, J., Hatch, G., Rubenstein, J. L. and Ekker, M. (2007). Distinct cis-regulatory elements from the Dlx1/Dlx2 locus mark different progenitor cell populations in the ganglionic eminences and different subtypes of adult cortical interneurons. J. Neurosci. 27, 5012-5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, W. A., Mehler, M. F. and Kessler, J. A. (2003). Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev. Biol. 255, 164-177. [DOI] [PubMed] [Google Scholar]
- Gonchar, Y. and Burkhalter, A. (1997). Three distinct families of GABAergic neurons in rat visual cortex. Cereb. Cortex 7, 347-358. [DOI] [PubMed] [Google Scholar]
- Gratacos, E., Checa, N., Perez-Navarro, E. and Alberch, J. (2001). Brain-derived neurotrophic factor (BDNF) mediates bone morphogenetic protein-2 (BMP-2) effects on cultured striatal neurones. J. Neurochem. 79, 747-755. [DOI] [PubMed] [Google Scholar]
- Gross, R. E., Mehler, M. F., Mabie, P. C., Zang, Z., Santschi, L. and Kessler, J. A. (1996). Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 17, 595-606. [DOI] [PubMed] [Google Scholar]
- Gulacsi, A. and Lillien, L. (2003). Sonic hedgehog and bone morphogenetic protein regulate interneuron development from dorsal telencephalic progenitors in vitro. J. Neurosci. 23, 9862-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Z. J., Kirkwood, A., Pizzorusso, T., Porciatti, V., Morales, B., Bear, M. F., Maffei, L. and Tonegawa, S. (1999). BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739-755. [DOI] [PubMed] [Google Scholar]
- Itami, C., Kimura, F. and Nakamura, S. (2007). Brain-derived neurotrophic factor regulates the maturation of layer 4 fast-spiking cells after the second postnatal week in the developing barrel cortex. J. Neurosci. 27, 2241-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, K. R., Farinas, I., Backus, C. and Reichardt, L. F. (1994). Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell 76, 989-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi, Y. and Kondo, S. (2002). Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J. Neurocytol. 31, 277-287. [DOI] [PubMed] [Google Scholar]
- Koenig, B. B., Cook, J. S., Wolsing, D. H., Ting, J., Tiesman, J. P., Correa, P. E., Olson, C. A., Pecquet, A. L., Ventura, F., Grant, R. A. et al. (1994). Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol. Cell. Biol. 14, 5961-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtz, J. D., Baker, D. P., Corte, G. and Fishell, G. (1998). Regionalization within the mammalian telencephalon is mediated by changes in responsiveness to Sonic Hedgehog. Development 125, 5079-5089. [DOI] [PubMed] [Google Scholar]
- Kubota, Y. and Kawaguchi, Y. (1994). Three classes of GABAergic interneurons in neocortex and neostriatum. Jpn J. Physiol. 44 Suppl. 2, S145-S148. [PubMed] [Google Scholar]
- Lavdas, A. A., Grigoriou, M., Pachnis, V. and Parnavelas, J. G. (1999). The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J. Neurosci. 19, 7881-7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Cogswell, C. A. and LoTurco, J. J. (1998). Neuronal differentiation of precursors in the neocortical ventricular zone is triggered by BMP. J. Neurosci. 18, 8853-8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, D. A., Tramontin, A. D., Trevejo, J. M., Herrera, D. G., Garcia-Verdugo, J. M. and Alvarez-Buylla, A. (2000). Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 28, 713-726. [DOI] [PubMed] [Google Scholar]
- Liodis, P., Denaxa, M., Grigoriou, M., Akufo-Addo, C., Yanagawa, Y. and Pachnis, V. (2007). Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J. Neurosci. 27, 3078-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F., Ventura, F., Doody, J. and Massague, J. (1995). Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol. Cell. Biol. 15, 3479-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Q. R., Sun, T., Zhu, Z., Ma, N., Garcia, M., Stiles, C. D. and Rowitch, D. H. (2002). Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109, 75-86. [DOI] [PubMed] [Google Scholar]
- Mabie, P. C., Mehler, M. F. and Kessler, J. A. (1999). Multiple roles of bone morphogenetic protein signaling in the regulation of cortical cell number and phenotype. J. Neurosci. 19, 7077-7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, X., Fujiwara, Y., Chapdelaine, A., Yang, H. and Orkin, S. H. (2001). Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood 97, 324-326. [DOI] [PubMed] [Google Scholar]
- Mishina, Y., Hanks, M. C., Miura, S., Tallquist, M. D. and Behringer, R. R. (2002). Generation of Bmpr/Alk3 conditional knockout mice. Genesis 32, 69-72. [DOI] [PubMed] [Google Scholar]
- Miyoshi, G., Butt, S. J., Takebayashi, H. and Fishell, G. (2007). Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J. Neurosci. 27, 7786-7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama, K., Ellis, P., Kimura, S. and Placzek, M. (2005). Directed differentiation of neural cells to hypothalamic dopaminergic neurons. Development 132, 5185-5197. [DOI] [PubMed] [Google Scholar]
- Patz, S., Grabert, J., Gorba, T., Wirth, M. J. and Wahle, P. (2004). Parvalbumin expression in visual cortical interneurons depends on neuronal activity and TrkB ligands during an Early period of postnatal development. Cereb. Cortex 14, 342-351. [DOI] [PubMed] [Google Scholar]
- Pleasure, S. J., Anderson, S., Hevner, R., Bagri, A., Marin, O., Lowenstein, D. H. and Rubenstein, J. L. (2000). Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons. Neuron 28, 727-740. [DOI] [PubMed] [Google Scholar]
- Powell, E. M., Campbell, D. B., Stanwood, G. D., Davis, C., Noebels, J. L. and Levitt, P. (2003). Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J. Neurosci. 23, 622-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta, J., Burke, G. M., McGuire, T., Pisarek, A. J., Mukhopadhyay, A., Mishina, Y. and Kessler, J. A. (2007). BMPR1a signaling determines numbers of oligodendrocytes and calbindin-expressing interneurons in the cortex. J. Neurosci. 27, 7397-7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai, Y., Lu, B., Steinbeisser, H. and De Robertis, E. M. (1995). Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 376, 333-336. [DOI] [PubMed] [Google Scholar]
- Sun, M., Thomas, M. J., Herder, R., Bofenkamp, M. L., Selleck, S. B. and O'Connor, M. B. (2007). Presynaptic contributions of chordin to hippocampal plasticity and spatial learning. J. Neurosci. 27, 7740-7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke, P., Yamashita, H., Sampath, T. K., Reddi, A. H., Estevez, M., Riddle, D. L., Ichijo, H., Heldin, C. H. and Miyazono, K. (1994). Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J. Biol. Chem 269, 16985-16988. [PubMed] [Google Scholar]
- Walsh, C. and Cepko, C. L. (1992). Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science 255, 434-440. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Toledo-Rodriguez, M., Gupta, A., Wu, C., Silberberg, G., Luo, J. and Markram, H. (2004). Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J. Physiol. 561, 65-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle, H., Garcia-Verdugo, J. M., Herrera, D. G. and Alvarez-Buylla, A. (1999). Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat. Neurosci. 2, 461-466. [DOI] [PubMed] [Google Scholar]
- Wichterle, H., Turnbull, D. H., Nery, S., Fishell, G. and Alvarez-Buylla, A. (2001). In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development 128, 3759-3571. [DOI] [PubMed] [Google Scholar]
- Wine-Lee, L., Ahn, K. J., Richardson, R. D., Mishina, Y., Lyons, K. M. and Crenshaw, E. B., III. (2004). Signaling through BMP type 1 receptors is required for development of interneuron cell types in the dorsal spinal cord. Development 131, 5393-5403. [DOI] [PubMed] [Google Scholar]
- Wonders, C. P. and Anderson, S. A. (2006). The origin and specification of cortical interneurons. Nat. Rev. Neurosci. 7, 687-696. [DOI] [PubMed] [Google Scholar]
- Wonders, C. P., Taylor, L., Welagen, J., Mbata, I. C., Xiang, J. Z. and Anderson, S. A. (2008). A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev. Biol. 314, 127-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Q., Cobos, I., De La Cruz, E., Rubenstein, J. L. and Anderson, S. A. (2004). Origins of cortical interneuron subtypes. J. Neurosci. 24, 2612-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Q., Wonders, C. P. and Anderson, S. A. (2005). Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development 132, 4987-4998. [DOI] [PubMed] [Google Scholar]
- Yi, S. E., Daluiski, A., Pederson, R., Rosen, V. and Lyons, K. M. (2000). The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development 127, 621-630. [DOI] [PubMed] [Google Scholar]
- Yung, S. Y., Gokhan, S., Jurcsak, J., Molero, A. E., Abrajano, J. J. and Mehler, M. F. (2002). Differential modulation of BMP signaling promotes the elaboration of cerebral cortical GABAergic neurons or oligodendrocytes from a common sonic hedgehog-responsive ventral forebrain progenitor species. Proc. Natl. Acad. Sci. USA 99, 16273-16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D., Mehler, M. F., Song, Q. and Kessler, J. A. (1998). Development of bone morphogenetic protein receptors in the nervous system and possible roles in regulating trkC expression. J. Neurosci. 18, 3314-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Feng, X., We, R. and Derynck, R. (1996). Receptor-associated Mad homologues synergize as effectors of the TGF-beta response. Nature 383, 168-172. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Musci, T. and Derynck, R. (1997). The tumor suppressor Smad4/DPC 4 as a central mediator of Smad function. Curr. Biol. 7, 270-276. [DOI] [PubMed] [Google Scholar]
- Zhao, Y., Flandin, P., Long, J. E., Cuesta, M. D., Westphal, H. and Rubenstein, J. L. (2008). Distinct molecular pathways for development of telencephalic interneuron subtypes revealed through analysis of Lhx6 mutants. J. Comp. Neurol. 510, 79-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. X., Zhao, M., Li, D., Shimazu, K., Sakata, K., Deng, C. X. and Lu, B. (2003). Cerebellar deficits and hyperactivity in mice lacking Smad4. J. Biol. Chem. 278, 42313-42320. [DOI] [PubMed] [Google Scholar]
- Zhu, G., Mehler, M. F., Mabie, P. C. and Kessler, J. A. (1999a). Developmental changes in progenitor cell responsiveness to cytokines. J. Neurosci. Res. 56, 131-145. [DOI] [PubMed] [Google Scholar]
- Zhu, G., Mehler, M. F., Zhao, J., Yu Yung, S. and Kessler, J. A. (1999b). Sonic hedgehog and BMP2 exert opposing actions on proliferation and differentiation of embryonic neural progenitor cells. Dev. Biol. 215, 118-129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.