Summary
In mammalian embryos, male and female external genitalia develop from the genital tubercle. Outgrowth of the genital tubercle is maintained by the urethral epithelium, and it has been reported that Fgf8 mediates this activity. To test directly whether Fgf8 is required for external genital development, we conditionally removed Fgf8 from the cloacal/urethral epithelium. Surprisingly, Fgf8 is not necessary for initiation, outgrowth or normal patterning of the external genitalia. In early genital tubercles, we found no redundant Fgf expression in the urethral epithelium, which contrasts with the situation in the apical ectodermal ridge (AER) of the limb. Analysis of Fgf8 pathway activity showed that four putative targets are either absent from early genital tubercles or are not regulated by Fgf8. We therefore examined the distribution of Fgf8 protein and report that, although it is present in the AER, Fgf8 is undetectable in the genital tubercle. Thus, Fgf8 is transcribed, but the signaling pathway is not activated during normal genital development. A phylogenetic survey of amniotes revealed Fgf8 expression in genital tubercles of eutherian and metatherian mammals, but not turtles or alligators, indicating that Fgf8 expression is neither a required nor a conserved feature of amniote external genital development. The results indicate that Fgf8 expression is an early readout of the genital initiation signal rather than the signal itself. We propose that induction of external genitalia involves an epithelial-epithelial interaction at the cloacal membrane, and suggest that the cloacal ectoderm may be the source of the genital initiation signal.
Keywords: Evolution, Fgf8, Genitalia, Wnt5a, Cloaca
INTRODUCTION
The genital tubercle is the developmental precursor to the male and female external genitalia. Its outgrowth and patterning have been likened to the vertebrate limb bud, but unlike the limb, the genital tubercle is composed of cells from all three germ layers (Seifert et al., 2008). Initiation of genital outgrowth begins at approximately E10.5, when paired genital swellings appear on either side of the cloacal membrane (Perriton et al., 2002). The paired swellings are joined by an anterior swelling a day later and collectively these form the genital tubercle. Signaling between the endoderm of the embryonic cloaca (which gives rise to the urethral epithelium) and the surrounding mesoderm is necessary for outgrowth and patterning of the genital tubercle. Mice lacking both copies of the sonic hedgehog (Shh) gene initiate outgrowth of the paired swellings, but these fail to form a genital tubercle and the mice exhibit complete agenesis of the external genitalia (Haraguchi et al., 2001; Perriton et al., 2002). Although Shh is required for the maintenance of genital outgrowth, it is not the early initiation signal.
In the vertebrate limb bud, fibroblast growth factors (Fgfs) 8 and 10 mediate the initiation of budding, and sustained outgrowth of the limb is controlled by Fgf signaling from the apical ectodermal ridge (AER) (Mariani and Martin, 2003; Ng et al., 1999). In the genital tubercle, Fgf8 is expressed in the distal urethral epithelium (Haraguchi et al., 2000; Perriton et al., 2002). Previous work identified Fgf8 as a genital outgrowth signal and compared its role to that during limb development (Haraguchi et al., 2000). It was reported that removal of the distal urethral epithelium causes the arrest of genital outgrowth in organ culture, and that application of Fgf8-loaded beads can restore (or at least augment) gene expression and tubercle outgrowth (Haraguchi et al., 2000). Furthermore, treatment of cultured tubercles with Fgf8-neutralizing antibody may inhibit development (Haraguchi et al., 2000). This and subsequent studies led to the suggestion that Fgf8 expression in the distal urethral epithelium is required for outgrowth of the genitalia (Haraguchi et al., 2000; Haraguchi et al., 2001; Haraguchi et al., 2007; Morgan et al., 2003; Ogino et al., 2001; Perriton et al., 2002; Satoh et al., 2004; Suzuki et al., 2003; Suzuki et al., 2008; Yamada et al., 2006). Although Fgf8 is widely held to be the outgrowth signal, its function in genital development has not been examined genetically, in part because Fgf8 null embryos die prior to genital tubercle initiation (Meyers et al., 1998). Here we provide a direct test of the hypotheses that Fgf8 is required for initiation, outgrowth and normal development of the external genitalia.
MATERIALS AND METHODS
Mice
Mouse strains used in this study have been described previously: ShhGFPcre (Harfe et al., 2004); Fgf8fl/fl (Lewandoski et al., 2000), Fgf4fl/fl (Sun et al., 2000) and Wnt5a-/- (Yamaguchi et al., 1999). We generated mice lacking Fgf8 in the genital tubercle by crossing ShhGFPcre;Fgf8fl/+males to Fgf8fl/fl females, and denote these mice as Fgf8 cKO (conditional knockout) mutants in the text. In one instance, a Fgf8 cKO embryo was recovered from a cross of a ShhGFPcre;Fgf8fl/+;Fgf4fl/+ male to a Fgf8fl/fl;Fgf4fl/fl female (Fgf4 is not expressed in the genital tubercle, and therefore its removal had no effect on the Fgf8 phenotype). Genitalia of ShhGFPcre;Fgf8fl/+ and Wnt5a+/- heterozygous animals were phenotypically normal and age-matched littermates with these genotypes were used as controls. Mice were genotyped as described previously (Harfe et al., 2004; Lewandoski et al., 2000; Yamaguchi et al., 1999).
In situ hybridization and analysis of cell death
In situ hybridization was performed as described previously (Perriton et al., 2002). In order to detect Fgf8 expression in Sus scrofa we used a pig-specific probe cloned and kindly provided by Brooke Armfield (NEOUCOM, Rootstown, OH, USA) and Zhengui Zheng (University of Florida, Gainesville, FL, USA); for detection in Monodelphis domesticus we used an opossum-specific probe kindly provided by Anna Keyte and Kathleen Smith (Duke University, Durham, NC, USA); for detection in both Trachemys scripta and Alligator mississippiensis we used an Fgf8 probe cloned from T. scripta and kindly provided by Scott Gilbert (Swarthmore College, Swarthmore, PA, USA). Mouse probes used in this study were Fgf8 (G. Martin, UCSF, San Francisco, CA, USA), Shh, Wnt5a (A. McMahon, Harvard University, Cambridge, MA, USA), Bmp4, Etv4, Etv5 (B. Hogan, Duke University, Durham, NC, USA), Dusp6 (J. C. Izpisua Belmonte, Salk Institute, La Jolla, CA, USA), Spry4 (B. Harfe, University of Florida, Gainesville, FL, USA) and Hoxd13 (D. Duboule, University of Geneva, Geneva, Switzerland). Cell death was assayed using Lysotracker Red (Invitrogen) according to the manufacturer's protocol.
Immunohistochemistry
Embryos were fixed in 2% paraformaldehyde overnight, washed in PBS, cryoprotected in 30% sucrose and embedded in OCT. Sections were cut at 10 μm. We performed immunohistochemistry as described previously (Thewissen et al., 2006). Briefly, we performed antigen retrieval by autoclaving in sodium citrate, followed by cooling on ice, washing with PBT, quenching of endogenous peroxidase with 2% hydrogen peroxide, blocking in 3% rabbit serum/PBT, and overnight incubation at 4°C with anti-fibroblast growth factor 8 (1:1000, Santa Cruz, sc-6958). Sections were washed in PBT, incubated with Vectastain HRP anti-rabbit ABC kit and detected with activated DAB. In addition, negative controls were performed without a primary antibody.
RESULTS
Fgf8 expression is associated with genital tubercle initiation in Wnt5a null mice
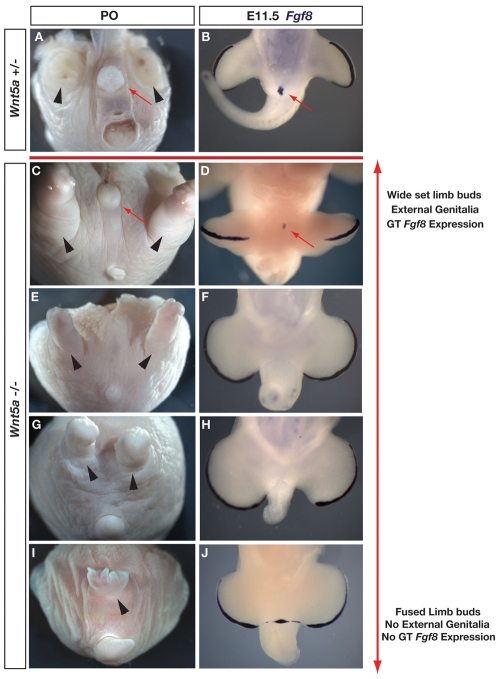
In order to address the role of Fgf8 during genital tubercle outgrowth, we first examined Wnt5a-/- mice, in which genital outgrowth is initiated but not maintained (Yamaguchi et al., 1999; Suzuki et al., 2003). Surprisingly, in newborn (P0) male and female Wnt5a-/- mice, we found that external genitalia can develop, although with variable penetrance (Fig. 1). Out of 15 Wnt5a-/- mice examined at birth, six had external genitalia with varying degrees of outgrowth (Fig. 1). We examined mice maintained on the original C57BL/6 background (Yamaguchi et al., 1999) and on C57BL/6 × CD1 mixed backgrounds. Wnt5a mutants on both backgrounds exhibited a similar range of phenotypes, and the phenotypic spectrum did not vary with the genetic sex of the animal (Fig. 1; data not shown). In Wnt5a null mice that developed external genitalia, the limbs were positioned normally (Fig. 1C). In the majority of cases in which external genitalia were absent, the hindlimbs were displaced medially (Fig. 1G), and in a few cases were fused into a single structure complete with digits (Fig. 1I). Although medial displacement of the limbs was generally associated with reduction of the genitalia, some Wnt5a-/- embryos exhibited complete agenesis of the external genitalia, despite having normally positioned hindlimbs (Fig. 1E).
Fig. 1.
Variably penetrant development of external genitalia in Wnt5a-/- mice correlates with Fgf8 expression. Wnt5a+/- (A,B) and Wnt5a-/- (C-J) mutants at P0 (A,C,E,G,I) and E11.5 (B,D,F,H,J). Ventral views; tail is at the bottom of each panel. Red arrows mark genitalia and black arrowheads mark positions of hindlimbs (tail and limbs dissected in A to show genitalia). (A,B) Wnt5a+/- mice showing normal position of external genitalia, hindlimbs, anus and tail (A), and normal Fgf8 expression in the distal genital tubercle (B). (C,E,G,I) Wnt5a-/- mice showing the range of phenotypes affecting the hindlimbs and external genitalia, which includes normally positioned hindlimbs and external genitalia (C), normally positioned hindlimbs and no external genitalia (E), medially displaced hindlimbs and no external genitalia (G) and a single fused hindlimb with digits and no external genitalia (I). No anal opening was found in all Wnt5a-/- mice examined. (D,F,H,J) Examination of Fgf8 expression in Wnt5a-/- embryos with varying degrees of hindlimb bud displacement shows Fgf8 expression in the distal genital tubercle (D) and no Fgf8 expression in mutants lacking genital tubercles (F,H,J). (J) Fgf8 expression in center of embryo marks continuous AER-like structure across the midline (also see Fig. S1 in the supplementary material).
Given the variable degree of genital outgrowth in Wnt5a-/- embryos, we asked whether the extent of outgrowth correlated with Fgf8 expression in the cloacal epithelium. Examination of Fgf8 expression and tubercle morphology in Wnt5a-/- embryos at E11.5 (when Fgf8 is expressed in normal embryos) revealed a range of expression patterns consistent with the morphological variation observed in mutants at postnatal day 0 (P0) (Fig. 1D,F,H,J). Fgf8 expression was detectable in Wnt5a-/- embryos that had a tubercle positioned centrally between the hindlimb buds (Fig. 1D) (Yamaguchi et al., 1999). In other embryos, the hindlimb buds were displaced medially and the tubercle was either absent or severely reduced (Fig. 1F,H,J). In these embryos, Fgf8 expression was undetectable in the genital region, although expression was robust in the AER (Fig. 1F,H,J). In the most extreme cases, medial displacement of the hindlimb buds was so severe that they appeared to meet in the midline, and in these cases we saw no evidence of genital outgrowth (Fig. 1J). Embryos with such medially displaced hindlimb buds showed a single contiguous AER that expressed Fgf8 and had a stratified or pseudostratified columnar epithelial character (Fig. 1J; see Fig. S1A-C in the supplementary material). In these mutants, the Shh domains were also contiguous between the two limb buds. Our finding that Fgf8 is expressed only in those Wnt5a mutants that initiate genital outgrowth raised the possibility that loss of Fgf8 expression might underlie the absence of external genitalia in the most severely affected mutants.
Fgf8 is induced at the endodermal-ectodermal boundary of the cloacal membrane
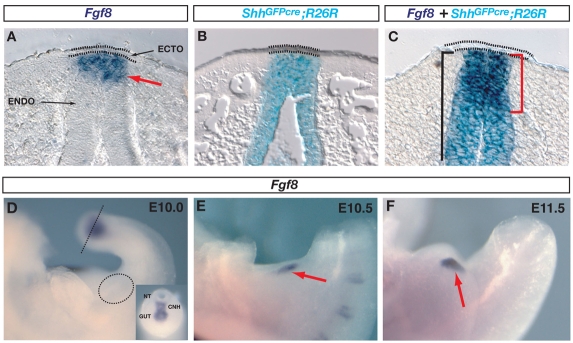
In order to conditionally remove Fgf8 from the genital tubercle, it was first necessary to map the origin of Fgf8-expressing cells. Previous reports have localized Fgf8 expression to the cloacal epithelium prior to genital tubercle outgrowth (Gofflot et al., 1997) and to the distal urethral epithelium following emergence of the tubercle (Haraguchi et al., 2000; Haraguchi et al., 2001; Morgan et al., 2003; Ogino et al., 2001; Perriton et al., 2002; Satoh et al., 2004). However, in the absence of cell lineage data, it has remained unclear to what extent Fgf8 is expressed in endoderm, ectoderm, or both cell types. We recently reported that the cloacal membrane consists of two lineage-restricted cellular compartments derived from endoderm and ectoderm (Seifert et al., 2008). To determine the origin of the epithelial cells that express Fgf8, we used LacZ expression in ShhGFPcre;R26R mice to mark the endodermal compartment, and in situ hybridization to detect Fgf8 expression (Fig. 2A-C). During initiation and early outgrowth of the genital tubercle, Fgf8 is restricted to the distal-most endoderm immediately beneath the surface ectoderm. The endodermal expression domain extended up to its boundary with the ectoderm, but the ectodermal cells themselves were negative for Fgf8 (Fig. 2A,C).
Fig. 2.
Fgf8 expression in normal genital tubercles is restricted to Shh-expressing cloacal endoderm. (A) Fgf8 expression (red arrow) in a section of an E11.5 normal embryo is localized exclusively to the cloacal endoderm (ENDO) and is excluded from the ectoderm (ECTO; dotted lines). (B) X-Gal-stained ShhGFPcre;R26R cells are restricted to the cloacal endoderm. (C) In situ hybridization and X-Gal staining of an E11.5 genital tubercle shows that Fgf8 expression (red bracket) is restricted to ShhGFPcre-expressing cloacal endodermal cells. Black bracket marks X-Gal-stained endoderm. (D-F) Dynamics of Fgf8 expression during initiation of genital outgrowth. (D) Fgf8 is not expressed in the cloacal endoderm at E10 (dotted circle), before initiation of genital outgrowth. Expression is detected in the tailbud (inset; position of section indicated by dotted line), where it localizes to the chordoneural hinge (CNH) and tailgut. NT, neural tube. (E,F) Fgf8 is expressed during initiation (E) and early outgrowth (F) of the genital swellings (red arrows).
We then mapped the dynamics of Fgf8 expression beginning at E10, and found that Fgf8 is not detectable in the cloacal endoderm before the onset of budding, although it was expressed in the endoderm and chordoneural hinge of the tailbud (Fig. 2D, and inset). From E10.5, Fgf8 is expressed in a broad ventral portion of the cloacal epithelium immediately subjacent to the ectoderm and, as the genital tubercle grows out, the domain becomes restricted to the distal urethral epithelium, disappearing after E14.5 (Fig. 2A,C,E,F) (Perriton et al., 2002). Thus, although Fgf8 is expressed within some primitive streak cells that give rise to the definitive endoderm (Crossley and Martin, 1995), it does not appear to be expressed continuously. Rather, its expression is switched back on in cloacal endodermal cells at the endoderm-ectoderm boundary of the cloacal membrane during initiation of genital outgrowth, in the cells fated to form the distal urethra (Seifert et al., 2008).
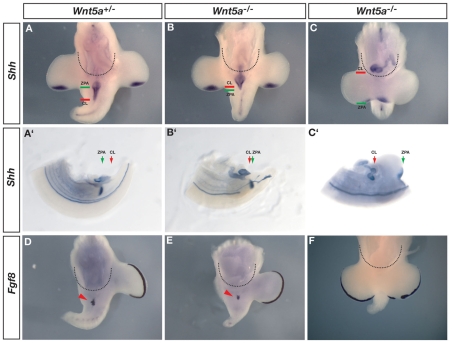
This comparison of Fgf8 expression and cell lineage shows that Fgf8 is activated in the cloacal endoderm that makes contact with the ectoderm, and it is therefore possible that Fgf8 expression is a response to an inductive signal from the adjacent ectoderm. As the embryonic cloaca becomes subdivided into the urogenital sinus (ultimately giving rise to the urethra and bladder) and the anorectal sinus (ultimately giving rise to the rectum and anus), the posterior-ventral endoderm remains in contact with the surface ectoderm at the cloacal membrane (Seifert et al., 2008). If endodermal-ectodermal signaling at the cloacal membrane is required for the activation of Fgf8, then one would expect embryos in which the cloacal endoderm fails to contact the ectoderm also to lack Fgf8 expression. We tested this hypothesis in Wnt5a-/- embryos in which anterior-posterior (AP) axis elongation is disrupted, which affects the posterior position of the embryonic cloaca. In order to identify the posterior limit of the gut we used the expression of Shh because it labels the entire gut endoderm prior to and during development of the genital tubercle (Fig. 3A-C′). Shh also marks the zone of polarizing activity (ZPA) in the limb buds, which provides a morphological landmark for the posterior limit of the hindlimb bud (Fig. 3A-C′). Comparisons of the position of the embryonic cloaca and ZPA at E10.5 in wild-type and heterozygous embryos (which are phenotypically normal) show that the cloacal endoderm is positioned posterior to the ZPA and that Fgf8 is expressed where the endoderm is in contact with the surface ectoderm (Fig. 3A,A′,D; see Fig. S2 in the supplementary material). In comparison, in Wnt5a-/- embryos that initiate genital outgrowth, the cloacal endoderm does not extend as far posteriorly but comes to lie anterior to the ZPA (Fig. 3B,B′; compare position of green and red lines relative to control embryos in Fig. 3A,A′). In these mutants the cloacal endoderm remains in contact with the ectoderm and Fgf8 is expressed, albeit in a smaller domain (Fig. 3B′,E; compare distance between black arrows in Fig. S2 in the supplementary material). In those Wnt5a-/- embryos that fail to initiate genital budding, the gut terminates anterior to the hindlimbs (Fig. 3C), the hindgut endoderm does not reach the cloacal ectoderm (Fig. 3C′; see Fig. S2 in the supplementary material) and Fgf8 is not expressed (Fig. 3F). Thus, Fgf8 is expressed in cases in which endodermal-ectodermal contact occurs, whereas in more extreme cases the gut terminates anterior to the cloacal ectoderm and Fgf8 is not expressed. These findings suggest that during initiation of genital tubercle outgrowth, Fgf8 expression is switched on only in the endodermal cells that contact cloacal ectoderm, perhaps in response to an ectodermally derived genital initiation signal.
Fig. 3.
Fgf8 is activated following endodermal-ectodermal contact at the cloacal membrane. (A-C′) In situ hybridization for Shh shows the posterior limit of the gastrointestinal tract (cloaca) in Wnt5a+/- and Wnt5a -/- mice. Dotted semicircles mark posterior boundary of the peritoneal cavity. (A′-C′) Cleared specimens reveal posterior limit of the embryonic cloaca (CL; red arrows) in relation to the zone of polarizing activity (ZPA; green arrows) of the hindlimb bud. The gut normally extends posterior to the ZPA (A′), but is anterior to the ZPA in some Wnt5a-/- mutants (B′) and is displaced anterior to the entire hindlimb buds in others (C′; compare position of green and red arrows). The endodermal-ectodermal boundary of the cloacal membrane has not formed in C,C′; also see Fig. S2 in the supplementary material. (D-F) Endodermal Fgf8 expression is detected in Wnt5a heterozygous embryos (D; red arrowhead) and in those Wnt5a null embryos where the cloacal endoderm is in contact with surface ectoderm at the cloacal membrane (E; red arrowhead). (F) Fgf8 expression is not detected in Wnt5a-/- embryos where the endoderm has failed to contact the cloacal ectoderm.
Fgf8 is not necessary for outgrowth of the genital tubercle
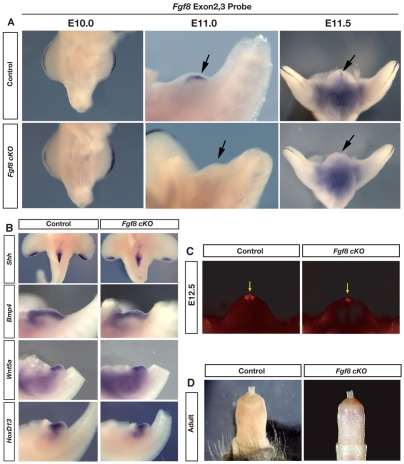
The observations that Fgf8 is expressed in Wnt5a-/- embryos that develop external genitalia, and that mutants lacking genital tubercles also lack Fgf8 expression, led us to ask whether Fgf8 is required for initiation and outgrowth of the external genitalia. In order to test this hypothesis directly, we conditionally removed Fgf8 from the cloacal endoderm prior to the initiation of the genital tubercle. Based on our finding that Fgf8 is expressed exclusively in Shh-expressing cells of the urethra, we used ShhGFPcre to inactivate floxed alleles of Fgf8 in the cloacal epithelium, and we refer to these mice as Fgf8 cKO (conditional knockout; see Materials and methods for details). Mice carrying ShhGFPcre and two floxed alleles of Fgf8 underwent initiation of the paired genital swellings and developed a morphologically normal genital tubercle (Fig. 4A). To confirm that this strategy results in the complete deletion of Fgf8 from the urethral epithelium, we used a riboprobe designed to detect exons 2 and 3, which are internal to the loxP sites in the conditional Fgf8 allele. Using this deletion probe, Fgf8 expression is undetectable in the genital tubercles of Fgf8 cKO mice before, during and after initiation of outgrowth (Fig. 4A). Fgf8 expression in the AER, which lacks Cre, served as a positive internal control, and the deletion probe revealed strong expression in the AER. In littermates lacking the ShhGFPcre allele, the deletion probe showed the normal pattern of Fgf8 expression in the genital tubercle and in the AER (Fig. 4A). The finding that Fgf8 cKO mice form a genital tubercle in the absence of Fgf8 demonstrates that Fgf8 is not required for initiation or outgrowth of the genital tubercle.
Fig. 4.
External genitalia develop normally in the absence of Fgf8. (A) An Fgf8 deletion probe specific to the floxed exons 2 and 3 shows absence of expression in both Fgf8 cKO and control embryos at E10.0. Expression is absent from the genital tubercle of Fgf8 cKO embryos during initiation (E11.0) and early outgrowth (E11.5), but is present in control embryos (arrow). Note expression in the AER of Fgf8 cKO embryos. Dark coloration of deep tissue at E11.5 is nonspecific background staining. (B) In situ hybridizations of Fgf8 cKO and control genitalia showing normal expression of Shh, Bmp4, Wnt5a and Hoxd13. (C) Analysis of cell death using Lysotracker Red in Fgf8 cKO and control littermates at E12.5 shows that apoptosis is not increased in the absence of Fgf8 (arrow). (D) Penis morphology is normal in Fgf8 cKO and control adults.
Does Fgf8 regulate Bmp4, Wnt5a, Hoxd13 and Shh expression in the genital tubercle?
Fgf8 has been placed at top of the genetic cascade that regulates genital tubercle outgrowth, and has been proposed to coordinate gene expression in the mesenchyme flanking the urethral epithelium (Haraguchi et al., 2000; Haraguchi et al., 2001; Morgan et al., 2003; Perriton et al., 2002; Satoh et al., 2004; Suzuki et al., 2003; Suzuki et al., 2008; Yamada et al., 2006). Manipulations of genital tubercles in organ culture suggested that the expression of Bmp4, Msx1 Fgf10 and Hoxd13 is regulated by Fgf8 from the distal urethral epithelium; removal of distal tissue led to varying degrees of downregulation, and implantation of Fgf8 beads augmented their expression (Haraguchi et al., 2000). Interestingly, genital tubercles cultured with an Fgf8-neutralizing antibody maintained normal expression of Msx1 and Fgf10, and Bmp4 showed only a minimal response (Haraguchi et al., 2000). Thus, it is unclear to what extent gene expression in the genital tubercle is regulated by Fgf8. To test this directly, we collected Fgf8 cKO embryos between E11.0 and E12.5 to examine whether deletion of Fgf8 affects the expression of Bmp4, Wnt5a, Hoxd13 or Shh. In Fgf8 cKO embryos, the expression domain of each of these genes in the genitalia was maintained (Fig. 4B), indicating that Fgf8 is not required for the expression of Bmp4, Wnt5a, Hoxd13 or Shh in the genital tubercle. These findings are consistent with the recent report that removal of β-catenin in the cloacal endoderm results in the loss of Fgf8 expression, but Hoxa13, Hoxd13, Msx2 and Wnt5a expression remains unaffected in the genital tubercle (Lin et al., 2008).
Fgf8 is not required for cell survival in the genital tubercle
Fgf8 functions as a cell survival factor in the limb bud (Sun et al., 2002), and it has been reported that Fgf8 inhibits apoptosis during outgrowth of the genitalia (Suzuki et al., 2003). Cell death is normally detected most prominently in the distal portion of the urethral epithelium and in the adjacent distal mesenchyme (Fig. 4C) (see also Morgan et al., 2003; Perriton et al., 2002; Suzuki et al., 2003). In contrast to the situation in the limb, we did not detect an increase in cell death at the distal tip of the genital tubercle following removal of Fgf8 (n=6; Fig. 4C). Cell death was still observed at the distal tip, although the apoptotic domain appeared smaller than that observed in wild-type littermates (Fig. 4C). Previous work in organ culture showed that antagonism of Shh results in the downregulation of Fgf8 and an increase in cell death (Haraguchi et al., 2000). Our finding that the genetic removal of Fgf8 in vivo results in neither increased cell death nor altered morphology of the tubercle indicates that Fgf8 is not required as a cell survival factor during genital development, and suggests that diminished Fgf8 expression in Shh-/- genitalia is unlikely to account for the increased apoptosis in those mutants. Why deletion of Fgf8 would result in a slight decrease in apoptosis is more difficult to explain, however, it is interesting that mice lacking noggin, a Bmp antagonist, also show reduced Fgf8 expression and decreased apoptosis in the distal genital tubercle (Suzuki et al., 2003).
Conditional removal of Fgf8 results in normal development of the penis
Fgf8 expression normally persists in the distal urethral epithelium through E14.5. Therefore, we wanted to determine whether Fgf8 plays a later role in genital development and patterning. To investigate this possibility we generated Fgf8 cKO mice and raised them to adulthood to assess the morphology of the penis. We found that outgrowth, axial patterning and urethral tube closure were unaffected by the removal of Fgf8 (Fig. 4D). In addition, we analyzed the bacula (os penis) of Fgf8 cKO males and found that they were indistinguishable from wild-type males (data not shown). The genitalia of Fgf8 cKO males are functional, as they are able to urinate, copulate and produce a semen plug, although they appear to be sterile. These results show that Fgf8 is not required for outgrowth, patterning or differentiation of the external genitalia.
Is Fgf8 signaling active during normal genital development?
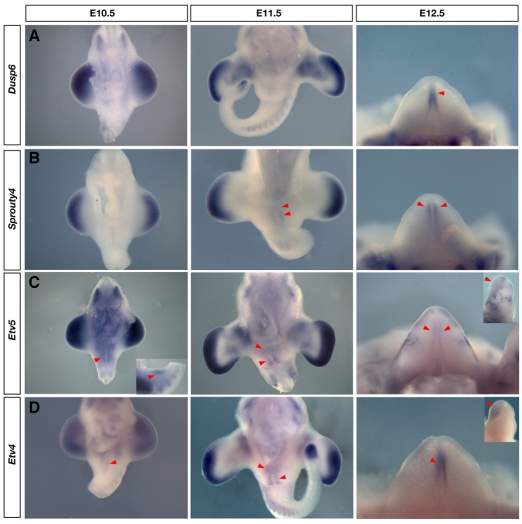
To determine whether other Fgfs might compensate for Fgf8 during genital initiation, we examined all 22 known Fgfs in the genitalia of wild-type embryos, however, none was expressed in the cloacal endoderm prior to E12.5 (data not shown; a complete description of all Fgf and Fgfr expression patterns in the genitalia will be described elsewhere). As an additional test for Fgf pathway activation, we monitored the expression of four targets of Fgf signaling during normal genital development. Dusp6, Spry4, Etv4 and Etv5 have been shown to be transcribed in response to Fgf signaling (Kawakami et al., 2003; Mao et al., 2009; Mariani et al., 2008; Minowada et al., 1999; Roehl and Nusslein-Volhard, 2001; Sharrocks, 2001; Taniguchi et al., 2007; Zhang et al., 2009). Dusp6 is induced through the phosphoinositide 3-kinase/Akt pathway in response to Fgfs expressed in the AER (Fgf4, -8, -9 and -17) and is particularly sensitive to Fgf8 (Kawakami et al., 2003; Mariani et al., 2008). Dusp6 expression was undetectable during early outgrowth of the genital tubercle (prior to E12.5), although robust expression was observed near other areas of Fgf8 expression, including the distal limb mesenchyme and dermamyotome (Fig. 5). We first observed Dusp6 expression in the urethral epithelium at E12.5, two days after initiation of genital outgrowth, in a domain that partially overlapped with the Fgf8 domain (Fig. 5).
Fig. 5.
Analysis of targets of Fgf signaling in the genital tubercle. (A-D) Expression of proposed targets of Fgf signaling: Dusp6, Spry4, Etv5 and Etv4 from E10.5 to E12.5. Red arrowheads mark expression in the external genitalia. (A,B) Neither Dusp6 nor Spry4 was detected during initiation of genital budding at E10.5, although both genes were expressed in the limb buds, somites and tailbud between E10.5 and E11.5. (C,D) By contrast, both Etv5 and Etv4 were expressed in broad domains around the cloacal membrane at E10.5 and E11.5. At E11.5, Dusp6 expression was not detected in the genital tubercle (A), whereas expression of Spry4 was detected distally (B). At E12.5, Dusp6 and Etv4 were expressed in the urethral plate endoderm (A,D), whereas Spry4 and Etv5 were expressed lateral to this domain (B,C). In addition, Etv5 and Etv4 expression covered the entire dorsal swelling (insets in C,D). Detection of these targets at E12.5 coincided with the expression of other Fgfs.
Sprouty 4 negatively regulates MAPK/ERK activation in response to Fgf signaling and has been used as a readout of Fgf8 expression in many cell populations during mouse embryogenesis (Minowada et al., 1999; Sasaki et al., 2003). We monitored the expression of Spry4 and, like Dusp6, it was undetectable in the genital tubercle at E10.5 (Fig. 5). Expression of Spry4 was first observed around the dorsal swelling of the tubercle at E11.5, with robust expression observed lateral to the urethral plate at E12.5 (Fig. 5; see Fig. S3 in the supplementary material). The absence of Spry4 and Dusp6 in early genital tubercles suggests an absence of Fgf8 activity during initiation and outgrowth of the external genitalia.
ETS transcription factors are expressed in response to ERK activation, and the expression of both Etv4 and Etv5 has been shown to mirror Fgf8 expression in zebrafish embryos and to be dependent on Fgf signaling in vertebrate limb buds (Mao et al., 2009; Roehl and Nusslein-Volhard, 2001; Zhang et al., 2009). We detected expression of both Etv4 and Etv5 at very low levels in a broad pattern surrounding the cloacal membrane at E10.5 (Fig. 5). By E11.5, both genes were expressed broadly in the genital mesenchyme lateral to the endoderm and in the dorsal swelling (Fig. 5; see Fig. S3 in the supplementary material). The expression of both ETS factors remained strong in the dorsal swelling at E12.5, with Etv5 expression now partially overlapping the Fgf8 domain in the urethral endoderm and Etv4 expression strongest in the mesenchyme lateral to the urethral plate (Fig. 5). To determine whether the activation of Etv4 and Spry4 was regulated by Fgf8, we assayed for the expression of both genes in Fgf8 cKO embryos. Both Etv4 and Spry4 were expressed in the appropriate patterns in Fgf8 cKO embryos at E11.5 (see Fig. S4 in the supplementary material). Taken together, the findings that neither Dusp6 nor Spry4 are activated prior to E11.5, and that Etv4 and Spry4 are unresponsive to the deletion of Fgf8, suggest that Fgf8 does not participate in initiation or outgrowth of the genital tubercle.
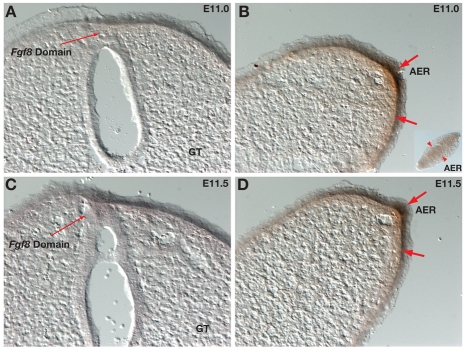
Given that Fgf8 is expressed robustly in the distal urethra but fails to activate genes reported to be Fgf targets, we next asked whether Fgf8 protein was present. We used an antibody against the N-terminal domain of Fgf8, which has been shown to detect Fgf8 protein in the limbs and fins of several vertebrate species (Freitas et al., 2006; Thewissen et al., 2006). Although Fgf8 protein was present in and immediately beneath the limb ectoderm, it could not be detected in the genital tubercle at either E11.0 or E11.5 (Fig. 6A-D). These results suggest that Fgf8 is not translated in the distal urethra, or that the protein is present at such low levels that it cannot be detected by immunohistochemistry.
Fig. 6.
Fgf8 protein is undetectable in the genital tubercle. (A-D) Immunohistochemical analysis of Fgf8 protein in the genital tubercle (A,C) and hindlimb buds (B,D) at E11.0 and E11.5. Fgf8 protein was detected in and near the AER of the hindlimb buds (thick arrows) but was undetectable in the genital tubercles (GT) of the same embryos. Thin arrows mark regions of Fgf8 mRNA expression but absence of detectable Fgf8 protein.
Phylogenetic distribution of Fgf8 in amniote genital tubercles
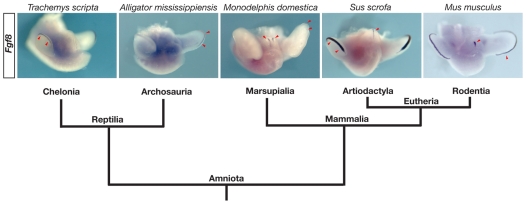
The results of our experiments indicate that Fgf8 is transcribed in response to, but is not required for, initiation of the genital tubercle in mouse embryos. External genitalia are found in every class of amniotes, and comparative developmental data indicate that amniote genitalia share a common embryonic origin and utilize a common suite of molecular developmental mechanisms (A.W.S. and M.J.C., unpublished observations). To determine whether Fgf8 expression is a conserved feature of amniote external genital development, we examined its expression in the genital tubercles of a diverse array of amniote embryos, including a rodent, an artiodactyl, a marsupial, an archosaur and a chelonian. Fgf8 expression was observed in the genital tubercles of the opossum Monodelphis domestica (a marsupial), the pig Sus scrofa (an artiodactyl) and the mouse (a rodent), indicating that Fgf8 expression is a conserved feature of mammalian external genital development (Fig. 7). Fgf8 expression in the pig genital tubercle was confined to a single domain in the urethral plate (Fig. 7). Opossums develop a bifid glans penis in which the distal urethral tube bifurcates into a pair of urethral grooves, and we observed two domains of Fgf8 at the distal end of the opossum genital tubercle (Fig. 7). In contrast to the three mammalian embryos, Fgf8 expression was not detectable in the genital tubercles of either the red-eared slider Trachemys scripta (a chelonian) or the alligator Alligator mississippiensis (an archosaur), although in both taxa we detected Fgf8 expression in the AER (Fig. 7). The finding that both turtles and alligators develop external genitalia in the absence of Fgf8 expression, along with our functional analysis of Fgf8 in the mouse, suggests that Fgf8 is not required for the development of external genitalia across amniotes.
Fig. 7.
Phylogenetic distribution of Fgf8 expression in amniote genitalia. Fgf8 was expressed in the genital tubercles of all mammalian embryos examined, including Monodelphis domestica (opossum), Sus scrofa (pig), and Mus musculus (mouse). By contrast, Fgf8 was undetectable in the genital tubercles of Trachemys scripta (turtle) and Alligator mississippiensis (alligator), although expression was detected in the AER of the limb buds. Phylogenetic relationships are based on Hedges and Poling and Beck et al. (Hedges and Poling, 1999; Beck et al., 2006). Red arrowheads indicate Fgf8 expression domains.
DISCUSSION
Conditional deletion of Fgf8 in the mouse genital tubercle demonstrates that Fgf8 is not required for any aspect of external genital development. Although Fgf8 is not necessary for outgrowth of the genitalia, its expression serves as a useful marker for genital induction, as illustrated in the variably penetrant phenotype of Wnt5a-/- mice. The analysis of Wnt5a-/- embryos suggests that Fgf8 expression is a readout of endodermal-ectodermal signaling at the cloacal membrane. As such, examination of Fgf8 in mouse mutants that exhibit cloacal membrane defects might be useful for elucidating the role of the cloacal ectoderm during induction of genital outgrowth. The conclusion that Fgf8 is simply a response to the initiation signal is consistent with the observation that in Shh-/- embryos Fgf8 expression is activated in the cloacal endoderm and external genital budding is initiated, although outgrowth is not maintained (Perriton et al., 2002). Thus, both Fgf8 and Shh can be eliminated as candidates for the genital initiation signal.
Previous work reported that surgical removal of the distal epithelium from genital tubercles in organ culture led to decreased growth, and that this can be reversed by the addition of Fgf8-loaded beads (Haraguchi et al., 2000). Because the distal urethral endoderm is contiguous with the distal ectoderm, surgical excision of the distal tip likely resulted in the removal of both ectoderm and endoderm, each of which expresses numerous signaling molecules and transcription factors that might be involved in outgrowth. Although the published interpretation of those manipulations is that Fgf8 produced from the distal tubercle is required for proliferation and outgrowth of the genital mensenchyme, our results indicate that those truncations cannot be explained by loss of Fgf8 alone.
During limb development several Fgfs are expressed in the AER, and this redundancy results in compensation following loss of any one Fgf (Boulet et al., 2004; Lewandoski et al., 2000; Mariani et al., 2008; Moon et al., 2000; Sun et al., 2000; Sun et al., 2002; Sun et al., 1999). We investigated whether such redundancy among Fgfs could account for the ability of Fgf8 cKO mice to form genital tubercles, but our in situ hybridization analysis of all known Fgfs during initiation and early outgrowth of the genitalia indicated that no other Fgf family member is expressed in a pattern similar to Fgf8 prior to E12.5 (although other Fgfs were detected in the mesenchyme and in the ectoderm). This highlights an interesting difference between the distal urethral epithelium of the genital tubercle and the AER of the limb bud. Whereas the AER is a site of extensive redundancy of Fgfs that synergistically control proximodistal outgrowth of the limb, the distal urethral epithelium shows no such redundancy during early stages of genital outgrowth. Therefore, the normal development of a genital tubercle in Fgf8 conditional mutants cannot be explained by compensatory Fgf signaling from the urethral endoderm.
After outgrowth is initiated, the expression of Spry4, Etv4 and Etv5 in the dorsal swelling coincides with the expression of several Fgfs in that region, suggesting that these target genes might be activated by other members of the Fgf family or other receptor tyrosine kinase pathways (Fig. 5, insets; data not shown). This is further supported by the finding that the expression of both Spry4 and Etv4 persists in the absence of Fgf8. When Fgf4/8 or Fgfr1/2 are inactivated during limb development, Etv4 and Etv5 expression is diminished but not completely abolished (Zhang et al., 2009). The expression of both genes can be completely abolished in zebrafish and chick limb buds following treatment with SU5402 (Mao et al., 2009; Roehl and Nusslein-Volhard, 2001). It is noteworthy that SU5402 does not specifically inhibit Fgfr signaling; previous studies have shown that it can inhibit both Pdgf and Vegf signaling (Mohammadi et al., 1997). In addition, several secreted signaling molecules are expressed in domains that overlap with Fgf8, including Bmp2, Bmp7 and Tgfb2, all of which are capable of ERK activation (Derynck and Zhang, 2003; Grijelmo et al., 2007; Jin et al., 2006). Thus, it is possible that ERK activation of Etv4 and Etv5 in the genital tubercle might occur in response to other factors present in or around the urethral epithelium.
The finding that Fgf8 protein is undetectable during initiation and early outgrowth of the genital tubercle suggests possible post-transcriptional regulation of Fgf8. Interestingly, analysis of the mouse Fgf8 sequence using MicroInspector software (Rusinov et al., 2005) revealed 155 miRNA binding sites, with 27 detected in exons 2 and 3, which are present in all of the potential Fgf8 splice variants (A.W.S. and M.J.C., unpublished observations). Further analysis of Fgf8 regulation, both at the transcriptional and translational levels, will be interesting in this context.
Why Fgf8 would be transcribed in the urethral epithelium of mammals but not reptiles is unclear. A major distinction between the genitalia of mammals and other amniotes is that only mammals undergo urethral tubulogenesis; in non-mammalian amniotes the urethral epithelium persists as an open sulcus. The phylogenetic distribution of an Fgf8 expression domain in the urethral epithelium suggests that the evolutionary origin of its expression domain coincided with the evolution of a urethral tube. The mechanism responsible for the evolutionary transition from a sulcus to a tube is unknown, but it is possible that Fgf8 expression is a reflection of this new developmental process. Whatever the evolutionary basis of this novel domain of expression, the results presented here suggest that Fgf8 expression is a consequence, rather than a cause, of external genital development. The nature of the external genital initiation factor remains to be discovered, and our findings raise the possibility that the cloacal ectoderm might be the source of this signal.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/15/2643/DC1
Supplementary Material
We are grateful to Rieko Ajima, Brian Harfe, Francesca Mariani, Gail Martin and Scott Stadler for helpful discussions. We thank Brian Harfe and Xin Sun for providing the ShhGFPcre and Fgf8fl/fl mouse lines; Brooke Armfield, Zhengui Zheng, Anna Keyte, Kathleen Smith, Teresa Bryant and Louis Guillette for sharing embryos and/or probes; and Eric Rubin, Heather Freiman and Christina Varvarikos for technical assistance. We gratefully acknowledge funding from the National Institutes of Health (5R01HD054554) and the National Science Foundation (0843590). Deposited in PMC for release after 12 months.
References
- Beck, R. M., Bininda-Emonds, O. R., Cardillo, M., Liu, F. G. and Purvis, A. (2006). A higher-level MRP supertree of placental mammals. BMC Evol. Biol. 6, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet, A. M., Moon, A. M., Arenkiel, B. R. and Capecchi, M. R. (2004). The roles of Fgf4 and Fgf8 in limb bud initiation and outgrowth. Dev. Biol. 273, 361-372. [DOI] [PubMed] [Google Scholar]
- Crossley, P. H. and Martin, G. R. (1995). The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 121, 439-451. [DOI] [PubMed] [Google Scholar]
- Derynck, R. and Zhang, Y. E. (2003). Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425, 577-584. [DOI] [PubMed] [Google Scholar]
- Freitas, R., Zhang, G. and Cohn, M. J. (2006). Evidence that mechanisms of fin development evolved in the midline of early vertebrates. Nature 442, 1033-1037. [DOI] [PubMed] [Google Scholar]
- Gofflot, F., Hall, M. and Morriss-Kay, G. M. (1997). Genetic patterning of the developing mouse tail at the time of posterior neuropore closure. Dev. Dyn. 210, 431-445. [DOI] [PubMed] [Google Scholar]
- Grijelmo, C., Rodrigue, C., Svrcek, M., Bruyneel, E., Hendrix, A., de Wever, O. and Gespach, C. (2007). Proinvasive activity of BMP-7 through SMAD4/src-independent and ERK/Rac/JNK-dependent signaling pathways in colon cancer cells. Cell. Signal. 19, 1722-1732. [DOI] [PubMed] [Google Scholar]
- Haraguchi, R., Suzuki, K., Murakami, R., Sakai, M., Kamikawa, M., Kengaku, M., Sekine, K., Kawano, H., Kato, S., Ueno, N. et al. (2000). Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development 127, 2471-2479. [DOI] [PubMed] [Google Scholar]
- Haraguchi, R., Mo, R., Hui, C., Motoyama, J., Makino, S., Shiroishi, T., Gaffield, W. and Yamada, G. (2001). Unique functions of Sonic hedgehog signaling during external genitalia development. Development 128, 4241-4250. [DOI] [PubMed] [Google Scholar]
- Haraguchi, R., Motoyama, J., Sasaki, H., Satoh, Y., Miyagawa, S., Nakagata, N., Moon, A. and Yamada, G. (2007). Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development 134, 525-533. [DOI] [PubMed] [Google Scholar]
- Harfe, B. D., Scherz, P. J., Nissim, S., Tian, H., McMahon, A. P. and Tabin, C. J. (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517-528. [DOI] [PubMed] [Google Scholar]
- Hedges, S. B. and Poling, L. L. (1999). A molecular phylogeny of reptiles. Science 283, 998-1001. [DOI] [PubMed] [Google Scholar]
- Jin, E. J., Lee, S. Y., Choi, Y. A., Jung, J. C., Bang, O. S. and Kang, S. S. (2006). BMP-2-enhanced chondrogenesis involves p38 MAPK-mediated downregulation of Wnt-7a pathway. Mol. Cells 22, 353-359. [PubMed] [Google Scholar]
- Kawakami, Y., Rodriguez-Leon, J., Koth, C. M., Buscher, D., Itoh, T., Raya, A., Ng, J. K., Esteban, C. R., Takahashi, S., Henrique, D. et al. (2003). MKP3 mediates the cellular response to FGF8 signalling in the vertebrate limb. Nat. Cell Biol. 5, 513-519. [DOI] [PubMed] [Google Scholar]
- Lewandoski, M., Sun, X. and Martin, G. R. (2000). Fgf8 signalling from the AER is essential for normal limb development. Nat. Genet. 26, 460-463. [DOI] [PubMed] [Google Scholar]
- Lin, C., Yin, Y., Long, F. and Ma, L. (2008). Tissue-specific requirements of beta-catenin in external genitalia development. Development 135, 2815-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, J., McGlinn, E., Huang, P., Tabin, C. J. and McMahon, A. P. (2009). Fgf-dependent Etv4/5 activity is required for posterior restriction of Sonic Hedgehog and promoting outgrowth of the vertebrate limb. Dev. Cell 16, 600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani, F. V. and Martin, G. R. (2003). Deciphering skeletal patterning: clues from the limb. Nature 423, 319-325. [DOI] [PubMed] [Google Scholar]
- Mariani, F. V., Ahn, C. P. and Martin, G. R. (2008). Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature 453, 401-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers, E. N., Lewandowski, M. and Martin, G. R. (1998). An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat. Genet. 18, 136-141. [DOI] [PubMed] [Google Scholar]
- Minowada, G., Jarvis, L. A., Chi, C. L., Neubuser, A., Sun, X., Hacohen, N., Krasnow, M. A. and Martin, G. R. (1999). Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development 126, 4465-4475. [DOI] [PubMed] [Google Scholar]
- Mohammadi, M., McMahon, G., Sun, L., Tang, C., Hirth, P., Yeh, B. K., Hubbard, S. R. and Schlessinger, J. (1997). Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276, 955-960. [DOI] [PubMed] [Google Scholar]
- Moon, A. M., Boulet, A. M. and Capecchi, M. R. (2000). Normal limb development in conditional mutants of Fgf4. Development 127, 989-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, E. A., Nguyen, S. B., Scott, V. and Stadler, H. S. (2003). Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development 130, 3095-3109. [DOI] [PubMed] [Google Scholar]
- Ng, J. K., Tamura, K., Buscher, D. and Izpisua-Belmonte, J. C. (1999). Molecular and cellular basis of pattern formation during vertebrate limb development. Curr. Top. Dev. Biol. 41, 37-66. [DOI] [PubMed] [Google Scholar]
- Ogino, Y., Suzuki, K., Haraguchi, R., Satoh, Y., Dolle, P. and Yamada, G. (2001). External genitalia formation: role of fibroblast growth factor, retinoic acid signaling, and distal urethral epithelium. Ann. New York Acad. Sci. 948, 13-31. [PubMed] [Google Scholar]
- Perriton, C. L., Powles, N., Chiang, C., Maconochie, M. K. and Cohn, M. J. (2002). Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev. Biol. 247, 26-46. [DOI] [PubMed] [Google Scholar]
- Roehl, H. and Nusslein-Volhard, C. (2001). Zebrafish pea3 and erm are general targets of FGF8 signaling. Curr. Biol. 11, 503-507. [DOI] [PubMed] [Google Scholar]
- Rusinov, V., Baev, V., Minkov, I. N. and Tabler, M. (2005). MicroInspector: a web tool for detection of miRNA binding sites in an RNA sequence. Nucleic Acids Res. 33, W696-W700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, A., Taketomi, T., Kato, R., Saeki, K., Nonami, A., Sasaki, M., Kuriyama, M., Saito, N., Shibuya, M. and Yoshimura, A. (2003). Mammalian Sprouty4 suppresses Ras-independent ERK activation by binding to Raf1. Nat. Cell Biol. 5, 427-432. [DOI] [PubMed] [Google Scholar]
- Satoh, Y., Haraguchi, R., Wright, T. J., Mansour, S. L., Partanen, J., Hajihosseini, M. K., Eswarakumar, V. P., Lonai, P. and Yamada, G. (2004). Regulation of external genitalia development by concerted actions of FGF ligands and FGF receptors. Anat. Embryol. 208, 479-486. [DOI] [PubMed] [Google Scholar]
- Seifert, A. W., Harfe, B. D. and Cohn, M. J. (2008). Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev. Biol. 318, 143-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks, A. D. (2001). The ETS-domain transcription factor family. Nat. Rev. Mol. Cell Biol. 2, 827-837. [DOI] [PubMed] [Google Scholar]
- Sun, X., Meyers, E. N., Lewandoski, M. and Martin, G. R. (1999). Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 13, 1834-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X., Lewandoski, M., Meyers, E. N., Liu, Y. H., Maxson, R. E., Jr and Martin, G. R. (2000). Conditional inactivation of Fgf4 reveals complexity of signalling during limb bud development. Nat. Genet. 25, 83-86. [DOI] [PubMed] [Google Scholar]
- Sun, X., Mariani, F. V. and Martin, G. R. (2002). Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature 418, 501-508. [DOI] [PubMed] [Google Scholar]
- Suzuki, K., Bachiller, D., Chen, Y. P., Kamikawa, M., Ogi, H., Haraguchi, R., Ogino, Y., Minami, Y., Mishina, Y., Ahn, K. et al. (2003). Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia development by concerted actions of BMP signaling. Development 130, 6209-6220. [DOI] [PubMed] [Google Scholar]
- Suzuki, K., Haraguchi, R., Ogata, T., Barbieri, O., Alegria, O., Vieux-Rochas, M., Nakagata, N., Ito, M., Mills, A. A., Kurita, T. et al. (2008). Abnormal urethra formation in mouse models of split-hand/split-foot malformation type 1 and type 4. Eur. J. Hum. Genet. 16, 36-44. [DOI] [PubMed] [Google Scholar]
- Taniguchi, K., Ayada, T., Ichiyama, K., Kohno, R., Yonemitsu, Y., Minami, Y., Kikuchi, A., Maehara, Y. and Yoshimura, A. (2007). Sprouty2 and Sprouty4 are essential for embryonic morphogenesis and regulation of FGF signaling. Biochem. Biophys. Res. Commun. 352, 896-902. [DOI] [PubMed] [Google Scholar]
- Thewissen, J. G., Cohn, M. J., Stevens, L. S., Bajpai, S., Heyning, J. and Horton, W. E., Jr (2006). Developmental basis for hind-limb loss in dolphins and origin of the cetacean bodyplan. Proc. Natl. Acad. Sci. USA 103, 8414-8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, G., Suzuki, K., Haraguchi, R., Miyagawa, S., Satoh, Y., Kamimura, M., Nakagata, N., Kataoka, H., Kuroiwa, A. and Chen, Y. (2006). Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev. Dyn. 235, 1738-1752. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, T. P., Bradley, A., McMahon, A. P. and Jones, S. (1999). A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126, 1211-1223. [DOI] [PubMed] [Google Scholar]
- Zhang, Z., Verheyden, J. M., Hassell, J. A. and Sun, X. (2009). FGF-regulated Etv genes are essential for repressing Shh expression in mouse limb buds. Dev. Cell 16, 607-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.