Abstract
HLA-G is an HLA class Ib gene that is highly expressed in human trophoblast cells. The single HLA-G mRNA is alternatively spliced to generate at least seven transcripts, three of which encode soluble isoforms. Many studies have shown that high levels of soluble antigens are associated with successful implantation and graft acceptance. To study expression, regulation and functions of two of the soluble isoforms, HLA-G5 and HLA-G6, we generated recombinant proteins in eukaryotic cells and developed monoclonal antibodies specific for each of the two proteins. In addition, we investigated the olive baboon Paan-AG gene as a potential functional correlate of HLA-G. Here, we present summaries of the studies that have been conducted in our laboratory using these tools and discuss the results within the context of the research on this topic that is ongoing in ours and other laboratories worldwide. Collectively, the data indicate that soluble HLA-G is a critical contributor to immune privilege in pregnancy and imply that this placenta-derived substance may impact other pathways leading to successful reproduction.
Keywords: baboon, HLA-G, human, immune privilege, Paan-AG, placenta, tolerance
INTRODUCTION
The HLA class Ib gene now known as HLA-G was identified by Geraghty et al in 1987 [1]. Subsequently, several unique features of the gene, its mRNAs and its proteins, were reported. These included (i) alternative splicing of a single mRNA to produce a spectrum of seven distinct mRNAs, three of which result in soluble isoforms and four of which encode membrane isoforms, (ii) a shortened cytoplasmic tail on membrane-bound isoforms which delays recycling of the antigens, (iii) a deletion in the promoter region that negatively impacts transcription enhancement by interferons (IFNs), (iv) multiple polymorphisms but few (five) alleles [reviewed in Refs. 2–6]. Although HLA-G mRNA is present in many tissues and organs, protein expression is highly restricted. The first normal tissues shown to express HLA-G were the placenta and chorion membrane, and the first tumor cells were trophoblastic tumor cell lines [7–9]. However, there is now considerable evidence for expression of this gene in other normal tissues such as the thymus and prostate [10, 11], as well as in a variety of tumors and tumor cell lines [12]. Regarding numbers of alleles and patterns of expression, HLA class Ib genes (HLA-E, -F, -G) differ significantly from HLA class Ia genes (HLA-A, -B, -C), which are highly polymorphic and expressed to a greater degree by all somatic cells.
The paragraphs below present current work from our laboratory within the context of ours and other reports, and point out controversial features of this interesting and apparently unique immune suppressive molecule. Topics include initiation of expression of HLA-G in embryonic tissues, conditions regulating expression of the mRNAs and protein isoforms and new views on the functions of HLA-G. Comments are included on future directions, including both research questions and utility in reproductive medicine.
HLA-G and gestational programming
Because of the results we obtained in an early study showing that soluble isoforms of HLA-G circulate in mothers during pregnancy [13], we have focused on two of these isoforms, HLA-G5 and HLA-G6. These two soluble isoforms are encoded by splice variants of the single HLA-G mRNA whose translation is truncated by a stop codon in intron 4, which eliminates translation of the transmembrane and cytoplasmic elements of the protein [reviewed in Ref. 4]. In order to study the expression, regulation and functions of these two isoforms we developed recombinant HLA-G5 (rHLA-G5) and rHLA-G6 in HEK293 cells, then generated monoclonal antibodies (mAb) to the recombinant proteins [14]. Others have generated mAb to HLA-G proteins using different strategies, and many mAb are now on the market. Not all of these have undergone the extensive characterization studies to which our mAb were subjected [14].
HLA-G in the preimplantation embryo
Gestational programming is evident in the observation that some if not all preimplantation embryos contain HLA-G mRNA and protein but that placentas from successful pregnancies are invariably positive. By using the mAbs mentioned above [14] in a collaborative study with C. Simon at the University of Valencia, we learned in a limited group of experiments that preimplantation human embryos may exhibit HLA-G5 but not HLA-G6 [5]. We have not studied HLA-G mRNAs in human preimplantation embryos, but this was explored in a 2005 article by Yao et al. [15], who concluded that both message and protein expression are stage-dependent and may be contributed by the mother. A notable observation in this study was that HLA-G is silenced in inner cell mass cells at the blastocyst stage, leaving the trophectoderm as the sole source of these proteins prior to implantation. Evidence for expression of this gene in the thymus, prostate and various tumor cells [10–12] suggests that the gene may be re-expressed in inner cell mass-derived cells later in gestation. The report from Yao et al. [15] conflicts with that of Hiby et al. [16], which was published in 1999 and which concluded that HLA-G mRNA is missing in two cell to eight cell embryos and in blastocysts.
Our results in the olive baboon [17], are in accord with the report of Yao et al. [15]; messages encoding the functional homologue of HLA-G5, Paan-AG5 [18], are readily identified in all stages of the preimplantation embryo (Fig. 1). Paan-AG5 is the only soluble isoform produced in the baboon. Whether the messages for this soluble isoform arise from maternal mRNA present in the oocyte or by transcription of embryonic DNA, or both, remains unknown. The question of Paan-AG protein expression in the preimplantation baboon embryo has not as yet been addressed.
Figure 1. Gestational programming of Paan-AG messages in the olive baboon.
Reverse transcriptase polymerase chain reaction (RT-PCR) was used to investigate Paan-AG transcripts in the olive baboon blastocyst, zygote and oocyte. The positive control was an extract of baboon placenta. All RNAs were acquired at the University of Illinois – Chicago (A. T. Fazleabas). Note that mRNA encoding the single soluble isoform of Paan-AG, Paan-AG5, is present at all stages. By contrast, Paan-AG1 mRNA, which encodes a membrane isoform, is detectable only in the placenta.
In striking contrast, mRNAs for the membrane isoform, Paan-AG1, are detectable only post-implantation, i.e., in the placenta. This observation has not been reported in humans, but strongly supports the idea of gestational programming of HLA-G isoforms.
HLA-G in extravillous cytotrophoblast cells
The placenta and its membranes invariably express HLA-G antigens. The initial observation that HLA-G is detectable in this organ was made by Ellis et al. [8, 9], who reported expression in chorionic membrane (extravillous) cytotrophoblast cells in term amniochorion and trophoblast tumor cells. This was followed by the finding of mRNA encoding this molecule in extravillous cytotrophoblast cells of early placentas that were contained in cytotrophoblast cell columns proximal to the decidua [18].
Investigators are now extremely interested in the structurally diverse isoforms of HLA-G that result from the mRNA splice variants. The functional implications of our collaborative study with C. Ober at the University of Chicago on women with a mutation in the HLA-G gene stimulated much of this activity. In these women, a single base pair deletion at nucleotide 1597 causes a frameshift at amino acid 130 that results in nonfunctional HLA-G1 and –G5 proteins [19]. Immunohistochemical and molecular indices for the expression of other HLA-G isoforms were present that we suggested might compensate for lack of HLA-G1 and –G5 because these women have viable pregnancies [19]. This idea has been adopted and supported by other investigators [20].
The isoform-specific mAb we generated have permitted identification of specific protein expression patterns. Although HLA-G5 is expressed in essentially all of the extravillous cytotrophoblast cells, those forming the trophoblastic shell distal to the villous and invading the decidua additionally express HLA-G1 and -G2/G6 [14]. The gradation of HLA-G antigens is illustrated in Figure 2. The extravillous cytotrophoblast cells in the shell also express the light chain for HLA class I, β2-microglobulin (β2m). Current studies in our laboratory indicate that even in late gestation, this pattern can be observed in the amniochorion, with HLA-G5 abundantly expressed throughout the chorion membrane while HLA-G1 and HLA-G2/G6 are detectable mainly in the cytotrophoblast cells directly adjacent to the decidua (J. S. Platt and J. S. Hunt, unpublished results). Thus, the chorion mirrors the cytotrophoblast column, which is not surprising since the chorion membrane cytotrophoblast cells are remnants of the extravillous cytotrophoblast cells that formed columns in early gestation.
Figure 2. HLA-G and β2m proteins expressed by trophoblast cells in the early to middle gestation placental villus, trophoblastic column, trophoblastic shell and decidua.
Although only HLA-G5 has been reported as positive in precursor cytotrophoblast cells and the proximal column, at least four isoforms (HLA-G1, -G2, -G5, -G6) and light chain, β2m, are expressed at the junction of the column and decidua as well as within the decidua. Revised and updated from Reference 6, Figure 4.
HLA-G isoforms in villous cytotrophoblast cells
Unexpectedly, recent studies have shown that soluble HLA-G is expressed not only in migrating extravillous cytotrophoblast cells but also in the precursor cytotrophoblast cells in the villous placenta [14, 21, 22]. Our laboratory has identified the isoform as HLA-G5 [14]. Since this cell layer does not contain β2m, we are currently investigating the idea that the villous cytotrophoblast cells are producing HLA-G5 that is β2m-free, a situation that is known to occur when β2m is scarce or absent. It may be noted that one group of investigators [23] reported failure of identification of HLA-G5 in villous placenta. However, three independent groups of investigators, all of whom have identified and reported HLA-G5 in placentas, took issue with this [24–26]. The differences remain to be resolved, as pointed out by Sargent [27].
HLA-G isoforms in syncytiotrophoblast
Although it is clear that syncytiotrophoblast does not express any of the membrane isoforms, defining the production of soluble isoforms has been difficult. First, soluble antigens tend to be found in many locations where they are not synthesized, and the cytotrophoblast cell layer immediately underlying the syncytium is a site of synthesis of HLA-G5, as noted above. Second, even in situ hybridizations can be deceiving due to contributions of mRNAs from the cytotrophoblast cells. Resolving this problem will obviously require innovative strategies.
In summary, (i) gestational programming of trophoblast HLA-G antigens is a central feature of placental development and differentiation, and (ii) anatomically distinct collections of cytotrophoblast cells are strictly patterned for expression of specific isoforms, which could indicate strict adherence to individual, specific or redundant isoform functions throughout gestation.
Receptors for HLA-G
Determination of HLA-G receptor expression on immune system and other types of cells as well as dissection of the interactions of these receptors with HLA-G is clearly of critical importance to understanding reproduction. HLA-G interacts with CD8 on T lymphocytes [28], and the HLA-G leader sequence may be associated with HLA-E, which binds to a lymphocyte inhibitory receptor, CD94/NKG2A, on natural killer (NK) cells [29, 30]. Yet another NK receptor which recognizes soluble HLA-G, KIR2DL4, has been reported in endosomes rather than on cell membranes [31]. KIR2DL4 is an activating receptor that stimulates NK cell production of inflammatory cytokines. HLA-G interacts with at least two of the leukocyte inhibitory receptors (LILRs) [reviewed in Refs. 2–7], which were first termed immunoglobulin-like transcripts (ILT) [32]. One of the LILRs is called LILRB1 (previously called ILT2), and is highly expressed on T and B lymphocytes and less prominently expressed on cells of the mononuclear phagocyte lineage. The second LILR is LILRB2 (previously called ILT4), and is exhibited mainly by monocytes, macrophages and similar cells. Activation of the LILR inhibitory receptors over-rides activating signals in immune cells by interfering with signal transduction pathways [32].
It is of the utmost importance to determine the natural structures of the HLA-G proteins produced in the various subpopulations of trophoblast cells as most studies on interactions between LILR and HLA-G have employed artificial constructs. Nonetheless, the results of the experiments conducted in vitro have been enlightening. For example, it is now known that disulfide bonding of HLA-G heavy chains is a feature of this molecule and occurs in cells such as the HEK293 cells we transfected [14]. Disulfide bonding is facilitated by interactions between cys42 in the α1 regions of the molecules [33]. Importantly, Shiroishi et al. have shown that the LILR1 and LILR2 bind these dimeric forms in preference to monomeric forms [34, 35]. Ongoing studies in our laboratory are designed to explore the quaternary structure of the HLA-G5 and –G6 produced in villous and extravillous cytotrophoblast cells and investigate the interactions of specific isoforms with LILR.
Although expression patterns of the LILRBs in decidua and placenta have not yet been thoroughly mapped, macrophages selectively harvested from early and late gestation decidua express both LILRB1 and LILRB2 receptors [36]. More studies on the distribution of these receptors among immune and other cells in health and disease are needed so as to predict target selection by HLA-G. Comments on the structural aspects of HLA-G5 as these molecules are tested for function are found below.
Conditions regulating HLA-G mRNA and protein expression
Early studies in ours and other laboratories showed that the usual promoters of transcription are not very effective for HLA-G. Two-fold inductions of HLA-G mRNA in response to interferons are average [37]. This is likely to be due to the deletion in the Enhancer A/ISRE region in the promoter that is a unique feature of the HLA-G gene distinguishing it from other HLA class I promoters. Furthermore, we have shown that the HLA-G gamma-activating sequence (GAS element) is crippled by a single nucleotide change [38], thus preventing enhanced transcription through this mechanism. However, other elements such as hypoxia response sequences are intact, and low oxygen levels are clearly strong enhancers of at least four alternatively spliced transcripts, HLA-G1, -G2, -G5 and –G6, from the single HLA-G mRNA [32]. Whether this carries through to the protein level remains uncertain although there is one report of no change in HLA-G expression in first trimester placental cytotrophoblast columns incubated under hypoxic conditions [40].
The impact of growth factors and substrates on expression of HLA-G5 and –G6 has also been a subject of study in our laboratory. Much remains to be learned about the effects of the multitude of growth and differentiation factors found in placentas, but it is clear that the substrate on which term cytotrophoblast cells are grown alters their HLA-G5. A recent review from our laboratory [6] contains a figure illustrating the alterations in the size of HLA-G-containing granules and increase in HLA-G5 protein in the term villous cytotrophoblast cells that results from culture on collagen IV-coated dishes in comparison with the commonly used uncoated plastic or glass dishes/slides. Thus, changes that take place in the matrix or basement membrane to which cytotrophoblast cells attach or upon which they migrate may play a major role in production and/or intracellular localization of HLA-G5. This would be consistent with the observation that as cytotrophoblast cells remove themselves from the villi and ultimately attach to the decidua, dramatic alterations take place in their spectra of detectable HLA-G (Fig. 2).
In summary, conditions that relate to stage of gestation, anatomic positioning relative to decidua or maternal blood, and driving toward invasion of the decidua either upregulate HLA-G mRNAs and antigens to detectable levels or permit translation of HLA-G messages that may already be in place but untranslated in trophectoderm-derived cells.
Functions of HLA-G molecules
In general, HLA-G is an immune suppressor. Its ability to diminish the immunological functions of various hematopoietic cells correlates well with the observation that following implantation, the human placenta repositions the uterus as a site of immune privilege, and supports the idea that HLA-G production is a central, pregnancy-specific feature of uteroplacental immune tolerance. These activities have been recently reviewed [4, 6] and are summarized in Figure 3, which displays the range of cells at the maternal-fetal interface whose functions are altered by HLA-G.
Figure 3. Multiple receptors for HLA-G are expressed on immune and other cells.
Many types of cells that participate in immune responses express receptors for HLA-G, including CD4+ and CD8+ T cells, B cells, NK cells, macrophages and dendritic cells. Newly added is the endothelial cell, which has been reported to bind HLA-G via CD160 [39]. Revised and updated from Reference 4, Figure 6..
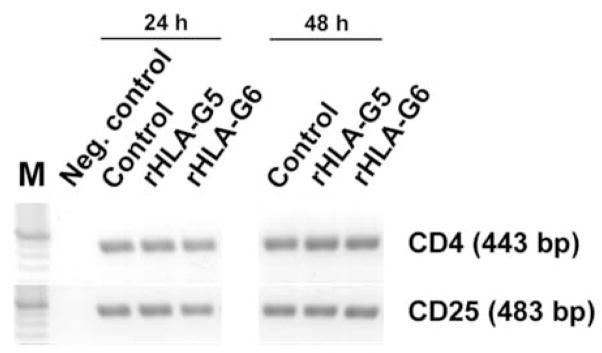
One of the first reports of HLA-G-mediated suppression documented death of phytohemagglutinin-activated, HLA-G-treated T lymphocytes via activation of the FasL/Fas cell death pathway [41]. This has been verified by Contini et al. [42], who describe increased Fas and decreased cytotoxicity by CD8+ T cells and NK cells. Le Rond et al. [43] reported induction of immunosuppressive T cells by HLA-G5, but concluded that these were not CD4+/CD25+ T regulatory (Treg) cells. A group of experiments in our laboratory designed to test whether markers of Treg are altered by rHLA-G5 and/or –G6 have also produced negative results (Fig. 4). After 24 hr and 48 hr of incubation with 50 nM of recombinant protein, levels of CD4 and CD25 mRNAs in magnetic bead-selected CD4+ blood lymphocytes tested by RT-PCR were not altered from control (no HLA-G) levels. Foxp3 expression was also unchanged (data not shown) but since change in this transcription factor has only been demonstrated when cells are activated with anti-CD3 and stimulated with TGF-β, conditions untested in our experiments, this was not surprising.
Figure 4. Neither CD4 nor CD25 mRNA is altered in CD4+ cells following incubation with HLA-G5 or –G6.
Mononuclear cells were harvested from healthy volunteers under a protocol approved by the Kansas Human Subjects Committee. CD4+ cells were selected by using magnetic bead technology. The cells were incubated in lymphocyte serum-free medium in the presence or absence (Control) of 50 nM of recombinant HLA-G5 or –G5 [14] for 24 hr or 48 hr. RNA was isolated and subjected to RT-PCR to detect CD4 and CD25 mRNAs. Although all signals were stronger at 48 hr than at 24 hr, recombinant HLA-G5 and –G6 had no major effect on levels of CD4 or CD25 in the CD4+ T lymphocytes. The negative control (water) yielded no signal.
The studies by Le Rond et al [43] were also of interest because they illustrated a point that we believe also to be of great importance, i.e., the effects of HLA-G aggregation on interaction with HLA-G receptors. In their experiments, association of HLA-G5 heavy chains with β2m did not dictate activity whereas aggregation promoted by adherence of the HLA-G5 proteins to microspheres significantly enhanced signaling in CD4+ suppressor T cells.
Mononuclear phagocytes are the target cell of greatest interest in our laboratory, mainly because both the cycling and pregnant uteri contain a stable population of these highly versatile cells. Experiments on polymyristate acetate (PMA)-differentiated, interferon-γ (IFNγ)-activated mononuclear phagocyte tumor cells (U937 cells) have shown that encounter with soluble isoforms of HLA-G stimulates the cells to produce the anti-inflammatory cytokine, transforming growth factor (TGF)-β1 [44]. The same is true of normal blood monocytes obtained from healthy volunteers (McIntire RH, Hunt JS, unpublished data). Interestingly, a second major anti-inflammatory cytokine that is prominent in decidual macrophages, interleukin-10 (IL-10), was not stimulated, and no inflammation-associated cytokines (tumor necrosis factor-α, interleukin-6) were detected. The U937 cells express both LILRB1 and LILRB2 and bind the soluble isoforms, HLA-G5 and –G6, via both receptors [44].
In addition to continued emphasis on the immunomodulatory properties of HLA-G, investigators are finding intriguing actions for these molecules in other systems. For example, HLA-G seems to affect endothelial cells. In 2001 Forte et al. described decreases in rolling adhesion of activated NK cells to endothelial cells by HLA-G [45]. More recently, Fons et al. have reported that soluble HLA-G1 inhibits angiogenesis via interactions with CD160 on endothelial cells [50]. Microarray experiments in our laboratory indicate that HLA-G has dramatic effects on the intracellular protein processing pathways in mononuclear phagocytes that could impact their motility and cytokine production as they near the placenta (McIntire RH, Hunt JS, unpublished results).
In summary, data are now emerging supporting the idea that trophoblast cell-derived HLA-G is a more versatile molecule than first suspected, and document the ability of other types of cells, including tumor cells, to produce this tolerizing molecule.
Perspectives
HLA-G is, uniquely, the only HLA molecule that has stimulated organization of its own biannual international meeting. This is because convincing evidence that HLA-G might serve as a general, natural immunosuppressant has emerged during the past half decade. It is exciting to think that HLA-G is not only associated with tolerance and immune privilege in human pregnancy, but participates in both beneficial and detrimental situations of tolerance in other contexts. For example, it appears that tumor cells have adapted production of HLA-G in an effort to circumvent host immune rejection [12]. Further, it has been suggested by many investigators that this immunosuppressive molecule could be used therapeutically to facilitate acceptance of allografts such as heart/lung transplants.
Before HLA-G can reach the marketplace, much needs to be learned. In conducting functional experiments, care is advised in selection of anti-HLA-G reagents and interpretation of binding patterns. RT-PCR needs to be conducted with well-established protocols [51], and amplified transcripts require sequencing for accuracy. Disparate results in the literature might be explained by the structure of the HLA-G protein being tested and/or by conditions used in different types of assays. The presence of lipopolysaccharide from bacterial fusion proteins or cellular antigens from HLA-G-expressing tumor cells contaminating the various preparations of HLA-G are potential sources of problems in functional experiments.
Definitive experimentation to support the postulate that HLA-G promotes human pregnancy by stimulating local tolerance has not been done. As a consequence, Koch’s postulates remain unfulfilled. The monkey or baboon model may be used to fill this gap, but there are potential problems stemming from differences between HLA-G and the primate correlates. For example, regulation of the baboon correlate of HLA-G, Paan-AG, more nearly resembles HLA class Ia genes than the class Ib gene, HLA-G [52].
It will be of considerable interest and importance to learn more about human variability in production of HLA-G mRNA and its isoforms, with the hope that remedies for infertility and/or recurrent pregnancy loss might be developed.
Acknowledgments
This work was supported by NIH grants RO1 HD33994 (Project IV), PO1 HD39878 and PO1 HD049480 to J.S.H., NIH grant HD42280 to A.T.F, and an NIH Center of Excellence for Minority Medical Education New Recruit Faculty Development Award to D.K.L. This publication was made possible by NIH grant number P20 RR016475 from the INBRE Program of the National Center for Research Resources. The authors appreciate the technical assistance of J. S. Platt and the assistance of S. Fernald, University of Kansas Medical Center Image Center, in generation of the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geraghty DE, Koller BH, Orr HT. A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc Natl Acad Sci USA. 1987;84:9145–9149. doi: 10.1073/pnas.84.24.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt JS, Orr HT. HLA and maternal-fetal recognition. FASEB J. 1992;6:2344–2348. doi: 10.1096/fasebj.6.6.1544544. [DOI] [PubMed] [Google Scholar]
- 3.Le Bouteiller P, Mallet V. HLA-G and pregnancy. Rev Reprod. 1997;2:7–13. doi: 10.1530/ror.0.0020007. [DOI] [PubMed] [Google Scholar]
- 4.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19:681–693. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 5.Hunt JS, McIntire RH, Petroff MG. Immunobiology of human pregnancy. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3. Vol. 2. Vol. 51. St Louis, MO: Elsevier/Academic Press; 2006. pp. 2759–2785. [Google Scholar]
- 6.Hunt JS. Stranger in a strange land. Immunol Rev. 2006;213:36–47. doi: 10.1111/j.1600-065X.2006.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovats S, Main EK, Librach C, Stubblebein M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 8.Ellis SA, Sargent IL, Redman CW, McMichael AJ. Evidence for a novel HLA antigen found on human extravillous trophoblast and a choriocarcinoma cell line. Immunol. 1986;59:595–601. [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis SA, Palmer MS, McMichael AJ. Human trophoblast and the choriocarcinoma cell line BeWo express a truncated HLA class I molecule. J Immunol. 1990;144:731–735. [PubMed] [Google Scholar]
- 10.Mallet V, Blaschitz A, Crisa L, Schmitt C, Fournel S, King A, Loke YW, Dohr G, Le Bouteiller P. HLA-G in the thymus: a subpopulation of medullary epithelial but not CD83(+) dendritic cells expresses HLA-G as a membrane-bound and soluble protein. Int Immunol. 1999;11:889–898. doi: 10.1093/intimm/11.6.889. [DOI] [PubMed] [Google Scholar]
- 11.Langat DK, Platt SJ, Tawfik O, Fazleabas AT, Hunt JS. Differential expression of human leukocyte antigen-G (HLA-G) messenger RNAs and proteins in normal human prostate and prostatic tumors. J Reprod Immunol. 2006;71:75–86. doi: 10.1016/j.jri.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Rouas-Freiss N, Moreau P, Ferrone S, Carosella ED. HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism? Cancer Res. 2005;65:10139–10144. doi: 10.1158/0008-5472.CAN-05-0097. [DOI] [PubMed] [Google Scholar]
- 13.Hunt JS, Jadhav L, Chu W, Geraghty DE, Ober C. Soluble HLA-G circulates in mothers during pregnancy. Am J Obstet Gynecol. 2000;183:682–688. doi: 10.1067/mob.2000.106762. [DOI] [PubMed] [Google Scholar]
- 14.Morales PJ, Pace JL, Platt JS, Phillips TA, Morgan K, Fazleabas AT, Hunt JS. Placental cell expression of HLA-G2 isoforms is limited to the invasive trophoblast phenotype. J Immunol. 2003;171:6215–6224. doi: 10.4049/jimmunol.171.11.6215. [DOI] [PubMed] [Google Scholar]
- 15.Yao YQ, Barlow DH, Sargent IL. Differential expression of alternatively spliced transcripts of HLA-G in human preimplantation embryos and inner cell masses. J Immunol. 2005;175:8379–8385. doi: 10.4049/jimmunol.175.12.8379. [DOI] [PubMed] [Google Scholar]
- 16.Hiby SE, King A, Sharkey A, Loke YW. Molecular studies of trophoblast HLA-G: polymorphism, isoforms, imprinting and expression in preimplantation embryo. Tissue Antigens. 1999;53:1–13. doi: 10.1034/j.1399-0039.1999.530101.x. [DOI] [PubMed] [Google Scholar]
- 17.Langat DK, Morales PJ, Fazleabas AT, Mwenda JM, Hunt JS. Baboon placentas express soluble and membrane-bound Paan-AG proteins encoded by alternatively spliced transcripts of the class Ib major histocompatibility complex gene, Paan-AG. Immunogenet. 2002;54:164–173. doi: 10.1007/s00251-002-0454-8. [DOI] [PubMed] [Google Scholar]
- 18.Hunt JS, Hsi BL, King CR, Fishback JL. Detection of class I MHC mRNA in subpopulations of first trimester cytotrophoblast cells by in situ hybridization. J Reprod Immunol. 1991;19:315–323. doi: 10.1016/0165-0378(91)90043-p. [DOI] [PubMed] [Google Scholar]
- 19.Ober C, Aldrich C, Rosinsky B, Robertson A, Walker MA, Willadsen S, Verp MS, Geraghty DE, Hunt JS. HLA-G1 protein expression is not essential for fetal survival. Placenta. 1998;19:127–132. doi: 10.1016/s0143-4004(98)90000-5. [DOI] [PubMed] [Google Scholar]
- 20.Menier C, Riteau B, Dausset J, Carosella ED, Rouas-Freiss N. HLA-G truncated isoforms can substitute for HLA-G1 in fetal survival. Hum Immunol. 2000;61:1118–1125. doi: 10.1016/s0198-8859(00)00194-4. [DOI] [PubMed] [Google Scholar]
- 21.Solier C, Aguerre-Girr M, Lenfant F, Campan A, Berrebi A, Rebmann V, Grosde-Wilde H, Le Bouteiller P. Secretion of pro-apoptotic intron-4-retaining soluble HLA-G1 by human villous trophoblast. Eur J Immunol. 2002;32:3576–3586. doi: 10.1002/1521-4141(200212)32:12<3576::AID-IMMU3576>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, Geraghty DE. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol. 2003;171:1376–1384. doi: 10.4049/jimmunol.171.3.1376. [DOI] [PubMed] [Google Scholar]
- 23.Blaschitz A, Juch H, Volz A, Hutter H, Daxboeck C, Desoye G, Dohr G. The soluble pool of HLA-G produced by human trophoblasts does not include detectable levels of the intron 4-containing HLA-G5 and HLA-G6 isoforms. Mol Hum Reprod. 2005;11:699–710. doi: 10.1093/molehr/gah185. [DOI] [PubMed] [Google Scholar]
- 24.Le Maoult J, Rouas-Freiss N, Carosella ED. HLA-G5 expression by trophoblast cells: the facts. Mol Human Reprod. 2005;11:719–722. doi: 10.1093/molehr/gah224. [DOI] [PubMed] [Google Scholar]
- 25.Le Bouteiller P. Human villous trophoblast and the lack of intron 4-retaining soluble HLA-G secretion: beware of possible methodological biases. Mol Hum Reprod. 2005;11:711–713. doi: 10.1093/molehr/gah211. [DOI] [PubMed] [Google Scholar]
- 26.Hunt JS, Geraghty DE. Soluble HLA-G isoforms: technical deficiencies lead to misinterpretations. Mol Human Reprod. 2005;11:715–717. doi: 10.1093/molehr/gah223. [DOI] [PubMed] [Google Scholar]
- 27.Sargent IL. Does “soluble” HLA-G really exist? Another twist to the tale. Mol Hum Reprod. 2005;11:695–698. doi: 10.1093/molehr/gah196. [DOI] [PubMed] [Google Scholar]
- 28.Sanders SK, Giblin PA, Kavathas P. Cell-cell adhesion mediated by CD8 and human histocompatibility leukocyte antigen G, a nonclassical major histocompatibility complex class 1 molecule on cytotrophoblasts. J Exp Med. 1991;174:737–740. doi: 10.1084/jem.174.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Llano M, Lee N, Navarro F, Garcia P, Albar JP, Geraghty DE, Lopez-Botet M. HLA-E-bound peptides influence recognition by inhibitory and triggering CD94/NKG2 receptors: preferential response to an HLA-G-derived nonamer. Eur J Immunol. 1998;28:2854–2863. doi: 10.1002/(SICI)1521-4141(199809)28:09<2854::AID-IMMU2854>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 30.King A, Allan DS, Bowen M, Powis SJ, Joseph S, Verma S, Hiby SE, McMichael AJ, Loke YW, Braud VM. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol. 2000;30:1623–1631. doi: 10.1002/1521-4141(200006)30:6<1623::AID-IMMU1623>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 31.Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, van der Meer A, Joosten I, Long EO. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. 2006;4:e9. doi: 10.1371/journal.pbio.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown D, Trowsdale J, Allen R. The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens. 2004;64:215–225. doi: 10.1111/j.0001-2815.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- 33.Boyson JE, Erskine R, Whitman MC, Chiu M, Lau JM, Koopman LA, Valter MM, Angelisova P, Horejsi V, Strominger JL. Disulfide bond-mediated dimerization of HLA-G on the cell surface. Proc Natl Acad Sci USA. 2002;99:16180–16185. doi: 10.1073/pnas.212643199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiroishi M, Kuroki K, Ose T, Rasubala L, Shiratori I, Arase H, Tsumoto K, Kumagai I, Kohda D, Maenaka K. Efficient leukocyte Ig-like receptor signaling and crystal structure of disulfide-linked HLA-G dimer. J Biol Chem. 2006;281:10439–10447. doi: 10.1074/jbc.M512305200. [DOI] [PubMed] [Google Scholar]
- 35.Shiroishi M, Kuroki K, Rasubala L, Tsumoto K, Kumagai I, Kurimoto E, Kato K, Kohda D, Maenaka K. Structural basis for recognition of the non-classical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d) Proc Natl Acad Sci USA. 2006;103:16412–16417. doi: 10.1073/pnas.0605228103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petroff MG, Sedlmayr P, Azzola D, Hunt JS. Decidual macrophages are potentially susceptible to inhibition by class Ia and class Ib HLA molecules. J Reprod Immunol. 2002;56:3–17. doi: 10.1016/s0165-0378(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Geraghty DE, Hunt JS. Cytokine regulation of HLA-G expression in human trophoblast cell lines. J Reprod Immunol. 1995;29:179–195. doi: 10.1016/0165-0378(95)00942-e. [DOI] [PubMed] [Google Scholar]
- 38.Chu W, Gao J, Murphy WJ, Hunt JS. A candidate interferon-gamma activated site (GAS element) in the HLA-G promoter does not bind nuclear proteins. Human Immunol. 1999;60:1113–1118. doi: 10.1016/s0198-8859(99)00091-9. [DOI] [PubMed] [Google Scholar]
- 39.Hunt JS, Langat DK, McIntire RH, Morales PJ. The role of HLA-G in pregnancy. Reprod Biol Endocrinol. 2006;4 (Suppl 1):S10. doi: 10.1186/1477-7827-4-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fournel S, Aguerre-Girr M, Huc X, Lenfant F, Alam A, Toubert A, Bensussan A, Le Bouteiller P. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol. 2000;164:6100–6104. doi: 10.4049/jimmunol.164.12.6100. [DOI] [PubMed] [Google Scholar]
- 42.Contini P, Ghio M, Merlo A, Poggi A, Indiveri F, Puppo F. Apoptosis of antigen-specific T lymphocytes upon the engagement of CD8 by soluble HLA class I molecules is Fas ligand/Fas mediated: evidence for the involvement of p56lck, calcium calmodulin kinase II, and Calcium-independent protein kinase C signaling pathways and for NF-kappaB and NF-AT nuclear translocation. J Immunol. 2005;175:7244–7254. doi: 10.4049/jimmunol.175.11.7244. [DOI] [PubMed] [Google Scholar]
- 43.Le Rond S, Azema C, Krawice-Radanne I, Durrbach A, Guettier C, Carosella ED, Rouas-Freiss N. Evidence to support the role of HLA-G5 in allograft acceptance through induction of immunosuppressive/regulatory T cells. J Immunol. 2006;176:3266–3276. doi: 10.4049/jimmunol.176.5.3266. [DOI] [PubMed] [Google Scholar]
- 44.McIntire RH, Morales PJ, Petroff MG, Colonna M, Hunt JS. Recombinant HLA-G5 and -G6 drive U937 myelomonocytic cell production of TGF-{beta}1. J Leukoc Biol. 2004;76:1220–1228. doi: 10.1189/jlb.0604337. [DOI] [PubMed] [Google Scholar]
- 45.Forte P, Pazmany L, Matter-Reissmann UB, Stussi G, Schneider MK, Seebach JD. HLA-G inhibits rolling adhesion of activated human NK cells on porcine endothelial cells. J Immunol. 2001;167:6002–6008. doi: 10.4049/jimmunol.167.10.6002. [DOI] [PubMed] [Google Scholar]
- 50.Fons P, Chabot S, Cartwright JE, Lemfant F, L’faqihi F, Giustiniani J, Herault JP, Gueguen G, Bono F, Savi P, Aguerre-Girr M, Fournel S, Malecaze F, Bensussan A, Plouet J, Le Bouteiller P. Soluble HLA-G1 inhibits angiogenesis through an apoptotic pathway and by direct binding to CD160 on endothelial cells. Blood. 2006;108:2608–2615. doi: 10.1182/blood-2005-12-019919. [DOI] [PubMed] [Google Scholar]
- 51.Pace JL, Morales PJ, Phillips TA, Hunt JS. Analysis of the soluble isoforms of HLA-G: mRNAs and proteins. In: Soares MJ, Hunt JS, editors. Placental and Trophoblast Methods and Protocols. Totowa: Humana Press; 2006. pp. 181–203. [DOI] [PubMed] [Google Scholar]
- 52.Langat DK, Morales PJ, Fazleabas AT, Hunt JS. Potential regulatory sequences in the untranslated regions of the baboon MHC class Ib gene, Paan-AG, more closely resemble those in the human MHC class Ia genes than those in the class Ib gene, HLA-G. Immunogenet. 2004;56:657–666. doi: 10.1007/s00251-004-0727-5. [DOI] [PubMed] [Google Scholar]