Abstract
A 77-year-old Asian man presented to the emergency department with bilateral pleural effusion and ascites accompanied with generalized weakness, dyspnea, tachycardia, and tachypnea. After an extensive workup that ruled out heart failure, pulmonary embolism, pneumonia, and malignancy—including extensive laboratory tests, electrocardiograms, chest x-ray, computed tomographic angiogram, computed tomography scans of the abdomen and pelvis, colonoscopy, thoracentesis, paracentesis, and exploratory laparoscopy—an elusive peritoneal tuberculosis was successfully identified. This case suggests that clinicians should consider extrapulmonary tuberculosis in their practice, given increasing immigration and the variety of populations present in our society. When tuberculosis is suspected, a negative smear for acid-fast bacillus, a lack of granulomas on histopathology, and failure to culture Mycobacterium tuberculosis do not exclude the diagnosis. Exploratory laparoscopy or minilaparotomy has a high level of sensitivity and specificity so should be considered.
A 77-year-old Asian man with a past medical history of diabetes, hyperlipidemia, and hypertension presented to the emergency department with complaints of weakness, fatigue, and shortness of breath for 2 weeks, symptoms that had worsened over the previous 3 to 4 days. The patient also complained of decreased appetite. He said he had had a nonproductive cough for 3 weeks, which had stopped 2 weeks earlier. He also had mild nausea and vomited once a day for 3 days. He had occasional mild abdominal pain, with his stomach becoming “hard and big.” The patient also complained of night sweating. He had no fever, no recent travel, no sick contacts, and no chest pain. He had lost 3 pounds in 2 weeks. He did not use alcohol or drugs but had a 40-pack-year smoking history; he had quit smoking 14 years earlier. In the 1960s, the patient stayed 10 years in a jungle jail in Asia.
In the emergency department, the patient's blood pressure was 144/85 mm Hg; heart rate, 108 beats per minute; respiratory rate, 28 breaths per minute; temperature, 97°F; weight, 58 kg. His oxygen saturation was 97% on room air. He had decreased breath sounds with rales in both lung bases, as well as mild right upper quadrant tenderness and moderate distension with decreased bowel sounds. The patient had no jugular venous distention, wheezing, precordial murmur, gallop, rub, jaundice, rash, clubbing, cyanosis, or edema. Laboratory results revealed no leukocytosis, no left shift, and mild anemia. The erythrocyte sedimentation rate was elevated to 42 mm/h (reference range, <20). Electrolytes were basically normal except for mild hyponatremia. Renal and liver function tests were within normal limits. The electrocardiogram showed normal sinus rhythm. The patient was given a point of care creatine kinase-MB test, which was 1.7 ng/mL (reference range, 1.0–7.0). Myoglobin was 110 ng/mL (reference range, 5–190); troponin, <0.05 ng/mL (reference range, 0.05–0.4); brain natriuretic peptide, 8 pg/mL (reference range, 5–100); and point of care D-dimer, 3740 ng/mL (reference range, 100–400). His chest x-ray showed bilateral pleural effusion with no infiltrates.
A computed tomography (CT) angiogram was subsequently performed. It showed no pulmonary embolism but confirmed moderate-to-large pleural effusion and revealed a moderate amount of ascites in the upper abdomen with omental thickening. The CT did not show any lung mass or hilar lymphadenopathy.
Thoracentesis generated 900 mL of grossly bloody fluid from the patient's right chest. Pleural fluid analysis was consistent with exudate, with 30,000 red blood cells and 1371 white blood cells consisting of 76% lymphocytes, 18% mesothelial cells, 1% neutrophils, 1% basinophils, and 1% unidentified cells. A cytologic study did not show malignant cells. Acid-fast staining of the pleural fluid was negative. Bacterial culture was negative as well.
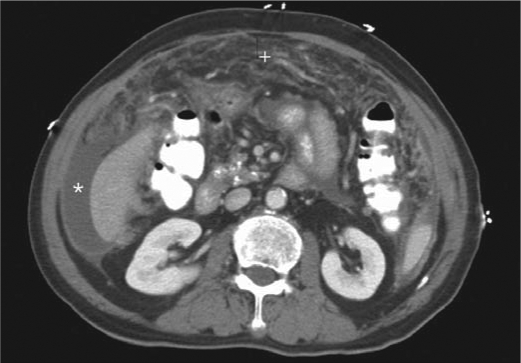
CT scans of the abdomen and pelvis confirmed moderate ascites and nodular omental thickening (Figure 1). No dominant mass was identified. Small punctate calcific deposits involving an uncinate process of the pancreas was slightly more prominent than previously. No underlying mass was seen. The cecum was not distended. An underlying mass could not be excluded. Cytologic analysis did not reveal any malignant cells.
Figure 1.
Computed tomography scan of the abdomen and pelvis shows moderate ascites (∗) and a thickened omentum with nodular appearance (+).
Ultrasound-guided paracentesis was subsequently conducted, which generated 230 mL of ascites. The ascites was exudate with 93% lymphocytes. Cytologic study again did not show malignant cells. Acid-fast staining was again negative. Aerobic and anaerobic cultures were negative. Fungal culture was sent for analysis, and there was no growth after 2 weeks. A colonoscopy was performed. Multiple benign polyps were resected.
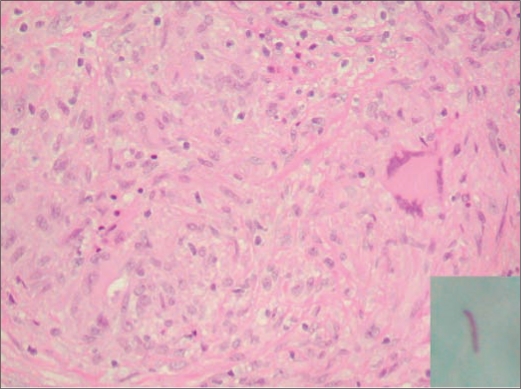
An exploratory laparoscopy was performed. Extensive nodularity and studding of the peritoneal surface throughout the entire abdominal cavity was noted, with innumerable soft, sheet-like small atypical nodules. Pathology showed granulomata, and acid-fast stain was positive (Figure 2). The diagnosis of peritoneal tuberculosis was made. PPD skin test and control were both negative.
Figure 2.
Pathological study of the thickened omentum showed granuloma, and acid-fast positive bacilli (inlet) was identified.
Treatment for tuberculosis was started with rifampin, isoniazid, ethambutol, and pyrazinamide. Isoniazid and rifampin were continued for 6 months, and ethambutol and pyrazinamide were continued for 2 months. The patient received a second ultrasound-guided thoracentesis. Acid-fast stains and cultures were again negative. His dyspnea improved significantly after the second thoracentesis, and heart rate normalized. His other symptoms of fatigue and night sweating started to improve 3 days after the initiation of treatment.
DISCUSSION
Tuberculosis remains a major worldwide health problem, with the global prevalence of infection estimated at 32% (1), although the USA has a much lower infection rate due to aggressive treatment and a strict monitoring system for infection. However, because of the prevalence of HIV infection, tuberculosis infection has become more common in the USA. In addition, the percentage of US cases that occurs among foreign-born persons is increasing (53% in 2003) (2). Hence, physicians should maintain a high order of suspicion of tuberculosis since more and more immigrants are coming to the USA.
Extrapulmonary tuberculosis has become more common among HIV-infected populations (3). In this case, the fact that this patient was HIV negative and his PPD test was inconclusive suggested that the elderly could be another group affected by extrapulmonary tuberculosis based on age-related immune decline.
Tuberculous peritonitis results from reactivation of latent foci in the peritoneum. It is more common in patients with cirrhosis and in those undergoing continuous ambulatory peritoneal dialysis, as well as in HIV-positive patients (4). As in this case, patients present with the insidious onset of ascites, abdominal pain, and fever. Peritoneal fluid is exudative, with a serum-ascites albumin gradient of <1.1 g/dL. Peritoneal fluid leukocyte count varies from 150 to 4000 per mm3 with a lymphocytic predominance (5), as seen in this case. A patient's tuberculosis-endemic country of origin should also raise a suspicion of tuberculosis (6).
The positive yield of an acid-fast bacillus smear and mycobacterial culture of pleural effusion or ascites could have been enhanced with centrifugation of 1 L of peritoneal fluid before conducting the test. It was reported that the peritoneal fluid adenosine deaminase level has a high sensitivity and specificity, with a cutoff value >33 U/L. However, the adenosine deaminase level in this patient's ascites was only 4.9 U/L. Polymerase chain reaction seems to have higher sensitivity and specificity, although large-scale evaluation needs to be done (7). Peritoneal biopsy under exploratory laparoscopy or minilaparotomy can be diagnostic in more than 95% of patients and should be strongly considered (6).
Numerous diseases may cause pleural effusion, including heart failure, lung cancer, pneumonia, tuberculosis, asbestosis, sarcoidosis, drug reaction, or any inflammatory process. This patient's pleural effusion seemed to be secondary to peritoneal tuberculosis since no pleural thickening or nodular change was noticed. However, thoracentesis is important for the relief of the patient's symptoms.
D-dimer has been widely used as a screening test for pulmonary embolism and deep vein thrombosis. A positive D-dimer indicates the presence of an abnormally high level of fibrin degradation products. It indicates significant thrombus formation and breakdown in the body, but it does not tell the location or cause. An elevated D-dimer may be due to a venous thromboembolism or disseminated intravascular coagulation, but it may also be due to a recent surgery, trauma, or infection. Elevated levels are also seen with liver disease, pregnancy, eclampsia, heart disease, and some cancers. In this particular case, elevated D-dimer was simply a result of bloody pleural effusion and ascites.
Treatment of tuberculosis in elderly and HIV patients is the same as for the general population. A 6- to 9-month regimen (2 months of isoniazid, rifampin, pyrazinamide, and ethambutol, followed by 4 to 7 months of isoniazid and rifampin) is recommended as initial therapy for all forms of extrapulmonary tuberculosis unless the organisms are known or suspected to be resistant to the first-line drugs (8). Physicians should consider noncompliance, malabsorption, and drug resistance as possible reasons for delayed or suboptimal response to appropriate therapy. Directly observed therapy is strongly recommended to encourage medication compliance (9). This case was reported to the infection control authority immediately after the diagnosis was made, and the Department of Health will directly observe therapy.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282(7):677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Trends in tuberculosis—United States, 1998–2003. MMWR Morb Mortal Wkly Rep. 2004;53(10):209–214. [PubMed] [Google Scholar]
- 3.Prieder HL, Snider DE, Jr., Cauthen GM. Extrapulmonary tuberculosis in the United States. Am Rev Respir Dis. 1990;141(2):347–351. doi: 10.1164/ajrccm/141.2.347. [DOI] [PubMed] [Google Scholar]
- 4.Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993;88(7):989–999. [PubMed] [Google Scholar]
- 5.Diagnostic standards and classification of tuberculosis in adults and children [Statement adopted by the American Thoracic Society and the Centers for Disease Control and Prevention and endorsed by the Council of the Infectious Disease Society of America] Am J Respir Crit Care Med. 2000;161(4 Pt 1):1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 6.Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72(9):1761–1768. [PubMed] [Google Scholar]
- 7.Nagesh BS, Sehgal S, Jindal SK, Arora SK. Evaluation of polymerase chain reaction for detection of Mycobacterium tuberculosis in pleural fluid. Chest. 2001;119(6):1737–1741. doi: 10.1378/chest.119.6.1737. [DOI] [PubMed] [Google Scholar]
- 8.American Thoracic Society. Centers for Disease Control and Prevention. Infectious Diseases Society of America Treatment of tuberculosis. MMWR Recomm Rep. 2003;52(RR-11):1–77. [PubMed] [Google Scholar]
- 9.Blumberg HM, Leonard MK, Jr., Jasmer RM. Update on the treatment of tuberculosis and latent tuberculosis infection. JAMA. 2005;293(22):2776–2784. doi: 10.1001/jama.293.22.2776. [DOI] [PubMed] [Google Scholar]