Abstract
Hematopoietic stem cells give rise to multiple lineages of cells. This process is governed by a tightly controlled signaling network regulated by cytokines and a direct cell-cell. Notch signaling represents one of the major pathways activated during direct interaction between hematopoietic progenitor cells and bone marrow stroma. A critical role of Notch signaling in differentiation of T and B lymphocytes has now been established. Until recently, the role of Notch signaling in the development of myeloid cells and particular dendritic cells remained unclear. In this review we discuss recent exciting findings that shed light on the critical role of Notch in differentiation and the function of dendritic cells and its impact on immune responses.
Keywords: hematopoietic progenitor cells, dendritic cells, macrophages, Notch
Main characteristics of Notch family of transcriptional regulators and receptors
The Notch family of transcriptional regulators is highly conserved from C. elegans to humans (reviewed in (1–3)). Notch signals influence multiple processes that govern normal morphogenesis, including the lineage specification among bipotent progenitor cells, programmed cell death, and cellular proliferation. Notch signaling is initiated by the binding of the extracellular domain of Notch to a Notch ligand. At present, two Notch ligand families, Delta and Jagged have been described (4). The Notch receptors family includes four members (Notch-1 to 4). Each member is a large single heterodimeric receptor comprised of noncovalently associated extracellular (ECN), transmembrane (TMN) subunits, and intracellular subunit (ICN). The extracellular subunits contain 11–36 tandem epidermal growth factor (EGF)-like repeats that bind ligand. The intracellular region contains a RAM domain that binds transcriptional repressor CSL/CBF-1, a series of cdc10/ankyrin repeats that participate in protein-protein interactions with CSL/CBF-1 and other polypeptides, and a C-terminal PEST sequence. Multiple lines of investigation have converged on a model in which the intracellular domain of Notch (ICN) translocates to the nucleus in a ligand-dependent fashion (5). Ligand/receptor interactions initiate two successive proteolytic cleavages within the TMN subunit. The protease responsible for the first cleavage, which occurs just external to the transmembrane domain, is an ADAM metalloprotease. Subsequently, a second cleavage within the transmembrane domain release ICN. This cleavage requires the function of two different classes of transmembrane proteins γ-secretase presenilins and nicastrin (6), which represent two components of a multisubunit complex that processes a number of transmembrane proteins in addition to Notch.
Intracellular Notch translocates to the nucleus where it interacts with a transcriptional repressor CSL also known as CBF-1 (RBP-J). In the absence of ICN, CSL/CBF-1 acts as a transcriptional repressor due to its ability to bind transcriptional corepressor (CoR) and histone deacetilase-1 (HDAC-1). Binding of ICN displaces co-repressor complexes, thereby derepressing transcription from promoters with CSL binding elements. In addition, the ankyrin repeats and C-terminal transcriptional activation domains of ICN recruit several different transcriptional coactivators, providing an additional stimulus for transcription (7, 8). One functionally conserved protein that may act as a Notch-specific coactivator is mastermind, an adaptor molecule that stabilizes CSL/ICN interaction and potentiates ICN stimulation of transcription from CSL-sensitive promoters. The more variable sequences C-terminal of the ankyrin repeats of Notch1 have been shown to interact with the general transcriptional coactivators p300, PCAF, and GCN5. Importantly, all four mammalian Notch receptors bind and activate CBF-1 leading to transactivation (Fig. 1). Targets of ICN/CSL signals in mammals include genes of the Hairy/Enhancer of Split (HES) family, which encode bHLH-type transcription factors, as well as the recently described HRT/HERP genes. Other ICN/CSL targets that may explain the effects of Notch on cell cycle kinetics in certain contexts are cyclin D1 and p21. Upregulated expression of cyclin D1 promotes G1 progression, and has been proposed to contribute to the transformation of BHK cells (9). In contrast, upregulation of p21 may promote the cell cycle exit of keratinocytes during differentiation (9). These opposing effects further illustrate the diverse context-specific responses induced by Notch signaling. A more detailed description of the Notch structure and function is provided in recent reviews (10, 11).
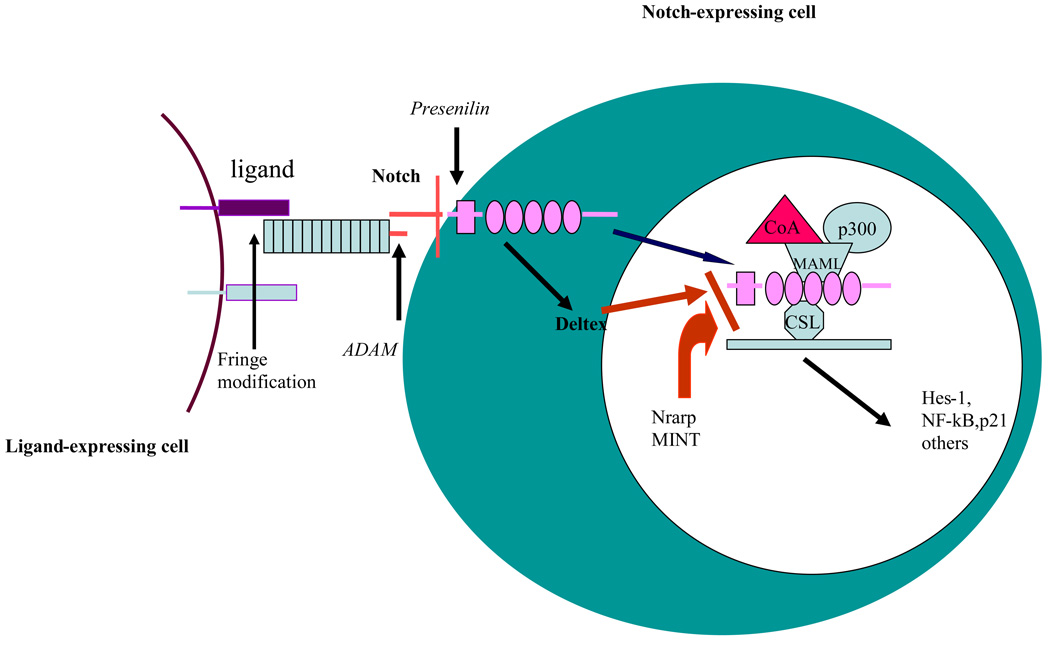
Figure 1. Schematics of Notch signaling pathway.
Notch signaling is initiated through binding of Notch ligands expressed an adjacent cell, followed by enzymatic cleavage and release of intercellular of Notch (ICN). The ICN then translocates to the nucleus, where it binds to CSL transcription repressor and converts it to activator by recruiting MAML (mastermind-like protein) to the complex including p300 and co-activator. Notch signaling is also regulated by other cellular proteins including Deltex, Nrarp (Notch-regulated ankyrin-repeat protein) and MINT (Msx2-interacting nuclear target protein).
Notch and myeloid cell differentiation
Maintenance of hematopoietic stem cells (HSC) and their differentiation are controlled by a combined effect of network of soluble factors and direct cell-cell contact between HSC and bone marrow stroma (12–14). HSCs expressing all four members of Notch family (15–17) are located in bone marrow where they constantly interact with surrounding stromal cells expressing Jagged-1 (13, 15, 18), Delta-1 (18), and Delta-4 (19). This results in an activation of Notch pathway in HSC. Most of the available data suggests that Notch activity in HSCs promotes self-renewal rather than lineage commitment (20). Bernstein and colleagues found that transduction of HSCs with ICN1 resulted in the outgrowth of immortalized, cytokine-dependent cell lines with the capacity to yield cells of lymphoid and myeloid lineages (21). Unlike long-term HSCs, however, these cells did not rescue radiation chimeras, suggesting that ICN1 activity had immortalized late-stage HSC. Varnum-Finney et al have shown that cell lines with constitutive Notch signaling could generate progeny with either lymphoid or myeloid characteristics both in vitro and in vivo indicating a role for Notch signaling in regulating HSC self-renewal (21). Consistent with this concept Calvi et al observed that osteoblasts expressing Jagged-1 were a regulatory component of the HSC niche in vivo that influenced stem cell function through Notch activation (13). Normally, HSCs express higher levels of Notch-2 than Notch-1 (15) raising the possibility that the individual Notch family members might have distinct roles in HSC self-renewal and differentiation.
The role of Notch signaling in myeloid cell differentiation remains controversial. Most in vitro experiments have shown that active Notch-1 expands the stem cell compartment but blocks or delays terminal myeloid cell differentiation. Overexpression of constitutively active Notch-1 (ICN1) in 32D myeloid progenitor cell line inhibited granulocytic differentiation and permitted expansion of undifferentiated cells (22–24). This was probably mediated through RBP-J-Hes1 pathway since overexpression of Hes-1 had similar to ICN1 effect on 32D cell differentiation (23–25). ICN1 inhibited erythroid differentiation of F5-5 mouse erythrolekemia cells through sustained activity of GATA2 transcription factor (24). ICN1 also blocked differentiation of human myeloid cell line HL60 to granulocytes induced by all-trans retinoic acid (ATRA) or to monocytes induced by tetradecanoylphorbol acetate (TPA) (26, 27). Consistent with these results inhibition of Notch-1 expression in K562 cells resulted in the induction of spontaneous erythroid maturation.(27). Block of hematopoietic cell differentiation and promotion of HSC self-renewal was observed when Notch signaling was activated via overexpression of Notch-2 (23, 28) or via interaction with Notch ligands Jagged-1 (13, 15, 25), Jagged-2 (26), Delta-1 (29, 30), or Delta-4 (31–33).
However, there are several reports that may suggest an opposite role of Notch signaling in myeloid cell differentiation. In 32D cells active form of Notch-1, transcriptionally active derivative of RBP-J, or activation of Notch by ligation of Jagged-1 increased myeloid differentiation (25). Notch induced activation of PU.1, a critical transcription factor involved in myeloid cell differentiation.(17). The similar phenomenon was observed in human CD34+ progenitor cells. Under serum-deprived conditions, soluble human Notch ligand Jagged-1 inhibited self-renewal of human cord blood CD34+ cell-derived macrophage progenitors (34). In the loss of function experiments using deficient mice in Notch-1 (35) and RBP-J (36), it has been reported that myeloid cell lineages are normal.
One possible mechanism of myeloid cell differentiation involving Notch signaling has been recently described. Mast cells are myeloid cells that are usually found in tissues in response to pathogens as first line of inflammation. Mast cells arise from the common myeloid progenitor (CMP) in bone marrow (37, 38). Several transcription factors and cytokines are involved in differentiation of these cells including GATA-1, GATA-2, PU.1, MITF, IL-3 and stem cell factor (39–41). Mast cells express specific pattern of Notch family members, low level of Notch-1, high level of Notch-2 and detectable level of Notch-3 (42). Mast cells also express Jagged-1 (43). It is well-known that GATA-3 and Notch are indispensable for T cell development, because deficiency of either of them blocks T cell development at earliest progenitor stage (35, 44). Recently, Taghon et al have found that overexpression of GATA-3 acts as antagonist of Notch, blocking pro-T cell survival. In the absence of Notch signaling, GATA-3 overexpression differentiates lymphoid progenitors from fetal liver and T precursors to mast cell through upregulation of GATA-1 and GATA-2 (45), the genes that are essential for mast cell development (41, 46).
Erythrocytes share with myeloid cells CMP for their lineage commitment (47). Erythroid colony forming cells (ECFC) generated from peripheral blood express Notch-1 and Notch-2, Delta-1, Delta-4, and Jagged-1 (48). Recently Lam et al have shown that Notch signaling inhibits erythroid development. In erythroleukemia K562 cell line ICN1 inhibited erythroid maturation. Interestingly, it did not affect GATA-1 and GATA-2 transcription factors (49). Consistent with these data downregulation of Notch-1 with antisense constructs caused differentiation of erythroid cells (27). Jagged-1 expressing feeder cells increased the erythroid colony formation from human CD34+ progenitor cells in the presence of stem cell factor (50). Delta-1 had a similar effect on erythroid cell differentiation from CD34+ HPC (19, 31). Tachikawa et al. also demonstrated that immobilized Delta-1 and Delta-4 inhibited erythroid cell maturation from ECFCs (48).
Thus, available data provide a consensus view on a critically important role of Notch signaling in myeloid cell differentiation. However, the exact nature of Notch effects remains controversial. Existing findings to date can be split into two groups: one demonstrating a critical role of Notch in maintenance of progenitor cells and block of terminal differentiation of myeloid cells and the other one showing requirements of Notch signaling for differentiation of mature myeloid cells. This controversy currently is not resolved. One possible explanation can be derived from the fact that the effect of Notch signaling on different cells is highly context dependent. It appears that impact of Notch signaling on myeloid cell differentiation depends on the stage of myeloid cell differentiation when Notch activation is triggered, the presence of specific cytokines, and on whether activation of Notch signaling was triggered by soluble or immobilized ligands. Elucidation of the specific conditions influencing the effect of Notch signaling on myeloid cells could be very important for understanding the overall biology cell differentiation and is a subject of ongoing and future studies.
Notch signaling and dendritic cell differentiation
Dendritic cells (DC) are professional antigen presenting cells that play a central role in the induction of immune responses against various pathogens, tumor cells, and self-antigen. DCs are differentiated in bone marrow mainly from common myeloid progenitors, although the proportion of DCs is also originated from common lymphoid progenitors. Two major subsets of DCs are currently recognized: conventional (myeloid) and plasmacytoid (lymphoid) DCs. The mechanisms of differentiation of these two subsets of DCs are different, although they may share some common pathways (51–53). In recent years clear evidence was obtained demonstrating an important role of Notch signaling in DC development. However, similar to a situation with other myeloid cells a great deal of controversy regarding specific impact of Notch signaling on DC differentiation remains.
We have previously shown that Notch-1 is necessary for DC development. Differentiation of DC was significantly impaired in Notch-1 anti-sense mice that have about half of the normal level of Notch-1 in HPC (54). These findings were further confirmed in an experimental model of DC differentiation of embryonic stem (ES) cells. ES cells lacking Notch-1 had dramatically reduced capacity to generate DCs (55). Different results were obtained by Radtke et al. They generated Notch-1 conditional knockout mice using Lox-Cre system and demonstrated that T cell development was blocked in these mice (56). However, the number of thymic DCs, conventional DCs, and Langerhans DCs in skin was normal. Detailed analysis of the effect of Notch signaling on DC development was recently performed by Caton et al. They used conditional deletion of RBP- J in bone marrow cells and DCs. Since RBP-J is an essential mediator of signaling by all Notch receptors this eliminated the potential redundancy in the effects of different members of the Notch family. Caton et al found a substantial reduction in the presence of conventional DCs in spleens of the knockout mice. This decrease affected primarily CD8− DC subset in the marginal zone of spleen (36). In a reconstituted chimeras, RBP-J−/− donor derived DCs in spleen were reduced to the same extent as in RBP-J−/− conditional knockout mice and a noticeable increase of plasmacytoid DC (pDC) was observed. Thus, it appears that Notch signaling controls the homeostasis of CD8-DC and plays an inhibitory role for pDC in spleen (36). Authors suggested that this DC loss might not necessarily reflect impaired DC lineage commitment. RBP-J–deficient bone marrow gave rise to all DC subsets, essentially similar phenotypes were caused by RBP-J deletion in the bone marrow hematopoietic progenitors (Mx1-Cre) and in committed DCs (CD11c-Cre), and immediate DC progenitors in the spleen were not affected.
Notch ligands Jagged-1 and Delta-1 both can activate Notch signaling through RBP-J. However, it appears that their effect on DC differentiation is different. Incubation of HPC on fibroblasts expressing Delta-1 induced DC differentiation, whereas Jagged-1 expressing fibroblasts had an opposite effect. They inhibit DC differentiation and instead promoted an accumulation of immature myeloid cells (57). The distinct effect of the two ligands may reflect their physiological function in the body. Bone marrow environment is conducive for generation of precursors for macrophages and DCs. About 40% of mouse bone marrow cells are Gr1+CD11b+ immature progenitor cells. In contrast, in spleens the environment promotes differentiation of mature myeloid cells. As a result less than 5% of splenocytes are Gr1+CD11b+ cells. The expression pattern of Notch ligands in bone marrow and splenic stroma cells is different. Bone marrow stroma expresses a substantially higher level of Jagged-1 than Delta-1 whereas splenic stroma has substantially higher level of Delta-1 expression than Jagged-1 (57). Delta-1 could be involved in loss of CD8− DCs observed in Caton’s study (36). CD8− DCs are located in the marginal zone of the spleens, the compartment shown to contain Delta-1-expressing cells. CD8− DCs in the marginal zone were found to reside in close contact with Delta-1-expressing cells, likely non-hematopoietic stromal cell types such as reticular fibroblasts and/or endothelium of the marginal sinus (36).
Effects of Notch ligands on DC differentiation were reported by several other groups. In a culture system containing IL-6, CD34+CD38− human cord blood HPC differentiate into CD14+ monocytes and CD15+ granulocytes. Delta-1 shifted cell differentiation towards CD14−CD1a+ DCs. These cells expressed DC-related markers CD11c, CD80, CD86 and HLA-DR (18). Ohishi et al have also shown that Delta-1 promotes DC differentiation. Monocyte precursors from blood can differentiate to macrophages and DCs depending on cytokine provided. GM-CSF supported primarily macrophages differentiation. Immobilized Delta-1 induced differentiation of DCs with characteristics similar to those observed in immature DCs derived from monocytes cultured with GM-CSF and IL-4 (58). The active form of Notch-1 increases IL-4 production through RBP-J binding to IL-4 promoter region (59). This may help to explain the observed effect in the shift in DC differentiation induced by Delta-1. The mechanism of the opposite effect of Jagged-1 on DC differentiation remains unclear. It is possible that this effect could be mediated by RBP-J independent pathway since RBP-J was equally activated by both ligands (57).
Plasmacytoid dendritic cells (pDC) are phenotypic and functionally distinct subset of DCs. Several reports described the possible effect of Notch signaling on their differentiation. Oliver et al reported that Notch signaling via one of the Notch ligands Delta-1 promote differentiation of pDC (60). Human CD34+ HPC and bone marrow CLP were cultured on Delta-1-expressing OP9 stroma in the presence of Flt-3 ligand and IL-7. These conditions provided for differentiation of BDCA-2+CD123+CD4+CD11c− cells with complete characteristics of pDC including morphology, expression of toll-like receptor-9 (TLR9), pre-T mRNAs, and secretion of IFN-α in response to CpG (60). Delta-1 enhanced the numbers of pDC by promoting the differentiation rather than affecting cell proliferation. Inhibition of Notch signaling by γ-secretase inhibitor blocked pDC development. A significant increased in pDC in this experimental system was observed at the expense of B cell development (60). T cell fate was not clear because they could not be generated under those experimental conditions. In a different study, stromal cells expressing Delta-1 promoted differentiation of CD34+CD1a− thymic progenitor cells to CD4+CD8+ T cells and blocked pDC development. In that study activation of Notch signaling through Delta-1 controlled the two alternative pathways of cell differentiation by preferentially activating GATA-3, a critical transcriptional factor for T-cell development and inhibiting the ETS family member Spi-B, a key regulator of pDC development. The Notch1-induced block in pDC development can be relieved through the ectopic expression of Spi-B (61). Inactivation of Notch-1 (62, 63) through the Mx-Cre recombinase did not affect the development of pDC suggesting that that this receptor is not essential for differentiation of these cells. In contrast, RBP-J deficient mice have shown an increased level of pDC (36) suggesting that Notch signaling may play an inhibitory role in the development of these cells.
Thus, most of the experiments with “gain of function” where Notch signaling is activated by Delta-1 demonstrate the induction of differentiation of pDCs. In contrast most of the experiments with “loss of function” based on knockout mice showed that Notch signaling either did not have the effect or inhibited pDC development. It is possible that redundancy in the function of Notch receptors may contribute to the results of these experiments. However, it is likely that lack of Notch-1 or RBP-J in knockout mice may result in the activation of RBP-J independent pathways of Notch signaling, which may have opposite functional consequences. The nature of these effects remains to be elucidated.
Notch and NF-κB are two evolutionally conserved pathways of gene regulation that determine fate and the survival of different cells. NF- κB family has five members (p50, p52, RelA, RelB and cRel) (64). These different family members can associate in various homo- or heterodimers through a highly conserved N-terminal Rel homology domain. In the cytoplasm of quiescent cells, they are associated with inhibitory molecules of the IκB family. Cell activation by various stimuli including TNFα, LPS, IL-1, and CD40 results in serine phosphorylation and degradation of IkB with subsequent nuclear translocation and specific DNA binding of NF-κB dimers (65). Mice deficient in NF-κB components have impaired DC differentiation (66). Interestingly, RelB deficient mice lacked CD11c+CD8− but not CD11c+CD8+ DCs in spleen marginal zones (67). This resembles the effect of RBP-J deletion (36). We have previously demonstrated that DNA binding of NF-κB as well as its ability to activate transcription was dramatically decreased in HPC from Notch-deficient mice (68). NF-κB driven transcriptional activity was completely restored after transduction of the cells with retroviral constructs containing activated Notch-1 gene. HPC from Notch deficient mice had decreased levels of several members of NF-κB family: p65, p50, RelB, and c-Rel and this was due to down-regulation of the gene expression (68). Differentiation of DCs inhibited in these mice was restored by transduction of activated Notch-1 into HPC. This data was consistent with observation made in previous studies that NF-κB is a putative Notch target gene, regulated by Notch signaling through RBP-J (69) and that Notch-3 sustained activity of NF-κB (70). Jagged-1 derived peptide activated Notch and induced IKKα mediated NF-κB activation (71). In addition, Notch-1 may physically interact with NF-κB subunits in T cells (71, 72). A recent study suggested that Notch and NF-κB signaling may synergize in the development of marginal zone B cells (73).
Thus, Notch signaling plays an important role in regulation of NF-κB. It is conceivable that Notch signaling may exert their effect on DC development through NF-κB. Since NF-κB is a major transcription factor involved in Toll-like receptor (TLR) signaling in DCs this may affect not only DC differentiation but also the function of these cells.
Notch signaling and dendritic cell function
As we mentioned above, the main function of DCs is the induction and/or activation of antigen-specific immune responses, which takes place during direct interaction of DCs with T cells. The quality and strength of immune responses depends in large degree on the type of surface molecules expressed on DCs. Several major types of CD4+ T cells with distinctive functions are currently recognized. Th1 cells are primarily involved in cellular immunity against intracellular pathogens and tumors and Th2 are primarily involved in humoral immunity against extracellular infection agents (74). Notch receptors are expressed on CD4+ T cells (75, 76). A number of studies have identified the expression of Notch ligands on the surface of DCs (Table 1). Those ligands can cause Notch activation in T cells during interaction between DCs and T cells. It has been proposed that polarizing T-cell responses can be controlled by Notch signaling. Recent studies support the hypothesis that Notch signaling instructs Th1/Th2 response switch although the nature of this regulation remains controversial (59, 75–78).
Table 1.
Expression of Notch receptors and ligands on the macrophages and dendritic cells
| Thymic DC | Splenic DC | BMDC | Mon-derived DC | Macrophage | |
|---|---|---|---|---|---|
| Jag1 (111) | (109) | (109), (99) | (59) | (82), (85) | (43), (110), (82), (109) |
| Jag2 | (109) | (109) | (59) | (85) | (111), (109) |
| Dll-1 | (109) | (109), (112) | (82), (85) | (43), (110), (82), (109) | |
| Dll-3 | (59) | (82) | |||
| Dll-4 | (112) | (82) | |||
| Notch-1 | (99) | (111) | |||
| Notch-2 | (111) | ||||
| Notch-4 | (111) |
BMDC – mouse bone marrow derived DCs, Mon-derived DCs – human monocyte derived DCs.
Regular numbers represent expression of mRNA; italic represents both protein and mRNA
Unstimulated macrophages and DCs express low level of Delta and Jagged. Under different conditions, the expression of Notch ligands can be induced towards preferential expression of either Jagged or Delta and their ratio may change in response to different stimuli. Lipopolysaccharide (LPS) can induce both Th1-type response that depends on MyD88 and Th2-type response independent on Myd88 (79–81). Stimulation of DCs with LPS resulted in up-regulation of Delta-4 and Jagged-1, which lead to Th1-type responses (59, 82, 83). The expression of Delta-4 depended on MyD88 since in MyD88−/− DCs LPS failed to induce up-regulation of the ligand and Th1 polarization (59, 75, 84). In mouse DC, the pattern of Notch ligands’ expression seems to be associated with pattern of Th1/Th2 responses. Surface expression of Delta-1 on DC stimulated Th1-type responses whereas Jagged-1 induces Th2-type responses (59). Stallwood et al used synthetic small interfering RNA (siRNA) sequences targeting the human Notch ligands Delta-1, Jagged-1, or Jagged-2. Transfection of these siRNAs into human primary CD4+ T cells and monocyte-derived DCs lead to the knockdown of endogenous Notch ligand message. Knockdown of any one of these three Notch ligands in DCs enhanced IFN-γ production from allogeneic CD4+ T cells. In contrast, Delta-1 knockdown in CD4+ T cells selectively enhanced production of IFN-γ, IL-2, and IL-5 in response to polyclonal stimulation, while Jagged-1 or Jagged-2 knockdown had no effect. The blockade of Notch cleavage with a γ-secretase inhibitor failed to affect cytokine production in this system, implying that Delta-1 can influence cytokine production via a Notch cleavage-independent mechanism (85). PGE2 and forskolin induces Th2 response by increasing only Delta-1 but not Jagged-2. Probiotic bacteria inducing Delta-1 or Delta-4 expression of DCs correlated with Th1 or Th2 activities (82).
There is enough evidence supporting the hypothesis that Notch signaling mediates primarily Th1-type differentiation. Co-culture of CD4 T cells with Delta-1-Fc soluble protein induced differentiation of IFN-γ secreted Th1 cells (76). In gain-of-function experiment, Notch-3 but not Notch-1overexpression in CD4 T cells directed Th1-type polarization. This was accompanied by the up-regulation of T-bet transcription factor critical for Th1 development.(86). In another study, CD4 Th1 polarization was blocked by γ-secretase inhibitors and Notch-1 antisense (78). Intercellular Notch-1 expression in T cells induced expression of Tbx21 encoding T-bet. There is also strong evidence in favor of the requirement of Notch signaling for Th2-type response. Amsen et al have shown abrogation of Th2-type response in CD4+ T cells that were deficient for Notch-1 and Notch-2. The role of Notch signaling in the development of Th2 cells was further supported using the conditional knockout of RBP-J, a factor involved in signaling from all four Notch family members. CD4+ T cells lacking RBP-J differentiated poorly into IL-4 producing Th2-type cells. In contrast, differentiation of IFN-γ producing Th1-type cells was enhanced (59, 75, 77). Consistent with these findings dominant-negative Mastermind abrogated Th2 response (87).
Both CD8+ and CD8− splenic DC can induce Th1 response but using different mechanisms. LPS induces IL-12 production of CD8+ DC, leading to Th1-type response. This response depends on TLR ligation and adaptor MyD88. However, LPS does not increase expression of Delta-4 on CD8+ DCs (84), a critical ligand accounting for Th1 polarization (59, 84). In contrast, Delta-4 expression on CD8−DCs rapidly increased when induced by LPS, and lead to Th1 response. Blockade the Notch–Delta interaction with soluble Delta-Fc mutant protein abolished induction of Th1 response. Using MyD88−/− and IL-12 deficient CD8− DCs, Skokos et al demonstrated that LPS-inducible Delta-4 expression on DCs depended on TLR-MyD88 pathway. (59, 84). DCs can direct Th polarization using different mechanism. Th1-type response can be induced by CD8+ DCs via TLR and production of IL-12 (88). However, Th1 response may also occur in the absence of IL-12 (89) via Notch signaling dependent pathway (84).
Taken together, this data provides a strong indication that polarization of Th-type responses indeed depends on the activation of Notch signaling. However, it is unlikely that there is a clear specialization in individual Notch ligands expressed on DCs that define one type of response rather than another. It appears that as it is the case with other experimental systems involving DCs the effect of Notch ligands on T-cell responses depends on the context of DC activation, the nature of other receptors expressed on their surface and cytokines produced.
In addition to defining the nature of the T-cell response Notch signaling may also be involved in the activation of DCs. Activation of Notch signaling by Jagged-derived peptide can induce the activation of human DCs (90). These DCs expressed maturation markers, produced IL-12, and were able to activate allogeneic T cells (90). As was discussed above Notch signaling might activate NF-κB and thus stimulate DC activation. However, DC activation was not observed in response to cell-bound ligands Jagged-1 and Delta-1 (18). Recent studies have demonstrated that Notch signaling may activate signal transducer and activator of transcription 3 (STAT3) in different cells (91, 92). STAT3 has been shown to block DC activation partially via regulation of NF-κB pathway (57, 93–95). These results may explain the lack of DC activation by Notch. Apparently, DC activation depends on the context and strength of Notch activation. It is conceivable that peptide-mediated Notch activation may provide a stronger signal than cell-bound ligands, which is sufficient to overcome negative regulatory mechanisms. However, whether the same mechanism exist in vivo is not clear at this time.
Antigen recognition by T cell in peripheral lymphoid organs induces either productive immune responses or tolerance. A number of studies have shown that Notch signaling may be required for activation of peripheral T cells. When CD4+ T cells incubated with antigen-presenting cells loaded with OVA peptide or stimulated with anti-CD3 and anti-CD28 antibody, Notch 1 expression was up-regulated and cleaved form of Notch-1 was detected (96, 97). Expression of Hes-1 was also induced (97, 98). Inhibition of Notch activation decreased T-cell proliferation and blocked both NF-κB activity and IFN-γ production in peripheral T cells (96, 97).
However, in recent years several studies have suggested that Notch signaling could also be involved in the induction of T-cell tolerance. Specifically, Notch was implicated in generation of regulatory T cells (Treg). Early evidence supporting the possible role of Notch signaling in Treg development came from studies by Hoyne et al. They showed that overexpression of Jagged-1 in splenic DC could direct CD4+ T cells to regulatory phenotype, inhibiting primary and secondary antigen-specific responses (99). These findings were confirmed by experiments from Yvon et al, in which they demonstrated that the overexpression of Jagged-1 in human B lymphoblastoid cell line transfected with Epstein-Barr virus could induce Tregs in response to virus-derived antigen (100). In a heart allograft mouse model, overexpression of Delta-1 on alloantigen-bearing cells could induce specific unresponsiveness to the same alloantigen (101). Furthermore, Notch ligands Delta-1 (102) and Jagged-1 (98, 102, 103) inhibited proliferation, and/or cytokine production, after stimulation of CD4+ T cells with different stimuli, which correlated with suppression of transcription factors NF-AT, AP-1 and NF-κB (102) and upregulation of GRAIL (gene related to anergy in lymphocytes) in CD4+ T cells (103). The inhibition of T cell proliferation was Notch signaling dependent because γ-secreatase significantly block this effect. CD4+CD25+ Treg isolated from human peripheral blood expressed significantly higher level of Deltex comparing with CD4+CD25− T cells (104).
The apparent discrepancy in possible role of Notch signaling in regulation of T-cell responses required further investigation. It is possible that different members of Notch family may have different effect on T-cell function. For instance transgenic mice with expression of active form of Notch-3 produce more CD4+CD25+ T reg (105). At the same time, Notch-1 activation showed an opposite effect (96, 97). Activation of Notch by Jagged-1 and Delta-1 inhibited T cell proliferation whereas Delta-4 enhanced T-cell activation and proliferation.(102). It is possible that the discrepancies could also be due to the existence of Notch-independent effects of some components of Notch signaling pathway such as Mastermind (106, 107) and RBP-J (108).
Conclusion
The wealth of available information clearly demonstrates an essential role of Notch signaling in regulation of DC differentiation and function. Notch is involved in DC differentiation via interaction with stromal cells, DC activation as well as in DC-mediated T-cell activation. However, the nature of this regulation remains largely unclear because of multitude of rather contradictory results describing the effect of individual members of Notch family and Notch ligands. These results are not surprising since the Notch effect is highly dependent on the environment and the type of the cells where activation is taking place. Elucidation of the exact nature of Notch effects on DC development and function is an arduous task but it is necessary for understanding the function of the immune system in physiological and pathological conditions.
References
- 1.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 2.Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268(5208):225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 3.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 4.Osborne B, Miele L. Notch and the immune system. Immunity. 1999;11:653–663. doi: 10.1016/s1074-7613(00)80140-5. [DOI] [PubMed] [Google Scholar]
- 5.Kopan R, Goate A. A common enzyme connects notch signaling and Alzheimer's disease. Genes Dev. 2000;14(22):2799–2806. doi: 10.1101/gad.836900. [DOI] [PubMed] [Google Scholar]
- 6.Yu G, Nishimura M, Arawaka S, et al. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature. 2000;407(6800):48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 7.Artavanis-Tsakonas SRM, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 8.Allman D, Punt J, Izon DJ, Aster JC, Pear WS. An invitation to T and more: Notch signaling in lymphopoiesis. Cell. 2002;109:S1–S11. doi: 10.1016/s0092-8674(02)00689-x. [DOI] [PubMed] [Google Scholar]
- 9.Ronchini C, Capobianco AJ. Induction of cyclin d1 transcription and cdk2 activity by Notch (IC): implication for cell cycle distribution in transformation by Notch (IC) Mol Cell Biol. 2001;21:5925–5934. doi: 10.1128/MCB.21.17.5925-5934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osborne BA, Minter LM. Notch signalling during peripheral T-cell activation and differentiation. Nature reviews. 2007;7(1):64–75. doi: 10.1038/nri1998. [DOI] [PubMed] [Google Scholar]
- 11.Miele L. Notch signaling. Clin Cancer Res. 2006;12(4):1074–1079. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81(11):2844–2853. [PubMed] [Google Scholar]
- 13.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 14.Lemischka IR, Moore KA. Stem cells: interactive niches. Nature. 2003;425(6960):778–779. doi: 10.1038/425778a. [DOI] [PubMed] [Google Scholar]
- 15.Varnum-Finney B, Purton LE, Yu M, et al. The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood. 1998;91(11):4084–4091. [PubMed] [Google Scholar]
- 16.Maeda T, Merghoub T, Hobbs RM, et al. Regulation of B versus T lymphoid lineage fate decision by the proto-oncogene LRF. Science. 2007;316(5826):860–866. doi: 10.1126/science.1140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeder T, Kohlhof H, Rieber N, Just U. Notch signaling induces multilineage myeloid differentiation and up-regulates PU.1 expression. J Immunol. 2003;170(11):5538–5548. doi: 10.4049/jimmunol.170.11.5538. [DOI] [PubMed] [Google Scholar]
- 18.Cheng P, Nefedova Y, Corzo CA, Gabrilovich DI. Regulation of dendritic-cell differentiation by bone marrow stroma via different Notch ligands. Blood. 2007;109(2):507–515. doi: 10.1182/blood-2006-05-025601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki M, Yamamoto M, Sugimoto A, Nakamura S, Motoda R, Orita K. Delta-4 expression on a stromal cell line is augmented by interleukin-6 via STAT3 activation. Exp Hematol. 2006;34(9):1143–1150. doi: 10.1016/j.exphem.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 20.Milner LA, Bigas A. Notch as a mediator of cell fate determination in hematopoiesis: evidence and speculation. Blood. 1999;93:2431–2448. [PubMed] [Google Scholar]
- 21.Varnum-Finney B, Xu L, Brashem-Stein C, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6(11):1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 22.Milner LA, Bigas A, Kopan R, Brashem-Stein C, Bernstein ID, Martin DI. Inhibition of granulocytic differentiation by mNotch1. Proc Natl Acad Sci U S A. 1996;93(23):13014–13019. doi: 10.1073/pnas.93.23.13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan-Pertel HT, Walker L, Browning D, Miyamoto A, Weinmaster G, Gasson JC. Notch signaling enhances survival and alters differentiation of 32D myeloblasts. J Immunol. 2000;165(8):4428–4436. doi: 10.4049/jimmunol.165.8.4428. [DOI] [PubMed] [Google Scholar]
- 24.Kumano K, Chiba S, Shimizu K, et al. Notch1 inhibits differentiation of hematopoietic cells by sustaining GATA-2 expression. Blood. 2001;98(12):3283–3289. doi: 10.1182/blood.v98.12.3283. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder T, Just U. Notch signalling via RBP-J promotes myeloid differentiation. Embo J. 2000;19(11):2558–2568. doi: 10.1093/emboj/19.11.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlesso N, Aster JC, Sklar J, Scadden DT. Notch1-induced delay of human hematopoietic progenitor cell differentiation is associated with altered cell cycle kinetics. Blood. 1999;93(3):838–848. [PubMed] [Google Scholar]
- 27.Lam LT, Ronchini C, Norton J, Capobianco AJ, Bresnick EH. Suppression of erythroid but not megakaryocytic differentiation of human K562 erythroleukemic cells by notch-1. J Biol Chem. 2000;275(26):19676–19684. doi: 10.1074/jbc.M002866200. [DOI] [PubMed] [Google Scholar]
- 28.Bigas A, Martin D, Milner LA. Notch1 and Notch2 inhibit myeloid differentiation in response to different cytokines. Mol Cell Biol. 1998;18:2324–2333. doi: 10.1128/mcb.18.4.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delaney C, Varnum-Finney B, Aoyama K, Brashem-Stein C, Bernstein ID. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106(8):2693–2699. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varnum-Finney B, Brashem-Stein C, Bernstein ID. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003;101(5):1784–1789. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- 31.Sugimoto A, Yamamoto M, Suzuki M, et al. Delta-4 Notch ligand promotes erythroid differentiation of human umbilical cord blood CD34+ cells. Exp Hematol. 2006;34(4):424–432. doi: 10.1016/j.exphem.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Dando JS, Tavian M, Catelain C, et al. Notch/Delta4 interaction in human embryonic liver CD34+ CD38-cells: positive influence on BFU-E production and LTC-IC potential maintenance. Stem Cells. 2005;23(4):550–560. doi: 10.1634/stemcells.2004-0205. [DOI] [PubMed] [Google Scholar]
- 33.Lauret E, Catelain C, Titeux M, et al. Membrane-bound delta-4 notch ligand reduces the proliferative activity of primitive human hematopoietic CD34+CD38low cells while maintaining their LTC-IC potential. Leukemia. 2004;18(4):788–797. doi: 10.1038/sj.leu.2403288. [DOI] [PubMed] [Google Scholar]
- 34.Masuya M, Katayama N, Hoshino N, et al. The soluble Notch ligand, Jagged-1, inhibits proliferation of CD34+ macrophage progenitors. Int J Hematol. 2002;75(3):269–276. doi: 10.1007/BF02982040. [DOI] [PubMed] [Google Scholar]
- 35.Radtke F, Wilson A, Stark G, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10(5):547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 36.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8-dendritic cells in the spleen. J Exp Med. 2007;204(7):1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winandy S, Brown M. No DL1 Notch ligand? GATA be a mast cell. Nature immunology. 2007;8(8):796–798. doi: 10.1038/ni0807-796. [DOI] [PubMed] [Google Scholar]
- 38.Gurish MF, Boyce JA. Mast cells: ontogeny, homing, and recruitment of a unique innate effector cell. J Allergy Clin Immunol. 2006;117(6):1285–1291. doi: 10.1016/j.jaci.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Walsh JC, DeKoter RP, Lee HJ, et al. Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity. 2002;17(5):665–676. doi: 10.1016/s1074-7613(02)00452-1. [DOI] [PubMed] [Google Scholar]
- 40.Kitamura Y, Oboki K, Ito A. Molecular mechanisms of mast cell development. Immunol Allergy Clin North Am. 2006;26(3):387–405. doi: 10.1016/j.iac.2006.05.004. v. [DOI] [PubMed] [Google Scholar]
- 41.Harigae H, Takahashi S, Suwabe N, et al. Differential roles of GATA-1 and GATA-2 in growth and differentiation of mast cells. Genes Cells. 1998;3(1):39–50. doi: 10.1046/j.1365-2443.1998.00166.x. [DOI] [PubMed] [Google Scholar]
- 42.Jonsson JI, Xiang Z, Pettersson M, Lardelli M, Nilsson G. Distinct and regulated expression of Notch receptors in hematopoietic lineages and during myeloid differentiation. Eur J Immunol. 2001;31(11):3240–3247. doi: 10.1002/1521-4141(200111)31:11<3240::aid-immu3240>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 43.Singh N, Phillips RA, Iscove NN, Egan SE. Expression of notch receptors, notch ligands, and fringe genes in hematopoiesis. Exp Hematol. 2000;28(5):527–534. doi: 10.1016/s0301-472x(00)00146-6. [DOI] [PubMed] [Google Scholar]
- 44.Ting CN, Olson MC, Barton KP, Leiden JM. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature. 1996;384(6608):474–478. doi: 10.1038/384474a0. [DOI] [PubMed] [Google Scholar]
- 45.Taghon T, Yui MA, Rothenberg EV. Mast cell lineage diversion of T lineage precursors by the essential T cell transcription factor GATA-3. Nature immunology. 2007;8(8):845–855. doi: 10.1038/ni1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai FY, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89(10):3636–3643. [PubMed] [Google Scholar]
- 47.Sonoda K, Kaku T, Hirakawa T, et al. The clinical significance of tumor-associated antigen RCAS1 expression in the normal, hyperplastic, and malignant uterine endometrium. Gynecol Oncol. 2000;79:424–429. doi: 10.1006/gyno.2000.5981. [DOI] [PubMed] [Google Scholar]
- 48.Tachikawa Y, Matsushima T, Abe Y, et al. Pivotal role of Notch signaling in regulation of erythroid maturation and proliferation. Eur J Haematol. 2006;77(4):273–281. doi: 10.1111/j.0902-4441.2006.t0-1-EJH2708.x. [DOI] [PubMed] [Google Scholar]
- 49.Iwasaki H, Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity. 2007;26(6):726–740. doi: 10.1016/j.immuni.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Walker L, Lynch M, Silverman S, et al. The Notch/Jagged pathway inhibits proliferation of human hematopoietic progenitors in vitro. Stem Cells. 1999;17(3):162–171. doi: 10.1002/stem.170162. [DOI] [PubMed] [Google Scholar]
- 51.Ishikawa F, Niiro H, Iino T, et al. The developmental program of human dendritic cells is operated independently of conventional myeloid and lymphoid pathways. Blood. 2007 doi: 10.1182/blood-2007-02-071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manz MG, Traver D, Miyamoto T, Weissman IL, Akashi K. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood. 2001;97(11):3333–3341. doi: 10.1182/blood.v97.11.3333. [DOI] [PubMed] [Google Scholar]
- 53.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404(6774):193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 54.Cheng P, Zlobin A, Volgina V, et al. Notch-1 regulates NF-kappaB activity in hemopoietic progenitor cells. J Immunol. 2001;167(8):4458–4467. doi: 10.4049/jimmunol.167.8.4458. [DOI] [PubMed] [Google Scholar]
- 55.Cheng P, Nefedova Y, Miele L, Osborne BA, Gabrilovich D. Notch signaling is necessary but not sufficient for differentiation of dendritic cells. Blood. 2003;102(12):3980–3988. doi: 10.1182/blood-2003-04-1034. [DOI] [PubMed] [Google Scholar]
- 56.Petrasch S, Reinacher-Schick A, Busemann B, et al. Neoadjuvant, hyperfractionated irradiation induces apoptosis and decreases proliferation in squamous cell cancer of the oral cavity. Int J Oral Maxillofac Surg. 2000;29:285–289. [PubMed] [Google Scholar]
- 57.Cheng P, Nefedova Y, Corzo CA, Gabrilovich DI. Regulation of dendritic cell differentiation by bone marrow stroma via different Notch ligands. Blood. 2007;109:507–515. doi: 10.1182/blood-2006-05-025601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohishi K, Varnum-Finney B, Serda RE, Anasetti C, Bernstein ID. The Notch ligand, Delta-1, inhibits the differentiation of monocytes into macrophages but permits their differentiation into dendritic cells. Blood. 2001;98(5):1402–1407. doi: 10.1182/blood.v98.5.1402. [DOI] [PubMed] [Google Scholar]
- 59.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117(4):515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 60.Olivier A, Lauret E, Gonin P, Galy A. The Notch ligand delta-1 is a hematopoietic development cofactor for plasmacytoid dendritic cells. Blood. 2006;107(7):2694–2701. doi: 10.1182/blood-2005-03-0970. [DOI] [PubMed] [Google Scholar]
- 61.Dontje W, Schotte R, Cupedo T, et al. Delta-like1-induced Notch1 signaling regulates the human plasmacytoid dendritic cell versus T-cell lineage decision through control of GATA-3 and Spi-B. Blood. 2006;107(6):2446–2452. doi: 10.1182/blood-2005-05-2090. [DOI] [PubMed] [Google Scholar]
- 62.Radtke F, Ferrero I, Wilson A, Lees R, Aguet M, MacDonald HR. Notch1 deficiency dissociates the intrathymic development of dendritic cells and T cells. J Exp Med. 2000;191(7):1085–1094. doi: 10.1084/jem.191.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferrero I, Held W, Wilson A, Tacchini-Cottier F, Radtke F, MacDonald HR. Mouse CD11c(+) B220(+) Gr1(+) plasmacytoid dendritic cells develop independently of the T-cell lineage. Blood. 2002;100(8):2852–2857. doi: 10.1182/blood-2002-01-0214. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 65.Verma IM, Stevenson JK, Scharz EM, Antwerp DV, Miyamoto S. Rel/NF-kB/IkB family: intimate tales of association and dissociation. Genes & Developm. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 66.Ouaaz F, Arron J, Zheng Y, Choi Y, Beg AA. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity. 2002;16(2):257–270. doi: 10.1016/s1074-7613(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 67.Chen C-H, Wu T-C. Experimental vaccine strategies for cancer immunotherapy. Journal of Biomedical Science. 1998;5:231–252. doi: 10.1007/BF02255855. [DOI] [PubMed] [Google Scholar]
- 68.Cheng P, Zlobin A, Volgina V, et al. Notch-1 regulates NF-kappa B activity in hematopoietic progenitor cells. J Immunol. 2001;167:4458–4467. doi: 10.4049/jimmunol.167.8.4458. [DOI] [PubMed] [Google Scholar]
- 69.Oswald F, Liptay S, Adler G, Schmid RM. NF-kappaB2 is a putative target gene of activated Notch-1 via RBP-Jkappa. Mol Cell Biol. 1998;18(4):2077–2088. doi: 10.1128/mcb.18.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bellavia D, Campese AF, Alesse E, et al. Constitutive activation of NF-kappaB and T-cell leukemia/lymphoma in Notch3 transgenic mice. Embo J. 2000;19(13):3337–3348. doi: 10.1093/emboj/19.13.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 2002;9(8):842–855. doi: 10.1038/sj.cdd.4401036. [DOI] [PubMed] [Google Scholar]
- 72.Guan E, Wang J, Laborda J, Norcross M, Baeuerle PA, Hoffman T. T cell leukemia-associated human Notch/translocation-associated Notch homologue has I kappa B-like activity and physically interacts with nuclear factor-kappa B proteins in T cells. J Exp Med. 1996;183(5):2025–2032. doi: 10.1084/jem.183.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moran ST, Cariappa A, Liu H, et al. Synergism between NF-kappa B1/p50 and Notch2 during the development of marginal zone B lymphocytes. J Immunol. 2007;179(1):195–200. doi: 10.4049/jimmunol.179.1.195. [DOI] [PubMed] [Google Scholar]
- 74.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nature reviews. 2003;3(12):984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 75.Amsen D, Antov A, Jankovic D, et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27(1):89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maekawa Y, Tsukumo S, Chiba S, et al. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19(4):549–559. doi: 10.1016/s1074-7613(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 77.Tanigaki K, Tsuji M, Yamamoto N, et al. Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity. 2004;20(5):611–622. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
- 78.Minter LM, Turley DM, Das P, et al. Inhibitors of gamma-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nature immunology. 2005;6(7):680–688. [PubMed] [Google Scholar]
- 79.Kaisho T, Hoshino K, Iwabe T, Takeuchi O, Yasui T, Akira S. Endotoxin can induce MyD88-deficient dendritic cells to support T(h)2 cell differentiation. Int Immunol. 2002;14(7):695–700. doi: 10.1093/intimm/dxf039. [DOI] [PubMed] [Google Scholar]
- 80.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196(12):1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nature immunology. 2001;2(10):947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 82.Wakui M, Nakano K, Matsushita S. Notch ligand mRNA levels of human APCs predict Th1/Th2-promoting activities. Biochem Biophys Res Commun. 2007;358(2):596–601. doi: 10.1016/j.bbrc.2007.04.175. [DOI] [PubMed] [Google Scholar]
- 83.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nature immunology. 2005;6(8):769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Skokos D, Nussenzweig MC. CD8-DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. J Exp Med. 2007;204(7):1525–1531. doi: 10.1084/jem.20062305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stallwood Y, Briend E, Ray KM, et al. Small interfering RNA-mediated knockdown of notch ligands in primary CD4+ T cells and dendritic cells enhances cytokine production. J Immunol. 2006;177(2):885–895. doi: 10.4049/jimmunol.177.2.885. [DOI] [PubMed] [Google Scholar]
- 86.Glimcher LH. Lineage commitment in lymphocytes: controlling the immune response. J Clin Invest. 2001;108(7):s25–s30. [PubMed] [Google Scholar]
- 87.Tu L, Fang TC, Artis D, et al. Notch signaling is an important regulator of type 2 immunity. J Exp Med. 2005;202(8):1037–1042. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nature immunology. 2000;1(3):199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 89.Jankovic D, Kullberg MC, Hieny S, Caspar P, Collazo CM, Sher A. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity. 2002;16(3):429–439. doi: 10.1016/s1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 90.Weijzen S, Velders MP, Elmishad AG, et al. The Notch ligand Jagged-1 is able to induce maturation of monocyte-derived human dendritic cells. J Immunol. 2002;169(8):4273–4278. doi: 10.4049/jimmunol.169.8.4273. [DOI] [PubMed] [Google Scholar]
- 91.Androutsellis-Theotokis A, Leker RR, Soldner F, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442(7104):823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 92.Kamakura S, Oishi K, Yoshimatsu T, Nakafuku M, Masuyama N, Gotoh Y. Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signalling. Nat Cell Biol. 2004;6(6):547–554. doi: 10.1038/ncb1138. [DOI] [PubMed] [Google Scholar]
- 93.Nefedova Y, Cheng P, Gilkes D, et al. Activation of dendritic cells via inhibition of Jak2/STAT3 signaling. J Immunol. 2005;175(7):4338–4346. doi: 10.4049/jimmunol.175.7.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172(2):989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 95.Wang JW, Gamsby JJ, Highfill SL, et al. Deregulated expression of LRBA facilitates cancer cell growth. Oncogene. 2004;23(23):4089–4097. doi: 10.1038/sj.onc.1207567. [DOI] [PubMed] [Google Scholar]
- 96.Adler SH, Chiffoleau E, Xu L, et al. Notch signaling augments T cell responsiveness by enhancing CD25 expression. J Immunol. 2003;171(6):2896–2903. doi: 10.4049/jimmunol.171.6.2896. [DOI] [PubMed] [Google Scholar]
- 97.Palaga T, Miele L, Golde TE, Osborne BA. TCR-mediated Notch signaling regulates proliferation and IFN-gamma production in peripheral T cells. J Immunol. 2003;171(6):3019–3024. doi: 10.4049/jimmunol.171.6.3019. [DOI] [PubMed] [Google Scholar]
- 98.Eagar TN, Tang Q, Wolfe M, He Y, Pear WS, Bluestone JA. Notch 1 signaling regulates peripheral T cell activation. Immunity. 2004;20(4):407–415. doi: 10.1016/s1074-7613(04)00081-0. [DOI] [PubMed] [Google Scholar]
- 99.Hoyne GF, Le Roux I, Corsin-Jimenez M, et al. Serrate1-induced notch signalling regulates the decision between immunity and tolerance made by peripheral CD4(+) T cells. Int Immunol. 2000;12(2):177–185. doi: 10.1093/intimm/12.2.177. [DOI] [PubMed] [Google Scholar]
- 100.Yvon ES, Vigouroux S, Rousseau RF, et al. Overexpression of the Notch ligand, Jagged-1, induces alloantigen-specific human regulatory T cells. Blood. 2003;102(10):3815–3821. doi: 10.1182/blood-2002-12-3826. [DOI] [PubMed] [Google Scholar]
- 101.Wong KK, Carpenter MJ, Young LL, et al. Notch ligation by Delta1 inhibits peripheral immune responses to transplantation antigens by a CD8+ cell-dependent mechanism. J Clin Invest. 2003;112(11):1741–1750. doi: 10.1172/JCI18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rutz S, Mordmuller B, Sakano S, Scheffold A. Notch ligands Delta-like1, Delta-like4 and Jagged1 differentially regulate activation of peripheral T helper cells. Eur J Immunol. 2005;35(8):2443–2451. doi: 10.1002/eji.200526294. [DOI] [PubMed] [Google Scholar]
- 103.Kostianovsky AM, Maier LM, Baecher-Allan C, Anderson AC, Anderson DE. Up-regulation of gene related to anergy in lymphocytes is associated with Notch-mediated human T cell suppression. J Immunol. 2007;178(10):6158–6163. doi: 10.4049/jimmunol.178.10.6158. [DOI] [PubMed] [Google Scholar]
- 104.Ng WF, Duggan PJ, Ponchel F, et al. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98(9):2736–2744. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 105.Anastasi E, Campese AF, Bellavia D, et al. Expression of activated Notch3 in transgenic mice enhances generation of T regulatory cells and protects against experimental autoimmune diabetes. J Immunol. 2003;171(9):4504–4511. doi: 10.4049/jimmunol.171.9.4504. [DOI] [PubMed] [Google Scholar]
- 106.Katada T, Ito M, Kojima Y, Miyatani S, Kinoshita T. XMam1, Xenopus Mastermind1, induces neural gene expression in a Notch-independent manner. Mech Dev. 2006;123(11):851–859. doi: 10.1016/j.mod.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 107.Shen H, McElhinny AS, Cao Y, et al. The Notch coactivator, MAML1, functions as a novel coactivator for MEF2C-mediated transcription and is required for normal myogenesis. Genes Dev. 2006;20(6):675–688. doi: 10.1101/gad.1383706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barolo S, Walker RG, Polyanovsky AD, Freschi G, Keil T, Posakony JW. A notch-independent activity of suppressor of hairless is required for normal mechanoreceptor physiology. Cell. 2000;103(6):957–969. doi: 10.1016/s0092-8674(00)00198-7. [DOI] [PubMed] [Google Scholar]
- 109.Yamaguchi E, Chiba S, Kumano K, et al. Expression of Notch ligands, Jagged1, 2 and Delta1 in antigen presenting cells in mice. Immunol Lett. 2002;81(1):59–64. doi: 10.1016/s0165-2478(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 110.Nomaguchi K, Suzu S, Yamada M, Hayasawa H, Motoyoshi K. Expression of Jagged1 gene in macrophages and its regulation by hematopoietic growth factors. Exp Hematol. 2001;29(7):850–855. doi: 10.1016/s0301-472x(01)00657-9. [DOI] [PubMed] [Google Scholar]
- 111.Monsalve E, Perez MA, Rubio A, et al. Notch-1 up-regulation and signaling following macrophage activation modulates gene expression patterns known to affect antigen-presenting capacity and cytotoxic activity. J Immunol. 2006;176(9):5362–5373. doi: 10.4049/jimmunol.176.9.5362. [DOI] [PubMed] [Google Scholar]
- 112.Kuroda K, Han H, Tani S, et al. Regulation of marginal zone B cell development by MINT, a suppressor of Notch/RBP-J signaling pathway. Immunity. 2003;18(2):301–312. doi: 10.1016/s1074-7613(03)00029-3. [DOI] [PubMed] [Google Scholar]