Abstract
The oxy-ferrous complex of cytochrome P450 2B4 (2B4) has been prepared at − 40°C with and without bound substrate (butylated hydroxytoluene, BHT), and radiolytically oneelectron cryoreduced at 77K. EPR shows that in both cases the observed product of cryoreduction is the hydroperoxo-ferriheme species, indicating that the microsomal P450 contains an efficient distal-pocket proton-delivery network. In the absence of substrate, two distinct hydroperoxo-ferriheme signals are observed, reflecting the presence of two major conformational substates in the oxy-ferrous precursor. Only one species is observed when BHT, is bound, indicating a more ordered active site. BHT binding also changes the g-tensor components of the hydroperoxo-ferric 2B4 intermediate, indicating that the substrate modulates the properties of this intermediate. Step-annealing the cryoreduced ternary 2B4 complex at 175K and above causes the loss of hydroperoxo-ferric 2B4 and the parallel appearance of high-spin ferri-2B4; LC-MS/MS analysis shows that in this process BHT is quantitatively converted to two products, hydroxymethyl BHT (1) and 3-hydroxy-t-butyl BHT (2). This implies that the hydroperoxo-ferric 2B4 prepared by cryoreduction is catalytically active, and that the high-spin state observed after annealing contains an enzymebound product of BHT monooxygenation. The ratio of products generated during cryoreduction/annealing, 1/2 ~ 6.2/1, is significantly different from the ratio, 2.5/1, at ambient temperature, and product coupling is significantly greater. This suggests that substrate is held more rigidly relative to the oxidizing species at low temperature, and/or that dissociation of FeOOH is inhibited. As in experiments under ambient conditions, product formation is not observed in the inactive F429H 2B4 mutant.
Keywords: Cytochrome P450, Cryoreduction, Electron Paramagnetic Resonance
Xenobiotic-metabolizing cytochromes P450 (P450) from mammalian liver microsomes function by oxidizing fat-soluble compounds, thereby converting them to excretable watersoluble compounds. Mammalian membrane-bound cytochromes P450 are able to react with a large variety of structurally diverse compounds because they have flexible substrate-binding sites that accommodate structurally diverse substrates (1–3).
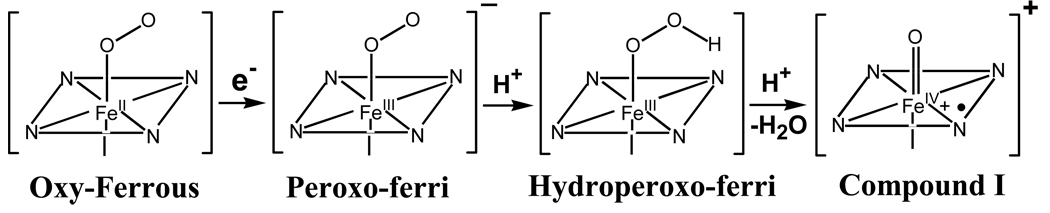
Monooxygenation reactions, such as those carried out by P450s, are described by the following mechanistic scheme: 1) substrate binding to the ferric protein; 2) reduction of the substrate-protein complex to the ferroheme form; 3) binding of dioxygen; 4) activation of the oxyferroheme by reduction with a second electron to form the peroxo intermediate (Fe(III)-[O2 2−]) (Scheme 1). In principle, this species can itself react (4, 5) or accept a proton to form the hydroperoxo-ferric intermediate (Fe(III)-[OOH−]), which in turn may either react or undergo proton-assisted heterolytic cleavage of the O-O bond to form Compound I (Cpd I), the oxo-Fe(IV) porphyrin π-cation radical(6). Which species is reactive depends on the active-site environment, as modulated by the substrate, and on the substrate reactivity(7, 8).
Scheme 1.
Recent cryoreduction EPR/ENDOR studies have characterized the elusive steps of the monooxygenase cycle subsequent to step 4 for the bacterial cytochrome P450cam (9), and in doing so confirmed the generally held view (4, 10) that Cpd I is the reactive species for this enzyme with camphor as substrate. However, analogous studies showed that methylene camphor also is oxidized by the hydroperoxo-ferric intermediate (7, 8).
In these experiments 77K γ-irradiation of the ternary complex of ferrous P450cam with O2 and camphor in frozen solution generates a trapped one-electron reduced oxy-P450 intermediate (step 4) whose rhombic EPR signal is characteristic of a hydropero-ferriheme. During stepannealing at temperatures of 180K and above, the cryotrapped intermediate converts to a state in which the hydroxy-camphor monooxygenation product is coordinated to the ferriheme through its hydroxyl. ENDOR studies showed that this process indeed occurs via Cpd I, although this intermediate was not directly detected in these experiments, probably because of its high reactivity and low steady-state concentration. In analogous crystallographic experiments with the ternary complex of camphor bound to oxy P450cam at temperatures greater than 100K, the hydroperoxy-ferric P450 intermediates were not detected but a state with an Fe-O bond and average bond length of ~1.67 A was revealed, and the authors considered the possibility that this might be Compound I (11).
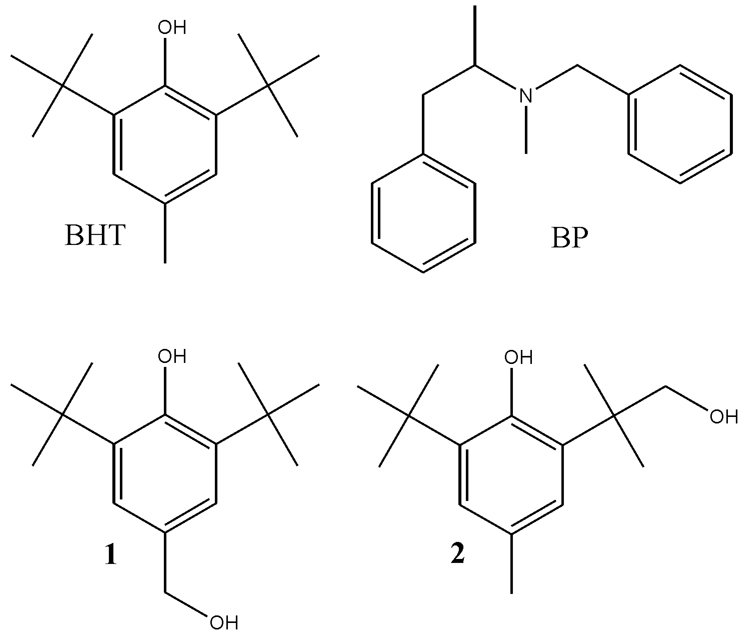
The present cryoreduction study of the intermediates in the monooxygenase cycle of human cytochrome P450 2B4 (2B4) is of importance as the first for a mammalian enzyme, and is of particular interest because the study of this enzyme helped initiate the idea that each of the three activated forms, peroxo, hydroperoxo, and Cpd I, might be a reactive species under the proper circumstances (12, 13). Application of cryoreduction methods to 2B4 has been hindered by two difficulties. One is rapid autooxidation of oxyferro 2B4, which makes it difficult to prepare this complex in high concentrations (0.5 – 1 mM) for study. We here report that oxy-2B4 prepared in 60% glycerol solutions in the presence of substrate at −40 °C at pH 8.0 offers relief from this difficulty. The second is that although the widely used model substrate benzphetamine (BP, Fig.1) binds tightly at ambient temperatures, the affinity of 2B4 for BP decreases upon cooling, to the extent that freezing at −40 °C or below does not trap the BP complex. This problem is overcome through use of butylated hydroxytoluene (BHT, Fig. 1) as substrate, as we have found that it remains bound to 2B4 at low temperatures in the presence of 0.3 M NaCl (Razeghifard, R.; Waskell, L; unpublished). These two approaches enable us to report the EPR study of an intermediate in the catalytic cycle of a mammalian cytochrome P450, the hydroperoxy-ferric 2B4 formed during cryoreduction of the oxy-2B4 and ternary oxy-P4502B4/BHT complexes, and to present an EPR investigation of its properties and step-annealing measurements of its reactivity. The catalytic competence of cryogenerated, one-electron reduced oxy-2B4/BHT complex is established by the determination that products of BHT monooxygenation form quantitatively during the cryoreduction and annealing of the ternary complex with wild-type 2B4, but not with the non-catalytic 2B4(F429H) variant.
Fig. 1.
Cytochrome P450 2B4 substrates, BHT and BP, and products, hydroxymethyl-BHT (1) and 3-hydroxy-t-butyl BHT (2).
Material and Methods
Chemicals
All organic solvents, BHT, hydroxymethyl-BHT (1 in Figure 1), BP (Figure 1), NADPH and sodium dithionite were from Sigma-Aldrich (St. Louis, MO). 3-hydroxy-t-butyl BHT (2) was a generous gift from Dr. John Thompson (School of Pharmacy, University of Colorado). High-purity glycerol was purchased from Roche Diagnostics (Indianapolis, IN). O2 and CO were obtained from Cryogenic Gas (Detroit, MI). Vivaspin-2 (MWCO 30,000) was from Biosciences (St. Louis, MO).
Full-length wild type 2B4 was expressed in E.coli and purified as previously described (14). The concentration of 2B4 was determined using an extinction coefficient of Δε450–490 = 91 mM−1 cm−1 for the 2B4-CO complex (14). To prepare oxy-2B4, the protein was concentrated at 4 °C using a Vivaspin-2 concentrator (30,000 MW cutoff) to 1 mM in a buffer consisting of 20% glycerol, 0.6 M NaCl, 0.1M Tris/HCl pH 8, at room temperature. To 200uL of the concentrated protein was added less than 1 µL of a ~1M stock solution of BP or BHT in methanol. Glycerol then was added to the P450-substrate complex to yield a final protein concentration of 0.5 mM P450, in 50 mM Tris/HCl pH 8 at room temperature, 0.3M NaCl, and 60% glycerol. The final concentration of BP in the sample was 2 mM, while the final BHT concentration was 0.5 mM or 2.0 mM. The 0.5 mM P450 solution was stored in liquid nitrogen until further use, at which time it was defrosted and placed in a glove box at 4 °C overnight to remove the oxygen. An aliquot of a standardized solution of sodium dithionite was added to reduce the ferric P450 by one electron, using a 10–20% excess of dithionite to remove possible residual O2. The reduced protein was cooled to −40 °C in a dry ice ethanol bath. Precooled O2 was bubbled (5–10 min) through the protein solution and mixed into the viscous 60% glycerol solution to form oxy-2B4. The oxygen was precooled in a stainless steel coil immersed in a dry ice ethanol bath at −70 °C. The oxy-P450 substrate complex content in the samples prepared in this way varied between 70–80% because part of the oxy P450 underwent autooxidation to the ferric protein during sample preparation.
Irradiation of the frozen solutions of oxy 2B4 at 77K typically was performed for 20–25 hours at a rate of 0.15 Mrad/hour (total dose ~3Mrad) using a Gammacell 220 60Co as described previously (15). Annealing at multiple temperatures over the range 77–270K was performed by placing the EPR sample in a bath at the desired temperature (usually n–pentane (T< 180K) or methanol (T>175K) cooled with liquid nitrogen) for the desired time and then refreezing in liquid nitrogen.
EPR spectroscopy
X-band continuous wave EPR spectra were recorded on a Bruker ESP 300 spectrometer equipped with an Oxford Instruments ESR 910 continuous He flow cryostat. The amount of hydroperoxo-ferric 2B4/BHT produced by cryoreduction was determined by comparing the EPR signal from this species to that of a 0.5mM solution of substrate free lowspin ferric 2B4 as standard. To decrease interference from radicals formed during the irradiation, the quantitations were performed on solutions that had first been annealed for 1 minute at 175K. The radical signal is greatly diminished in this step, while this is at the highest temperature at which the hydroperoxo-ferric state does not react. Quantitations of multiple samples showed that a 3Mr dose at 77K reduces approximately 40% of the oxy-2B4/BHT present, generating 0.14 (3) mM hydroperoxo-ferric 2B4/BHT in the samples as prepared.
Product Analysis
The oxidation of BP to its N-demethylated metabolite, norbenzphetamine, was measured by a LC-MS/MS assay as previously described (14). Quantification of the BHT oxygenation products, 1 (major product) and 2, was performed with LC-MS/MS. For quantifying BHT metabolites, 100 µL of each EPR sample was spiked with 0.3 mM of 2,4-di-t-butyl phenol as the internal standard. The solution was saturated with NaCl and extracted twice with an equal volume of hexane. The two hexane phases were recovered, pooled and dried under a stream of nitrogen gas before re-dissolving in 600 µL of acetonitrile. LC-MS/MS analysis was performed on a Thermo Finnegan TSQ Quantum Ultra AM mass spectrometer (ThermoFinnigan, San Jose, CA). BHT and its metabolites were separated on a 4.6 × 250 mm Luna column (Phenomenex, CA) at a flow rate of 1.0 ml/min. Mobile phases were water (solvent A) and acetonitrile (solvent B). The HLPC running conditions were: initial conditions 60:40 (A:B) followed by three linear gradients of 40–70% B in 20 min and 70–90% B in 30 min and 90–100% B in 10 min. The column was then returned to the initial conditions 60:40 (A:B) in 1 min and reequilibrated for 9 min, resulting in a total run time of 70 min. Elution of 1, 2, internal standard, and BHT occurred at 18, 26, 29 and 37 min, respectively. Since under these experimental conditions they were easily deprotonated during electrospray ionization to form negative pseudomolecular ions, the negative ion mode was employed for detection.
Results and Discussion
EPR of ferric 2B4
We have studied the effect of the substrates and products on the EPR spectra of ferric 2B4 to characterize their binding to the protein, to generate reference spectra for assignment of ferric species in the EPR spectra of the irradiated samples of oxy-2B4, and to help in the identification of product complexes formed during cryoreduction/step-annealing.
Resting-state, ferric 2B4 contains a low-spin aquoferriheme whose rhombic EPR signal has g = [2.43, 2.25, 1.92] (Table 1), in agreement with previous studies (16). Optical spectroscopy shows that addition of saturating amounts of BP to a 2B4 solution at ambient temperature causes the majority of enzyme to convert to the five-coordinate, high-spin, substrate-bound form. However, the amount of high-spin form decreases upon cooling, and it is mostly gone by −40 °C (17); upon freezing, one primarily observes the low-spin aquoferriheme EPR signal, with only ~5–10 % of the enzyme in the high-spin BP-bound form, g = [8.1, 3.5, ~2]. It is likely that this reflects a virtually complete loss of substrate during the cooling/freezing process, although the same phenomena would be observed if BP shifts to a more distant position in the heme pocket, thereby permitting the retention of Fe-bound water. For simplicity, we mostly discuss solutions frozen in the presence of BP as containing ‘substrate-free’ enzyme to distinguish them from solutions without the addition of any substrate.
Table 1.
g-Values for low-spin ferric cytochrome P450 2B4 and its complexes with substrates.a
| Substrate | g1 | g2 | g3 |
|---|---|---|---|
| None | 2.43 | 2.25 | 1.91 |
| BHT | 2.43 | 2.24 | 1.92 |
| 2.39 | 2.24 | 1.97 | |
| 1 minor | 2.54 | 2.26 | 1.87 |
| major | 2.42 | 2.24 | 1.92 |
| minor | 2.39 | 2.24 | 1.97 |
The sample preparation and measurement of EPR spectra are described in Methods.
Under ambient conditions (25 °C), BHT binds stoichiometrically to ferric 2B4 in 60% glycerol and 0.3 M NaCl, and it remains ≅ 90% bound at − 40 °C. Frozen solutions (77 K) of ferric 2B4 in the presence of BHT show a majority high-spin signal from BHT-bound enzyme, with the same g-values as for the BP-bound form. In addition, there are signals from two minority, unidentified low-spin species. The g-values of one of these minority species are close to those for substrate-free ferric 2B4 (Table 1) while those of the other, with g = [2.39, 2.24, 1.97], resemble very much those for substrate-bound P450cam in which camphor was proposed to act as the sixth ligand to the ferric heme (18). This suggests that at low temperature the hydroxyl group of BHT can coordinate to the ferric iron of 2B4.
Under ambient conditions (25 °C, pH 7.4, 0.3 M NaCl) optical spectroscopy shows that in the presence of an excess of 1, the major product of BHT metabolism, 2B4 is ~ 64% high spin, ~ 36% low spin. The former presumably has 1 bound in the heme pocket, while the latter does not. However, the EPR spectrum of a frozen solution of ferri-2B4 in the presence of 1 shows a largely low spin signal resembling substrate-free protein (Table 1), suggesting that 1, like BP, either is lost from the heme pocket or moves within it during freezing. In addition, there is a new minor rhombic low-spin EPR signal that has g = [2.54, 2.26, 1.87] that may be attributed to enzyme with the hydroxymethyl group of 1 coordinated to the ferric heme iron, plus one minor low-spin signal and one minor high-spin signal (Table 1).
EPR of Cryoreduced oxy-2B4/BHT and oxy- 2B4 (2B4 + BP)
As reported previously, the oxy-2B4 complex is much less stable than that of oxy P450cam (19). The lifetime of the oxy-2B4 is significantly increased by substrate binding, increases in pH, and lower temperatures (19). To optimize conditions for preparation of samples, we carried out kinetic studies on the decay of oxy P450 with and without substrate as a function of pH and temperature using stopped-flow spectrophotometry. These results indicate that autooxidation of 0.5 mM oxy-2B4 to the ferric form is minimized if BP (2–3 mM) or stoichiometric BHT are present in a solution containing 60 % glycerol/50mM Tris buffer pH 8.0–8.2/0.3M NaCl (measured at 25 °C), and the sample is oxygenated at −40 °C. As noted above, the frozen sample prepared with BHT is largely the ternary complex, and it contained ~20–30% ferric form. Whether BP is indeed excluded from the active site or merely adopts an alternate conformation within it, the presence of the BP nonetheless serves to assist the trapping of the oxy-complex; even so, the samples contain ~ 20–30 % ferric form. In the absence of any substrate, the amount of ferri-2B4 in a frozen sample is significantly higher.
oxy-2B4/BHT Ternary Complex
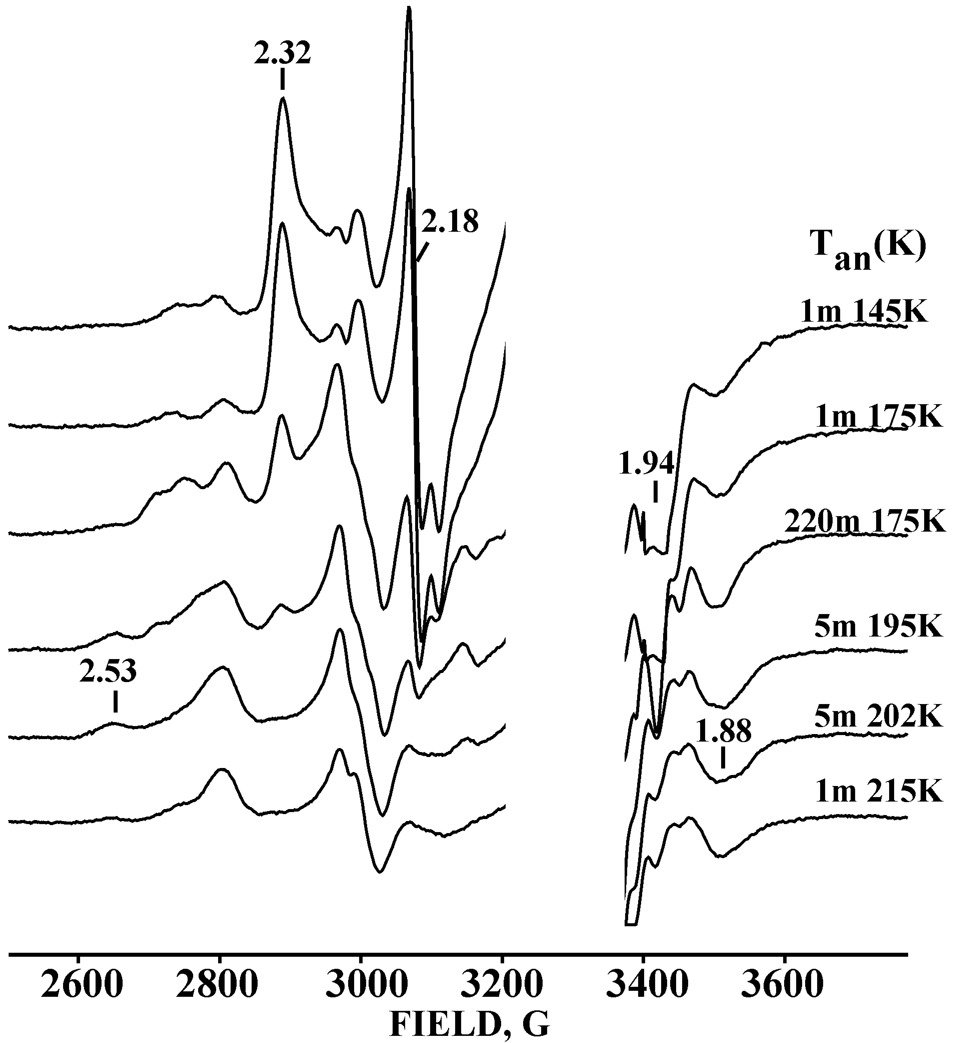
Figure 2 presents the EPR spectrum of the oxy-2B4/BHT ternary complex after exposure to γ-irradiation at 77 K. The main signal from the cryoreduced ternary complex has g = [2.32, 2.176, ~1.94]. These values, in particular g1 ≥ 2.27, show that 77K cryoreduction has produced the hydroperoxo-ferriheme state (9) (Table 2). At a dose of 3Mr, the yield of this species is ~40% of total ternary complex (~ 0.14 mM).
Fig. 2.
EPR spectra of cryoreduced ternary oxy-2B4/BHT complex annealed at the indicated temperatures for the specified times in minutes (m). Conditions: microwave frequency, 9.361 GHz, 30K, 10G modulation amplitude.
Table 2.
g-Values for hydroperoxoferri-2B4 formed by cryoreduction of oxy-2B4, with and without substrate.a
| Substrate | Species | g1 | g2 | g3 |
|---|---|---|---|---|
| noneb | A | 2.29 | 2.16 | 1.94 |
| B | 2.34 | 2.19 | nd | |
| BHT | 2.32 | 2.18 | 1.94 |
Sample preparation and measurement of EPR spectra are described in Methods.
Prepared in presence of BP; see text.
It has been shown that the primary product of 77K cryoradiolytic reduction of oxy hemoproteins is the peroxo ferriheme, which then accepts a proton to generate the hydroperoxo state (20). The heme-containing monooxygenases studied previously, P450cam (9) and heme oxygenase (HO) (21), each contain an efficient distal-pocket proton-delivery network that can supply this proton even at 77K; indeed, proton delivery in heme oxygenase has been seen at liquid helium temperatures (22). In contrast, the D251N mutation of P450cam perturbs this proton delivery network to the extent that protonation of the cryogenerated peroxo-ferric intermediate occurs only at a much higher temperature (> 170K) (9). The observation that 77K cryoreduction of oxy-2B4 generates the hydroperoxo-ferric heme indicates that this mammalian enzyme also has an efficient proton delivery network. We surmise that for oxy-monooxygenase complexes that exhibit such a network, reduction under physiological conditions commonly will involve proton-coupled electron transfer to form the hydroperoxy-ferric species.
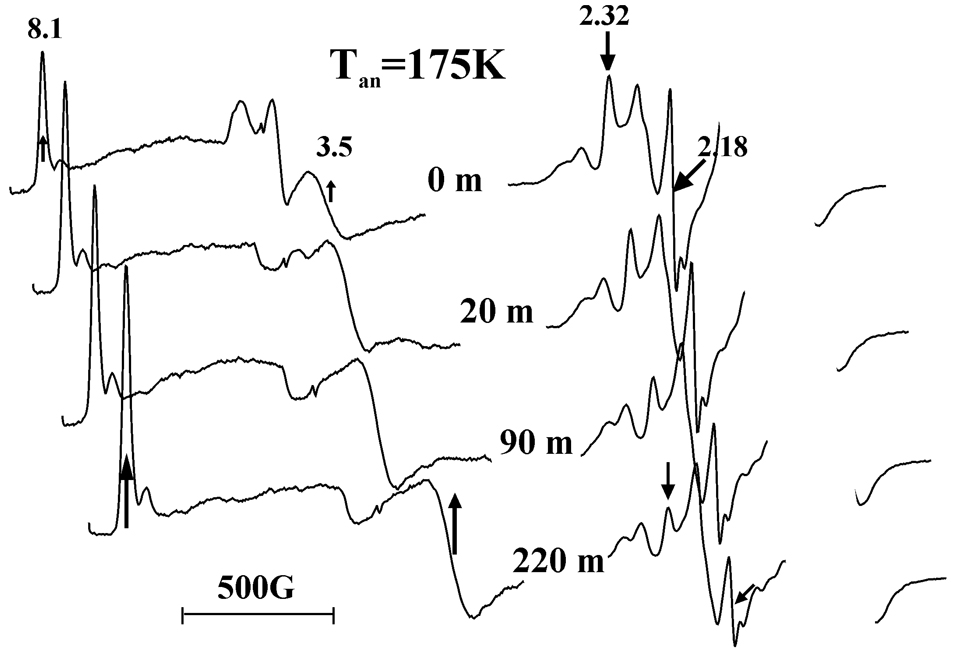
Figure 2 shows EPR spectra taken during annealing of the cryoreduced oxy-2B4/BHT ternary complex at successively higher temperatures. During this process the hydroperoxoferric 2B4/BHT intermediate decays at T ~ 175K and above. Figure 3 shows that the decay of the hydroperoxo-ferric 2B4/BHT during annealing at 175K is accompanied by a parallel increase of the high-spin ferri-2B4 signal, g = [8,1, 3.5, ~2] (Fig. 3), seen as the minority (10%) signal from a solution of ferri-2B4 frozen in the presence of the product, 1. We propose that this product state likewise contains 1 that remains trapped in the distal pocket of the frozen sample, without coordination to the ferriheme. Thus, it is proposed to be analogous to the high-spin form with bound 1 that is observed by optical spectroscopy at room temperature.
Fig. 3.
EPR spectra of cryoreduced ternary oxy-2B4/BHT complex, showing loss of hydroperoxoferric signal and growth of high-spin ferric signal during annealing at 175K for indicated times (minutes, m). Spectra other than that taken at t = 0 are arbitrarily offset to higher field to permit a better perspective on the annealing behavior. Measurement temperatures: low-field spectra, 8K; high-field, 30 K. Conditions: microwave frequency, 9.365 GHz; 10G modulation amplitude;
This supposition is supported by the product analysis presented below, and by the fact that high-spin ferriheme is not formed when the cryoreduced ‘substrate-free’ oxy-2B4 is annealed (see below). The loss of hydroperoxo-ferriheme and appearance of high-spin product state have approximately the same half-times (not shown), consistent either with direct reaction of the hydroperoxo-ferric species, or with formation of Cpd I, which reacts promptly to form product without accumulating. We further propose that the weak ferriheme signal with g1 = 2.54 that appears during subsequent annealing at 195–205K reflects a relaxation of the heme pocket to the equilibrium product complex of 1, discussed above. This relaxation competes with dissociation of the product complex, which can occur in glycerol solutions at these low temperatures (23), and ultimately the g1 = 2.54 signal disappears with further annealing.
‘Substrate-free’ oxy-2B4
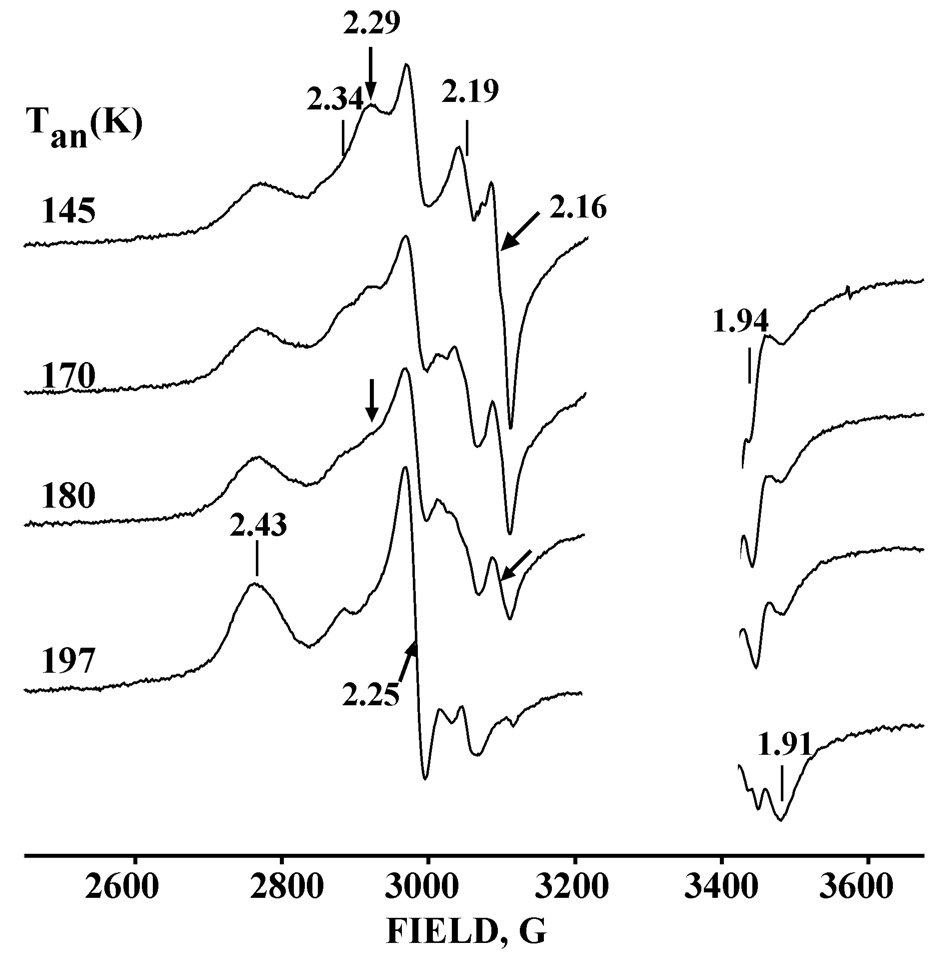
Figure 4 presents the EPR spectrum of the binary, oxy-2B4 complex (prepared in the presence of 2mM BP) after exposure to γ-irradiation at 77 K. Although the spectrum is complicated by signals from the ferriheme formed by autooxidation, (Table 1), it nonetheless clearly reveals two EPR signals from hydroperoxoferri-2B4 generated by cryoreduction of oxy-2B4, a major conformer (A) with g = [2.29, 2.16, 1.942] and minor conformer (B) with g = [~2.34, 2.19, nd] (Table 2), in addition to a decreased low-spin ferric 2B4 signal (in the irradiated samples ~ 40% of available ferric 2B4 contamination is reduced to the EPR silent ferrous state). As conformational rearrangements are quenched at 77K, the observation of two signals in the product of cryoreduction means that the active site of the parent oxy-2B4 adopts at least two different major conformational substates. This is likely the explanation for the biphasic kinetics of autooxidation of oxy-2B4 at ambient temperature (24).
Fig. 4.
EPR spectra of cryoreduced binary oxy-2B4 complex (prepared in presence of BP) annealed at indicated temperatures. The features associated with principal g-values for species of particular interest are offset and color coded. Conditions: as in Fig 2.
The g-values of both the cryogenerated hydroperoxo-ferric centers of ‘substrate-free’ 2B4 are different from that of the BHT-bound enzyme, which indicates that the active-site structure is modulated by the substrate, as reported previously for cytochrome P450cam (7). However, the observation of the hydroperoxo-ferriheme after 77K cryoreduction means that in the majority conformation(s) the 2B4 proton delivery pathway remains effective in the ‘substrate-free’ enzyme.
At a dose of 3Mr, the yield of the cryoreduced species is ~40% of total oxy P450 complex (~ 0.14 mM). Interestingly, the yield of the species increases only slightly at a dose of 7–8 Mr. This is probably due to further cryoreduction of cryogenerated hydroperoxo species at doses above 7–8 Mrad as occurs with the cryogenerated hydroperoxo-ferric HO intermediate (25).
During progressive annealing from 145 to 170 K, the intensity of signal A (g = 2.29, 2.16) decreases while signal B (g = 2.34, 2.19) increases (Fig. 4), suggesting a possible conversion of A to B; both signals then decay during annealing at 180 K (Fig. 4). This process is accompanied by an increase of the resting, low-spin aquo-ferriheme signal (g= 2.43, 2.25, 1.93) (Fig. 4), in contrast to the appearance of the high-spin product signal upon annealing the hydroperoxo-ferriheme formed in the ternary complex.
Product formation following cryoreduction and under steady-state conditions
To correlate the cryoreduction measurements of microsomal oxy-2B4/BHT complexes with processes that occur under ambient conditions, and using physiological redox partners, product formation was measured following annealing of the cryoreduced ternary complex.
Table 3 presents data showing that the cryogenerated hydroperoxo-ferric 2B4-BHT complex is catalytically competent. Application of the liquid chromatography-mass spectrometry assay to cryoreduced samples subsequent to annealing reveals the formation of 1 and 2, consistent with the products of BHT oxygenation as observed during catalysis in solution at 25 °C under steady-state conditions. The absence of these metabolites in control experiments in which the ferri-2B4 BHT complex was irradiated excludes the possibility that product was generated during warming, through reaction of ferric 2B4 with radiolytically generated H2O2 (26). Likewise, cryoreduction and annealing of oxy-2B4 prepared in the presence of BP do not form the product norbenzphetamine, further substantiating our conclusion based on spectroscopic data that BP does not bind to oxy-2B4 at low temperatures. This negative result is noteworthy because at ambient temperatures, BP oxidation occurs with a rate constant ≅ 38% faster than that of BHT (14).
Table 3.
Concentration of product (mM) generated by step-annealing the cryogenerated hydroperoxoferri-2B4/BHT complex.
| Protein substrate complex | [Fe-OOH]a | [1]b | [2]b | [1] + [2]b |
|---|---|---|---|---|
| oxy-2B4/BHT (1:8)c | 0.14 (3) | 0.101 | 0.016 | 0.117 |
| oxy-2B4/BHT (1:1)c | 0.14 (3) | 0.101 | 0.016 | 0.117 |
| oxy-2B4(F429H)/BHT (1:8)c | 0.16 (3) | 0.007 | nd | 0.007 |
Hydroperoxoferri-2B4/BHT generated by 3 Mr radiation dose, as determined by EPR spin quantitations. Numbers in parentheses are uncertainties in last digit.
Product formation as measured by LC-MS/MS assay as described.
Total enzyme, 0.5 mM; initial concentration of oxy-2B4, 0.4 mM. Numbers in parentheses are the molar ratio, [oxy-2B4]/[BHT]
According to our estimates, the maximal concentration of the cryogenerated hydroperoxo intermediate is 0.14±0.3 mM, whereas the LC-MS/MS assay showed the formation of ~0.12 mM BHT metabolites (Table 3). We thus infer that the hydroperoxoferric 2B4/BHT species prepared by cryoreduction of the ternary oxy-2B4/BHT complex quantitatively converts BHT to 1 (major product) and 2 (minor product) in a reaction that is characterized by relatively high coupling. The product distribution and coupling for BHT hydroxylation in the cryoreduction experiment differs somewhat from those obtained under steady-state conditions at 30 °C with NADPH cytochrome P450 reductase as the electron donor. Conversion of BHT into product by the cryogenerated hydroperoxo intermediate is characterized by higher “coupling” ~80% vs 32% and a higher molar ratio 1/2 = 6.2 as opposed to a ratio of 2.5 measured at ambient temperature and with the physiological electron donor. These differences suggest that at these lower temperatures, the substrate is held more rigidly in proximity to the active oxidizing species, and/or that the low temperature inhibits side-product formation and dissociation of the protonated FeOOH species that result in uncoupling.
Table 3 presents a further example of the agreement between ambient and cryoreduction conditions. When the oxyferrous, BHT-bound 2B4(F429H) variant is cryoreduced, the hydroperoxo species accumulates to the same extent as with the wild type enzyme. The subsequent annealing of the hydroperoxo intermediate of the F429H mutant, however, exhibits markedly decreased product formation. Only ≅ 4% of the hydroperoxoferric 2B4(F429H) intermediate forms product compared to ≅ 80% with the native protein. This result agrees with our finding under ambient conditions, where the 2B4(F429H) mutant only has ≅ 5% of the activity of the native protein.
In conclusion, our results demonstrate the feasibility and the promise of cryoreduction/EPR and ENDOR spectroscopy in the study of the dioxygen activation by mammalian cytochromes P450. Cryoreduction at 77 K generates the hydroperoxo-ferriheme intermediate, which oxygenates substrate with efficiency comparable to that seen during steady-state catalysis under ambient conditions. Future cryoreduction ENDOR experiments with isotope-labeled substrates, carried out with oxy-2B4 samples prepared by rapid freeze-quench, combined with step-annealing kinetic measurements conducted in H2O/D2O, will be carried out to determine the nature of active species in the catalytic cycle (hydroperoxo-ferri P450 or compound I) and the details of the rate-limiting catalytic step(s).
Acknowledgement
We thank Professor Howard Halpern (University of Chicago) for providing access to the Gammacell irradiator. We thank Drs. Kate Noon and Yoichi Osawa from the Biomedical Mass Spectrometry facility at the University of Michigan for Assistance with the LC-MS/MS assay.
Abbreviations
- P450
cytochrome P450
- 2B4
cytochrome P450 2B4
- BHT
butylated hydroxy-toluene
- BP
benzphetamine
Footnotes
This work has been supported by the NIH (HL 13531, BMH; GM35533 and VA, Grants, LW).
References
- 1.Johnson EF, Stout CD. Structural diversity of human xenobiotic-metabolizing cytochrome P450 monooxygenases. Biochem. Biophys. Res. Commun. 2005;338:331–336. doi: 10.1016/j.bbrc.2005.08.190. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Halpert JR. Structure-function analysis of cytochromes P 450 2B. Biochimica et Biophysica Acta, General Subjects. 2007;1770:402–412. doi: 10.1016/j.bbagen.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Scott EE, White MA, He YA, Johnson EF, Stout CD, Halpert JR. Structure of Mammalian Cytochrome P450 2B4 Complexed with 4-(4-Chlorophenyl)imidazole at 1.9-Å Resolution: Insight into the Range of P450 Conformations and the Coordination of Redox Partner Binding. J. Biol. Chem. 2004;279:27294–27301. doi: 10.1074/jbc.M403349200. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz de Montellano PR, De Voss JJ. Oxidizing Species in the Mechanism of Cytochrome P450. Nat. Prod. Rep. 2002;19:477–493. doi: 10.1039/b101297p. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Silverman RB. Revisiting Heme Mechanisms. A Perspective on the Mechanisms of Nitric Oxide Synthase (NOS), Heme Oxygenase (HO), and Cytochrome P450s (CYP450s) Biochemistry. 2008;47:2231–2243. doi: 10.1021/bi7023817. [DOI] [PubMed] [Google Scholar]
- 6.Newcomb M, Hollenberg PF, Coon MJ. Multiple mechanisms and multiple oxidants in P 450-catalyzed hydroxylations. Arch. Biochem. Biophys. 2002;409:72–79. doi: 10.1016/s0003-9861(02)00445-9. [DOI] [PubMed] [Google Scholar]
- 7.Davydov R, Perera R, Jin S, Yang T-C, Bryson TA, Sono M, Dawson JH, Hoffman BM. Substrate Modulation of the Properties and Reactivity of the Oxy-Ferrous and Hydroperoxoferric Intermediates of Cytochrome P450cam as hown by Cryoreduction- EPR/ENDOR Spectroscopy. J. Am. Chem. Soc. 2005;127:1403–1413. doi: 10.1021/ja045351i. [DOI] [PubMed] [Google Scholar]
- 8.Jin S, Makris TM, Bryson TA, Sligar SG, Dawson JH. Epoxidation of Olefins by Hydroperoxo-Ferric Cytochrome P450. J. Am. Chem. Soc. 2003;125:3406–3407. doi: 10.1021/ja029272n. [DOI] [PubMed] [Google Scholar]
- 9.Davydov R, Makris TM, Kofman V, Werst DW, Sligar SG, Hoffman BM. Hydroxylation of Camphor by Reduced oxy-Cytochrome P450cam: Mechanistic Implications of EPR and ENDOR of Catalytic Intermediates in Native and Mutant Enzymes. J. Am. Chem. Soc. 2001;123:1403–1415. doi: 10.1021/ja003583l. [DOI] [PubMed] [Google Scholar]
- 10.Hlavica P. Models and mechanisms of O-O bond activation by cytochrome P450. A critical assessment of the potential role of multiple active intermediates in oxidative catalysis. Eur. J. Biochem. 2004;271:4335–4360. doi: 10.1111/j.1432-1033.2004.04380.x. [DOI] [PubMed] [Google Scholar]
- 11.Schlichting I, Berendzen J, Chu K, Stock AM, Maves SA, Benson DE, Sweet BM, Ringe D, Petsko GA, Sligar SG. The catalytic pathway of cytochrome P450cam at atomic resolution. Science. 2000;287:1615–1622. doi: 10.1126/science.287.5458.1615. [DOI] [PubMed] [Google Scholar]
- 12.Vaz ADN, Pernecky SJ, Raner GM, Coon MJ. Peroxo-iron and oxenoid-iron species as alternative oxygenating agents in cytochrome P450-catalyzed reactions: switching by threonine-302 to alanine mutagenesis of cytochrome P450 2B4. Proc. Natl. Acad. Sci. U. S. A. 1996;93:4644–4648. doi: 10.1073/pnas.93.10.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coon MJ. Multiple oxidants and multiple mechanisms in cytochrome P450 catalysis. Biochem. Biophys. Res. Commun. 2003;312:163–168. [PubMed] [Google Scholar]
- 14.Zhang H, Im S-C, Waskell L. Cytochrome b5 Increases the Rate of Product Formation by Cytochrome P450 2B4 and Competes with Cytochrome P450 Reductase for a Binding Site on Cytochrome P450 2B4. J. Biol. Chem. 2007;282:29766–29776. doi: 10.1074/jbc.M703845200. [DOI] [PubMed] [Google Scholar]
- 15.Davydov R, Kofman V, Nocek J, Noble RW, Hui H, Hoffman BM. Conformational Substates of the Oxyheme Centers in α and β Subunits of Hemoglobin as Disclosed by EPR and ENDOR Studies of Cryoreduced Protein. Biochemistry. 2004;43:6330–6338. doi: 10.1021/bi036273z. [DOI] [PubMed] [Google Scholar]
- 16.LeLean JE, Moon N, Dunham WR, Coon MJ. EPR Spectrometry of Cytochrome P450 2B4: Effects of Mutations and Substrate Binding. Biochem. Biophys. Res. Commun. 2000;276:762–766. doi: 10.1006/bbrc.2000.3539. [DOI] [PubMed] [Google Scholar]
- 17.Harris DL, Park J-Y, Gruenke L, Waskell L. Theoretical study of the ligand-CYP2B4 complexes: Effect of structure on binding free energies and heme spin state. Proteins: Struct., Funct., Bioinf. 2004;55:895–914. doi: 10.1002/prot.20062. [DOI] [PubMed] [Google Scholar]
- 18.Lipscomb JD. Electron paramagnetic resonance detectable states of cytochrome P-450cam. Biochemistry. 1980;19:3590–3599. doi: 10.1021/bi00556a027. [DOI] [PubMed] [Google Scholar]
- 19.Bonfils C, Debey P, Maurel P. Highly-purified microsomal P-450: the oxyferro intermediate stabilized at low temperature. Biochem. Biophys. Res. Commun. 1979;88:1301–1307. doi: 10.1016/0006-291x(79)91122-7. [DOI] [PubMed] [Google Scholar]
- 20.Davydov R, Satterlee JD, Fujii H, Sauer-Masarwa A, Busch DH, Hoffman BM. A Superoxo-Ferrous State in a Reduced Oxy-Ferrous Hemoprotein and Model Compounds. J. Am. Chem. Soc. 2003;125:16340–16346. doi: 10.1021/ja037037e. [DOI] [PubMed] [Google Scholar]
- 21.Davydov R, Kofman V, Fujii H, Yoshida T, Ikeda-Saito M, Hoffman B. Catalytic Mechanism of Heme Oxygenase through EPR and ENDOR of Cryoreduced Oxy-Heme Oxygenase and its Asp140 Mutants. J. Am. Chem. Soc. 2002;124:1798–1808. doi: 10.1021/ja0122391. [DOI] [PubMed] [Google Scholar]
- 22.Davydov R, Chemerisov S, Werst DE, Rajh T, Matsui T, Ikeda-Saito M, Hoffman BM. Proton Transfer at Helium Temperatures during Dioxygen Activation by Heme Monooxygenases. J. Am. Chem. Soc. 2004;126:15960–15961. doi: 10.1021/ja044646t. [DOI] [PubMed] [Google Scholar]
- 23.Denisov IG, Makris TM, Sligar SG. Cryotrapped reaction intermediates of cytochrome P450 studied by radiolytic reduction with phosphorus-32. J. Biol. Chem. 2001;276:11648–11652. doi: 10.1074/jbc.M010219200. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Gruenke L, Arscott D, Shen A, Kasper C, Harris DL, Glavanovich M, Johnson R, Waskell L. Determination of the Rate of Reduction of Oxyferrous Cytochrome P450 2B4 by 5-Deazariboflavin Adenine Dinucleotide T491V Cytochrome P450 Reductase. Biochemistry. 2003;42:11594–11603. doi: 10.1021/bi034968u. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Serres R, Davydov RM, Matsui T, Ikeda-Saito M, Hoffman BM, Huynh BH. Distinct Reaction Pathways Followed upon Reduction of Oxy-Heme Oxygenase and Oxy-Myoglobin as Characterized by Moessbauer Spectroscopy. J. Am. Chem. Soc. 2007;129:1402–1412. doi: 10.1021/ja067209i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spinks JWT, Woods RJ. An Introduction to Radiation Chemistry. 3rd Ed 1990. [Google Scholar]