Summary
Objectives
Pregnant women in malaria-endemic communities are susceptible to Plasmodium falciparum infections, with adverse consequences including maternal anaemia, placental malaria parasitaemia and infant low birth weight (LBW). We sought to assess the prevalence, incidence, and clinical markers of pregnancy-associated malaria (PAM) in a rural district of Ghana.
Methods
A total of 294 pregnant women were enrolled and followed passively and actively, monthly and weekly until delivery. Haemoglobin levels, malaria parasitaemia and Hb electrophoresis were done from peripheral blood samples. At delivery, placental smears were examined for malaria parasites.
Results
Prevalence of peripheral blood P. falciparum parasitaemia at enrolment was 19.7% and related to parity. Incidence rate of parasitaemia was 0.06 infections/ person/month [95% confidence interval (CI): 0.04 to 0.08]. Symptomatic infections rose sharply from the first trimester to the last. Prevalence of malaria parasites in the placenta was 35.9% (61/170) and highest among primigravidae (P(χ2)=0.006). Incidence of LBW infants was 17.7% (30/170), most common among those with placental P. falciparum infection (P(χ2)=0.005) corresponding to a relative risk of 2.8 [1.4 to 5.2]. Median infant birth weight in those with placental infection was significantly lower than in those without infections (P(χ2)=0.001). Maternal haemoglobin levels were lower (9.7 [9.3-10.1] g/dL) at enrolment, among women who subsequently had placental P. falciparum infection than among those who did not have placental infection at delivery (10.5 [10.2–10.8] g/dL) (P (t)=0.003).
Conclusion
Primigravidae and secundigravidae are significantly at risk of developing PAM, and low haemoglobin during pregnancy is a clinical indicator of placental P. falciparum infection.
Keywords: Placenta, pregnancy, malaria, haemoglobin
Introduction
Pregnant women are twice as likely to become infected with P. falciparum malaria as non-pregnant women living under the same conditions.1 This risk of pregnancy-associated malaria (PAM) is highest among primigravid women, with lower incidence and severity in women of higher parity. The adverse consequences of PAM include infant low birth weight and anaemia in newborn babies, as well as maternal anaemia.2–8 Other parasitic infections, especially hookworm, and micronutrient deficiencies, particularly of iron and folate, also contribute to anaemia in pregnant women.9–12
Malaria due to P. falciparum infection is a major cause of morbidity and remains the biggest cause of loss of number of days of healthy life.11 Malaria is mainly a childhood disease in Ghana because adults enjoy some level of protection due to acquired immunity. However, pregnant women constitute an important exception to the rule of clinical protection against P. falciparum malaria in adults in endemic areas. Recognition of malaria during pregnancy is often difficult clinically, yet such information is necessary for the evaluation of effective interventions against PAM as well as for health policy planning at all levels including the district level. The present study therefore sought to characterise the PAM situation and to identify specific markers of importance during pregnancy in a rural, coastal community of Ghana (Dangme West district) to allow for implementation of effective and knowledge-based intervention against this major cause of maternal and child morbidity and mortality.
Methods
The Dangme-West district is a rural coastal district in the Greater Accra region of Ghana with an estimated, 2001 mid-year population of one hundred and thirteen thousand. Malaria transmission in the district is perennial with an estimated 20 infectious bites per person/year.13
However, the prevalence of malaria shows considerable seasonal variation, peaking during and immediately after the rains. P. falciparum constitutes 98% of all infections.14
The study was a longitudinal design involving 294 pregnant women, who were recruited early in pregnancy. On the first day of enrolment, a detailed questionnaire was completed after explaining the study to the women in their languages and receiving written informed consent to participate. Personal information (age, parity, educational background, marital status, educational background and occupation of partner) was obtained. Each participant was visited once weekly by a trained field worker and also monitored in the laboratory of the district health centre every month for clinical symptoms and for the presence of malaria parasites.
Each participant was clinically examined at enrolment at the Dodowa Health Centre and 5 ml of venous blood was collected using butterfly needles into EDTA vacutainer tubes for measurement of haemoglobin (Hb) levels using an automated haematology analyser (Sysmex, KX-21, Germany). Malaria parasite species and density were assessed using thin and thick blood smears respectively. Blood smears on glass slides were air-dried and thin smears fixed in methanol and both smears subsequently stained with 10% Giemsa in phosphate buffer. An individual was considered malaria positive if malaria parasites were detected in her blood smear. On the other hand, if parasites were not detected after examining 200 oil-immersion fields of the thick smear, then the blood smear was considered negative. Parasitaemia (parasite density) was determined by counting the number of asexual parasites per 200 leukocytes and converting this to the number of parasites per micro-litre of blood.
ABO blood typing was done using commercial antisera (Biotec Laboratories, UK). Participants were also screened for sickling status using the metabisulphite method and Hb electrophoresis was carried out on those found to be sickle cell positive, using commercially available kits at the Noguchi Memorial Institute for Medical Research.
Recruitment began in January 2003 and by January 2004 all the participants had delivered. The surveillance period was from the day of recruitment to the day of delivery. There were both active and passive follow-up where participants were visited once every week by a field assistant, who measured the body temperature with a digital thermometer, and filled in a questionnaire containing a set of parameters used to access malaria morbidity. If the individual had a body temperature higher than 38°C and also gave positive responses to any of the anamnestic questions, then she was referred to the Health Centre for laboratory confirmation of the diagnosis of malaria. All those with slide-positive P. falciparum parasitaemia were referred to the physician in charge of the Health Centre who then prescribed the appropriate anti-malarial drug free of charge to the participant. The field assistants ensured that all the participants came to the laboratory which was open 7 days a week for participants, on days three and seven where finger-prick blood samples were taken and blood smears prepared, stained and examined for malaria parasitaemia. A standard regimen of chloroquine at a dose of 25mg/kg for a three-day period, which was at that time the approved drug for the treatment of uncomplicated malaria, was used.
Placental tissues were collected at delivery from all the participants who delivered at the Dodowa Health Centre and any of the two major hospitals (Atua Government Hospital and Agomenya St Martin's Hospital) nearby, to which all complicated cases were referred. Blood smears were also prepared from maternal blood and blood flushed from the placenta. Impression smears were prepared from the placental tissues. Birth weight of infant(s) for each participant was recorded. The Institutional Review Board of the Noguchi Memorial Institute for Medical Research and the Ethical Committee of the Ministry of Health, Ghana approved this study.
Statistical analysis
All statistical analyses were performed using SigmaStat 3.3 software (USA). Pair-wise comparisons were performed with student's t-test for data passing normality and equal-variance tests. Mann-Whitney Rank sum test was used for data failing one or both. Confidence intervals (95%) for parameter estimates are given in square brackets where relevant. Differences in proportions and relative risks were analysed by the χ2-test. Differences were considered significant if p<0.05.
Results
Of the 294 women recruited into the study between January and June 2003, 116 (39.5%) were primigravidae and 93 (31.6%) secundigravidae. Eighteen women (6.1%) had sickle-cell trait (HbAS) with no significant difference (P (χ2)=0.30) between parity groups. Haemoglobin levels at recruitment were significantly related to parity (1-way ANOVA, P=0.02), being lowest in primigravidae (9.7 [9.3 to 10.1] g/dL), intermediate in secundigravidae (10.1 [9.7 to 10.5] g/dL), and highest in multigravidae (10.5 [9.7 to 10.9] g/dL). Prevalence of peripheral P. falciparum parasitaemia was also related to parity, the highest among primigravidae (30/116), followed by secundigravidae (16/93) and lowest in multigravidae (12/85) (P (χ2)=0.09).
All women who were parasitaemic at enrolment received anti-malarial treatment using chloroquine. All patients recovered completely and were microscopically confirmed negative.
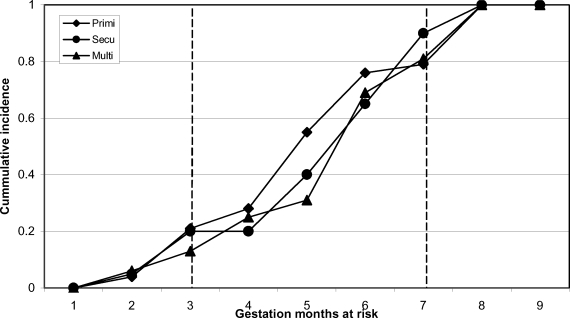
During the follow-up period, we observed 65 clinical episodes of P. falciparum malaria which translates into an infection rate of 0.06 [0.04 to 0.08] infections/person/month. Most of the clinical episodes occurred in the second trimester, while the remaining episodes were distributed equally between first and third trimesters. The rate of acquisition of new infections was similar in all parity groups (Figure 1).
Figure 1.
Cumulative incidence of symptomatic Plasmodium falciparum infections in pregnant women with different gravidity over a nine months gestation period (March – December 2003) in the Dangme West district of Ghana
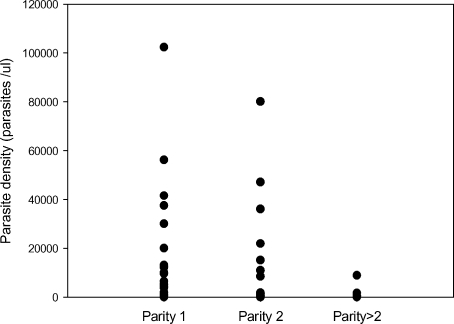
Sixty-one (35.9%) of the 170 placentas that were obtained at delivery showed evidence of P. falciparum infection. The prevalence (32/64, 19/59 and 10/47 in primigravidae, secundigravidae and multigravidae, respectively) (P(χ2)=0.006) and intensity (Figure 2) of placental infection were both related to parity, being most common among primigravidae.
Figure 2.
Density of placental Plasmodium falciparum
The proportion of women with placental infection at delivery was lower (P(χ2)=0.001) among women who had a clinical episode during pregnancy (13/65) than among those who did not (48/105), corresponding to a relative risk of 0.7 [0.5 to 0.8] of placental infection if treated for malaria during gestation. There were fewer cases of placental infections among HbAS women (0/8) than among HbAA women (61/162) (P (χ2)=0.07).
Thirty of 170 of the cohort gave birth to an underweight (Birth weight <2,500 g) singleton baby. Low birth weight (LBW) was related to parity (19/62, 9/60, 2/48 among primigravidae secundigravidae, and multigravidae, respectively) (P (χ2)=0.001).
Parasites in relation to gravidity among a group of Ghanaian women living in the Dangme West district in 2004. The proportion of LBW singleton deliveries was higher (P (χ2)=0.005) among women with placental P. falciparum infection (18/61) than among women without (12/109), corresponding to a relative risk of 2.7 [1.4 to 5.2] of delivering a LBW baby if carrying a placental P. falciparum infection at term. Similarly, the median birth weight of singletons (2.9 [2.7–3.0] Kg) was lower among women with placental infection than among women without a parasitaemic placenta at delivery (3.1[3.0–3.2] Kg) (P (χ2)=0.001). Importantly, maternal haemoglobin levels were lower (9.7 [9.3–10.1] g/dL) at enrolment, among those women who subsequently had placental P. falciparum infection than among those who did not have placental infection at delivery (10.5 [10.2–10.8] g/dL) (P (t)=0.003).
Discussion
The accumulation of Plasmodium falciparum parasites in the placental intervillous space referred to as pregnancy-associated malaria (PAM) is associated with maternal anaemia, low birth weight and mortality.15,16 Prevalence of parasitaemia and parasite densities in placental infections is high in primigravidae from malaria endemic communities with different levels of transmission. Additionally, the presence of P. falciparum parasites in the peripheral blood of clinically symptomatic pregnant women is an indication of on-going placental infection.17 Shulman & Dorman18 have shown that peripheral and placental parasitaemia decrease with increasing parity among pregnant women, a finding that is also supported by the results of the present study.
The prevalence of peripheral blood P. falciparum parasitaemia among pregnant women on enrolment into the study was 19.7%, much lower than the 35.1% reported for pregnant women in the Sekyere West district of the Ashanti region10, perhaps reflecting the higher malaria transmission associated with the forest belt of Ghana in which the Ashanti region is located, compared to the relatively drier coastal savannah region in which the present study was carried out. Malaria parasitaemia was most common among primigravidae but secundigravidae were also equally at risk, indicating that any measure to protect women against PAM in this community will have to give priority to first and second pregnancies.
These women were followed actively and passively, on a weekly basis, in addition to monthly parasitological monitoring at the laboratory throughout pregnancy until delivery. Despite this, the incidence of clinically symptomatic P falciparum infections was 6% per month. This is a significant amount of infection in an area of low to moderate transmission of malaria.13 Most of the symptomatic infections occurred between the end of the first trimester and the beginning of the last trimester, a finding that is important for targeting the control of PAM.
There was no difference in the cumulative incidence in terms of parity with respect to occurrence (Figure 1).
All the techniques available in investigating placental malaria by microscopy, which included preparation of smears from the maternal side of the placenta, from flushed red blood cells from the placenta and from tissue obtained from the placenta (impression smear) were employed. A placental malaria prevalence of 35% was quite high, after the strict monitoring and antimalarial treatment of most of the participants during pregnancy. The prevalence was lower among those who developed clinical disease as compared with those who did not, in other words, those who were treated for malaria during pregnancy were better protected from developing placental malaria. This finding, therefore, supports the recommended use of intermittent preventive treatment during pregnancy (IPTP) as it will eliminate malaria parasites and prevent their accumulation in the placenta. The prevalence and intensity of placental parasitization were both parity-related, being most common in primigravidae. It is also possible that the relatively high placental malarial infections could have been partly due to the ineffectiveness of chloroquine, which at that time was known to be associated with very high treatment failure rates in the country 19 and has since been replaced by artemisinin-based combination therapy (ACT) for the treatment of malaria. The data, nonetheless, are comparable with data from studies done in other African countries with similar levels of malaria transmission.4,20,21 It is important to note that women with microscopically detectable placental P falciparum malaria had a higher risk of delivering a LBW baby than those without detectable placental P. falciparum (P (χ2)=0.001) corresponding to a relative risk of 2.7, supporting the strong association between P. falciparum and P vivax malaria and the risk of LBW22, 23.
Although there is growing evidence that blood group types may play a role in the pathogenesis of placental malaria, especially blood group A, which has been implicated in severe disease24, this study did not show any relationship between placental infection and blood group A type, perhaps because we did not have a large enough sample size. The majority of women with placental malaria were of blood group O (42%) with only 21% of blood group A. The prevalence of HbAS phenotype was lower among infected women in the study cohort and HbAS may protect against placental malaria. This study has defined in detail PAM in a typical rural and coastal savannah community in Ghana, which is necessary for effective and knowledge-based intervention against this major cause of mother/child morbidity and mortality. For practical reasons, microscopy rather than histology, which is considered the classical method in the study of placental malaria was employed. Despite this limitation, it is has been shown clearly that PAM is a major public health problem in this community in Ghana.
The association of maternal Hb levels with prevalence of placental P. falciparum infection is an important finding. It suggests that reduced Hb level at any time in pregnancy could serve as a clinical indicator of possible placental P. falciparum infection. However, the analysis did not correct for the effect of socio-economic factors which could also influence the Hb-levels in such a setting.
In conclusion, PAM is a major public health problem in the Dangme West district of Ghana. Primigravidae and secundigravidae are most at risk of developing placental malaria. Placental malaria is associated with increased risk of LBW as well as reduced levels of maternal Hb during pregnancy. Increased public education, the use of insecticide-treated bed nets (ITNs) and intermittent preventive treatment as well as adequate treatment of malaria with effective anti-malarial drugs during pregnancy can help to control PAM.
Acknowledgments
We thank all the pregnant women who participated in this study, all the staff of the Dodowa Health Centre and the field workers. We are also grateful to the staff of the Immunology Department for technical support. This study was funded by the Enhancement of Research Capacity in Developing Countries (ENRECA) program of the Danish International Development Assistance (DANIDA) grant number 104. Dan. 8. L. 306.
References
- 1.Lindsay S, Ansell J, Selman C, Cox V, Hamilton K, Walraven G. Effect of pregnancy on exposure to malaria mosquitoes. Lancet. 2000;355(9219):1972. doi: 10.1016/S0140-6736(00)02334-5. [DOI] [PubMed] [Google Scholar]
- 2.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Hlth Org. 1983;61:1005–1016. [PMC free article] [PubMed] [Google Scholar]
- 3.Brabin BJ. The risks and severity of malaria in pregnant women. Applied Field Research Report No. 1, UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, Geneva. 1991:1–52. [Google Scholar]
- 4.Cot M, le Hesran JY, Miailhes P, et al. Effect of chloroquine prophylaxis during pregnancy on maternal haematocrit. Ann Trop Med Parasitol. 1998;92:37–43. doi: 10.1080/00034989860157. [DOI] [PubMed] [Google Scholar]
- 5.McGregor IA, Wilson ME, Billewicz NZ. Malaria infection of the placenta in The Gambia, West Africa, its incidence and relationship to stillbirth, birth weight and placental weight. Trans R Soc Trop Med Hyg. 1986;33:517–525. doi: 10.1016/0035-9203(83)90081-0. [DOI] [PubMed] [Google Scholar]
- 6.Shulman CE, Graham WJ, Jilo H, et al. Malaria is an important cause of anaemia in primigravidae: evidence from a district hospital in coastal Kenya. Trans R Soc Trop Med Hyg. 1996;90:535–539. doi: 10.1016/s0035-9203(96)90312-0. [DOI] [PubMed] [Google Scholar]
- 7.Steketee RW, Wirima JJ, Hightower AW, et al. The effect of malaria and malaria prevention in pregnancy on offspring birth weight, prematurity and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg. 1996;55:33–41. doi: 10.4269/ajtmh.1996.55.33. [DOI] [PubMed] [Google Scholar]
- 8.Steketee RW, Wirima JJ, Bloland PB, et al. Impairment of a pregnant woman's acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg. 1996;55:42–49. doi: 10.4269/ajtmh.1996.55.42. [DOI] [PubMed] [Google Scholar]
- 9.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 10.Glover-Amengor M, Owusu WB, Akanmori BD. Determinants of anaemia in pregnancy in Sekyere West District, Ghana. Ghana Med J. 2005;39:102–107. [PMC free article] [PubMed] [Google Scholar]
- 11.Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121(Suppl):S23–S38. doi: 10.1017/s0031182000006491. [DOI] [PubMed] [Google Scholar]
- 12.Gilles HM, Lawson JB, Sibelas M, et al. Malaria, anaemia and pregnancy. Ann Trop Med Parasitol. 1969;63:245–263. doi: 10.1080/00034983.1969.11686625. [DOI] [PubMed] [Google Scholar]
- 13.Afari EA, Appawu M, Dunyo S, Baffoe-Wilmot A, Nkrumah FK. Malaria infection, morbidity and transmission in two ecological zones in southern Ghana. Afr J Health Sci. 1995;2:312–316. [PubMed] [Google Scholar]
- 14.Ministry of Health, author. Malaria Action Plan 1993–1997. 1992 Prepared by Epidemiology Division Ministry of Health, Ghana, Accra.
- 15.Guyatt HL, Snow RW. Malaria in pregnancy as an indirect cause of infant mortality in sub-Saharan Africa. Trans R Soc Trop Med Hyg. 2001;95:569–576. doi: 10.1016/s0035-9203(01)90082-3. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt HL, Snow RW. The Epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-saharan Africa. Am J Trop Med Hyg. 2001;64:36–44. doi: 10.4269/ajtmh.2001.64.36. [DOI] [PubMed] [Google Scholar]
- 17.Ofori MF, Staalsoe T, Bam V, et al. Expression of variant surface antigens by Plasmodium falciparum parasites in the peripheral blood of clinically immune pregnant women indicates ongoing placental infection. Infect Immun. 2003;71:1584–1586. doi: 10.1128/IAI.71.3.1584-1586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shulman CE, Dorman EK. Importance and prevention of malaria in pregnancy. Trans R Soc Trop Med Hyg. 2003;97:30–35. doi: 10.1016/s0035-9203(03)90012-5. [DOI] [PubMed] [Google Scholar]
- 19.Abuaku BK, Koram KA, Binka FN. Antimalarial drug use among caregivers in Ghana. Afr J Health Sci. 2004;4:171–4177. [PMC free article] [PubMed] [Google Scholar]
- 20.Menendez C, Ordi J, Ismail MR, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–1745. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- 21.Nosten F, ter Kuile F, Maelankiri L, et al. Mefloquine prophylaxis prevents malaria during pregnancy: a double-blind, placebo-controlled study. J Infect Dis. 1994;169:595–603. doi: 10.1093/infdis/169.3.595. [DOI] [PubMed] [Google Scholar]
- 22.Nosten F, McGready R, Simpson JA, et al. The effects of Plasmodium vivax in pregnancy. Lancet. 1999;354:546–549. doi: 10.1016/s0140-6736(98)09247-2. [DOI] [PubMed] [Google Scholar]
- 23.Rogerson SJ, Mkundika P, Kanjala MK. Diagnosis of Plasmodium falciparum malaria at delivery: comparison of blood film preparation methods and of blood films with histology. J Clin Microbiol. 2003;41:1370–1374. doi: 10.1128/JCM.41.4.1370-1374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer PR, Boone P. Short report: severe malaria associated with blood group. Am J Trop Med Hyg. 1998;58:122–123. doi: 10.4269/ajtmh.1998.58.122. [DOI] [PubMed] [Google Scholar]