Abstract
Response inhibition is a hallmark of executive control. The concept refers to the suppression of no-longer required or inappropriate actions, which supports flexible and goal-directed behavior in ever-changing environments. The stop-signal paradigm is most suitable for the study of response inhibition in a laboratory setting. The paradigm has become increasingly popular in cognitive psychology, cognitive neuroscience and psychopathology. We review recent findings in the stop-signal literature with the specific aim of demonstrating how each of these different fields contributes to better understanding of the processes involved in inhibiting a response and monitoring stopping performance, and more generally, discovering how behavior is controlled.
People can readily stop talking, walking, typing, etc., in response to changes in internal states or changes in the environment. This ability to inhibit inappropriate or irrelevant responses is a hallmark of executive control. The role of inhibition in many experimental paradigms is debated, but most researchers agree that some kind of inhibition is involved in deliberately stopping a motor response. In this article, we focus on the stop-signal paradigm [1], which has proven to be a useful tool for the study of response inhibition in cognitive psychology, cognitive neuroscience and psychopathology. We review recent developments in the stop-signal paradigm in these different fields. The focus is primarily on the inhibition of manual responses. Studies of oculomotor inhibition are discussed in Box 1.
Box 1: Inhibitory control and monitoring of eye movements
Important insights into the cognitive and neural mechanisms involved in stopping have come from stop-signal studies of eye movements in macaque monkeys while recording single-cell activity [for a review, see 54]. The fine temporal resolution of single-cell recording allows stronger inferences about the role of different brain regions in response inhibition and monitoring: Cells that modulate before SSRT can contribute directly to response inhibition; cells that modulate after SSRT cannot contribute directly and may contribute to monitoring and control instead. Several studies have shown that activity of movement- and fixation-related neurons in frontal eye fields in dorsolateral prefrontal cortex [55] and superior colliculus in midbrain [56], was modulated before SSRT on successful stop trials (Figure Ia). This suggests that certain neurons in these regions are part of a circuit that directly controls the inhibition of eye movements [55, 56]. By contrast, activity of neurons in supplementary eye fields (SEF), which are an ocularmotor extension of SMA, and anterior cingulate cortex (ACC) in medial frontal cortex did not modulate until after SSRT (Figure Ib). Instead, neurons in SEF and ACC signal feedback about errors, rewards and possibly, conflict. This suggests that SEF and ACC are not directly involved in the inhibition of movements, but instead are involved in monitoring performance [57, 58]. Consistent with this idea, unsuccessful inhibition is associated with negatively polarized local-field potentials recorded in ACC of monkeys stopping eye movements [59].
Stopping of eye movements is also studied in humans. Behavioral studies showed that stopping eyes and hands is qualitatively similar, although SSRT is shorter for eye movements [60, 61], An fMRI study showed greater activation in FEF on stop-signal trials and greater activation in SEF on unsuccessful stop trials [62]. However, this study used only one stop-signal delay in the fMRI phase of the experiment, which may have influenced performance [2].
Successful stopping: Inhibition and performance monitoring
In the stop-signal paradigm, subjects perform a go task, such as reporting the identity of a stimulus. Occasionally, the go stimulus is followed by a stop signal, which instructs subjects to withhold the response (see Figure 1). Stopping a response requires a fast control mechanism that prevents the execution of the motor response [1]. This process interacts with slower control mechanisms that monitor and adjust performance [2].
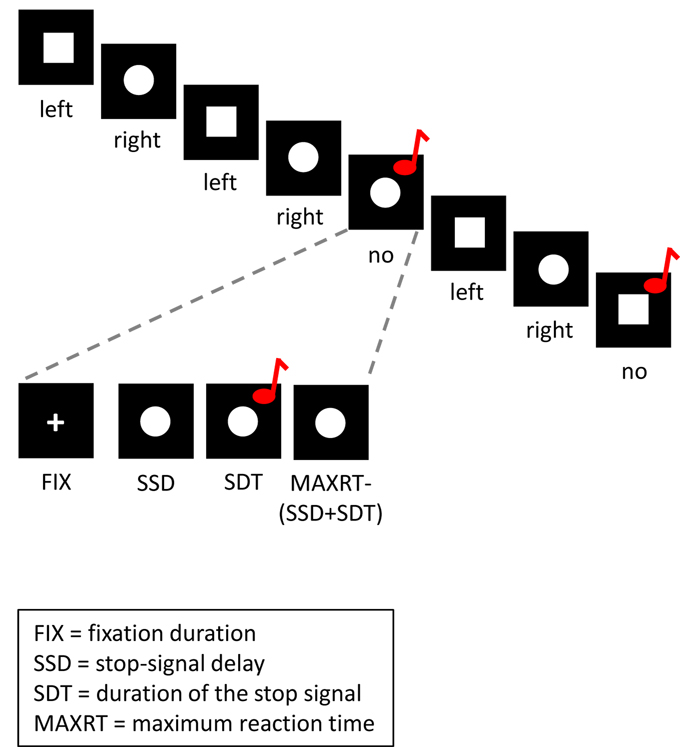
Figure 1.
Depiction of a trial course in the stop-signal paradigm. Tasks and task parameters in this figure are adapted from STOP-IT, which is a free-to-use stop-signal task program [74]. In the go task, subjects respond to the shape of a stimulus (a ‘square’ requires a left response and a ‘circle’ requires a right response). On one fourth of the trials, the go stimulus is followed by an auditory stop signal after a variable stop-signal delay (SSD). Subjects are instructed to respond as quickly and accurately as possible to the go stimulus on no-stop-signal trials. They are instructed to try to withhold their response on stop-signal trials, but not to wait for the stop signal to occur. On both no-stop-signal trials and stop-signal trials, the stimulus remains on the screen until subjects respond or until the maximal RT has elapsed.
The race between going and stopping
Performance in the stop-signal paradigm is modeled as a race between a go process, which is triggered by the presentation of the go stimulus, and a stop process, which is triggered by the presentation of the stop signal. When the stop process finishes before the go process, the response is inhibited; when the go processes finishes before the stop process, the response is emitted. The latency of the stop process (stop-signal reaction time; SSRT) is covert and must be estimated from a stochastic model, such as the independent race model [3; Box 2]. SSRT has proven to be an important measure of the cognitive control processes that are involved in stopping. Cognitive neuroscientists use SSRT as a criterion to determine whether neural processes participate directly in response inhibition (see Box 1). Psychopathologists use SSRT to study inhibitory deficits in different patient groups (see below). Developmental scientists found that SSRT is elevated in younger children and older adults compared to young adults, and a comparison of SSRT and go reaction time (RT) showed that going and stopping develop and decline independently [4–6].
Box 2: The independent and interactive race models of response inhibition
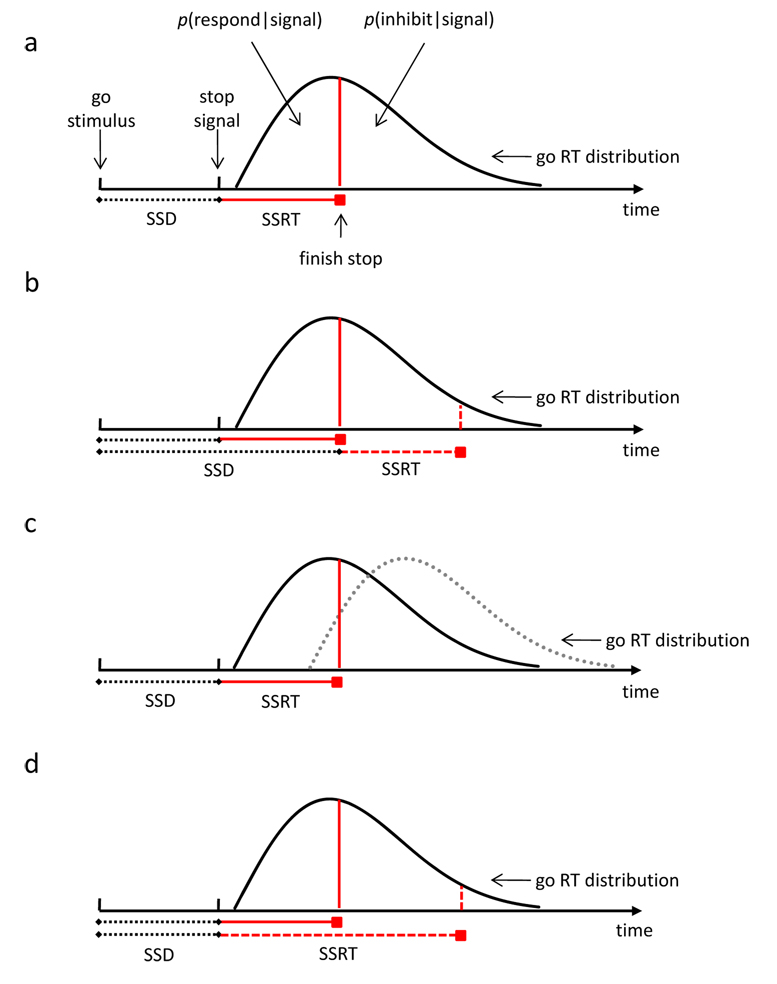
Logan and Cowan [3] developed an independent race model (Figure IIa) that described the probability of responding on a stop-signal trial, p(respond|signal), the latency of go RTs that escape inhibition, and SSRT. According to the model, p(respond|signal) depends primarily on three factors: SSD, go RT and SSRT. First, increasing SSD increases p(respond|signal): the stop process starts later and therefore, finishes later relative to the go process (Figure IIb). Second, for every SSD, increasing go RT decreases p(respond|signal) because the probability that the stop process finishes before the go process increases (Figure IIc). Third, for every SSD, increasing SSRT increases p(respond|signal) because the probability that the stop process finishes after the go process increases (Figure IId). Importantly, the independent race model provides methods for estimating SSRT. The model assumes that the stop process begins at SSD, which is observed. The point at which the stop process finishes can be estimated from the observed go-RT distribution on no-signal trials and the observed p(respond|signal) for a given SSD (see Figure IIa). SSRT can be estimated by subtracting SSD from the finishing time [for reviews of estimation methods of SSRT, see 1, 63].
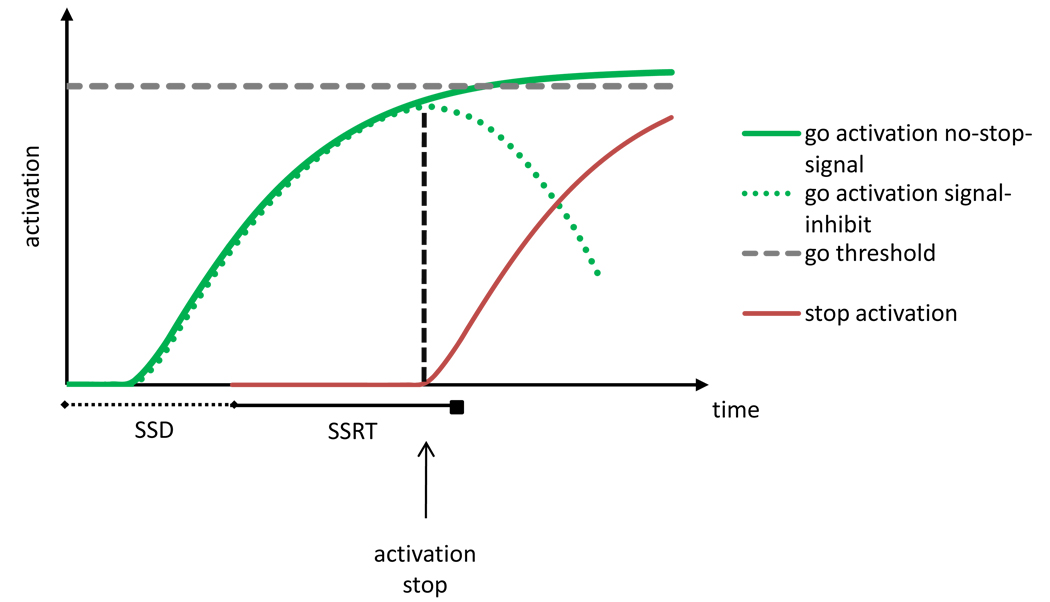
The independent race model assumes stochastic independence between the go and stop process. However, complete independence between the go and stop process is unlikely. Boucher et al. [64] proposed an interactive race model, in which the go and stop process are independent for much of their latencies and interact strongly near the end (Figure III). The go process is initiated by the go stimulus and a go unit is activated after an afferent delay. The stop process is initiated by the stop signal and a stop unit is activated after an afferent delay. Once the stop unit is activated, it inhibits go processing strongly and quickly. In this model, SSRT primarily reflects the period before the stop unit is activated, during which stop and go processing are independent, so its predictions correspond to those of the independent race model [64].
Monitoring and adjusting go and stop performance
Successful performance in the stop-signal paradigm also involves monitoring go and stop performance and adjusting response strategies to find an optimal balance between the conflicting demands of the go task (“respond as quickly as possible”) and the stop task (“stop the response”). Several studies suggest that subjects change response strategies proactively when they expect stop signals to occur, trading speed in the go task for success in the stop task [2, 7]. Many studies suggest that subjects also change response strategies reactively after stop-signal trials [8–11]. Some show that go RT increases after unsuccessful inhibition, reminiscent of the post-error slowing observed in choice reaction tasks. Others show that go RT increases after successful stopping, which is inconsistent with error-correction but suggests a shift in priority to the stop task after a stop signal. Recent studies show that stimulus repetition may be a critical variable: responding after successful stopping is typical slower when the stimulus from the stop trial is repeated, as if the stimulus was associated with stopping, and retrieval of that association impaired go performance [8]. This stimulus-specific slowing can persist over many intervening trials [10] and may support the development of automatic inhibition [12].
Interim conclusions
Cognitive psychologists have identified the computational mechanisms underlying performance in the stop-signal paradigm, identifying a fast-acting stop process that produces immediate inhibition and slower monitoring and adjustment processes that optimize performance. In recent years, many insights into the underlying cognitive and neural mechanisms of response inhibition have come from cognitive neuroscience and psychopathology. In the next sections, we review the most important findings in these fields and show how they contribute to a better understanding of control processes involved in stopping and monitoring stop performance.
Neural substrates of stopping and monitoring
Going is associated with activation of a cortico-basal-ganglia-thalamocortical circuit [13]. Recent studies using a variety of methods suggest that stopping is associated with activation of a fronto-basal-ganglia circuit that includes inferior frontal gyrus (IFG; ventrolateral prefrontal cortex), middle frontal gyrus (dorsolateral prefrontal cortex), medial frontal gyrus (MFG), and basal ganglia [14–18]. Results are sometimes inconsistent between studies, possibly because they used different methods for isolating inhibition-related regions.
A fronto-basal-ganglia circuit for response inhibition
Recent neuroimaging research has suggested that right IFG is involved in stopping [e.g., 14, 15, 18], and possibly, other kinds of inhibition (cf. Box 3). This region shows increased activation when stopping is successful, and the magnitude of the activation correlates negatively with SSRT [14, 17, 19]. Some studies showed that right IFG is activated to some degree on unsuccessful stop trials [e.g., 17], but not on no-stop-signal trials [e.g., 17, 18]. Successful stopping is also associated with pre-SMA activation, but unlike right IFG, the magnitude of activation in pre-SMA did not correlate with SSRT [14]. According to some researchers, these findings suggest that right IFG contributes to response inhibition and not to monitoring performance or adjusting behavior [18], whereas pre-SMA is involved in monitoring or resolving the conflict between the opposing task demands in the stop-signal paradigm [14, 20]. However, the poor temporal resolution of fMRI makes it difficult to determine the specific role of right IFG and pre-SMA (cf. Box 1).
Box 3: One or many inhibitory mechanisms?
An important issue is whether the inhibitory mechanism that is involved in the stop-signal paradigm is also involved in other inhibitory paradigms. Behavioral results suggest a functional relation between stop-signal inhibition and interference control in the Stroop task and the flanker task. Incongruent trials produce interference and prolong SSRT in these tasks [65, 66]. Individual-difference studies show correlations between stop-signal inhibition and interference control [67]. Neuroimaging studies show activation in right IFG and pre-SMA in different inhibition tasks [68, 69]. However, this need not imply that the same inhibitory circuit is involved. rTMS of the right IFG influenced response inhibition but not interference control in a flanker task with stop signals [21]. Future research should clarify whether functional dependence between different kinds of inhibition implies similar neural mechanisms.
A related issue is whether the same inhibitory mechanism is involved in stopping responses with different effectors. SSRTs are similar for interrupting speech and interrupting manual responses [70] but SSRT is typically shorter for eye movements than for hand movements (see Box 1). fMRI data suggest that right IFG and pre-SMA are involved in inhibition of hand movements and suppression of speech but STN was activated only for inhibition of hand movements [70]. However, activation of STN is hard to detect in fMRI. One fMRI study compared inhibition of eye and hand movements directly, and found common activation in right IFG and medial frontal regions (among other regions) [71]. Inhibition of hand movements was associated with activation in more ventral and posterior parts of right IFG, whereas inhibition of eye movements was associated with activation in more dorsal and anterior parts of right IFG. However, no eye movements were recorded in the scanner, so it is not clear whether the common activation is due to inhibition or performance monitoring. Future research should clarify how general the inhibitory circuits are.
The involvement of right IGF and pre-SMA in stopping is further supported by results of transcranial magnetic stimulation (TMS) and lesion studies. Repetitive TMS (rTMS) of right IFG (but not left IFG or right middle frontal gyrus) impaired stopping but not going [21, 22]. By contrast, rTMS over right dorsal premotor cortex influenced going but not stopping. These findings support the theoretical distinction between stop and go processes in formal race models. Response inhibition is impaired in patients with lesions to right IFG but not left IFG [23]; moreover, the magnitude of the lesion to right IFG correlated with SSRT but not with go RT. Similarly, lesions to right SMA and pre-SMA impaired stopping without influencing going very much [24].
Several subcortical regions may also play an important role in stopping. fMRI studies showed inhibition-related activation in basal ganglia, including the subthalamic nucleus [STN; 17] and striatum [18, 25]. Lesions to the basal ganglia impaired stop performance for both humans and rodents [26–28], whereas deep-brain stimulation of STN in Parkinson patients enhanced inhibitory control [29]. Lesions to STN and stimulation of STN in Parkinson patients influence both go RT and SSRT [29, 30]. However, the effects of STN stimulation on go RT and SSRT may be functionally independent [29].
Combined, these studies suggest that right IFG, pre-SMA, and basal ganglia are part of a fronto-basal-ganglia inhibition network, although the exact role of these regions is debated. Some researchers proposed that activation in right IFG or pre-SMA leads to a suppression of motor output through a projection to STN [14, 17, 31]. When STN is activated, the internal segment of the globus pallidus becomes activated and motor output is suppressed. In most stop-signal situations, this suppression is very general and may affect all response tendencies including activation in muscles that are irrelevant to the task [32–34].
Neural substrates of monitoring
Unsuccessful inhibition is associated with an error-related negativity (ERN) in the electroencephalogram [35], which is reminiscent of the ERN that is typically observed after choice errors in reaction tasks. Event-related fMRI studies showed that (mainly) parietal and frontal brain regions are more activated when inhibition is unsuccessful [15, 16, 18, 19]. Unsuccessful inhibition is associated with greater activation of medial frontal regions, including anterior cingulate cortex (ACC) and pre-SMA, and middle frontal regions. Some studies report that ACC is also activated on successful stop-signal trials [e.g., 17], which suggests that this region is involved in monitoring of stopping performance. Consistent with this idea, single-cell studies show that ACC modulation occurs after SSRT, which is too late to be involved directly in inhibiting the response; instead, the neurons signal reward and error (Box 1). Medial frontal regions are commonly associated with detection of errors and detection of conflict between responses and action plans (monitoring behavior), whereas middle frontal regions are commonly associated with adjusting behavior after conflict or errors [36]. Some researchers proposed that activation of middle frontal regions reflects adjusting response strategies to balance the opposing demands of the go and stop tasks [18].
Combined, behavioral data (post-error slowing) and neural data (ERN and activation of medial and middle frontal regions) suggest that monitoring and adjusting performance in the stop-signal paradigm may be similar to monitoring and adjusting performance in paradigms that do not involve inhibition of motor responses. Moreover, the neural mechanisms involved in monitoring can be distinguished from the neural mechanisms involved directly in stopping (cf. Box 1). However, it is unclear to which extent activation associated with monitoring actually reflects memory-retrieval effects (cf. supra).
Inhibitory disorders and psychopathology
Response-inhibition deficits have been linked to several psychopathological and neurological disorders. Some disorders, such as autism [37] and schizophrenia [38], are associated with general cognitive impairments as well as inhibitory deficits. Other disorders, such as attention-deficit/hyperactivity disorder [ADHD; for a metanalysis, see 39], and compulsive disorders [40], are described specifically as inhibitory disorders. In the following sections, we review stop-signal studies of inhibitory disorders. These reveal important insight into the underlying cognitive and neural mechanisms of response inhibition and monitoring.
Attention-deficit/hyperactivity disorder
Probably the most studied clinical group in the stop-signal literature is children with ADHD. ADHD is typically associated with poor control of impulses. Many stop-signal studies have shown slower SSRT in people with ADHD [39] and in their relatives [41]. These findings led researchers to propose that stop-signal inhibition may be an endophenotype of ADHD [41, 42]. In children with ADHD, slower SSRT is often accompanied by slower go RT, suggesting a general deficit in cognitive control [38]. By contrast, in adults with ADHD, SSRT is impaired but go RT is not, suggesting a selective deficit in inhibition. The inhibitory deficit ADHD is linked to functional and structural differences in the fronto-basal-ganglia inhibitory network [for reviews, see 42, 43]. However, the amplitude of non-inhibitory (attentional) components in the electroencephalogram is also reduced in ADHD , which suggests that the response-inhibition deficit may have multiple origins [44].
Performance monitoring may also be impaired in ADHD. Children with ADHD slow their go RTs less after unsuccessful stopping than control subjects [9]. Post-error adjustments were not correlated with SSRT, suggesting a dissociation of response inhibition and monitoring or adjustment. It is not clear whether the behavioral impairment reflects deficits in monitoring or deficits in adjustment. However, neural data suggest a monitoring deficit: Unsuccessful stopping in ADHD is associated with a reduced amplitude of the ERN [45] and a reduced magnitude of activation in posterior and anterior parts of the cingulate gyrus and in left ventrolateral prefrontal cortex [46, 47]. Combined, behavioral and neural data suggest an error-monitoring deficit in ADHD that can be dissociated from the response-inhibition deficit.
Poor inhibitory control over obsessions, compulsions, tics and urges
Inhibitory deficits are associated with disorders other than ADHD. SSRT is prolonged in people with obsessive-compulsive disorder (OCD) and their first-degree relatives, people with trichotillomania (repetitive hair pulling), and people with Tourette’s syndrome [e.g., 40, 48, 49]. These inhibitory deficits result in poor control of behavior, which is characteristic of these disorders. Typically, go RT is not affected, suggesting a selective deficit in inhibition. The inhibitory deficit in OCD has been linked to reduced grey matter in the orbitofrontal and right inferior frontal regions [49]. A recent fMRI study of people with OCD found reduced activation in inferior and orbital fronto-striatothalamic brain regions when inhibition was successful and reduced activation in mesial and dorsolateral prefrontal cortices when inhibition was unsuccessful. This suggests that response inhibition and performance monitoring are both impaired in OCD [50]. However, these results should be interpreted cautiously because sample size was small.
Poor inhibitory control is also characteristic of substance-abuse disorders. SSRT is prolonged in chronic cocaine users [51], chronic methamphetamine users [52] and alcohol-dependent subjects [48], compared with normal control subjects, suggesting a response-inhibition deficit. It is not clear whether these SSRT differences reflect pre-morbid differences in inhibitory control, post-morbid abnormalities due to chronic chemical abuse, or both. However, prolonged SSRTs in high-risk adolescents predict alcoholism and other substance-abuse disorders [53], which suggests that prolonged SSRTs in chronic substance users may reflect pre-morbid differences in inhibitory control.
Conclusions
The stop-signal paradigm has become a popular tool for the study of response inhibition in cognitive psychology, cognitive neuroscience and psychopathology. Through this paradigm, findings from different fields of research have stimulated each other, leading to integrative, converging conclusions about cognitive control processes involved in stopping and performance monitoring. Cognitive psychologists modeled response inhibition as a race between a go process and a stop process. Cognitive neuroscientists showed that these processes may activate brain regions differently, and psychopathologists showed selective deficits in inhibition. Studies in each field suggest that successful stop performance requires effective performance monitoring and behavior adjustment, as well as an efficient stop process, to find an optimal balance between the opposing task demands of the stop-signal paradigm.
Inhibitory processes and monitoring have been dissociated behaviorally and neurally. The most important challenge for the future is to determine how inhibitory processes and monitoring jointly contribute to successful stop performance and how the neural substrates of these processes carry out the required computation. Successful stopping may require some general processes that are not unique to response inhibition as well as some specific processes that are unique. Future research should further differentiate these processes and their associated brain circuits. This will require combining different methods and using techniques that have sufficient temporal resolution to distinguish between processes that occur before and after SSRT.
Box 4: Open questions and future directions
Recent stop-signal studies provided important insights in how stopping may be achieved. Several open questions remain.
How do working memory and long-term memory contribute to successful stopping? Short-term memory may be necessary for maintaining task rules and action plans. Retrieval of short-term and long-term associations may also contribute to stop performance. After many repetitions, associations between stimuli and stopping may allow automatic response inhibition, which reduces the need for cognitive control processes [11]. Automatic inhibition is more likely to develop in the go/no-go paradigm, where stimuli are consistently associated with going and stopping, than in the stop-signal paradigm, where stimuli are inconsistently associated with going and stopping [11]. It will be useful to link the neural substrates of inhibition to the neural substrates of memory, and to explore the links between inhibition deficits and memory deficits.
How does the ability to switch contribute to successful stopping? Successful stopping implies shifting attention from the go signal to the stop signal, and upon detection of the stop signal, activating an alternative task goal (the stop goal) or action plan [20, 72, 73]. The links between neural substrates of response inhibition, shifting attention, and replacing task goals and action plans should be explored.
How selective can stopping be? This article focused on non-selective stopping, in which subjects inhibit all responses when a stop signal occurs. Some research has examined selective stopping, in which subjects inhibit only some responses or inhibit only when some signals occur [e.g., 34, 60]. Much more is known about non-selective stopping. Future research should focus on selective forms of stopping and determine how one response can be stopped without inhibiting all other concurrent processing.
The further development of formal models. The independent race model is very general, addressing only finishing times of stop and go processes without addressing how the processes unfold computationally or neurally. The interactive race model is constrained by neural data but it is currently specific to stopping eye movements. No current models address the monitoring processes involved in the stop-signal paradigm. Future research should focus on the development of general models that account for stopping and monitoring computationally and neurally.
Figure II (Box 2).
(A) Graphic representation of the assumptions of the independent race model [3], indicating how the probability of responding [p(respond|signal)] and the probability of inhibiting [p(inhibit|signal)] depend on stop-signal delay (SSD) (B), the distribution of go reaction times (C), and stop-signal reaction time (SSRT) (D). P(respond|signal) is represented by the area under the curve to the left of each red vertical line.
Figure III (Box 2).
Graphic representation of the assumptions of the interactive race model [64], indicating how go activation on a signal-inhibit trial is inhibited when the stop unit is activated.
Acknowledgments
The authors thank Jeff Schall, Tom Palmeri, Leanne Boucher, Arnaud Szmalec, Matt Crump and three anonymous reviewers for their useful comments on this manuscript, and Jeff Shall for providing the data for Figure I. Frederick Verbruggen is a Postdoctoral Fellow of the Research Foundation—Flanders (FWO-Vlaanderen). The work in Gordon Logan’s lab is supported by grant BCS 0646588 from the National Science Foundation, grant FA9550-07-1-0192 from the Air Force Office of Scientific Research, and grant R01-MH073879-01 from the National Institute of Mental Health
Figure I (Box 1).
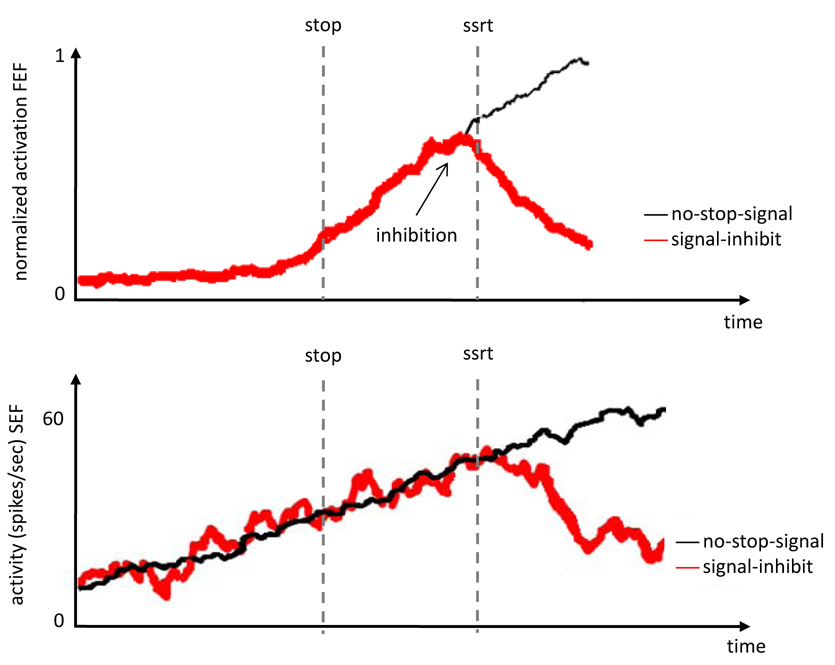
(A) Neural activity of FEF movement neurons on no-stop-signal trials and signal-inhibit trials [55]. (B) Neural activity in SEF neurons on no-stop-signal trials and signal-inhibit trials [Data provided by J.D. Schall]
References
- 1.Logan GD. On the ability to inhibit thought and action: A user’s guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory and language. Academic; 1994. [Google Scholar]
- 2.Verbruggen F, Logan GD. Proactive adjustments of response strategies in the stop-signal paradigm. Journal of Experimental Psychology: Human Perception and Performance. doi: 10.1037/a0012726. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological Review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- 4.van den Wildenberg WPM, van der Molen MW. Developmental trends in simple and selective inhibition of compatible and incompatible responses. Journal of Experimental Child Psychology. 2004;87:201–220. doi: 10.1016/j.jecp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Williams BR, et al. Development of inhibitory control across the life span. Developmental Psychology. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- 6.Rush BK, et al. Accounting for cognitive aging: Context processing, inhibition or processing speed? Aging Neuropsychology and Cognition. 2006;13:588–610. doi: 10.1080/13825580600680703. [DOI] [PubMed] [Google Scholar]
- 7.Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nature Neuroscience. 2006;9:925–931. doi: 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- 8.Verbruggen F, et al. Short-term aftereffects of response inhibition: Repetition priming or between-trial control adjustments? Journal of Experimental Psychology: Human Perception and Performance. 2008;34:413–426. doi: 10.1037/0096-1523.34.2.413. [DOI] [PubMed] [Google Scholar]
- 9.Schachar RJ, et al. Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2004;32:285–293. doi: 10.1023/b:jacp.0000026142.11217.f2. [DOI] [PubMed] [Google Scholar]
- 10.Verbruggen F, Logan GD. Long-term aftereffects of response inhibition: Memory retrieval, task goals, and cognitive control. Journal of Experimental Psychology: Human Perception and Performance. doi: 10.1037/0096-1523.34.5.1229. in press. [DOI] [PubMed] [Google Scholar]
- 11.Emeric EE, et al. Influence of history on saccade countermanding performance in humans and macaque monkeys. Vision Research. 2007;47:35–49. doi: 10.1016/j.visres.2006.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verbruggen F, Logan GD. Automatic and controlled response inhibition: Associative learning in the go/no-go and stop-signal paradigms. Journal of Experimental Psychology: General. doi: 10.1037/a0013170. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander GE, et al. Basal Ganglia-Thalamocortical Circuits - Parallel Substrates for Motor, Oculomotor, Prefrontal and Limbic Functions. Progress in Brain Research. 1990;85:119–146. [PubMed] [Google Scholar]
- 14.Aron AR, et al. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. Journal of Neuroscience. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubia K, et al. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- 16.Li CSR, et al. Imaging response inhibition in a stop-signal task: Neural correlates independent of signal monitoring and post-response processing. Journal of Neuroscience. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chevrier AD. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Human Brain Mapping. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubia K. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior Cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nachev P, et al. The role of the pre-supplementary motor area in the control of action. Neuroimage. 2007;36:T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chambers CD, et al. Dissociable mechanisms of cognitive control in prefrontal and premotor cortex. Journal of Neurophysiology. 2007;98:3638–3647. doi: 10.1152/jn.00685.2007. [DOI] [PubMed] [Google Scholar]
- 22.Chambers CD. Executive "brake failure" following deactivation of human frontal lobe. Journal of Cognitive Neuroscience. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- 23.Aron AR, et al. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 24.Floden D, Stuss DT. Inhibitory control is slowed in patients with right superior medial frontal damage. Journal of Cognitive Neuroscience. 2006;18:1843–1849. doi: 10.1162/jocn.2006.18.11.1843. [DOI] [PubMed] [Google Scholar]
- 25.Vink M, et al. Function of striatum beyond inhibition and execution of motor responses. Human Brain Mapping. 2005;25:336–344. doi: 10.1002/hbm.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauggel S, et al. Inhibition of ongoing responses in patients with Parkinson’s disease. Journal of Neurology Neurosurgery and Psychiatry. 2004;75:539–544. doi: 10.1136/jnnp.2003.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rieger M, et al. Inhibition of ongoing responses following frontal, nonfrontal, and basal ganglia lesions. Neuropsychology. 2003;17:272–282. doi: 10.1037/0894-4105.17.2.272. [DOI] [PubMed] [Google Scholar]
- 28.Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: Effects of lesions of the medial striatum and d-amphetamine. Behavioral Neuroscience. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- 29.van den Wildenberg WPM, et al. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. Journal of Cognitive Neuroscience. 2006;18:626–636. doi: 10.1162/jocn.2006.18.4.626. [DOI] [PubMed] [Google Scholar]
- 30.Eagle DM, et al. Stop-signal reaction-time task performance: Role of prefrontal cortex and subthalamic nucleus. Cerebral Cortex. 2008;18:178–188. doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- 31.Mostofsky SH, Simmonds DJ. Response inhibition and response selection: Two sides of the same coin. Journal of Cognitive Neuroscience. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- 32.Coxon JP, et al. Intracortical inhibition during volitional inhibition of prepared action. Journal of Neurophysiology. 2006;95:3371–3383. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- 33.Coxon JP, et al. Selective inhibition of movement. Journal of Neurophysiology. 2007;97:2480–2489. doi: 10.1152/jn.01284.2006. [DOI] [PubMed] [Google Scholar]
- 34.Aron AR, Verbruggen F. Stop the presses: Dissociating a selective from a global mechanism for stopping. Psychological Science. doi: 10.1111/j.1467-9280.2008.02216.x. in press. [DOI] [PubMed] [Google Scholar]
- 35.Kok A, et al. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology. 2004;41:9–20. doi: 10.1046/j.1469-8986.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 36.Ridderinkhof KR, et al. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Geurts HM, et al. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? Journal of Child Psychology and Psychiatry. 2004;45:836–854. doi: 10.1111/j.1469-7610.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- 38.Enticott PG. Response inhibition and impulsivity in schizophrenia. Psychiatry Research. 2008;157:251–254. doi: 10.1016/j.psychres.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Lijffijt M, et al. A meta-analytic review of stopping performance in attention-deficit/ hyperactivity disorder: Deficient inhibitory motor control? Journal of Abnormal Psychology. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- 40.Chamberlain SR, et al. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. American Journal of Psychiatry. 2006;163:1282–1284. doi: 10.1176/ajp.2006.163.7.1282. [DOI] [PubMed] [Google Scholar]
- 41.Schachar RJ, et al. Inhibition of motor responses in siblings concordant and discordant for attention deficit hyperactivity disorder. American Journal of Psychiatry. 2005;162:1076–1082. doi: 10.1176/appi.ajp.162.6.1076. [DOI] [PubMed] [Google Scholar]
- 42.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: Relevance for genetic research in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 43.Kieling C, et al. Neurobiology of attention deficit hyperactivity disorder. Child and Adolescent Psychiatric Clinics of North America. 2008;17:285–307. doi: 10.1016/j.chc.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Bekker EM, et al. Disentangling deficits in adults with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2005;62:1129–1136. doi: 10.1001/archpsyc.62.10.1129. [DOI] [PubMed] [Google Scholar]
- 45.Liotti M, et al. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41:377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- 46.Rubia K, et al. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. American Journal of Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- 47.Pliszka SR, et al. Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. American Journal of Psychiatry. 2006;163:1052–1060. doi: 10.1176/ajp.2006.163.6.1052. [DOI] [PubMed] [Google Scholar]
- 48.Goudriaan AE, et al. Neurocognitive functions in pathological gambling: a comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction. 2006;101:534–547. doi: 10.1111/j.1360-0443.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- 49.Menzies L, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–3236. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- 50.Woolley J, et al. Brain activation in paediatric obsessive-compulsive disorder during tasks of inhibitory control. British Journal of Psychiatry. 2008;192:25–31. doi: 10.1192/bjp.bp.107.036558. [DOI] [PubMed] [Google Scholar]
- 51.Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug and Alcohol Dependence. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- 52.Monterosso JR. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Nigg JT. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- 54.Schall JD, Boucher L. Executive control of gaze by the frontal lobes. Cognitive Affective & Behavioral Neuroscience. 2007;7:396–412. doi: 10.3758/cabn.7.4.396. [DOI] [PubMed] [Google Scholar]
- 55.Hanes DP. Role of frontal eye fields in countermanding saccades: Visual, movement, and fixation activity. Journal of Neurophysiology. 1998;79:817–834. doi: 10.1152/jn.1998.79.2.817. [DOI] [PubMed] [Google Scholar]
- 56.Pare M, Hanes DP. Controlled movement processing: Superior colliculus activity associated with countermanded saccades. Journal of Neuroscience. 2003;23:6480–6489. doi: 10.1523/JNEUROSCI.23-16-06480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ito S, et al. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- 58.Stuphorn V, et al. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- 59.Emeric EE, et al. Performance monitoring local field potentials in the medial frontal cortex of primates: Anterior cingulate cortex. Journal of Neurophysiology. 2008;99:759–772. doi: 10.1152/jn.00896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boucher L, et al. Stopping eye and hand movements: Are the processes independent? Perception & Psychophysics. 2007;69:785–801. doi: 10.3758/bf03193779. [DOI] [PubMed] [Google Scholar]
- 61.Logan GD, Irwin DE. Don’t look! Don’t touch! Inhibitory control of eye and hand movements. Psychonomic Bulletin & Review. 2000;7:107–112. doi: 10.3758/bf03210728. [DOI] [PubMed] [Google Scholar]
- 62.Curtis CE, et al. Canceling planned action: An fMRI study of countermanding saccades. Cerebral Cortex. 2005;15:1281–1289. doi: 10.1093/cercor/bhi011. [DOI] [PubMed] [Google Scholar]
- 63.Band GPH, et al. Horse-race model simulations of the stop-signal procedure. Acta Psychologica. 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- 64.Boucher L, et al. Inhibitory control in mind and brain: An interactive race model of countermanding saccades. Psychological Review. 2007;114:376–397. doi: 10.1037/0033-295X.114.2.376. [DOI] [PubMed] [Google Scholar]
- 65.Verbruggen F, et al. The interaction between stop signal inhibition and distractor interference in the flanker and Stroop task. Acta Psychologica. 2004;116:21–37. doi: 10.1016/j.actpsy.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 66.Verbruggen F, et al. Effects of stimulus-stimulus compatibility and stimulus-response compatibility on response inhibition. Acta Psychologica. 2005;120:307–326. doi: 10.1016/j.actpsy.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 67.Friedman NP, Miyake A. The relations among inhibition and interference control functions: A latent-variable analysis. Journal of Experimental Psychology-General. 2004;133:101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- 68.Aron AR, et al. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 69.Derrfuss J, et al. Cognitive control in the posterior frontolateral cortex: Evidence from common activations in task coordination, interference control, and working memory. Neuroimage. 2004;23:604–612. doi: 10.1016/j.neuroimage.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 70.Xue G, et al. Common neural substrates for inhibition of spoken and manual responses. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhm220. [DOI] [PubMed] [Google Scholar]
- 71.Leung HC, Cai WD. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. Journal of Neuroscience. 2007;27:9893–9900. doi: 10.1523/JNEUROSCI.2837-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Camalier CR. Dynamics of saccade target selection: Race model analysis of double step and search step saccade production in human and macaque. Vision Research. 2007;47:2187–2211. doi: 10.1016/j.visres.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verbruggen F, et al. How to stop and change a response: The role of goal activation in multi-tasking. Journal of Experimental Psychology: Human Perception and Performance. doi: 10.1037/0096-1523.34.5.1212. in press. [DOI] [PubMed] [Google Scholar]
- 74.Verbruggen F, et al. STOP-IT: Windows executable software for the stop-signal paradigm. Behavior Research Methods. 2008;40:479–483. doi: 10.3758/brm.40.2.479. [DOI] [PubMed] [Google Scholar]