Abstract
We used shotgun proteomics to identify plasma membrane and lipid raft proteins purified from B cells obtained from mantle cell lymphoma (MCL) patients in leukemic phase. Bioinformatics identified 111 transmembrane proteins, some of which were profiled in primary MCL cases, MCL-derived cell lines, and normal B cells using RT-PCR and Western blotting. Several transmembrane proteins, including CD27, CD70, and CD31 (PECAM-1), were overexpressed when compared with normal B cells. CD70 was up-regulated (>10-fold) in three of five MCL patients along with its cognate receptor CD27, which was up-regulated (4–9-fold) in five of five patients, suggesting that MCL cells may undergo autocrine stimulation via this signaling pathway. Activated calpain I and protein kinase C βII were also detected in the plasma membranes, suggesting that these proteins are constitutively active in MCL. Protein kinase C βII has been associated with lipid rafts, and shotgun proteomics/protein profiling revealed that key lipid raft proteins, raftlin (four of five patients) and CSK (C-terminal Src kinase)-binding protein (Cbp)/phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG) (four of four patients) were down-regulated in MCL. Levels of other known lipid raft proteins, such as Lyn kinase and flotillin 1, were similar to normal B cells. However, 5-lipoxygenase (5-LO), a key enzyme in leukotriene biosynthesis, was associated with lipid rafts and was up-regulated ∼7-fold in MCL compared with normal B cells. Significantly inhibitors of 5-LO activity (AA861) and 5-LO-activating protein (FLAP) (MK886, its activating enzyme) induced apoptosis in MCL cell lines and primary chronic lymphocytic leukemia cells, indicating an important role for the leukotriene biosynthetic pathway in MCL and other B cell malignancies. Thus, using shotgun proteomics and mRNA and protein expression profiling we identified a subset of known and unknown transmembrane proteins with aberrant expression in MCL plasma membranes. These proteins may play a role in the pathology of the disease and are potential therapeutic targets in MCL.
Mantle cell lymphoma (MCL)1 accounts for ∼5% of adult non-Hodgkin lymphoma in the United States and Europe and is characterized by malignant transformation of the mantle zone cells surrounding the germinal centers. This B cell non-Hodgkin lymphoma has a poor prognosis and a median survival time of ∼3–5 years (1). It predominantly affects older, male adults, and at the time of diagnosis the majority of patients have malignant cell invasion of spleen, bone marrow, and particularly the gastrointestinal tract (2). As MCL responds poorly to therapy, there is a need to develop new or improved therapeutic strategies (3). MCL is characterized by the t(11;14)(q31;q32) translocation resulting in the up-regulation of cyclin D1 (4), although cyclin D1 overexpression alone cannot induce lymphoma (5). Gene expression profiling of mantle cell lymphoma has identified differentially expressed genes involved in apoptosis, cell cycle, and metastasis that may contribute to its distinctive pathology (6–8). Although RNA microarray studies of many diseases have identified gene markers that may be valuable as prognostic or diagnostic tools, there are very few studies that have validated and correlated the gene and protein expression data. Such information is necessary for developing rational mechanism-based therapy and also identifying potential surface membrane or antigen proteins that could be potential therapeutic targets. Significantly several studies have shown a discordant correlation between mRNA transcript and protein levels (9, 10). For example during cell stimulation or differentiation the correlation between the differential expression of mRNA and protein expression is no greater than 40% (11), and during stem cell differentiation, 46% of 145 changes observed in protein expression were not detected at the mRNA level (12).
Many studies have only compared global mRNA and protein expression in whole cells, and this approach ignores the effects of translational regulation, post-translational processing, and protein degradation. For plasma membrane-associated proteins additional factors such as internalization, recycling, and post-translocation modifications can also affect protein localization in the plasma membrane. Consequently membrane proteins can show an even greater discordance between mRNA transcript and protein levels; for example, in a human mesenchymal stem cell line undergoing osteoblast differentiation, there was no correlation (except for alkaline phosphatase) between the gene and protein expression (13). Similarly in chronic lymphocytic leukemia (CLL) and multiple myeloma, although there was good correlation between gene and protein expression of CD19, CD20, CD23, and CD138 cell surface markers, other proteins including immunoglobulin light chain, CD38, and CD79b (CLL) and CD45 and CD52 (multiple myeloma) showed no correlation between gene and protein expression (14).
To understand the pathogenic mechanisms underlying MCL it is important to characterize the protein differences at a cellular and more specifically at a plasma membrane level. Thus, two-dimensional gel electrophoresis has been used to compare the protein profiles of lymph node tissue in MCL patients and normal control samples (15). A protein (antibody) microarray study compared proteins in CD19+ purified B cells obtained from normal tonsils and histologically confirmed MCL patients (16). This approach identified proteins that had not been identified by earlier gene expression profiling approaches. However, protein microarrays are limited by the size and composition of the antibody array, and in this respect, mass spectrometry has an advantage as it can unequivocally identify both known and novel proteins. Furthermore mass spectrometry coupled with cell fractionation and protein purification techniques enables a functional proteomics approach to be applied in which protein expression can be linked directly to biochemical/pathological changes in the affected cells or tissue.
In the current study we focused on characterizing the differential expression of proteins in the MCL plasma membrane as compared with normal B cells and also identifying novel MCL plasma membrane proteins with novel defined as either (i) a protein hitherto unknown in B cells, (ii) a protein identified within genomics databases but uncharacterized, or (iii) a known protein not normally believed to be associated with the plasma membrane. Therefore, we purified plasma membranes from B cells isolated from MCL patients in leukemic phase of disease (to derive sufficient numbers of purified MCL B cells). The plasma membrane proteins were then separated by 1D SDS-PAGE and identified by LC-MS/MS shotgun proteomics. This approach overcomes the limitations of using two-dimensional gels for membrane proteins and also provides information on post-translational modifications. Shotgun proteomics can be used to estimate the abundance of proteins in complex mixtures as the number of spectra (spectral counts) identified for each protein varies according to relative protein abundance within the sample (17). The spectral count technique has been used, for example, in studies to identify and compare protein and mRNA transcript changes (18). Using this approach we identified 423 proteins, including 111 transmembrane proteins, and we used immunoblotting and RT-PCR to profile selected proteins in normal and MCL-derived B cells. Significant changes were detected in a number of proteins associated with plasma membranes and lipid raft domains of MCL cells. Some of these proteins may be potential targets for therapy, and for example one protein, 5-lipoxygenase (5-LO), was markedly overexpressed in MCL cells and cell lines. Significantly inhibitors of 5-LO and the leukotriene biosynthesis pathway are potent inducers of apoptotic cell death of malignant B cells, suggesting a new therapeutic approach.
EXPERIMENTAL PROCEDURES
Cell Purification and Cell Lysate Preparation
MCL peripheral blood samples were derived from patients attending the CLL clinic at Leicester Royal Infirmary after ethics committee review and after obtaining informed, written consent. Five patients with lymphocyte counts greater than 50 × 106/ml were selected for this study to minimize possible contamination of tumor cells by normal hemopoietic cells. Clinical characteristics of these patients are detailed in supplemental Table 1, and confirmation of MCL diagnosis was obtained by immunophenotyping, metaphase cytogenetics, interphase fluorescence in situ hybridization, and high expression of cyclin D1. MCL leukemic cells were purified as described previously (19) to a purity of >95% as determined by flow cytometry. Purified CD19+ B cells from normal individuals were purchased from Yorkshire Bioscience (York, UK). MCL-derived cell lines and their characteristics have been described previously (20) and were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ). Cell line cultures were grown in RPMI 1640 medium, 10% fetal calf serum, and 2 mm GlutaMAX™. Whole cell lysates of MCL cells or CD19+ selected lymphocytes were prepared in 1D lysis buffer without reducing agent, glycerol, and bromphenol blue (62.5 mm Tris-HCl, pH 6.8, 2% (w/v) SDS) and heated for 5 min at 95 °C. The whole cell lysate was centrifuged at 100,000 × g for 1 h, and the protein was assayed using the BCA™ protein assay kit (Pierce).
Cell Fractionation and Plasma Membrane Preparation
Cells were fractionated, and plasma membranes were purified as described previously (21) in buffers containing 0.5 ml of protease inhibitor mixture (Sigma-Aldrich) in 20 ml of purified MCL cells (109), which were washed three times in PBS, resuspended (1 × 109 cells/20 ml) in homogenization buffer (0.25 m sucrose, 10 mm HEPES, pH 7.4, 1 mm EDTA, 0.02% sodium azide), and disrupted with a ball-bearing homogenizer (Isobiotec, Heidelberg, Germany). Cell membranes/nuclei were removed by sequential centrifugation at 1000 × g for 10 min and 3000 × g for 10 min, and the postnuclear supernatant was layered onto a 2-ml (60%, w/v) sucrose cushion before centrifuging at 100,000 × g for 1 h. The floating crude plasma membranes were harvested and adjusted to 15% (w/v) sucrose in 10 mm HEPES, pH 7.4 buffer and then purified by sucrose density centrifugation (21). Aliquots from the fractionated sucrose gradient were separated by 4–20% 1D SDS-PAGE (Invitrogen) and analyzed by Western blotting for plasma membrane marker proteins. Fractions containing CD20 and transferrin receptor (plasma membrane markers) but not oxidoreductase II (mitochondrial) or calnexin (endoplasmic reticulum) were pooled and constitute the plasma membrane fraction.
Lipid Raft Generation
Lipid rafts were prepared from 100 × 106 cells that were solubilized in 1 ml of 1% Triton X-100, 1 mm EDTA, 50 mm Tris-HCl, pH 7.4 on ice for 30 min and purified by sucrose density gradient purification (22). The whole cell lysate was diluted with 80% sucrose (in homogenization buffer) to a final concentration of 40% sucrose (4 ml) and was overlaid with 30% (5 ml) and 5% (4 ml) sucrose buffer (without Triton X-100). The discontinuous sucrose gradient was centrifuged for 17 h at 100,000 × g at 4 °C, and lipid rafts were harvested from the interphase of the 5 and 30% sucrose bands and centrifuged at 100,000 × g for 1 h at 4 °C. The lipid raft pellet was then solubilized in SDS sample buffer and analyzed as described below.
Shotgun Proteomics
Solubilized plasma membrane fractions were separated by 4–20% 1D SDS-PAGE and stained with colloidal Coomassie Blue, and the gel lanes were sequentially sliced into 1 × 3-mm sections covering a molecular mass range between 20 and 200 kDa. Gel slices were subjected to in-gel tryptic digestion using an In-gel Digest ZP kit (Millipore). Tandem electrospray spectra were obtained using a Q-TOF hybrid quadrupole/orthogonal acceleration TOF spectrometer (Waters) interfaced to a CapLC chromatography system (Waters). Sample digests dissolved in 5% formic acid were applied (6 μl) to a 3-μm Atlantis™ dC18 column (Waters). Mass spectrometry data were acquired using MassLynx 4.0 with automatic precursor ion selection of doubly and triply charged ions. The acquired mass spectra were processed by ProteinLynx Global Software 2.2, and data files (.pkl format) were directly submitted to the MASCOT search engine for MS/MS ion database searching. The database searched was the non-redundant protein sequence database UniProtKB/Swiss-Prot (release 52.5 with a total of 267,354 sequence entries). The parameters used for MASCOT searches were as follows: up to three missed cleavages; 0.4-Da mass accuracy for the parent and fragment ions, respectively; and variable oxidization of the methionine (variable modification). Scaffold (Proteome Software Inc., Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95% probability as specified by the Peptide Prophet algorithm (23). Protein identifications were accepted if they could be established at greater than 90% probability and contained at least two identified peptides with MASCOT scores of >35. In some cases where the protein was identified by a single peptide with a MASCOT score >30 the spectrum was interpreted manually. For quantitative purposes, protein abundance was assessed by calculating the spectral count values for each protein as described earlier (17). In this analysis the spectral count is defined as the total number of spectra identified from a protein with the proviso that each peptide had a MASCOT score >30. In addition MS/MS spectra were also searched against a corresponding random sequence (decoy) database (MASCOT), and the false discovery rate (FDR = number of validated decoy hits/(number of validated target hits + number of decoy hits) × 100)) calculated for all samples was <2%. Significant proteins or proteins of special interest were validated further by expression profiling using RT-PCR and immunoblotting (see below).
Western Blot Analysis
Aliquots (10 μg) of cell lysate or plasma proteins were separated by 4–20% 1D SDS-PAGE, transferred onto nitrocellulose membranes, and probed with the appropriate antibodies before detection by enhanced chemiluminescence (ECL or ECL plus Western blotting Detection Reagent, GE Healthcare, Amersham Biosciences) as described previously (19). Primary antibodies were used at the following dilutions: anti-actin (1:1000) from Sigma-Aldrich; anti-transferrin (1:1000) from Zymed Laboratories Inc.; anti-oxidoreductase II (1:2000) and anti-calnexin (1:1000) from Affinity Bioreagents (Cambridge Bioscience, Cambridge, UK); anti-PKCβ phospho-Thr-500 (1:1000), anti-PAG (MEM-255) (1:1000), and anti-PAG (ab14989) (1:1000) from Abcam PLC (Cambridge, UK); anti-CD22 (1:1000), anti-CD31 (1:1000) and anti-CD27 (1:1000) from R&D Systems Europe Ltd. (Abingdon, Oxfordshire, UK); anti-cyclin D1 (1:200) from NeoMarkers (Stratech, Newmarket, Suffolk, UK); anti-Lyn (1:2000), anti-flotillin 1 (1:1000), and anti-PKCβ (both isoforms) (1:1000) from BD Biosciences; anti-CD27L (1:500), anti-PKCβI (1:1000), anti-PKCβII (1:1000) from Santa Cruz Biotechnology; and anti-CD20 (1:1000) from Dako UK (Ely, Cambridgeshire, UK). Anti-raftlin antibody (1:1000) was a kind gift provided by Dr. A Yoshimura, Division of Molecular and Cellular Immunology, Medical Institute of Bioregulation, Kyushu University, Fukuoka, Japan. Additional antibodies obtained from other sources are as indicated in the legends to the figures. For normalization purposes, actin was used as the loading control. The blots were detected with ECL using multiple film exposures and densitometry to determine the volume of each band (arbitrary units) using ImageQuant TL software (GE Healthcare). The samples were then normalized using their respective actin loading control.
Analysis of Apoptosis
Apoptotic cell death in MCL cell lines and primary CLL cells was assessed by flow cytometry of annexin V-fluorescein isothiocyanate/propidium iodide-stained cells as described previously (24). Cells with increased annexin V binding to phosphatidylserine (PS+) were analyzed using a BD FACSCalibur flow cytometer and CellQuest software (BD Biosciences). Additional experiments were carried out using the DNA dye Hoechst 33342 to stain normal and apoptotic nuclei in permeabilized cells. Images were collected and analyzed by confocal microscopy on a Zeiss LSM510 with Axiovert 200 microscope and 405 nm laser. Samples for Western blot analysis of caspase-9 and caspase-3 activation were processed and run as described previously (24).
Real Time RT-PCR Quantification of mRNA for Selected Membrane-associated Proteins
RNA was prepared from cultured cells or freshly isolated cells using the RNeasy minikit (Qiagen). RNA was quantitated by absorbance (260/280 nm), and purity was assessed by gel electrophoresis. The SuperScript III first strand synthesis system (Invitrogen) was used to synthesize cDNA from RNA by extension with random hexamer primers and reverse transcriptase. PCR primers were designed with primer express software (Applied Biosystems), and where possible, amplicons that span over two or more exons were selected. The PCR primers used were as follows: Cbp/PAG, sense 5′-GCCGTCCGCCAGGAA-3′ and antisense 5′-CCAACTGCTGAAATAGGAGAAAAAT-3′;PKC-βII, sense 5′-TTGTGGGCGAAATGCTGAA-3′ and antisense 5′-CTGGTCGGGAGGTGTTAGGA-3′;PKC-βI, sense 5′-AGCCAAGCGTATGTATCAATTCTAGTC-3′ and antisense 5′-GACAAGCTTTCCACATGTTGAATG-3′;CD70, sense 5′- TCTGCCTCGTGGTGTGCAT-3′ and antisense 5′-GCTGAGGTCCTGTGTGATTCAG-3′; CD27, sense 5′-CGGCACTGTAACTCTGGTCTTC-3′ and antisense 5′-CGACAGGCACACTCAGCATT-3′; Cyclin D1, sense 5′-CCGTCCATGCGGAAGATC-3′ and antisense 5′-GAAGACCTCCTCCTCGCACT-3′; Raftlin, sense 5′-GGACTTCACACTAAATGATGCT-3′ and antisense 5′-GAGCTGGAAGATCACGCAAAG-3′. PCRs containing cDNA, SYBR Green sequence detection reagents (PE Biosystems), Taq polymerase, and sense and antisense primers were assayed on an ABI7000 sequence detection system (PE Biosystems). The PCR conditions were one cycle at 50 °C for 2 min, one cycle at 95 °C for 10 min, and 40 cycles at 95 °C for 15 s and 60 °C for 1 min. PCR products were measured in real time by the increase in SYBR Green fluorescence, and data were analyzed using the Sequence Detector program (PE Biosystems). Relative amounts of mRNA in two samples, for instance normal B lymphocytes and MCL cells, were quantified using the comparative Ct method (25). The TATA box-binding protein was used as the housekeeping gene, and the amplification efficiencies of the target and TATA box-binding protein genes were approximately equal.
RESULTS
Identification of Transmembrane Proteins in MCL Plasma Membranes
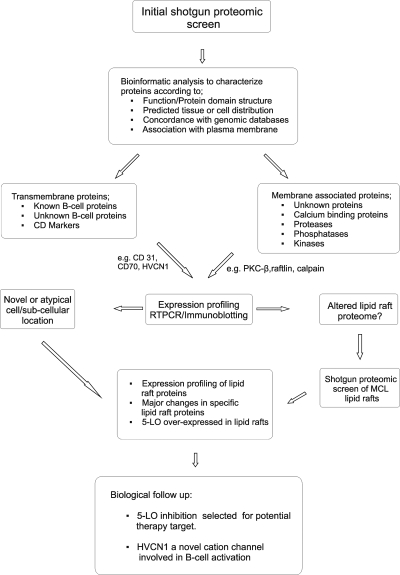
A major aim of this study was to identify previously uncharacterized cell surface proteins in MCL plasma membranes. Using shotgun proteomics we identified extrinsic and intrinsic proteins in plasma membranes purified from primary B cells obtained from two patients (MCL-P2 and MCL-P5) and from two MCL-derived cell lines (Z138 and JVM2). In this initial screen, a total of 423 proteins were identified, and from this global list, several proteins were selected for expression profiling using Western blotting and RT-PCR. Proteins were selected for expression profiling and further analysis according to the criteria described in Fig. 1. Potential transmembrane and plasma membrane-associated proteins were identified using in silico analysis of cell or tissue expression (26) and lymphoid specific-cell expression of mRNA in microarray data from naïve B lymphocyte and MCL cells (6, 27).
Fig. 1.
Flow chart detailing how proteins were selected for expression profiling and further follow-up studies. The flow chart details how selected proteins were identified, characterized, and selected for further studies using bioinformatics analysis to characterize the proteins in terms of structure and cellular and tissue localization (see also Fig. 2).
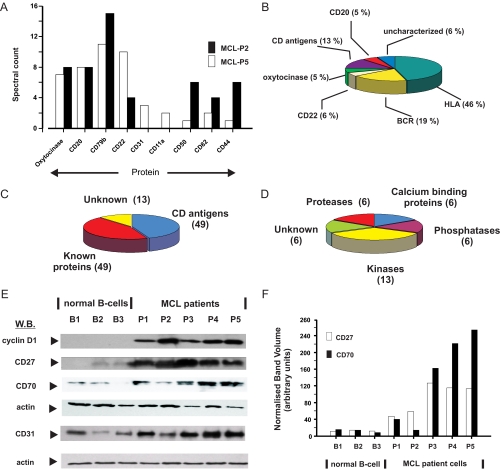
The spectral count values of selected proteins that were identified in patient plasma samples are shown in Fig. 2A. For example, CD20, CD22, and CD79a/CD79b are abundant proteins in B cell plasma membranes and were readily identified by LC-MS/MS in both patient samples with high spectral counts (Fig. 2A). Other less abundant cell surface proteins (e.g. CD82 and CD44) were also identified with correspondingly lower scores (Fig. 2A). The spectral count data also give a good estimate of the relative abundance of the individual proteins or class of proteins (Fig. 2B). The B cell receptor (BCR) and cell surface membrane and HLA proteins were all highly expressed and identified by LC-MS/MS. Atypically and of potential interest, oxytocinase appeared to be a highly abundant protein in MCL plasma membranes as it was detected by LC-MS/MS (with high spectral counts) in both patient samples (Fig. 2A). Highly abundant proteins such as oxytocinase, CD22, and CD20 accounted for 16% of the total number of peptides identified (Fig. 2B).
Fig. 2.
Identification and characterization of transmembrane and membrane-associated proteins in MCL plasma membranes. MCL plasma membranes from two patients, MCL-P2 and MCL-P5, and two MCL cell lines, JVM2 and Z138, were analyzed using shotgun proteomics. A, the abundance of selected proteins was assessed by determining the spectral count for each protein as described under “Experimental Procedures.” B, the relative abundance of individual and membrane protein types in the MCL-P5 patient plasma membrane preparation is shown as a pie chart. The abundance of each protein/class was calculated by dividing individual scores by the total number of identified spectra obtained for all proteins with a known or predicted transmembrane region. Using the criteria listed under “Experimental Procedures” we identified 111 transmembrane proteins that were subcharacterized as shown (C) and 37 peripheral proteins that were classified according to known enzymic activity (D). E, selected proteins were profiled by Western blotting (W.B.) with specific antibodies as described under “Experimental Procedures.” CD70, CD27, CD22, cyclin D1, and actin (loading control) expression are shown for B cells isolated from normal subjects (B1–B3) and from five MCL patients (P1–P5). F, CD27 and CD70 immunoblots of B cells isolated from normal subjects and MCL cells were quantitated by densitometry with the band volumes (arbitrary units) normalized to their respective actin loading controls. The mean ± S.E. for CD27 and CD70 for the normal B cells (n = 3) was 12.3 ± 0.6 and 12.52 ± 2.3, respectively. The corresponding values for CD27 and CD70 in the MCL patient cells (n = 5) were 92.2 ± 16.3 and 138.4 ± 47.9, respectively.
From the initial list of proteins, we selected 111 transmembrane proteins that were characterized further by bioinformatics analysis and subdivided into CD and known and unknown (previously or not previously identified in B cells, for e.g. HVCN1) transmembrane proteins, including HLA class I, HLA class II, and cell surface immunoglobulins (Fig. 2C and supplemental Table 2). HLA class proteins were not analyzed further. Cyclin D1 was used as a signature MCL marker protein, and as expected this was very high in all five MCL patients but was virtually undetectable in the normal B cells (Fig. 2E). A large number of CD marker proteins were identified, and representative proteins were expression-profiled in MCL whole cell lysates and compared with control, peripheral B cells that were isolated from normal, healthy age-matched patients.
Thus, for example CD70 was detected by LC-MS/MS in MCL cell line plasma membranes (supplemental Table 1) and is a type II transmembrane glycoprotein expressed on a small subset of circulating B cells (28). Western blotting with an antibody raised against the N terminus of CD70 revealed that CD70 was up-regulated in primary MCL cells in four of five patients when compared with normal CD19+ B cells (Fig. 2E). Densitometric quantitation using actin as a loading control showed that there was an approximately 3- to >10-fold increase in CD70 content in MCL cells compared with normal B cells (Fig. 2F). Analysis of MCL and normal B cell RNA also showed that CD70 mRNA levels were markedly up-regulated in agreement with the increased protein expression (data not shown). CD27 is the receptor for CD70 and has been reported previously to be up-regulated in MCL cells (29); we confirmed this by Western blotting, which detected CD27 in MCL membranes migrating with an apparent molecular mass of 50–55 kDa on SDS-PAGE (Fig. 2E). CD27 was up-regulated (4–9-fold) in primary MCL cells compared with normal B cells (Fig. 2F), and RT-PCR analysis also showed a good correlation between the increase in CD27 mRNA levels and protein expression (data not shown). In conclusion both CD27 receptor and CD70 ligand were strongly up-regulated in four of five MCL patients.
CD31 (PECAM-1), which was identified in a gel slice corresponding to a molecular mass of approximately 140 kDa (Fig. 2A and supplemental Table 2), is a transmembrane member of the immunoglobulin superfamily that is expressed in normal B cell subpopulations (30). We therefore profiled the expression of CD31, which was detected by Western blotting in both normal B and MCL patient cells. CD31 was up-regulated in three of five of the MCL patient cells as compared with the normal B cells (Fig. 2E). CD70 and CD31 showed a similar pattern of up-regulation in that both these CD markers were strongly up-regulated in patients 3, 4, and 5.
Other proteins of interest were also identified as transmembrane proteins that hitherto had not been detected in either B or MCL cells. Thus, TRPV2 and HVCN1 are ion channels with unknown function in B cells. TRPV2 was identified in Z138 plasma membranes by LC-MS/MS of tryptic digests of gel slices corresponding to a molecular mass of >150 kDa (supplemental Table 2). TRPV2 is a calcium-permeable, non-selective cation channel that has not been reported to be expressed in B cells before, although gene expression analysis indicated that it was also expressed in other hematopoietic cells such as T cells, monocytes, and dendritic cells (26). Finally HVCN1 (MGC15619; supplemental Table 2) was detected in MCL-P2 plasma membranes, and expression profiling using RT-PCR data showed similar levels of mRNA for HVCN1 in both normal B and MCL cells, whereas T cells and monocytes had little or no mRNA expression (data not shown). The exact function of HVCN1 is unknown, although recent reports have characterized the HVCN1 protein as a voltage-gated proton-selective channel with high expression in lymph node tissues (31). The role of HVCN1 in MCL and B cells is being investigated, and ongoing experiments show that HVCN1 co-localizes with the B cell receptor and is involved in class switch recombination (32).2
Aberrant Localization of Proteins in the MCL Plasma Membranes
In addition to the transmembrane proteins we also identified 37 peripheral membrane-associated proteins that were subcategorized as phosphatases, kinases, calcium-binding proteins, proteases, and unknown proteins (Fig. 2D and supplemental Table 3). Calpain 1 for example is a protein that upon activation is transported to the plasma membrane where it facilitates actin cytoskeleton reorganization (33, 34). Calpain 1 was detected in MCL-P5 plasma membranes by LC-MS/MS mass spectrometry (supplemental Table 2). The calpain 1 proenzyme (80 kDa) undergoes calcium-dependent autoproteolysis to remove the first 26 N-terminal amino acids to yield the processed enzyme (76 kDa). Formation of the 76-kDa subunit is specifically carried out by active calpain, and its presence can be used to confirm calpain activation. Using immunoblotting we detected the 76-kDa active calpain subunit in MCL plasma membranes, whereas both the 80-kDa proform and the 76-kDa form were detected in whole cell lysates from all five patients (data not shown). Interestingly in three normal B cell lysates that were analyzed only the 80-kDa proenzyme was detected.
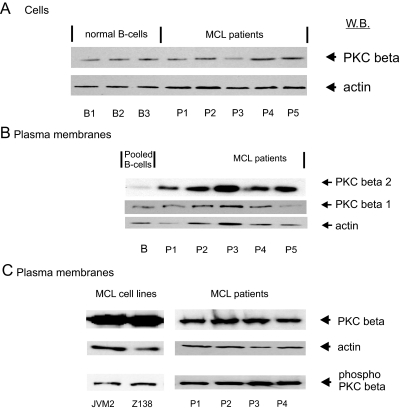
PKCβ is another enzyme that on activation is translocated to the plasma membrane and was one of the most abundant of the 13 kinases identified by LC-MS/MS (supplemental Table 2). Expression profiling carried out using a specific PKCβ antibody and an anti-phospho-PKCβ antibody (Thr-500) showed that PKCβ was phosphorylated and catalytically competent (data not shown). Overall cellular levels of levels of PKCβ were similar in normal B and MCL primary cells (Fig. 3A). However, isoform-specific antibodies to PKCβI and -II showed the PKCβII isoform was specifically elevated (approximately 8-fold) in the plasma membrane of primary MCL cells as compared with normal B cells (Fig. 3B). Furthermore immunoblotting with the anti-phospho-PKCβ antibody showed that PKCβ associated with the plasma membrane in both MCL cell lines and primary cells was in the phosphorylated active form (Fig. 3C). Thus, the change in PKCβII at the MCL plasma membrane (Fig. 3B) appears to be due to an increase in translocation/association of the active phosphorylated kinase to the plasma membrane.
Fig. 3.
Protein kinase C β immunoreactivity in normal B lymphocytes and MCL whole cell lysates or cellular fractions. In A, the PKCβ (I and II isoforms) content of MCL primary (P1–P5) and normal B cells (B1–B3) is shown. In B, plasma membrane fractions isolated from MCL cell lines and primary cells were immunoblotted for the specific PKCβ isoforms I and II. In C, the plasma membranes from MCL cell lines and primary cells were immunoblotted with a phosphospecific antibody to PKCβ. W.B., Western blotting.
Characterization of the MCL Lipid Raft Proteome
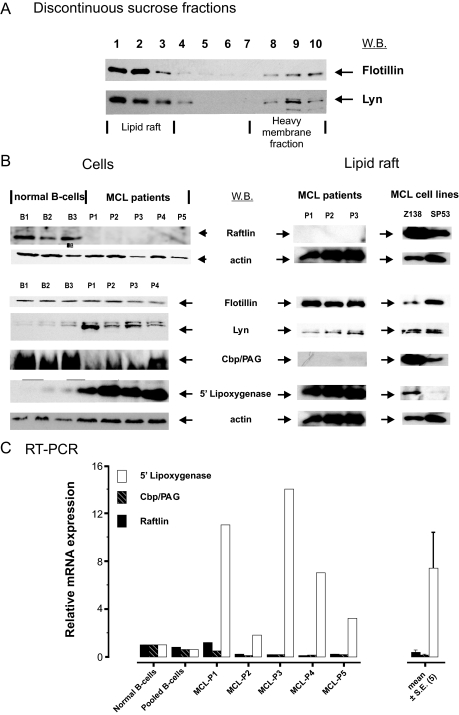
Activated PKCβII has been reported to be recruited to lipid rafts during BCR signaling and to control IκB kinase lipid raft recruitment and activation (35). C-terminal Src kinase (CSK) is another kinase associated with lipid rafts (36), and interestingly we also identified raftlin (not listed in the defined categories shown supplemental Table 3), which is reported to be a B cell-specific lipid raft protein (22). These findings and the fact that activated PKCβII was detected in the plasma membrane indicated that in MCL the protein composition and functioning of lipid rafts was altered. To investigate this possibility we purified lipid rafts from MCL primary cells and cell lines by sucrose density centrifugation. The fractionated gradients were immunoblotted for known constitutive lipid raft proteins, Lyn kinase, and flotillin 1 (36), which sedimented in fractions 1, 2, and 3, the interphase segment of the discontinuous 5–30% sucrose gradient (Fig. 4A). Similar preparations were then obtained from the indicated MCL primary cells and cell lines and analyzed by LC-MS/MS and shotgun proteomics. Over 100 proteins were detected by mass spectrometry (supplemental Table 4), although surprisingly PKCβII was not detected, indicating that the increased amounts of PKCβII associated with the plasma membrane was not due to its recruitment to lipid rafts.
Fig. 4.
Altered protein composition in lipid rafts obtained from mantle cell plasma membranes. In A, Western blotting (W.B.) was used to confirm the distribution of flotillin 1 and Lyn in MCL P1 lipid rafts that had been purified by sucrose density gradient purification of Triton X-100 cell lysates as described under “Experimental Procedures.” The discontinuous sucrose gradient was fractionated as indicated, and fractions 1, 2, and 3 represent regions in the density gradient where lipid rafts should be found. This method was then used to purify lipid rafts from MCL cell lines and MCL patient cells, and the lipid rafts were separated by SDS-PAGE and analyzed by shotgun proteomics as described in Fig. 1. Selected proteins that are typical of lipid rafts are detailed in Table I. In B, Western blotting was used to compare the expression of raftlin, flotillin, Lyn, Cbp/PAG, 5-lipoxygenase, and actin in normal B cell (B1–B3) and MCL patient (P1–P5) whole cell lysates (Cells). Lipid rafts prepared from three patients and two MCL cell lines were also immunoblotted for these proteins. In C, RT-PCR was used to compare the mRNA expression of raftlin, 5-LO, and Cbp/PAG in normal B cells and cells derived from five MCL patients. Results are shown as mean ± S.E. (n = 5).
However, characterization of the MCL lipid raft proteome by mass spectrometry analysis identified Lyn kinase, flotillin, ezrin, Cbp/PAG, CSK, and raftlin (Table I), which are known constituents of lipid rafts (36, 37). Raftlin, which was detected in Z138 and JVM2 cell lines (Table I), is a myristoylated lipid raft B cell-specific associated protein and is required for the integrity of lipid rafts and BCR signal transduction (22). However, unlike the lipid rafts prepared from the cell lines, raftlin peptides were not detected in lipid raft preparations derived from patient cells (Table I). This suggested that primary MCL cells have little or no raftlin present in the lipid raft fractions, and this was confirmed by Western blotting of total cell lysates obtained from normal B and primary MCL cells. Thus, raftlin was detected as an 85-kDa protein in normal B cell preparations but not in the patient MCL samples (Fig. 4B). Furthermore although raftlin was strongly detected in lipid raft fractions isolated from MCL cell lines, it was difficult to detect in lipid rafts isolated from primary (three patients) MCL cells (Fig. 4B). Thus, the immunoblotting results correlated with the LC-MS/MS data and confirmed that primary MCL cells are deficient in raftlin, which is abundant in normal B cells and MCL cell lines. Furthermore RT-PCR analysis revealed that raftlin mRNA in MCL patients was strongly down-regulated (≅6-fold) in four of five MCL patient samples as compared with normal B cells (Fig. 4C). One patient (MCL-P1) exhibited normal levels of raftlin mRNA.
Table I. Membrane-associated proteins identified in MCL lipid rafts.
Membrane-associated proteins identified by LC-MS/MS spectrometry in MCL plasma membrane preparations are shown. Proteins were identified as described under “Experimental Procedures,” and for those proteins where multiple peptides were identified only the number of peptides and percentage sequence coverage are given. For those proteins where a single peptide was identified, the m/z value, error, percentage of peptide sequence, and MASCOT ion score are given. In some cases, multiple independent spectra for the same peptide were acquired and identified. For the single peptide identifications for CSK and 5-lipoxygenase, MS/MS spectra are provided in the supplemental single peptide spectra.
| Accession number | Protein | Sample | Unique peptides | Sequence coverage | m/z | Peptide | Score |
|---|---|---|---|---|---|---|---|
| % | |||||||
| P07948 | Lyn | MCL-P1 | 8 | 20 | |||
| MCL-P2 | 6 | 16 | |||||
| MCL-P5 | 9 | 26 | |||||
| Z138 | 5 | 15 | |||||
| HBL2 | 5 | 14 | |||||
| P07948 | Flotillin-1 | MCL-P1 | 9 | 30 | |||
| MCL-P2 | 7 | 27 | |||||
| MCL-P5 | 11 | 36 | |||||
| Z138 | 8 | 28 | |||||
| HBL2 | 8 | 29 | |||||
| P41240 | CSK | MCL-P1 | 1 | 3 | 839.54 | R↓HSNLVQLLGVIVEEK↓G | 41 |
| Z138 | 2 | 6 | |||||
| P09917 | 5-Lipoxygenase | MCL-P1 | 2 | 5 | |||
| MCL-P2 | 1 | 2 | 827.98 | K↓GVVTIEQIVDTLPDR↓G | 46 | ||
| MCL-P5 | 2 | 6 | |||||
| Q7Z7P2 | Raftlin | Z138 | 4 | 12 | |||
| JVM2 | 3 | 12 | |||||
| Q9NWQ8 | Cbp/PAG | Z138 | 3 | 8 |
The very low levels of raftlin in MCL cells indicated that in primary tumor cells the lipid raft proteome is altered or abnormal, and immunoblotting was used to profile the lipid raft fractions for the presence of selected known lipid raft proteins. Thus, relatively large amounts of the lymphoid transmembrane adaptor CSK-binding protein (Cbp)/phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG) (38, 39) were identified in Z138 lipid rafts by mass spectrometry (Table I). PAG migrated with an apparent molecular mass of ∼70 kDa on 1D SDS-PAGE (Fig. 4B). We confirmed this result using two different antibodies raised against Cbp/PAG and showed that >90% of the cellular protein was localized in the MCL cell line Z138 lipid raft fraction (data not shown). However, mass spectrometry did not detect Cbp/PAG in lipid raft samples derived from primary MCL patients (Fig. 4B), although Western blotting with long ECL exposures did reveal an immunoreactive band in all MCL lipid raft samples obtained from patients. These results indicated that Cbp/PAG, a known lipid raft protein, was underexpressed in primary MCL cells, and RT-PCR showed that mRNA levels in MCL patients were also markedly down-regulated (up to 5-fold) in four of four MCL patient samples. So in the case of this particular lipid raft protein, primary MCL cells exhibit marked differences when compared with MCL cell lines.
5-LO Aberrantly Localizes in Lipid Rafts in MCL
Mass spectrometry identified 5-LO in lipid rafts isolated from one MCL cell line and three MCL patients (Table I). Immunoblotting also showed a significant difference in expression between normal B and MCL cells. Thus, 5-LO (molecular mass, ∼80 kDa) was markedly up-regulated in cells isolated from all five patients (Fig. 4B). In two of three normal B cell lysates it was also weakly detected by immunoblotting but at very much lower levels than in the MCL cells. The mRNA levels of 5-LO as measured by RT-PCR were elevated (∼3–14-fold) in primary MCL cells as compared with normal B cells (Fig. 4C). High levels of 5-LO were also detected by Western blotting in purified lipid rafts obtained from three patients and one MCL cell line (Z138), although in comparison the SP53 cell line lipid raft preparation contained only a small amount of 5-LO.
Inhibitors of 5-LO Induce Apoptosis in MCL Cell Lines and Primary CLL Cells
5-LO catalyzes the stereospecific oxygenation of arachidonic acid to form 5-hydroperoxyeicosatetraenoic acid, which is metabolized to either 5-HETE or the highly unstable allylic epoxide leukotriene LTA4. The latter is hydrolyzed to LTB4 or sequentially converted into LTC4, LTD4, and LTE4 (for a review, see Ref. 40). In the unactivated cell 5-LO is located either in the cytosol or the nucleus, and after activation 5-LO migrates to the nuclear membrane where 5-LO-activating protein (FLAP) facilitates docking of arachidonic acid to 5-LO. Nuclear translocation is critical for 5-LO activity as it is at this location that it binds to FLAP and encounters phospholipase A2. Thus, the active form of 5-LO should be located in the nucleus, and our finding that 5-LO is a component of the lipid rafts of MCL cells is unexpected.
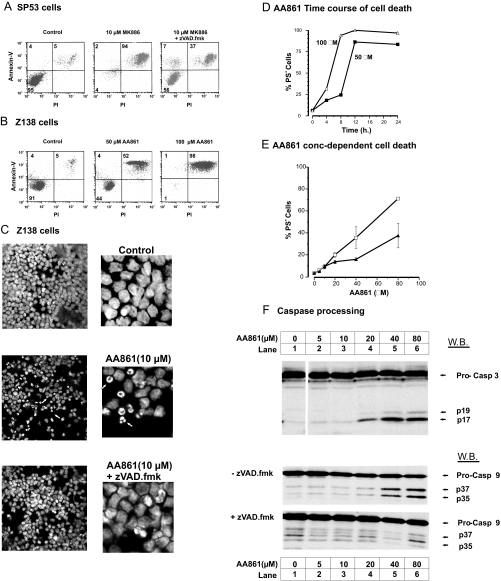
Aberrant expression of 5-LO has been observed in a variety of cancer cells (for reviews, see Refs. 40–42) and appears to promote cell proliferation while suppressing apoptosis. 5-LO overexpression has been correlated with increased resistance to apoptosis of Epstein-Barr virus-infected lymphomas (43), and 5-LO inhibitors induce apoptosis that can be antagonized by 5-LO metabolites (i.e. 5-HETE and 15-HETE). We therefore investigated the effect of lipoxygenase inhibitors in MCL cell lines using AA861 (5-LO) and MK886 (FLAP) inhibitors (44). Both these compounds were potent inducers of cell death in Z138 and SP53 MCL cell lines as assessed by an increase in the numbers of cells with high annexin V binding (Fig. 5, A and B). Apoptotic cells normally exhibit high annexin binding (due to phosphatidylserine externalization) and low PI staining, whereas (secondary) necrotic cells usually exhibit high annexin V and PI fluorescence due to the increased permeability of the plasma membranes. In many cases the apoptotic phase of cell death is followed by secondary necrosis that is seen in fluorescence-activated cell sorting analysis as two populations of PS+ cells with high (necrotic) and low (apoptotic) levels of PI fluorescence. Both AA861 and MK886 induced high levels of PS+ cells as judged by increased annexin V binding in a time-dependent and concentration-dependent manner (Fig. 5, D and E). The transition from apoptotic to necrotic phenotype appeared to be very rapid with both AA861 and MK886 inhibitors (Fig. 5, A and B) as most of the cells exhibited high PS+ and PI staining. However, Hoechst 33342 staining clearly showed that AA861 induces typical apoptotic nuclei morphology (Fig. 5C). A key feature of apoptotic death is the induction of the caspase cascade, which can be inhibited by Z-VAD-fmk, a polycaspase inhibitor. Significantly Z-VAD-fmk abolished the appearance of apoptotic nuclei in AA861-treated Z138 cells (Fig. 5C) and also inhibited MK886-induced cell death in SP53 cells as shown by the increased number of viable cells (Fig. 5A, lower left quadrant) and a decrease in PS+ cells. In the latter case the presence of Z-VAD-fmk revealed the tracking of the apoptotic cells to the secondary necrotic phenotype. Furthermore AA861 induced in Z138 cells a time-dependent (data not shown) and concentration-dependent processing of caspase-9 and caspase-3 to yield the signature cleavage products. Caspase-9 and -3 processing was inhibited by Z-VAD-fmk, clearly demonstrating induction of the intrinsic (mitochondrial) cell death pathway (Fig. 5F). Finally AA861 was also a potent inducer (EC50 ∼ 50 μm) of apoptotic cell death in primary CLL cells that was inhibited by Z-VAD-fmk (Fig. 5E), demonstrating that inhibitors of the 5-LO pathway induce cell death in MCL cell lines and CLL primary cells.
Fig. 5.
Inhibitors of 5-LO induce apoptosis in MCL cell lines and CLL primary cells. The effect of AA861 (5-LO inhibitor) and MK886 (FLAP inhibitor) were investigated using SP53 and Z138 MCL cell lines and primary CLL cells. In A, SP53 cells were treated for 24 h with 10 μm MK886 plus or minus the polycaspase inhibitor Z-VAD-fmk (100 μm), and apoptosis was assessed by flow cytometry with annexin V binding/PI staining as described under “Experimental Procedures.” In B, Z138 cells were treated with AA861 (50 and 100 μm) for 24 h before quantifying the PS+ (high annexin V binding) cells. Numbers in the lower left, upper left, and right quadrants refer to the percentage of cells that were viable, apoptotic, and necrotic, respectively. In C, Z138 cells were treated for 24 h with AA861 (10 μm) plus and minus Z-VAD-fmk (100 μm). Nuclei were stained with Hoechst 33342 and visualized by confocal microscopy using a Zeiss LSM 510 microscope and a 405 nm laser (blue diode) at 40× magnification with a 1.3 zoom. The blown up images are taken from the center of each cell field, and representative apoptotic nuclei are indicated with arrows. In D, the time course for AA861 induction of cell death (PS+) in SP53 cells is shown with 50 and 100 μm AA861. In E, concentration-effect curves for AA861-inducible cell death in primary CLL cells (after 18 h; n = 3; ±S.E.) are shown plus (▴) or minus (□) 200 μm Z-VAD-fmk. In F, the immunoblots show AA861-induced concentration-dependent caspase-9 and caspase-3 processing in Z-138 cells (E). The pro- and cleaved forms of caspase (Casp)-9 and -3 are indicated with arrows, and the effects of Z-VAD-fmk (200 μm) on caspase-9 processing are also shown. W.B., Western blotting.
DISCUSSION
Ideally potential candidates for antibody therapy should be highly expressed antigen/proteins at the outer surface of the cell membrane and have low expression in normal B cells and a corresponding high and universal expression in MCL tumor cells. Although microarray studies can provide sensitive and quantitative information on the relative expression of most genes, such studies do not provide information on post-translational modifications or change in plasma membrane and organelle composition. The LC-MS/MS and shotgun proteomics approach used in the current study overcomes these limitations. The data presented here are the first comprehensive analysis of the plasma membrane proteome of MCL primary cells and cell lines. A large number of CD markers were identified in mantle cell plasma membranes (Fig. 2 and supplemental Table 2). For example, CD70 was identified in MCL cell lines, and three of five patients exhibited marked up-regulation of CD70 as compared with normal B cells. CD27, its cognate receptor, was barely detectable in normal B cells but was markedly up-regulated in all patient plasma membrane samples (Fig. 2E). The RT-PCR data also confirmed that in the patient MCL cells CD27 and CD70 mRNA were overexpressed. In normal B cells, CD70 has a limited and tightly regulated expression in peripheral B cells and germinal centers in tonsils (28, 45). CD27-CD70 costimulation promotes B cell expansion, germinal center formation, and plasma cell differentiation (46). Gene expression studies have shown that CD70 is up-regulated (22–80%) in MCL (6, 8, 47, 48). A subset of CLL cells exhibited a vigorous proliferative response to a CD70 monoclonal antibody indicating that CD70 can act as a receptor (28). Anti-CD70 antibody-drug conjugates containing Aurostatin derivatives are cytotoxic in CD70-expressing renal cell carcinoma cells (49), and an antihuman CD70 monoclonal antibody induces tumor cell lysis in B lineage cancers (28). Thus, CD70 meets many of the criteria required for a therapeutic antigen, and this protein may be a suitable target for antibody therapy in mantle cell lymphoma.
The elevated levels of PKCβII in MCL plasma membranes indicated that this kinase was constitutively activated. The pathological significance of this in malignant B cells is unclear, but in normal B cells BCR-mediated PKC activation leads to NF-κB activation and B cell survival (for reviews, see Refs. 50 and 51). Triggering of the B cell receptor generates diacylglycerol and release of Ca2+ that both bind to phosphorylated PKCβ, thereby increasing its affinity for plasma membrane phospholipids. BCR signaling in CLL cells is regulated by overexpressed active PKCβII (52), and plasma membrane expression of PKCβII in diffuse large B cell lymphoma (DLBCL) is correlated with a poor response to chemotherapy and survival (53). The specific PKCβ inhibitor Ly379I96 induces apoptosis in DLBCL cell lines (35). In DLBCL PKCβ expression is inversely correlated with survival, indicating that activation/overexpression of the kinase acts as a survival signal (53). PKCβ and the IκB kinase complex are recruited into lipid rafts (35), and after ligation the BCR rapidly associates with lipid raft domains and is in close contact with the Src family kinases Lyn, Fyn, and Blk (54). Therefore, given its localization and abundance it is possible that PKCβII has a key role in promoting MCL and CLL cell survival.
The association of PKCβII with the MCL plasma membranes suggested constitutive activation of the BCR and recruitment into the lipid rafts, and analysis of MCL lipid rafts by LC-MS/MS revealed significant proteomic changes. Raftlin, a B cell-specific raft protein (22), was strongly down-regulated in patient MCL lipid rafts. Disruption of the raftlin gene in the DT40 B lymphocyte cell line reduces the content of specific raft proteins (e.g. Lyn kinase) and results in reduced cell proliferation and attenuated BCR signaling (22). The RT-PCR data also showed down-regulation of raftlin mRNA in MCL cells. Interestingly although the protein profiling data (Fig. 3) revealed that raftlin is markedly down-regulated in lipid rafts of primary MCL cells it is still expressed in the lipid rafts of MCL cell lines. The down-regulation of raftlin could have important consequences for MCL cell signaling as the BCR receptor is composed of the antigen-binding immunoglobulin molecule and associated heterodimer Igα-Igβ, which contains cytoplasmic immunoreceptor tyrosine-based activation motifs. Ligated BCR is believed to associate with lipid rafts and initiate signaling by Lyn-dependent phosphorylation of immunoreceptor tyrosine-based activation motifs, which then bind and activate the protein tyrosine kinase Syk, thereby triggering the signaling cascade. Lyn kinase is believed to be a key component of lipid rafts, and Lyn was detected in both MCL cells and cell lines by LC-MS/MS. Current evidence indicates that B cells during their development are regulated at different checkpoints by BCR signaling that executes crucial cell fate decisions including cell death and differentiation (55). Activation of Syk leads to activation of the phosphatidylinositol 3-kinase/AKT pathways and the production of inositol 1,4,5-trisphosphate, which in turn leads to Ca2+ release and diacylglycerol production; both Ca2+ and diacylglycerol are involved in PKC activation. Both Ca2+ and PKC are crucial for the activation of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), and transcription factors such as NF-κB and nuclear factor of activated T cells (NFAT). The consequences for MCL cells of these deregulated cell survival pathways may be to give MCL cells a survival advantage, which allows the malignant cells to survive and proliferate.
MCL is thought to be derived from naïve pregerminal B cells localized in primary follicles or in the mantle region of secondary follicles. However, increasing evidence suggests that MCL is derived from preactivated B cells (56). Analysis of B cell lymphomas assumes that malignant B cells keep many of the characteristics of the normal B cell (56). Consequently the malignant B cell should retain the protein characteristics of the B cell at that stage of development at which the malignant transformation occurred. In this study CD70, CD27, and CD31 were up-regulated in MCL cells. Although germinal center cells express CD27 (57) and CD31 (30), only a subset of germinal center cells express CD70 (28, 45). PAG/Cbp has been reported to be strongly expressed in germinal B cells (58) but not in mantle cells. In contrast, in our study both raftlin and PAG/Cbp were down-regulated in the MCL cells, and PAG/Cbp has also been reported to be down-regulated in MCL cells (59), which is in agreement with the current results. This suggests that PAG/Cbp may be a promising negative prognostic marker for MCL. The expression of raftlin and 5-LO during B cell development has not been fully characterized. Although in the case of 5-LO (while the current study was under review), an advance publication has reported that in tonsil tissue 5-LO is strongly expressed in the mantle zone but only weakly expressed in the germinal center (66). These data validate our findings and strongly suggest that the mantle zone cells have up-regulated levels of 5-LO. Thus, some proteins (i.e. PAG/Cbp and 5-LO) found in the circulating leukemic cells can be directly related to the lymphoid mantle zone, whereas other proteins such as CD70, CD27, and CD31 appear to originate from the germinal cells. These results suggest that the MCL has a more complex origin than hitherto believed.
The ability of “shotgun proteomics” to identify novel proteins provides the possibility of uncovering unexpected changes in the biochemistry of malignant cells. In this respect the design of follow-up experiments depends on the type of proteins identified or in what cellular/subcellular location the proteins were identified. A totally uncharacterized protein will require the generation of antibodies and/or affinity-tagged constructs that can be used to identify interacting partners and thereby gain possible insights into the function/role of the novel protein. Clearly the differential expression and immunohistochemical localization of such proteins in lymph node biopsies would need to be profiled across a large cohort of patient samples and compared with normal tonsils. In some cases the novelty of the protein that has been detected is related to the location of the protein within the cell, and a very good example is the marked up-regulation of 5-LO and its localization in lipid rafts in primary MCL cells. The function of leukotriene biosynthesis in B lymphocytes is unclear, but it appears to be important in B cell malignancies as gene expression studies have shown that 5-LO is one of the most abundantly overexpressed genes (≅6-fold increase) in CLL (60). In MCL cells we found a similar overexpression of 5-LO both at the protein and mRNA levels, and inhibitors of 5-LO and FLAP were potent inducers of apoptosis in MCL cell lines and primary CLL cells (Fig. 5). This implies that 5-LO and leukotriene synthesis confer a survival advantage to CLL and MCL cells, and there is increasing evidence that 5-LO overexpression is procarcinogenic in many cell types (for reviews, see Refs. 42 and 61). Both 5-LO expression and LT biosynthesis are tightly regulated, and in resting cells, 5-LO is located either in the cytosol or in the nucleus. After activation, 5-LO co-migrates with cytosolic phospholipase A2 to the nuclear membrane where cytosolic phospholipase A2 produces arachidonic acid from phospholipids that is transferred by FLAP to 5-LO, thereby stimulating LT biosynthesis. Thus, the activation of 5-LO is dependent on it being associated with a membrane, and the presence of large amounts of 5-LO associated with lipid rafts is of interest. Leukotriene synthesis in response to the calcium ionophore A32187 is inhibited by methyl-β-cyclodextrin, a cholesterol-depleting drug that disrupts lipid raft and cell signaling pathways (62). Co-localization of FLAP with flotillin 1 was observed in lipid raft fractions isolated from rat peripheral blood basophilic leukemia cells (62). Therefore, the presence of 5-LO in the plasma membranes and lipid rafts of MCL cells may indicate that this is a hitherto unknown site for 5-LO activation.
The downstream effects of the products of 5-LO activity are complex, but 5-HETE and LTA4 can protect cancer cells from apoptosis (61), and it has been suggested that eicosanoids act as agonists of the ligand-activated peroxisome proliferator-activated receptors (PPARs). Three isotypes, namely PPARα, PPARβ/δ, and PPARγ, mediate the transcriptional effects of fatty acids and metabolites (for a review, see Ref. 63). The transcriptional and metabolic effects of PPARs can have both pro- and antiapoptotic effects. Thus, peroxisome proliferators suppress spontaneous and induced apoptosis in rodent but not human hepatocytes probably by agonist activation of PPARα (64). In contrast PPARγ activation appears to have antitumorigenic effects, and PPARγ agonists have been shown to induce apoptosis in normal and malignant B lineage cells (65). The natural PPARγ agonist 15-deoxy-Δ12,14-prostaglandin J2 and the synthetic ligands pioglitazone and rosiglitazone induce apoptosis in MCL cell lines (67). Interestingly MK886 has been reported to induce apoptosis in lung cancer cell lines accompanied by up-regulation of PPARα and PPARγ (68). Furthermore the combination of MK886 and PPAR ligands produces superadditive inhibition of growth and induction of apoptosis. Thus, there is increasing evidence for the involvement of the 5-LO pathway in tumor cell proliferation and survival, suggesting that it could be a viable anticancer target. Our finding that 5-LO is overexpressed in MCL cells and the susceptibility of MCL cell lines and primary CLL cells to 5-LO and FLAP inhibitors indicate that this could be a promising therapeutic strategy for MCL and CLL.
Footnotes
* This work was funded in part by a grant from the Lymphoma Research Foundation.
 The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
‡ Senior authors.
2 Brown, M. J. S. Dyer, and K. Cain, unpublished observations.
1 The abbreviations used are:
- MCL
- mantle cell lymphoma
- 5-LO
- 5-lipoxygenase
- FLAP
- 5-LO-activating protein
- CLL
- chronic lymphocytic leukemia
- BCR
- B cell receptor
- TRPV2
- transient receptor potential cation channel, subfamily V member 2
- HVCN1
- hydrogen voltage-gated channel 1
- Syk
- spleen tyrosine kinase
- PAG
- phosphoprotein associated with glycosphingolipid-enriched microdomains
- CSK
- C-terminal Src kinase
- Cbp
- CSK-binding protein
- PKC
- protein kinase C
- LT
- leukotriene
- HETE
- hydroxyeicosatetraenoic acid
- 1D
- one-dimensional
- PS
- phosphatidylserine
- Z
- benzyloxycarbonyl
- fmk
- fluoromethyl ketone
- PI
- propidium iodide
- DLBCL
- diffuse large B cell lymphoma
- PPAR
- peroxisome proliferator-activated receptor
REFERENCES
- 1.Weisenburger D. D., Armitage J. O. ( 1996) Mantle cell lymphoma: an entity comes of age. Blood 87, 4483– 4494 [PubMed] [Google Scholar]
- 2.Cohen P. L., Kurtin P. J., Donovan K. A., Hanson C. A. ( 1998) Bone marrow and peripheral blood involvement in mantle cell lymphoma. Br. J. Haematol. 101, 302– 310 [DOI] [PubMed] [Google Scholar]
- 3.Witzig T. E. ( 2005) Current treatment approaches for mantle-cell lymphoma. J. Clin. Oncol. 23, 6409– 6414 [DOI] [PubMed] [Google Scholar]
- 4.Yatabe Y., Suzuki R., Matsuno Y., Tobinai K., Ichinohazama R., Tamaru J., Mizoguchi Y., Hashimoto Y., Yamaguchi M., Kojima M., Uike N., Okamoto M., Isoda K., Ichimura K., Morishima Y., Seto M., Suchi T., Nakamura S. ( 2001) Morphological spectrum of cyclin D1-positive mantle cell lymphoma: study of 168 cases. Pathol. Int. 51, 747– 761 [DOI] [PubMed] [Google Scholar]
- 5.Bodrug S. E., Warner B. J., Bath M. L., Lindeman G. J., Harris A. W., Adams J. M. ( 1994) Cyclin D1 transgene impedes lymphocyte maturation and collaborates in lymphomagenesis with the myc gene. EMBO J. 13, 2124– 2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ek S., Högerkorp C. M., Dictor M., Ehinger M., Borrebaeck C. A. ( 2002) Mantle cell lymphomas express a distinct genetic signature affecting lymphocyte trafficking and growth regulation as compared with subpopulations of normal human B cells. Cancer Res. 62, 4398– 4405 [PubMed] [Google Scholar]
- 7.Korz C., Pscherer A., Benner A., Mertens D., Schaffner C., Leupolt E., Döhner H., Stilgenbauer S., Lichter P. ( 2002) Evidence for distinct pathomechanisms in B-cell chronic lymphocytic leukemia and mantle cell lymphoma by quantitative expression analysis of cell cycle and apoptosis-associated genes. Blood 99, 4554– 4561 [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y., Hollmén J., Räty R., Aalto Y., Nagy B., Elonen E., Kere J., Mannila H., Franssila K., Knuutila S. ( 2002) Investigatory and analytical approaches to differential gene expression profiling in mantle cell lymphoma. Br. J. Haematol. 119, 905– 915 [DOI] [PubMed] [Google Scholar]
- 9.Gygi S. P., Rochon Y., Franza B. R., Aebersold R. ( 1999) Correlation between protein and mRNA abundance in yeast. Mol. Cell. Biol. 19, 1720– 1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G., Gharib T. G., Huang C. C., Taylor J. M., Misek D. E., Kardia S. L., Giordano T. J., Iannettoni M. D., Orringer M. B., Hanash S. M., Beer D. G. ( 2002) Discordant protein and mRNA expression in lung adenocarcinomas. Mol. Cell. Proteomics 1, 304– 313 [DOI] [PubMed] [Google Scholar]
- 11.Tian Q., Stepaniants S. B., Mao M., Weng L., Feetham M. C., Doyle M. J., Yi E. C., Dai H., Thorsson V., Eng J., Goodlett D., Berger J. P., Gunter B., Linseley P. S., Stoughton R. B., Aebersold R., Collins S. J., Hanlon W. A., Hood L. E. ( 2004) Integrated genomic and proteomic analyses of gene expression in Mammalian cells. Mol. Cell. Proteomics 3, 960– 969 [DOI] [PubMed] [Google Scholar]
- 12.Unwin R. D., Pierce A., Watson R. B., Sternberg D. W., Whetton A. D. ( 2005) Quantitative proteomic analysis using isobaric protein tags enables rapid comparison of changes in transcript and protein levels in transformed cells. Mol. Cell. Proteomics 4, 924– 935 [DOI] [PubMed] [Google Scholar]
- 13.Foster L. J., Zeemann P. A., Li C., Mann M., Jensen O. N., Kassem M. ( 2005) Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stem cell line undergoing osteoblast differentiation. Stem Cells 23, 1367– 1377 [DOI] [PubMed] [Google Scholar]
- 14.Zent C. S., Zhan F., Schichman S. A., Bumm K. H., Lin P., Chen J. B., Shaughnessy J. D. ( 2003) The distinct gene expression profiles of chronic lymphocytic leukemia and multiple myeloma suggest different anti-apoptotic mechanisms but predict only some differences in phenotype. Leuk. Res. 27, 765– 774 [DOI] [PubMed] [Google Scholar]
- 15.Antonucci F., Chilosi M., Parolini C., Hamdan M., Astner H., Righetti P. G. ( 2003) Two-dimensional molecular profiling of mantle cell lymphoma. Electrophoresis 24, 2376– 2385 [DOI] [PubMed] [Google Scholar]
- 16.Ghobrial I. M., McCormick D. J., Kaufmann S. H., Leontovich A. A., Loegering D. A., Dai N. T., Krajnik K. L., Stenson M. J., Melhem M. F., Novak A. J., Ansell S. M., Witzig T. E. ( 2005) Proteomic analysis of mantle-cell lymphoma by protein microarray. Blood 105, 3722– 3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H., Sadygov R. G., Yates J. R., 3rd ( 2004) A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 76, 4193– 4201 [DOI] [PubMed] [Google Scholar]
- 18.Cox B., Kislinger T., Emili A. ( 2005) Integrating gene and protein expression data: pattern analysis and profile mining. Methods 35, 303– 314 [DOI] [PubMed] [Google Scholar]
- 19.Boyd R. S., Adam P. J., Patel S., Loader J. A., Berry J., Redpath N. T., Poyser H. R., Fletcher G. C., Burgess N. A., Stamps A. C., Hudson L., Smith P., Griffiths M., Willis T. G., Karran E. L., Oscier D. G., Catovsky D., Terrett J. A., Dyer M. J. ( 2003) Proteomic analysis of the cell-surface membrane in chronic lymphocytic leukemia: identification of two novel proteins, BCNP1 and MIG2B. Leukemia 17, 1605– 1612 [DOI] [PubMed] [Google Scholar]
- 20.Drexler H. G., MacLeod R. A. ( 2006) Mantle cell lymphoma-derived cell lines: unique research tools. Leuk. Res. 30, 911– 913 [DOI] [PubMed] [Google Scholar]
- 21.Boyd R. S., Dyer M. J., Cain K. ( 2006) Proteomic analysis of cell surface membrane proteins in leukemic cells, in Adhesion Protein Protocols ( Coutts A. S., ed) 2nd Ed., pp. 135– 146, Human Press Inc, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 22.Saeki K., Miura Y., Aki D., Kurosaki T., Yoshimura A. ( 2003) The B cell-specific major raft protein, Raftlin, is necessary for the integrity of lipid raft and BCR signal transduction. EMBO J. 22, 3015– 3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller A., Nesvizhskii A. I., Kolker E., Aebersold R. ( 2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383– 5392 [DOI] [PubMed] [Google Scholar]
- 24.Almond J. B., Snowden R. T., Hunter A., Dinsdale D., Cain K., Cohen G. M. ( 2001) Proteasome inhibitor-induced apoptosis of B-chronic lymphocytic leukaemia cells involves cytochrome c release and caspase activation, accompanied by formation of an approximately 700 kDa Apaf-1 containing apoptosome complex. Leukemia 15, 1388– 1397 [DOI] [PubMed] [Google Scholar]
- 25.Livak K. J., Schmittgen T. D. ( 2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402– 408 [DOI] [PubMed] [Google Scholar]
- 26.Su A. I., Wiltshire T., Batalov S., Lapp H., Ching K. A., Block D., Zhang J., Soden R., Hayakawa M., Kreiman G., Cooke M. P., Walker J. R., Hogenesch J. B. ( 2004) A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. U.S.A. 101, 6062– 6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basso K., Liso A., Tiacci E., Benedetti R., Pulsoni A., Foa R., Di Raimondo F., Ambrosetti A., Califano A., Klein U., Dalla Favera R., Falini B. ( 2004) Gene expression profiling of hairy cell leukemia reveals a phenotype related to memory B cells with altered expression of chemokine and adhesion receptors. J. Exp. Med. 199, 59– 68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lens S. M., Drillenburg P., den Drijver B. F., van Schijndel G., Pals S. T., van Lier R. A., van Oers M. H. ( 1999) Aberrant expression and reverse signalling of CD70 on malignant B cells. Br. J. Haematol. 106, 491– 503 [DOI] [PubMed] [Google Scholar]
- 29.Dong H. Y., Shahsafaei A., Dorfman D. M. ( 2002) CD148 and CD27 are expressed in B cell lymphomas derived from both memory and naive B cells. Leuk. Lymphoma 43, 1855– 1858 [DOI] [PubMed] [Google Scholar]
- 30.Stacchini A., Chiarle R., Antinoro V., Demurtas A., Novero D., Palestro G. ( 2003) Expression of the CD31 antigen in normal B-cells and non Hodgkin's lymphomas. J. Biol. Regul. Homeost. Agents 17, 308– 315 [PubMed] [Google Scholar]
- 31.Ramsey I. S., Moran M. M., Chong J. A., Clapham D. E. ( 2006) A voltage-gated proton-selective channel lacking the pore domain. Nature 440, 1213– 1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capasso M., Bhamrah M., Boyd R. S., Cain K., Pulford K., Musset B., Cherny V. V., Morgan D., DeCoursey T. E., Gascoyne R. D., Dyer M. J.2008The voltage-gated proton channel HVCN1 co-localises with B-cell receptor and is involved in class switch recombination. Abstract from the 50th meeting of the American Society of Hematology. Blood 112, 707 (abstr.) [Google Scholar]
- 33.Hood J. L., Brooks W. H., Roszman T. L. ( 2006) Subcellular mobility of the calpain/calpastatin network: an organelle transient. BioEssays 28, 850– 859 [DOI] [PubMed] [Google Scholar]
- 34.Goll D. E., Thompson V. F., Li H., Wei W., Cong J. ( 2003) The calpain system. Physiol. Rev. 83, 731– 801 [DOI] [PubMed] [Google Scholar]
- 35.Su T. T., Guo B., Kawakami Y., Sommer K., Chae K., Humphries L. A., Kato R. M., Kang S., Patrone L., Wall R., Teitell M., Leitges M., Kawakami T., Rawlings D. J. ( 2002) PKC-beta controls I kappa B kinase lipid raft recruitment and activation in response to BCR signaling. Nat. Immunol. 3, 780– 786 [DOI] [PubMed] [Google Scholar]
- 36.Foster L. J., De Hoog C. L., Mann M. ( 2003) Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc. Natl. Acad. Sci. U.S.A. 100, 5813– 5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta N., Wollscheid B., Watts J. D., Scheer B., Aebersold R., DeFranco A. L. ( 2006) Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor-mediated lipid raft dynamics. Nat. Immunol. 7, 625– 633 [DOI] [PubMed] [Google Scholar]
- 38.Kawabuchi M., Satomi Y., Takao T., Shimonishi Y., Nada S., Nagai K., Tarakhovsky A., Okada M. ( 2000) Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature 404, 999– 1003 [DOI] [PubMed] [Google Scholar]
- 39.Brdicka T., Pavlistová D., Leo A., Bruyns E., Korínek V., Angelisová P., Scherer J., Shevchenko A., Hilgert I., Cerný J., Drbal K., Kuramitsu Y., Kornacker B., Horejsí V., Schraven B. ( 2000) Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J. Exp. Med. 191, 1591– 1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romano M., Claria J. ( 2003) Cyclooxygenase-2 and 5-lipoxygenase converging functions on cell proliferation and tumor angiogenesis: implications for cancer therapy. FASEB J. 17, 1986– 1995 [DOI] [PubMed] [Google Scholar]
- 41.Rådmark O., Werz O., Steinhilber D., Samuelsson B. ( 2007) 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem. Sci. 32, 332– 341 [DOI] [PubMed] [Google Scholar]
- 42.Fürstenberger G., Krieg P., Müller-Decker K., Habenicht A. J. ( 2006) What are cyclooxygenases and lipoxygenases doing in the driver's seat of carcinogenesis? Int. J. Cancer 119, 2247– 2254 [DOI] [PubMed] [Google Scholar]
- 43.Belfiore M. C., Natoni A., Barzellotti R., Merendino N., Pessina G., Ghibelli L., Gualandi G. ( 2007) Involvement of 5-lipoxygenase in survival of Epstein-Barr virus (EBV)-converted B lymphoma cells. Cancer Lett. 254, 236– 243 [DOI] [PubMed] [Google Scholar]
- 44.Steele V. E., Holmes C. A., Hawk E. T., Kopelovich L., Lubet R. A., Crowell J. A., Sigman C. C., Kelloff G. J. ( 1999) Lipoxygenase inhibitors as potential cancer chemopreventives. Cancer Epidemiol. Biomarkers Prev. 8, 467– 483 [PubMed] [Google Scholar]
- 45.Lens S. M., de Jong R., Hooibrink B., Koopman G., Pals S. T., van Oers M. H., van Lier R. A. ( 1996) Phenotype and function of human B cells expressing CD70 (CD27 ligand). Eur. J. Immunol. 26, 2964– 2971 [DOI] [PubMed] [Google Scholar]
- 46.Borst J., Hendriks J., Xiao Y. ( 2005) CD27 and CD70 in T cell and B cell activation. Curr. Opin. Immunol. 17, 275– 281 [DOI] [PubMed] [Google Scholar]
- 47.Rizzatti E. G., Falcão R. P., Panepucci R. A., Proto-Siqueira R., Anselmo-Lima W. T., Okamoto O. K., Zago M. A. ( 2005) Gene expression profiling of mantle cell lymphoma cells reveals aberrant expression of genes from the PI3K-AKT, WNT and TGFbeta signalling pathways. Br. J. Haematol. 130, 516– 526 [DOI] [PubMed] [Google Scholar]
- 48.Tracey L., Pérez-Rosado A., Artiga M. J., Camacho F. I., Rodríguez A., Martínez N., Ruiz-Ballesteros E., Mollejo M., Martinez B., Cuadros M., Garcia J. F., Lawler M., Piris M. A. ( 2005) Expression of the NF-kappaB targets BCL2 and BIRC5/Survivin characterizes small B-cell and aggressive B-cell lymphomas, respectively. J. Pathol. 206, 123– 134 [DOI] [PubMed] [Google Scholar]
- 49.Law C. L., Gordon K. A., Toki B. E., Yamane A. K., Hering M. A., Cerveny C. G., Petroziello J. M., Ryan M. C., Smith L., Simon R., Sauter G., Oflazoglu E., Doronina S. O., Meyer D. L., Francisco J. A., Carter P., Senter P. D., Copland J. A., Wood C. G., Wahl A. F. ( 2006) Lymphocyte activation antigen CD70 expressed by renal cell carcinoma is a potential therapeutic target for anti-CD70 antibody-drug conjugates. Cancer Res. 66, 2328– 2337 [DOI] [PubMed] [Google Scholar]
- 50.Guo B., Su T. T., Rawlings D. J. ( 2004) Protein kinase C family functions in B-cell activation. Curr. Opin. Immunol. 16, 367– 373 [DOI] [PubMed] [Google Scholar]
- 51.Spitaler M., Cantrell D. A. ( 2004) Protein kinase C and beyond. Nat. Immunol. 5, 785– 790 [DOI] [PubMed] [Google Scholar]
- 52.Abrams S. T., Lakum T., Lin K., Jones G. M., Treweeke A. T., Farahani M., Hughes M., Zuzel M., Slupsky J. R. ( 2007) B-cell receptor signaling in chronic lymphocytic leukemia cells is regulated by overexpressed active protein kinase C beta II. Blood 109, 1193– 1201 [DOI] [PubMed] [Google Scholar]
- 53.Espinosa I., Briones J., Bordes R., Brunet S., Martino R., Sureda A., Prat J., Sierra J. ( 2006) Membrane PKC-beta 2 protein expression predicts for poor response to chemotherapy and survival in patients with diffuse large B-cell lymphoma. Ann. Hematol. 85, 597– 603 [DOI] [PubMed] [Google Scholar]
- 54.Pierce S. K. ( 2002) Lipid rafts and B-cell activation. Nat. Rev. Immunol. 2, 96– 105 [DOI] [PubMed] [Google Scholar]
- 55.Niiro H., Clark E. A. ( 2002) Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2, 945– 956 [DOI] [PubMed] [Google Scholar]
- 56.Sara E., Borrebaeck C. A. ( 2007) Parallel gene expression profiling of mantle cell lymphoma: how do we transform omics data into clinical practice. Curr. Genomics 8, 171– 179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Oers M. H., Pals S. T., Evers L. M., van der Schoot C. E., Koopman G., Bonfrer J. M., Hintzen R. Q., von dem Borne A. E., van Lier R. A. ( 1993) Expression and release of CD27 in human B-cell malignancies. Blood 82, 3430– 3436 [PubMed] [Google Scholar]
- 58.Tedoldi S., Paterson J. C., Hansmann M. L., Natkunam Y., Rüdiger T., Angelisova P., Du M. Q., Roberton H., Roncador G., Sanchez L., Pozzobon M., Masir N., Barry R., Pileri S., Mason D. Y., Marafioti T., Horejsí V. ( 2006) Transmembrane adaptor molecules: a new category of lymphoid-cell markers. Blood 107, 213– 221 [DOI] [PubMed] [Google Scholar]
- 59.Svec A., Velenská Z., Horejsí V. ( 2005) Expression pattern of adaptor protein PAG: correlation between secondary lymphatic follicle and histogenetically related malignant lymphomas. Immunol. Lett. 100, 94– 97 [DOI] [PubMed] [Google Scholar]
- 60.Stratowa C., Löffler G., Lichter P., Stilgenbauer S., Haberl P., Schweifer N., Döhner H., Wilgenbus K. K. ( 2001) CDNA microarray gene expression analysis of B-cell chronic lymphocytic leukemia proposes potential new prognostic markers involved in lymphocyte trafficking. Int. J. Cancer 91, 474– 480 [DOI] [PubMed] [Google Scholar]
- 61.Werz O., Steinhilber D. ( 2006) Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol. Ther. 112, 701– 718 [DOI] [PubMed] [Google Scholar]
- 62.You H. J., Seo J. M., Moon J. Y., Han S. S., Ko Y. G., Kim J. H. ( 2007) Leukotriene synthesis in response to A23187 is inhibited by methyl-beta-cyclodextrin in RBL-2H3 cells. Mol. Cells 23, 57– 63 [PubMed] [Google Scholar]
- 63.Michalik L., Desvergne B., Wahli W. ( 2004) Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat. Rev. Cancer 4, 61– 70 [DOI] [PubMed] [Google Scholar]
- 64.Roberts R. A., James N. H., Woodyatt N. J., Macdonald N., Tugwood J. D. ( 1998) Evidence for the suppression of apoptosis by the peroxisome proliferator activated receptor alpha (PPAR alpha). Carcinogenesis 19, 43– 48 [DOI] [PubMed] [Google Scholar]
- 65.Padilla J., Kaur K., Cao H. J., Smith T. J., Phipps R. P. ( 2000) Peroxisome proliferator activator receptor-gamma agonists and 15-deoxy-delta(12,14)(12,14)-PGJ(2) induce apoptosis in normal and malignant B-lineage cells. J. Immunol. 165, 6941– 6948 [DOI] [PubMed] [Google Scholar]
- 66.Mahshid Y., Lisy M. R., Wang X., Spanbroek R., Flygare J., Christensson B., Björkholm M., Sander B., Habenicht A. J., Claesson H. E.High expression of 5-lipoxygenase in normal and malignant mantle zone B lymphocytes. BMC Immunol. 2009102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eucker J., Sterz J., Krebbel H., Zavrski I., Kaiser M., Zang C., Heider U., Jakob C., Elstner E., Sezer O. ( 2006) Peroxisome proliferator-activated receptor-gamma ligands inhibit proliferation and induce apoptosis in mantle cell lymphoma. Anticancer Drugs 17, 763– 769 [DOI] [PubMed] [Google Scholar]
- 68.Avis I., Martínez A., Tauler J., Zudaire E., Mayburd A., Abu-Ghazaleh R., Ondrey F., Mulshine J. L. ( 2005) Inhibitors of the arachidonic acid pathway and peroxisome proliferator-activated receptor ligands have superadditive effects on lung cancer growth inhibition. Cancer Res. 65, 4181– 4190 [DOI] [PubMed] [Google Scholar]