Abstract
Transient receptor potential A1 (TRPA1) is expressed in a subset of nociceptive sensory neurons where it acts as a sensor for environmental irritants, including acrolein, and some pungent plant ingredients such as allyl isothiocyanate and cinnamaldehyde. These exogenous compounds activate TRPA1 by covalent modification of cysteine residues. We have used electrophysiological methods and measurements of intracellular calcium concentration ([Ca2+]i) to show that TRPA1 is activated by several classes of endogenous thiol-reactive molecules. TRPA1 was activated by hydrogen peroxide (H2O2; EC50, 230 μm), by endogenously occurring alkenyl aldehydes (EC50: 4-hydroxynonenal 19.9 μm, 4-oxo-nonenal 1.9 μm, 4-hydroxyhexenal 38.9 μm) and by the cyclopentenone prostaglandin, 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2, EC50: 5.6 μm). The effect of H2O2 was reversed by treatment with dithiothreitol indicating that H2O2 acts by promoting the formation of disulfide bonds whereas the actions of the alkenyl aldehydes and 15d-PGJ2 were not reversed, suggesting that these agents form Michael adducts. H2O2 and the naturally occurring alkenyl aldehydes and 15d-PGJ2 acted on a subset of isolated rat and mouse sensory neurons [∼25% of rat dorsal root ganglion (DRG) and ∼50% of nodose ganglion neurons] to evoke a depolarizing inward current and an increase in [Ca2+]i in TRPA1 expressing neurons. The abilities of H2O2, alkenyl aldehydes and 15d-PGJ2 to raise [Ca2+]i in mouse DRG neurons were greatly reduced in neurons from trpa1−/− mice. Furthermore, intraplantar injection of either H2O2 or 15d-PGJ2 evoked a nocifensive/pain response in wild-type mice, but not in trpa1−/− mice. These data demonstrate that multiple agents produced during episodes of oxidative stress can activate TRPA1 expressed in sensory neurons.
Keywords: TRPA1, DRG, hydrogen peroxide, 4-hydroxynonenal, 15d-PGJ2, oxidative stress
Introduction
The transient receptor potential A1 (TRPA1) channel is a nonselective cation channel expressed by a subset of primary afferent nociceptive neurons where it acts as a sensory receptor for some pungent chemicals found in plants, including allyl isothiocyanate (from mustard and wasabi), cinnamaldehyde (from cinnamon), and allicin (in garlic). TRPA1 can also be activated by some other agents including methylsalicylate, icilin, and the environmental irritant, acrolein (Bandell et al., 2004; Jordt et al., 2004; Bautista et al., 2005, 2006) and mediates some responses to proinflammatory mediators, such as bradykinin (Bandell et al., 2004; Jordt et al., 2004; Bautista et al., 2006).
Site directed mutagenesis studies have shown that allyl isothiocyanate (AITC), cinnamaldehyde and acrolein activate TRPA1 by covalently reacting with cysteine residues in the cytoplasmic N terminus of the channel (Hinman et al., 2006; Macpherson et al., 2007a). TRPA1 can therefore act as a sensor of reactive, electrophilic chemicals. It has been unclear if there are endogenous electrophilic activators of TRPA1. To address this question, we have investigated whether endogenous thiol reactive agents activate TRPA1. For these studies we have studied the responsiveness of heterologously expressed TRPA1 channels and sensory neurons from wild-type rats and mice and TRPA1-null mice as well as the pain behaviors of TRPA1-null mice. Our studies have focused on several thiol reactive chemicals that are produced during oxidative stress and inflammation.
Oxidative stress occurs during many pathophysiological conditions including inflammation and reperfusion after ischemia and results in the production of a range of highly reactive chemicals including hydrogen peroxide (H2O2), lipid peroxidation products such as 4-hydroxynonenal (4-HNE), and 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) (Hyslop et al., 1995; Sprong et al., 1997; Chen et al., 1999; Gao et al., 2003; Uchida, 2003). H2O2, which stimulates a subset of capsaicin-sensitive sensory nerves innervating the heart, lungs and gastrointestinal tract (Stahl et al., 1993; Ustinova and Schultz, 1994b; Soukhova et al., 1999; Ruan et al., 2006), is known to oxidize cysteine residues in proteins to form either cysteine sulfenic acids or disulfides (Poole et al., 2004). 4-HNE and related lipids, 4-oxo-2-nonenal (4-ONE) and 4-hydroxyhexenal (4-HHE), are highly reactive products of lipid peroxidation that contain an electrophilic α,β-unsaturated carbonyl moiety similar to that found in the TRPA1 agonist, cinnamaldehyde. These lipid peroxidation products can form adducts with lysine, histidine, and cysteine residues (Uchida, 2003). Finally cyclopentenone prostaglandins (cyPGs), produced from arachidonic acid via cyclooxygenase and prostaglandin D2 (PGD2) synthase or nonenzymatically during oxidative stress (Chen et al., 1999; Gao et al., 2003), also contain electrophilic α,β-unsaturated carbonyl moieties and are known to be thiol reactive compounds (Levonen et al., 2004).
Materials and Methods
Cell culture.
Untransfected Chinese hamster ovary (CHO) cells and CHO cells expressing mouse TRPA1, mouse TRPM8, rat TRPV4, or human TRPV1 were grown in MEM-α medium supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), l-glutamine (2 mm), and FCS (10%).
Dorsal root ganglion (DRG) and nodose ganglion neurons were prepared from adult (∼200 g) male or female Wistar rats using methods described previously (Bevan and Winter, 1995). TRPA1-null mice and wild-type littermates were bred from heterozygotic mice kindly provided by Drs. Kelvin Kwan (Harvard Medical School, Boston, MA) and David Corey (Harvard Medical School, Boston, MA) (Kwan et al., 2006). DRG neurons from TRPA1-null and TRPV1-null mice and their respective wild-type littermates were prepared using the protocol used for rat neurons. The chemosensitivities of DRG and nodose neurons were investigated 18–48 h after plating the cells on laminin/poly-d-lysine-coated coverslips.
Imaging of intracellular calcium levels.
CHO cells and DRG neurons were loaded with 2 μm Fura-2 AM (Invitrogen, Carlsbad, CA) in the presence of 1 mm probenecid for ∼1 h. The dye loading and the subsequent experiments were performed in a physiological saline solution containing (in mm) 140 NaCl, 5 KCl, 10 glucose, 10 HEPES, 2 CaCl2, and 1 MgCl2, buffered to pH 7.4 (NaOH). Compounds were applied to cells by local continuous microperfusion of solution through a fine tube placed very close to the cells being studied. Experiments were conducted at 30°C. Images of a group of cells were captured every 2 s using 340 and 380 nm excitation wavelengths with emission measured at 520 nm with a microscope based imaging system (PTI, Birmingham, NJ). Analyses of emission intensity ratios at 340 nm/380 nm excitation (R; in individual cells) were performed using the ImageMaster suite of software.
Electrophysiology.
CHO cells expressing TRPA1 were studied under voltage-clamp conditions using an Axopatch 200B amplifier and pClamp 10.0 software (Molecular Devices, Sunnyvale, CA). Whole-cell recordings from CHO cells were performed at a holding potential of −60 mV, unless stated otherwise. Drugs and solutions were applied by local superfusion using a rapid solution changer (Bio-Logic, Claix, France). Borosilicate glass pipettes (2–5 MΩ, 75–80% series resistance compensation) were filled with (in mm) 140 KCl, 1 CaCl2, 2 MgATP, 10 EGTA, and 10 HEPES buffered to pH 7.4 (KOH). The external solution was as described above for imaging of intracellular Ca2+ concentrations. In experiments with Ca2+-free external solutions, 1 mm EGTA was included and CaCl2 was omitted. Cell-attached single-channel recordings were performed using the Ca2+-free external solution both in the pipette and for superfusion. Inside-out patches were superfused on the cytoplasmic side with a solution containing (in mm) 110 KCl, 10 Na5P3O10, 1 CaCl2, 1 MgCl2,10 HEPES, and 10 EGTA, pH 7.4 (KOH). Na5P3O10 was included to maintain channel activity in isolated patches (Kim and Cavanaugh, 2007). For inside-out patch recordings, pipettes were filled with the external solution described above for Ca2+-imaging experiments. All single-channel currents records were sampled at 10 kHz and filtered online at 5 kHz. The displayed single-channel records have been low pass filtered at 1 kHz. For the inside-out and cell-attached patch experiments we used higher resistance glass pipettes (8–12MΩ) than in the whole-cell experiments.
DRG neurons were studied using a CsCl based internal solution [containing (in mm): 140 CsCl, 1 CaCl2, 2 MgATP, 10 EGTA, and 10 HEPES, pH 7.4 (CsOH)] to block potassium currents. The external medium contained (in mm) 140 NaCl, 5 KCl, 10 glucose, 10 HEPES, 0.015 CaCl2, and 1 MgCl2 buffered to pH 7.4 (NaOH). The use of a reduced calcium concentration eliminated significant calcium flux through voltage-gated calcium channels and prevented sodium movements through the calcium channels, which can occur in calcium-free solutions.
The voltage sensitivity of membrane currents were investigated using either a voltage ramp protocol (1 s duration, −100 to +100 mV, CHO cells; or 2 s, −40 to +40 or +60 mV, DRG neurons) or depolarizing voltage steps up to +180 mV followed by repolarization to the holding potential of −60 mV. Because TRPA1-mediated currents showed a rapid inactivation in calcium-containing solutions, measurements of voltage sensitivity were performed in the calcium-free solutions noted above for CHO cells and DRG neurons.
Ninety-six-well plate intracellular calcium concentration assays.
In some experiments, changes in intracellular calcium ([Ca2+]i) were determined in TRPA1 expressing CHO cells grown in 96-well black-walled plates (Costar, Cambridge, MA) using a Flexstation 3 (Molecular Devices). Cells were loaded with Fura 2-AM and assays were performed at 25°C. Basal emission ratios with excitation wavelengths of 340 and 380 nm were measured and changes in dye emission ratio determined at various times after compound addition.
Behavioral responses.
All animal studies were performed according to the UK Home Office Animal Procedures Act (1986). Data shown are from male and female homozygote trpa1−/− and wild-type littermates. Intraplantar injections of hydrogen peroxide (0.3% [2.2 μmol] in saline) or 15-deoxy-Δ12,14-prostaglandin J2 (30 nmol [10 μg] in 10%DMSO/saline) were used to induce and compare nocifensive/pain responses in trpa1−/− and wild-type mice. These doses were based on dose–response relationships for hydrogen peroxide and 15-deoxy-Δ12,14-prostaglandin J2 determined in wild-type mice. Injections (25 μl) were made subcutaneously into the plantar surface of one of the hind paws using a 50 μl luer-syringe (Hamilton, Reno, NV) fitted with a 26-gauge by ⅜ inch intradermal needle. Immediately after injection, mice were placed inside a Perspex chamber and the duration of the pain-related behaviors (licking and biting or flinching and shaking of the injected paw) recorded using a digital stop-watch. Observation periods of 1 min were used and behavior recorded for up to 10 min after injection. Total pain response times over the first 3 min were used for analysis as the pain behaviors were largely confined to this period. Groups of six animals were used for each agent.
Drugs.
15-Deoxy-Δ12,14-prostaglandin J2 was from Biomol (Exeter, UK). PGA2, 9,10-dihydro-15d-PGJ2, 4-ONE, 4-HNE, and 4-HHE were obtained from Cayman Chemical (Ann Arbor, MI). Hydrogen peroxide was from VWR International (Lutterworth, UK). All other reagents were from Sigma (Poole, UK).
Results
H2O2 activates TRPA1 in CHO cells
Electrophysiology
H2O2 evoked an inward current in TRPA1-expressing CHO cells at a holding potential of −60 mV (Fig. 1A). In contrast, concentrations of H2O2 up to 100 mm failed to evoke currents in untransfected CHO cells (data not shown). The time course of the current was influenced by the presence or absence of Ca2+ in the external medium. In Ca2+-free external solutions the current developed slowly and persisted in the continued presence of H2O2. When Ca2+ was subsequently added to the H2O2-containing solution there was a rapid increase in current amplitude, consistent with a calcium-mediated potentiation, followed by a rapid inactivation (Fig. 1A). When H2O2 was applied in the presence of external Ca2+, the current activated with a concentration-dependent latency and then inactivated. The current occurred with a short latency with high concentrations of H2O2 but developed after a delay of up to a minute with low concentrations (Fig. 1B). A similar waveform and calcium dependence of TRPA1 mediated currents has been noted with either AITC or cinnamaldehyde as the agonist (Nagata et al., 2005; Doerner et al., 2007).
Figure 1.
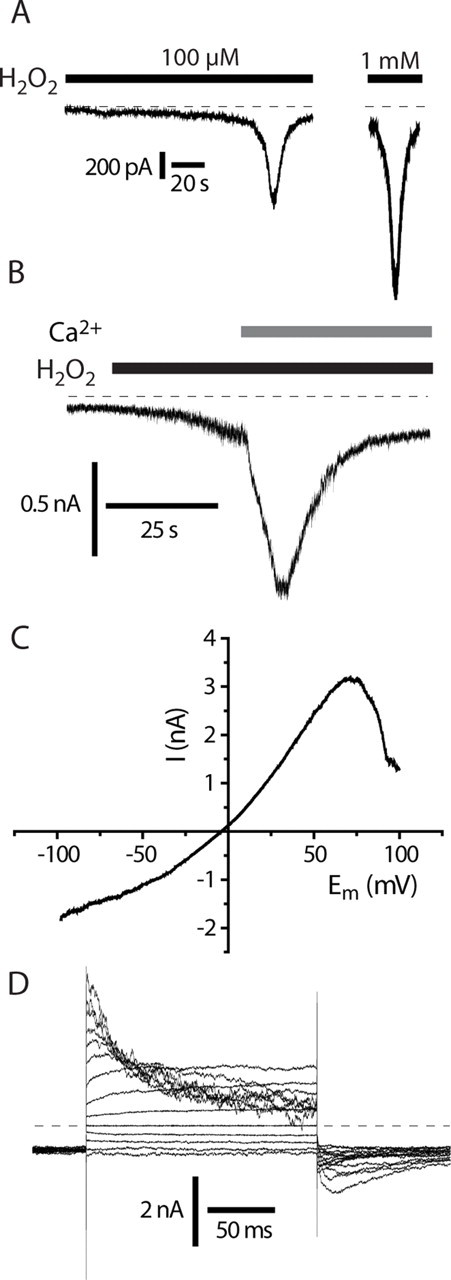
H2O2 activates TRPA1 expressed in CHO cells. A, H2O2 activates TRPA1 with a concentration-dependent latency in Ca2+-containing solutions. Currents recorded in calcium- containing solution showing characteristic “threshold” with a sudden increase in membrane current. B, Ca2+ potentiates H2O2-induced TRPA1 currents. Current response to 10 mm H2O2 in a TRPA1 CHO cell, −60 mV. Note the slow increase in current in calcium-free solution followed by a rapid current increase when Ca2+ (2 mm) is added. C, Current–voltage relationship of H2O2-evoked current with 2 s voltage ramp in a TRPA1 CHO cell in calcium-free solution revealed a reduced current at positive potentials. D, Kinetics of H2O2-evoked TRPA1 current in calcium free solution. Note the time and voltage-dependent inactivation at more positive potentials that accounts for reduced conductance seen with voltage ramp protocols (C) (Figs. 2C, 4C). Holding potential −60 mV with 20 mV interval steps to from −80 to +180 mV.
Under physiological conditions, the cytosol usually contains millimolar concentrations of glutathione, which acts as an antioxidant. It was possible that the intracellular glutathione levels were depleted during whole-cell recording, rendering the cells more sensitive to oxidative agents. Experiments were therefore performed with 10 mm reduced glutathione in the intracellular, pipette filling solution. Inclusion of exogenous glutathione did not reduce the amplitude of the H2O2 induced current or alter the current waveform (data not shown).
The H2O2 evoked current had a reversal potential close to 0 mV (Fig. 1C) and the current–voltage relationship obtained with ramp changes in voltage showed a characteristic decreased conductance at positive (>70 mV) membrane potentials (Fig. 1C). The reduction in conductance at positive membrane potentials was caused by a time-dependent inactivation that was revealed using a voltage step protocol (Fig. 1D). In the presence of H2O2 the current–voltage relationship was linear over the range −80 to 0 mV. The outward current showed an initial small, time-dependent growth at more positive potentials (0 to +60 mV), which was associated with an instantaneous, rapidly decaying inward “tail” current (deactivation) seen when the membrane was repolarized to −60 mV. A similar time-dependent growth at positive membrane potentials and subsequent deactivation on hyperpolarization has been reported for TRPV1 and TRPM8 in the absence of any added agonists as well in the presence of their respective agonists, capsaicin and menthol (Voets et al., 2004; Nilius et al., 2005). With more positive step potentials (≥+80 mV), the outward current showed an additional time-dependent inactivation during the depolarizing voltage step and the inward tail current seen on repolarization to −60 mV was more complex with an initial time-dependent growth followed by a decay (deactivation) to the initial holding current (Fig. 1D). The simplest explanation for these observations is that the TRPA1 channels continue to show a time-dependent increase in open probability at the more positive membrane potentials but this is overlaid by a block of the ion channels by some unknown mechanism. The growth in the tail current on repolarization probably represents unblocking of the channels, which then close. Very similar voltage-dependent properties were seen with AITC-evoked currents (supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
A voltage-dependent inactivation of the H2O2-evoked currents at positive membrane potentials was also evident in H2O2-evoked single-channel currents recorded in membrane attached patches (Fig. 2A–C). A single-channel chord conductance of 94 ± 3 pS was noted over the voltage range −100 to +100 mV. The single-channel current activity was clearly reduced at the more positive potentials, but no reduction in single-channel conductance was noted.
Figure 2.
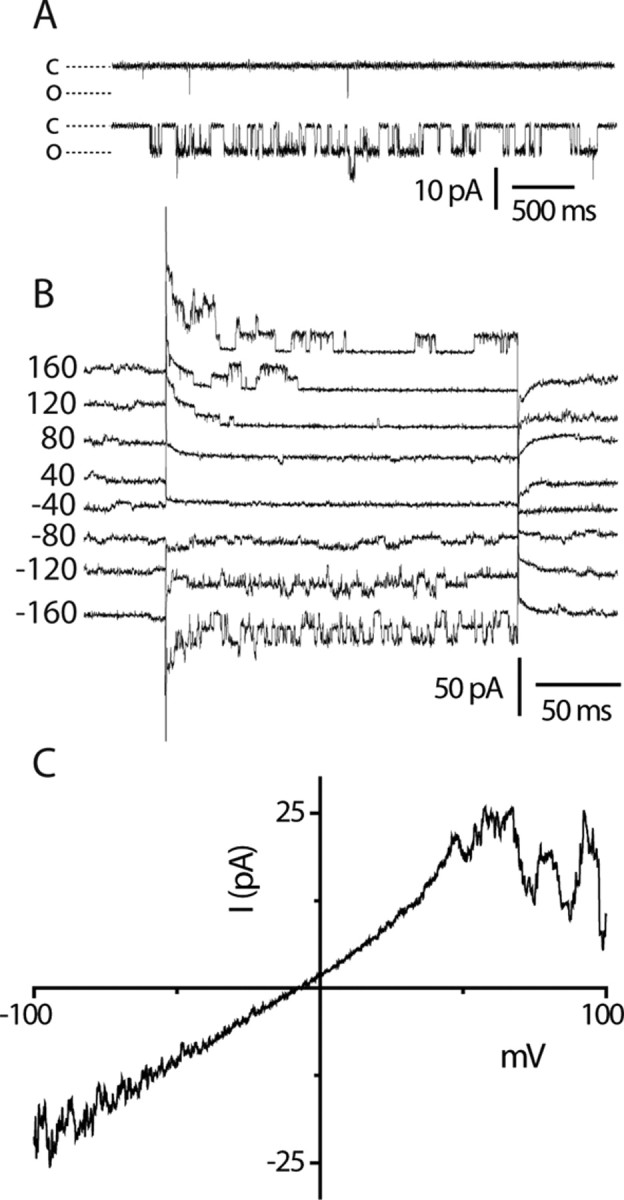
Single-channel activity evoked by H2O2. Cell attached TRPA1 single-channel currents. A, Few brief openings seen in the absence of H2O2 (top trace), but robust channel activity elicited by 1 mm H2O2 (lower trace) at −100 mV. B, Voltage-dependent single-channel current activity showing inactivation at positive membrane potentials. Holding potential, −60 mV; traces are offset for clarity. C, Voltage ramp illustrating single-channel inactivation at positive membrane potentials (the trace shown is the average of 5 sweeps).
Measurements of [Ca2+]i
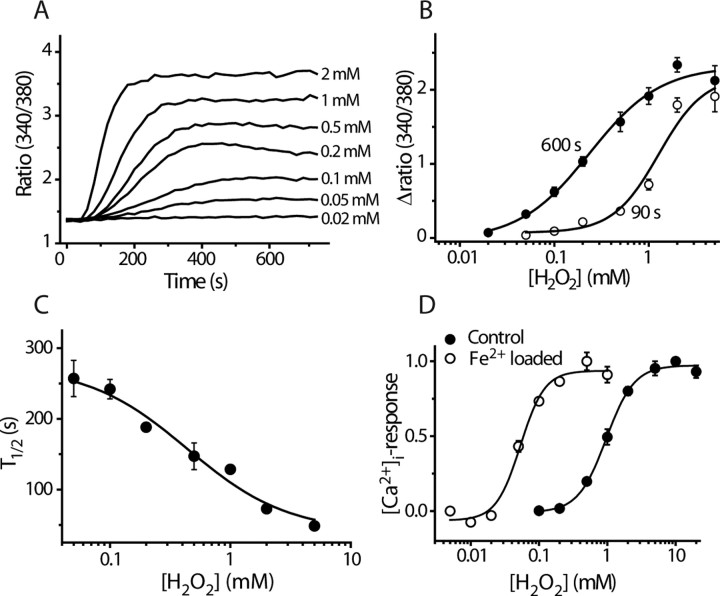
We used agonist-evoked changes in [Ca2+]i to quantify the effects of TRPA1 ligands. H2O2 evoked an increase in [Ca2+]i in TRPA1-expressing CHO cells but not in untransfected CHO cells or in cells expressing TRPV1, TRPV4, or TRPM8 (data not shown). The effect of H2O2 was time- and concentration-dependent with larger and faster responses seen at higher H2O2 concentrations (Fig. 3A). Concentration–response curves obtained using changes in [Ca2+]i as an index of channel activation yielded an EC50 value of 230 μm after 600 s exposure to H2O2 when the responses to each concentration had reached a plateau (Fig. 3B). The time dependence of the response is illustrated in Figure 3C where the time to half maximal response at each concentration is plotted against H2O2 concentration. Higher EC50 concentrations were calculated after shorter exposure times and an EC50 value of 1.2 ± 0.4 mm was estimated after 90 s exposure (Fig. 3B).
Figure 3.
Concentration-dependent effect of H2O2 on TRPA1. A, Concentration-dependent time course of [Ca2+]i-responses to stimulation with H2O2 in CHO cells expressing TRPA1. Traces are mean ratios from quadruplicate wells. B, Concentration–response curves constructed from the experiment shown in (A) 90 and 600 s after addition of H2O2 (mean ± SEM). C, The time required for half-maximal activation (T1/2) is concentration dependent (data points are mean ± SEM of 4 measurements). D, Fe2+ potentiates the effect of H2O2, suggesting that H2O2 acts via intracellular production of hydroxyl radicals. Concentration–response curves for H2O2-evoked increase in [Ca2+]i in normal and Fe2+-loaded TRPA1 cells are shown. Fe2+-loaded cells were incubated with 100 μm FeSO4 for 1 h and then washed so that no extracellular FeSO4 was present during the experiment (mean ± SEM; n = 4).
H2O2 can act on some proteins via the production of OH• radicals, which are generated at an accelerated rate when the intracellular concentration of iron is raised because Fe2+ acts as a catalyst in the Fenton reaction. We therefore examined the H2O2 sensitivity of TRPA1 expressing cells that had been preloaded with FeSO4 (Fig. 3D). The EC50 value in Fe2+-loaded cells (53 ± 6 μm, after 90 s) was ∼20-fold lower than in untreated cells, which indicates that H2O2 exerts its effect, at least in part, by the generation of intracellular OH• (Fig. 3D). TRPA1 was also activated by 5 μm Rose Bengal, which generates another reactive oxygen species (ROS), singlet oxygen, and by the oxidizing agent chloramine T (supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
H2O2 activates TRPA1-expressing sensory neurons
We examined whether H2O2 stimulated isolated sensory neurons using changes in intracellular free Ca2+ concentration ([Ca2+]i) as an indicator of neuronal activation. H2O2 evoked a robust increase in [Ca2+]i in ∼25% of rat DRG neurons, which typically were of small diameter (20–25 μm) (Fig. 4A). In whole-cell voltage-clamp experiments on DRG neurons, H2O2 evoked an inward current at −60 mV and increased membrane conductance (Fig. 4B). When the inward current was allowed to develop in solutions containing 15 μm Ca2+, subsequent addition of 2 mm Ca2+ rapidly inactivated the current (Fig. 4B). Using the same solutions, a similar current inactivation (without the surge in current shown in Fig. 1B) was evident in TRPA1 CHO cells when 2 mm Ca2+ was added (data not shown). A voltage ramp protocol revealed that the H2O2-evoked current had a reversal potential close to 0 mV (−6 mV), which suggested that the response was mediated by a nonselective cation channel, such as one of the TRP channels. The current–voltage relationship showed a characteristic reduction in conductance at positive membrane potentials similar to that seen for TRPA1 in CHO cells (Fig. 4C), but markedly different from the behavior of TRPV1–4 and TRPM8 mediated currents which do not exhibit any current inactivation at membrane potentials up to +200 mV (Nilius et al., 2005).
Figure 4.
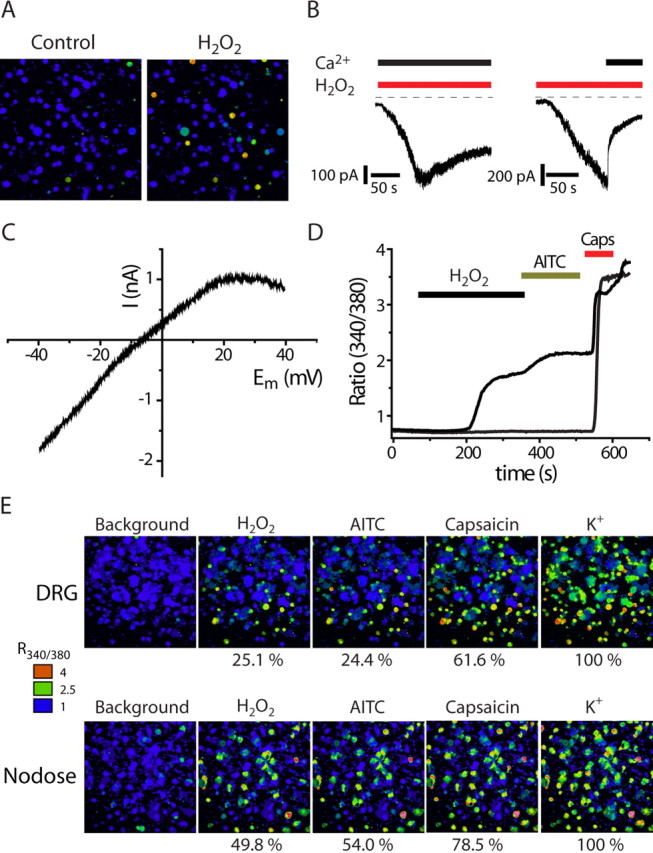
H2O2 activates TRPA1-containing DRG and nodose neurons. A, Pseudocolored images illustrating [Ca2+]i responses evoked by H2O2 (5 mm) measured with Fura-2 in rat DRG neurons. B, Currents evoked by H2O2 (1 mm) in DRG neurons in the presence (left) and absence (right) of extracellular calcium (at a holding potential of −60 mV). The current rapidly inactivated when Ca2+ was applied after an initial current has developed in Ca2+-free conditions. C, Current–voltage plot for the H2O2 response in a DRG neuron generated by a slow 2 s voltage ramp in Ca2+-free solution. Note the characteristic reduced conductance at positive membrane potential. D, Change in [Ca2+]i (340/380 ratio) of typical DRG neurons in response to sequential applications of H2O2, AITC, and capsaicin (Caps) showing H2O2-sensitive and H2O2-insensitive TRPV1 expressing neurons. E, [Ca2+]i responses in DRG (top sequence) and nodose neurons (bottom sequence) to sequential application of H2O2 (5 mm), AITC (50 μm), and capsaicin (1 μm). All neurons in the culture were identified by the [Ca2+]i increase elicited by application of 50 mm K+. H2O2, AITC and capsaicin stimulated a larger proportion of neurons dissociated from nodose than dorsal root ganglia. The number of neurons tested in each group was between 363 and 744.
To gain an insight into the molecular identity of the H2O2-activated channels in the native cells, we used changes in [Ca2+]i to examine the responses of rat DRG and nodose neurons to a sequence of agonists that activate different TRP channels and differentiate subpopulations of DRG neurons. In initial experiments, we found no correspondence between H2O2- and menthol-sensitivity (100 μm), which was expected given the relatively low percentage (∼8%) of menthol sensitive DRG neurons in DRG cultures (Andersson et al., 2007). Therefore, for most experiments we used AITC and capsaicin to activate TRPA1 and TRPV1, respectively. Any cell responding to a given stimulus with a [Ca2+]i-increase of at least 15% of the response to a subsequent challenge with 50 mm KCl, was considered “positive.” There was a striking correspondence between H2O2- and AITC-sensitive neurons (Fig. 4E). This was evident from the very similar percentage of DRG neurons responding to these agents (H2O2, 25.1%; AITC, 24.4% in DRG neurons; H2O2, 49.8%; AITC, 54.0% in nodose neurons) and from investigations of the chemosensitivities of individual neurons. All the H2O2-sensitive DRG neurons responded to AITC and treatment with AITC always occluded the response to a subsequent challenge with H2O2. As TRPA1 is expressed in a subpopulation of TRPV1 neurons we found neurons that responded to both H2O2 and capsaicin but ∼50% of capsaicin-sensitive DRG neurons and ∼30% of capsaicin-sensitive nodose neurons did not respond to H2O2 (Fig. 4D,E). Furthermore, the proportion of H2O2-sensitive DRG neurons was unchanged in trpv1−/− mice (31.8%, 113 of 355) compared with wild-type mice (30.1%, 134 of 445), ruling out TRPV1 as a mediator of DRG H2O2 responses.
TRPA1 is activated by products of lipid peroxidation
Oxidative stress generates other reactive chemicals, including products of lipid peroxidation, some of which have an electrophilic α,β-unsaturated carbonyl moiety like that found in the TRPA1 agonist, cinnamaldehyde. We therefore examined the effects on TRPA1 of three major lipids produced during episodes of oxidative stress (Uchida, 2003): 4-HNE, 4-HHE, and 4-ONE.
In calcium imaging experiments, 4-HNE (30 μm) and 4-ONE (3 μm) increased [Ca2+]i in ∼25% of DRG neurons, similar to the percentage that responded to AITC or H2O2. Approximately 50% of the capsaicin-sensitive neurons responded to 4-HNE and 4-ONE and there was an exact correspondence between the 4-HNE/4-ONE responsive and AITC sensitive neurons (Fig. 5A). This pattern suggests that TRPA1 mediates the response to 4-HNE and 4-ONE in DRG neurons.
Figure 5.
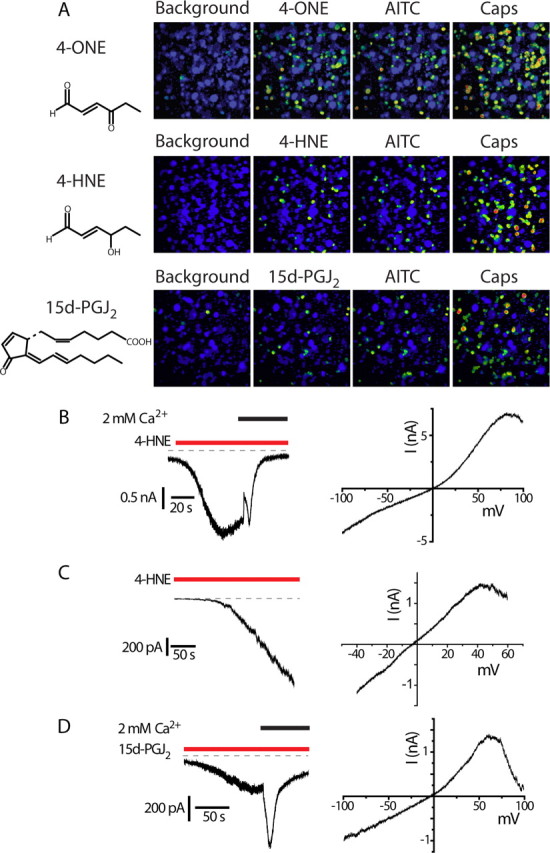
Lipid peroxidation products and 15d-PGJ2 are TRPA1 agonists. A, [Ca2+]i increases evoked by 4-ONE (3 μm), 4-HNE (30 μm), and 15d-PGJ2 (20 μm) in DRG neurons. Sequential applications of AITC and capsaicin (Caps) show that 4-ONE, 4-HNE, and 15d-PGJ2 activate the same subset of TRPV1-expressing, capsaicin-sensitive neurons as AITC. B, Left, 4-HNE-evoked current in a TRPA1 CHO cell initially in calcium-free solution. Admission of calcium led to rapid inactivation. Right, Current–voltage relationship of 4-HNE-evoked current. C, Left, 4-HNE-evoked current in a DRG neuron (external solution containing 15 μm Ca2+, −60 mV). Right, Current–voltage relationship of the 4-HNE-evoked current in the same neuron. D, Time course (left) and voltage-dependent kinetics (right) for 15d-PGJ2-evoked TRPA1 current in a CHO cell. Right, Current–voltage relationship of 15d-PGJ2-evoked current.
To examine the effects of 4-HNE on TRPA1 further, we studied its effect on heterologously expressed channels. 4-HNE evoked membrane currents and a robust [Ca2+]i increase in TRPA1 CHO cells, but not in untransfected cells or in TRPV1 expressing CHO cells (data not shown). The characteristics of the TRPA1-evoked currents were similar, although not identical, to those found with AITC or H2O2 activation (Fig. 5B). A relatively sustained current developed after several seconds delay in a Ca2+-free external solution. Addition of Ca2+ evoked a sudden decrease in current, not seen with the other agonists, followed by a transient increase in current and rapid inactivation.
In DRG neurons, application of 4-HNE evoked a sustained inward current in solutions containing 15 μm Ca2+. Furthermore, the 4-HNE current showed a voltage-dependent inactivation at positive membrane potentials as shown by the current responses to a voltage ramp in TRPA1 expressing CHO cells and DRG neurons (Fig. 5B,C). These results are consistent with the independent previous reports by two other groups that 4-HNE activates TRPA1 (Macpherson et al., 2007b; Trevisani et al., 2007).
All three lipid peroxidation products tested (4-HHE, 4-HNE, and 4-ONE) evoked increases in [Ca2+]i in TRPA1-expressing CHO cells (Fig. 6A). Concentration–response curves for the agonist induced increases in [Ca2+]i (Fig. 6A) revealed that 4-HHE and 4-HNE were approximately equipotent (EC50 values: 4-HNE, 19.9 ± 2.7 μm; 4-HHE, 39.9 ± 12.0 μm) whereas 4-ONE was more potent (EC50 value, 1.9 ± 0.7 μm). These EC50 values are consistent with the greater thiol reactivity of 4-ONE (Lin et al., 2005).
Figure 6.
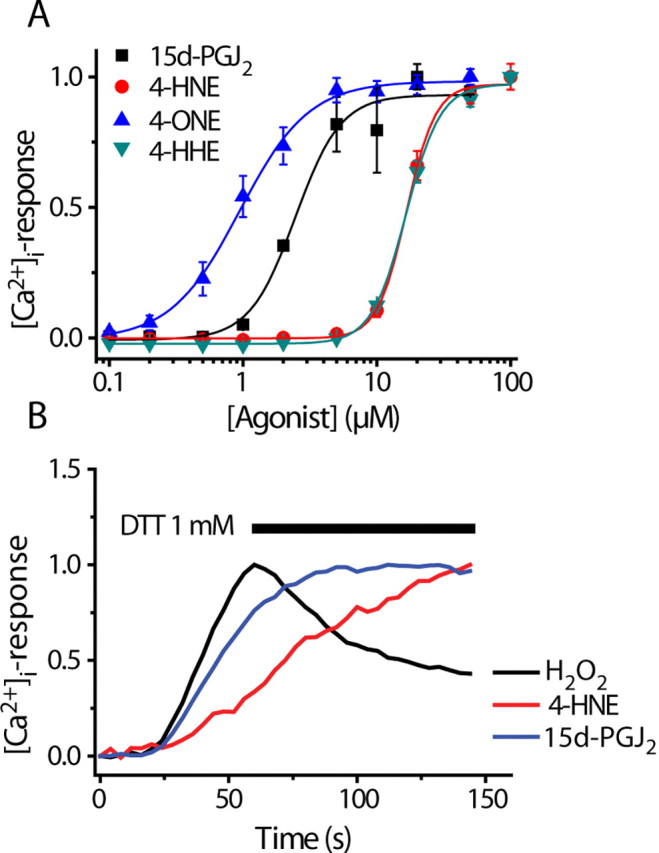
Concentration dependence of lipid mediators and dithiothreitol sensitivity. A, Concentration–response curves for 4-ONE, 4-HNE, 4-HHE, and 15d-PGJ2 in TRPA1 CHO cells (mean ± SEM, n = 4, representative of at least 3 experiments). B, Application of DTT reverse [Ca2+]i-responses induced by application of H2O2, but not 4-HNE or 15d-PGJ2.
Cyclopentenone prostaglandins are TRPA1 agonists
15d-PGJ2 is a major cyclopentenone prostaglandin produced nonenzymatically by dehydration of the labile prostaglandin, PGD2. The electrophilic 15d-PGJ2 is thiol reactive, induces intracellular production of reactive oxygen species and contributes to oxidative stress (Kondo et al., 2001; Levonen et al., 2004). In calcium-imaging experiments, we found that 20 μm 15d-PGJ2 elicited an increase in [Ca2+]i in AITC-sensitive DRG neurons (Fig. 5A). To investigate whether 15d-PGJ2 could activate TRPA1, we examined the effects of 15d-PGJ2 on TRPA1 CHO cells. 15d-PGJ2 evoked inward currents and an increase in [Ca2+]i in TRPA1 CHO cells (Fig. 5D), but had no effect on either TRPV1 or untransfected CHO cells (data not shown). The effect of 15d-PGJ2 was concentration dependent with an EC50 value of 5.6 ± 1.1 μm calculated from increases in [Ca2+]i (Fig. 6A). Importantly, the structurally related, but chemically less-reactive analogs 9,10-dihydro-15d-PGJ2 and PGA2 failed to elicit any significant [Ca2+]i-increase at concentrations <50 μm (data not shown).
Reversibility of TRPA1 activation by DTT
Reactive oxygen species and the lipid activators of TRPA1 are able to modify cysteine, lysine, and histidine residues. H2O2 may promote the formation of disulfide bonds between cysteine residues, whereas the lipid activators are likely to form Michael adducts and may cross-link vicinal reactive groups. We therefore examined whether the effects of these agonists could be reversed by dithiothreitol (DTT) (1 mm), which will reduce disulfide bonds but is unable to hydrolyze Michael adducts. DTT reversed the effects of H2O2, but not the responses to 4-HNE or 15d-PGJ2 (Fig. 6B).
Effect of H2O2, 4-ONE, and 15d-PGJ2 on TRPA1 in isolated patches
Compounds with electrophilic or oxidative properties are likely to modify the activity of many cellular proteins and processes in addition to TRPA1. To examine the possibility that the novel endogenous ligands activate TRPA1 by interacting with cytoplasmic proteins, we investigated whether H2O2, 4-ONE, and 15-PGJ2 were able to activate TRPA1 in isolated inside-out membrane patches. As shown in Figure 7, application of H2O2 (Fig. 7A), 4-ONE (Fig. 7B), and 15d-PGJ2 (Fig. 7C) opened TRPA1 channels in isolated inside-out patches, consistent with a membrane-delimited site of action that is not dependent on cytosolic mechanisms. In some patches, we noted that TRPA1 could be repeatedly activated and inactivated by H2O2 and DTT, respectively, further suggesting a direct action of these compounds on the channel (supplemental Fig. 3, available at www.jneurosci.org as supplemental material).
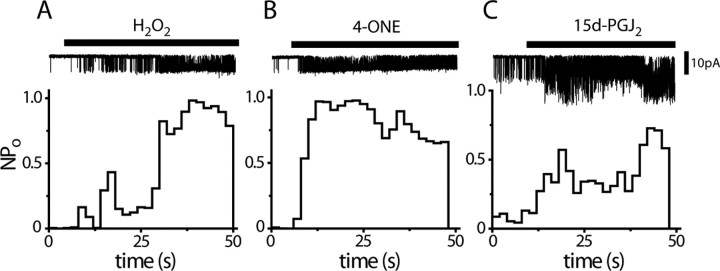
Figure 7.
H2O2, 4-ONE and 15d-PGJ2 activate TRPA1 in a membrane-delimited manner. A–C, Application of 5 mm H2O2 (A), 5 μm 4-ONE (B) and 20 μm 15d-PGJ2 (C) evoke single-channel activity in excised inside-out patches (−100 mV, each trace is representative of at least 3 experiments).
Effects of oxidative stress products on DRG neurons from trpa1−/− mice
The correspondence between AITC-sensitivity and sensitivities to H2O2, 4-HNE, 4-ONE and 15d-PGJ2 in DRG neurons and the abilities of these agents to activate TRPA1 in CHO cells is consistent with TRPA1 acting as the neuronal sensor. To confirm this hypothesis we examined the responses of DRG neurons from wild-type and TRPA1-null allele mutant mice using calcium imaging. The results of these experiments are shown in Table 1. The percentage of responsive DRG neurons from wild-type mice was very similar to the percentages seen for rat DRG neurons. First, we confirmed the loss of TRPA1 in the trpa1−/− DRG neurons using AITC as an agonist. Robust responses were noted in neurons from wild-type mice but responses to AITC were largely absent in trpa1−/− mouse neurons as previously described (Kwan et al., 2006; Macpherson et al., 2007b). Similarly, the responses to H2O2, 4-HNE, 4-ONE, and 15d-PGJ2 were essentially eliminated in the DRG neurons from knock-out mice. When present, the residual responses in trpa1−/− neurons were usually slow in onset, small and showed oscillating changes in [Ca2+]i, unlike the sustained responses in wild-type neurons.
Table 1.
Comparison of [Ca2+] responses in DRG neurons from trpa1+/+ and trpa1−/− mice
| Agonist |
trpa1+/+ |
trpa1−/− |
||
|---|---|---|---|---|
| Responding neuronsa | Response amplitude (% of K+)b | Responding neuronsa | Response amplitude (% of K+)b | |
| AITC | 29% | 86% | 2% | 21% |
| (133 of 466) | (4 of 181) | |||
| H2O2 | 33% | 70% | 3% | 31% |
| (91 of 274) | (12 of 403) | |||
| 4-ONE | 31% | 89% | 7% | 34% |
| (97 of 310) | (27 of 364) | |||
| 4-HNE | 28% | 61% | 1% | 34% |
| (98 of 356) | (4 of 469) | |||
| 15d-PGJ2 | 32% | 63% | 10% | 47% |
| (96 of 304) | (15 of 148) | |||
aNeurons responding with a [Ca2+]i increase of at least 15% of the response to a subsequent challenge with 50 mm KCl.
b[Ca2+]i increase expressed as percentage of the response to a challenge with 50 mm KCl. The weak residual [Ca2+]i responses in DRG neurons from trpa1−/− mice were qualitatively different from the responses seen in neurons from wild-type mice and showed delayed, oscillating signals. Similar weak responses in trpa1 −/− DRG neurons were noted for AITC in our study and by Kwan et al. (2006). These evoked changes in [Ca2+]i probably represent actions of the reactive compounds on other cellular mechanisms.
H2O2 and 15d-PGJ2 produce pain-related behavior through activation of TRPA1
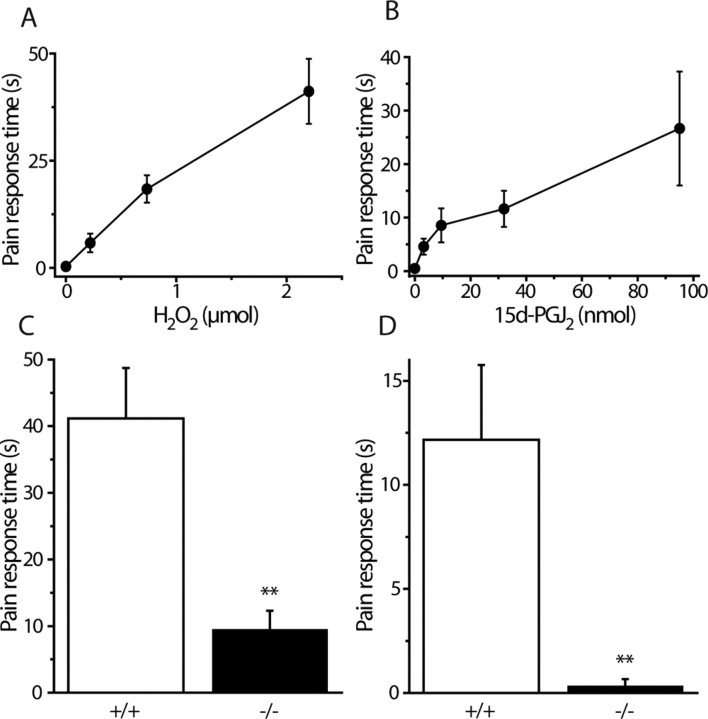
We examined whether TRPA1 is required for pain responses elicited by H2O2 and 15d-PGJ2 in vivo. Intraplantar injections of either compound evoked dose-dependent pain related behaviors in wild-type mice (licking, biting, flinching or shaking of the injected paw) that lasted for at least 3 min after injection (Fig. 8A,B). Doses that evoked reproducible robust responses were used to test the effects on trpa1−/− mice and wild-type littermates. Injections of either 2.2 μmol of H2O2 or 32 nmol of 15d-PGJ2 (in 25 μl) induced marked nocifensive behaviors in wild-type mice that were greatly reduced or absent in trpa1−/− mice (Fig. 8C,D).
Figure 8.
H2O2 and 15d-PGJ2 induce pain-related behavior in vivo by activating TRPA1. A, B, Duration of nocifensive (licking/flinching) behavior in wild-type mice evoked by intraplantar hindpaw injections of H2O2 in saline (A) or 15d-PGJ2 in saline containing 10% DMSO (B). The injection volume was 25 μl. Pain-related behavior was recorded over 5 min (mean ± SEM, n = 6 for each group). C, D, Wild-type (+/+) and TRPA1-deficient mice (−/−) were injected in the hind paw with H2O2 (C) (2.2 μmol/25 μl) or 15d-PGJ2 (D) (32 nmol/25 μl). Pain-related behavior (licking, biting, flinching, or shaking of the injected paw) was recorded for 3 min after injection. The nocifensive responses induced by H2O2 and 15d-PGJ2 were dramatically reduced or absent in mice lacking TRPA1 (mean ± SEM; n = 6 in each group; **p < 0.01).
Discussion
Our results demonstrate that reactive oxygen species, alkenyl aldehydes and 15d-PGJ2, which are generated during oxidative stress, activate TRPA1 in sensory neurons. All the agents activated TRPA1 expressed in CHO cells and evoked increases in [Ca2+]i in ∼50% of capsaicin sensitive DRG neurons. There was a close correspondence between the sensitivity of individual sensory neurons to the known TRPA1 agonist, AITC, and responsiveness to either H2O2, 4-HNE, 4-ONE, or 15d-PGJ2. Importantly, the responses to H2O2, 4-HNE, 4-ONE, and 15d-PGJ2 were almost absent in DRG neurons from trpa1−/− mice. Furthermore, our in vivo experiments with trpa1−/− mice demonstrated that TRPA1 was required for the pain-related behavioral responses evoked by H2O2 and 15d-PGJ2.
Several studies have suggested that TRPV1 mediates the sensory neuron responses to H2O2 (Schultz and Ustinova, 1998; Ruan et al., 2005, 2006; Yoshida et al., 2006). However, the mismatch between H2O2- and capsaicin-sensitivity and the loss of H2O2 responses in trpa1−/− mice indicate that TRPV1 is not primarily responsible for H2O2 activation of sensory nerve fibers. Unlike Yoshida et al. (2006), we found no evidence for direct activation of TRPV1 by H2O2 in our experiments. Furthermore, we observed no loss of H2O2 responses in DRG neurons from mice lacking TRPV1 ruling out the possibility that responses to H2O2 are mediated by a splice variant of TRPV1.
TRPA1 activation by H2O2 is likely to be an important pathway for neuronal stimulation in vivo. Single-unit recording from afferent nerve fibers innervating the heart showed that ∼50% of capsaicin sensitive units were activated by H2O2 via production of OH• (Ustinova and Schultz, 1994). This corresponds well with our findings that a similar percentage of isolated, capsaicin sensitive DRG neurons were activated by H2O2 in calcium imaging experiments and that activation was, at least in part, mediated by OH• radicals.
The finding that H2O2, 4-HNE, or 15d-PGJ2 activated TRPA1 in isolated membrane patches is consistent with direct chemical modification of TRPA1, although we cannot rule out effects on closely associated interacting proteins. Six cysteine residues located in the N-terminal segment have been identified as potential sites for oxidation and activation of TRPA1 by exogenous electrophilic reagents (AITC, cinnamaldehyde), whereas other molecular mechanisms appear to operate for agonists such as icilin and THC (Hinman et al., 2006; Macpherson et al., 2007a). DTT, which can reduce disulphide bonds but does not affect Michael adducts (Macpherson et al., 2007a), did not affect the responses to 4-HNE or 15d-PGJ2, suggesting that these lipid mediators form covalent adducts with TRPA1. This conclusion is consistent with reports that the actions of 15d-PGJ2 on Kelch-like ECH-associated protein 1 (KEAP1) and peroxisome proliferator-activated receptor-γ (PPAR-γ) are exerted by Michael additions to redox-sensitive cysteine thiols and that 4-HNE and 4-ONE form adducts with cysteine residues (Shibata et al., 2003; Levonen et al., 2004; Sayre et al., 2006). The finding that DTT reversed the effects of H2O2 indicates the formation of disulfide bonds between vicinal cysteine residues. Although actions on cysteine residues in TRPA1 are therefore likely, it is premature to conclude that all the effects reported in this study result exclusively from cysteine modifications. Hinman et al. (2006) showed that one lysine residue (K708) also influenced the activity of AITC. The alkenyl aldehydes studied here preferentially react with cysteine residues (Petersen and Doorn, 2004), but can also form Michael adducts with histidine, and 4-ONE has been reported to form a Schiff base with ε-amino groups of lysine residues (Lin et al., 2005). In TRPA1 CHO cells the waveform of the membrane currents evoked by increasing the extracellular Ca2+ concentration from Ca2+-free to 2 mm Ca2+ were agonist dependent. The current suddenly increased and then inactivated with H2O2, 15d-PGJ2, or AITC as the agonist, whereas with 4-HNE, the current showed a sudden decrease before increasing and then inactivating. These findings suggest some agonist-dependent differences in the interactions with TRPA1. Identification of the residues and mechanisms responsible for TRPA1 activation by H2O2, alkenyl aldehydes and 15d-PGJ2 remains to be explored.
Our studies show that H2O2 and 15d-PGJ2 activate sensory neurons in vivo to evoke pain responses. The responses were almost completely absent in trpa1−/− mice, demonstrating that sensory stimulation by these agents was mediated by TRPA1. These results complement the previous finding that 4-HNE evokes pain responses by activating TRPA1 (Trevisani et al., 2007). The concentrations of H2O2, 4-HNE, and 15d-PGJ2 required to stimulate TRPA1 and pain responses are higher than those that normally occur in tissues, but all the agents are produced at much higher concentrations during periods of oxidative stress.
NADPH oxidase-derived ROS, such as H2O2, have been proposed to play a role in local cell signaling by affecting the function of various kinases, phosphatases, phospholipases, and transcription factors, at low submicromolar concentrations (Lambeth, 2004; Bedard and Krause, 2007). ROS occur at higher concentrations in conditions of oxidative stress when their production exceeds the antioxidant activity of the cell. H2O2 concentrations of 10–100 μm have been measured in situations of physiological stress such as inflammation and reperfusion after ischemia (Hyslop et al., 1995; Sprong et al., 1997; Stone and Yang, 2006), which is similar to concentrations that activate TRPA1 (50–100 μm). The intracellular H2O2 concentration is probably 7–10 times lower than the extracellularly applied concentration (Stone and Yang, 2006), suggesting that TRPA1 can be activated when the intracellular H2O2 concentration reaches tens of micromolar. TRPA1 activation may be mediated directly by H2O2, but the finding that the potency of H2O2 was increased by 20-fold in Fe2+ loaded cells indicates that the effect is mediated, at least in part, by OH• produced from H2O2 by the Fenton reaction. Our data fit well with the observations that cardiac reperfusion after a brief experimental ischemia generates reactive oxygen species (O'Neill et al., 1996) and activates H2O2-sensitive sensory nerves. This activation is inhibited by deferoxamine pretreatment consistent with an effect mediated by OH• radicals (Ustinova and Schultz, 1994a; Huang et al., 1995b).
TRPA1 was activated by several naturally occurring alkenyl aldehydes produced by lipid peroxidation (4-HNE, 4-ONE, and 4-HHE). The concentrations of 4-HNE (EC50, 19.9 μm) and 4-ONE (EC50, 1.9 μm) required to activate TRPA1 are within the concentration range of 10 μm to >100 μm attained during oxidative stress (Esterbauer et al., 1991; Sayre et al., 2006). These electrophilic chemicals form adducts with many cellular molecules and the presence of these adducts is often used as an index of oxidative stress and damage. The finding that 4-HNE evokes pain responses by activating TRPA1 in vivo (Trevisani et al., 2007) supports a role for alkenyl aldehydes in signaling potentially damaging conditions of oxidative stress.
Cyclopentenone prostaglandins of the A and J series are produced in vivo by dehydration of the pentane ring of the prostaglandins, PGE2 and PGD2. The parent prostaglandins are usually produced enzymatically by the cyclooxygenase pathway. In conditions of oxidative stress, the cis-isomers of PGE2 and PGD2 (iso-PGE2 and iso-PGD2) are also generated nonenzymatically in vivo at high concentrations as products of free radical-induced peroxidation of arachidonoyl lipids (Chen et al., 1999; Gao et al., 2003). These isoprostanes are unstable and undergo epimerization to form PGE2 and PGD2, which in turn leads to an increased production of PGA2 and PGJ2 and their dehydration products, including 15d-PGJ2 (Gao et al., 2003). At low concentrations, 15d-PGJ2 can exert an anti-inflammatory effect and protect against oxidative stress by activation of PPARγ and KEAP1 (Landar et al., 2006; Lin et al., 2006; Ou et al., 2006; Napimoga et al., 2008), but at higher concentrations, the reactive cyclopentenone prostaglandins can cause tissue damage (Koh et al., 2005; Musiek et al., 2007). Low micromolar concentrations of 15d-PGJ2 activated TRPA1. The estimated EC50 value for TRPA1 agonism (5.6 μm) compares well with the concentrations of 15d-PGJ2 usually used to activate PPARγ (Forman et al., 1995; Kliewer et al., 1995).
Our studies show that TRPA1 can be activated by several different agents produced during conditions of oxidative stress. We propose that these chemicals activate TRPA1 and stimulate sensory neurons to elicit pain and to promote immediate protective responses either by local release of neuropeptides from the peripheral sensory nerve terminals or by sympathetically and vagally mediated neuronal reflexes. For example, activation of cardiac afferents evokes the symptoms of angina pectoris and reflex changes in blood pressure and heart rate (see e.g., Huang et al., 1995a). Similarly ROS activation of afferents innervating the airways causes a reflex increase in respiratory rate and bronchial vasodilation that increases airway blood flow (Soukhova et al., 1999; Ruan et al., 2006).
Footnotes
This work was supported by the Medical Research Council. We thank Dr. Terry Hart for discussion.
References
- Andersson DA, Nash M, Bevan S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci. 2007;27:3347–3355. doi: 10.1523/JNEUROSCI.4846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bevan S, Winter J. Nerve growth factor (NGF) differentially regulates the chemosensitivity of adult rat cultured sensory neurons. J Neurosci. 1995;15:4918–4926. doi: 10.1523/JNEUROSCI.15-07-04918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Morrow JD, Roberts LJ., II Formation of reactive cyclopentenone compounds in vivo as products of the isoprostane pathway. J Biol Chem. 1999;274:10863–10868. doi: 10.1074/jbc.274.16.10863. [DOI] [PubMed] [Google Scholar]
- Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- Gao L, Zackert WE, Hasford JJ, Danekis ME, Milne GL, Remmert C, Reese J, Yin H, Tai HH, Dey SK, Porter NA, Morrow JD. Formation of prostaglandins E2 and D2 via the isoprostane pathway: a mechanism for the generation of bioactive prostaglandins independent of cyclooxygenase. J Biol Chem. 2003;278:28479–28489. doi: 10.1074/jbc.M303984200. [DOI] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Stahl GL, Longhurst JC. Cardiac-cardiovascular reflexes induced by hydrogen peroxide in cats. Am J Physiol. 1995a;268:H2114–H2124. doi: 10.1152/ajpheart.1995.268.5.H2114. [DOI] [PubMed] [Google Scholar]
- Huang HS, Pan HL, Stahl GL, Longhurst JC. Ischemia- and reperfusion-sensitive cardiac sympathetic afferents: influence of H2O2 and hydroxyl radicals. Am J Physiol. 1995b;269:H888–H901. doi: 10.1152/ajpheart.1995.269.3.H888. [DOI] [PubMed] [Google Scholar]
- Hyslop PA, Zhang Z, Pearson DV, Phebus LA. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H2O2 in vitro. Brain Res. 1995;671:181–186. doi: 10.1016/0006-8993(94)01291-o. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Kim D, Cavanaugh EJ. Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: role of inorganic polyphosphates. J Neurosci. 2007;27:6500–6509. doi: 10.1523/JNEUROSCI.0623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Koh SH, Jung B, Song CW, Kim Y, Kim YS, Kim SH. 15-Deoxy-delta12,14-prostaglandin J2, a neuroprotectant or a neurotoxicant? Toxicology. 2005;216:232–243. doi: 10.1016/j.tox.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Kondo M, Oya-Ito T, Kumagai T, Osawa T, Uchida K. Cyclopentenone prostaglandins as potential inducers of intracellular oxidative stress. J Biol Chem. 2001;276:12076–12083. doi: 10.1074/jbc.M009630200. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Landar A, Zmijewski JW, Dickinson DA, Le Goffe C, Johnson MS, Milne GL, Zanoni G, Vidari G, Morrow JD, Darley-Usmar VM. Interaction of electrophilic lipid oxidation products with mitochondria in endothelial cells and formation of reactive oxygen species. Am J Physiol Heart Circ Physiol. 2006;290:H1777–H1787. doi: 10.1152/ajpheart.01087.2005. [DOI] [PubMed] [Google Scholar]
- Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, Morrow JD, Darley-Usmar VM. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defenses in response to electrophilic lipid oxidation products. Biochem J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Lee HG, Liu Q, Perry G, Smith MA, Sayre LM. 4-Oxo-2-nonenal is both more neurotoxic and more protein reactive than 4-hydroxy-2-nonenal. Chem Res Toxicol. 2005;18:1219–1231. doi: 10.1021/tx050080q. [DOI] [PubMed] [Google Scholar]
- Lin TN, Cheung WM, Wu JS, Chen JJ, Lin H, Chen JJ, Liou JY, Shyue SK, Wu KK. 15d-prostaglandin J2 protects brain from ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:481–487. doi: 10.1161/01.ATV.0000201933.53964.5b. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007a;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. J Neurosci. 2007b;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, McLaughlin B, Morrow JD. Electrophilic cyclopentenone isoprostanes in neurodegeneration. J Mol Neurosci. 2007;33:80–86. doi: 10.1007/s12031-007-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napimoga MH, Souza GR, Cunha TM, Ferrari LF, Clemente-Napimoga JT, Parada CA, Verri WA, Jr, Cunha FQ, Ferreira SH. 15d-prostaglandin J2 inhibits inflammatory hypernociception: involvement of peripheral opioid receptor. J Pharmacol Exp Ther. 2008;324:313–321. doi: 10.1124/jpet.107.126045. [DOI] [PubMed] [Google Scholar]
- Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T. Gating of TRP channels: a voltage connection? J Physiol (Lond) 2005;567:35–44. doi: 10.1113/jphysiol.2005.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill CA, Fu LW, Halliwell B, Longhurst JC. Hydroxyl radical production during myocardial ischemia and reperfusion in cats. Am J Physiol. 1996;271:H660–H667. doi: 10.1152/ajpheart.1996.271.2.H660. [DOI] [PubMed] [Google Scholar]
- Ou Z, Zhao X, Labiche LA, Strong R, Grotta JC, Herrmann O, Aronowski J. Neuronal expression of peroxisome proliferator-activated receptor-gamma (PPARgamma) and 15d-prostaglandin J2–mediated protection of brain after experimental cerebral ischemia in rat. Brain Res. 2006;1096:196–203. doi: 10.1016/j.brainres.2006.04.062. [DOI] [PubMed] [Google Scholar]
- Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Poole LB, Karplus PA, Claiborne A. Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol. 2004;44:325–347. doi: 10.1146/annurev.pharmtox.44.101802.121735. [DOI] [PubMed] [Google Scholar]
- Ruan T, Lin YS, Lin KS, Kou YR. Sensory transduction of pulmonary reactive oxygen species by capsaicin-sensitive vagal lung afferent fibres in rats. J Physiol (Lond) 2005;565:563–578. doi: 10.1113/jphysiol.2005.086181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan T, Lin YS, Lin KS, Kou YR. Mediator mechanisms involved in TRPV1 and P2X receptor-mediated, ROS-evoked bradypneic reflex in anesthetized rats. J Appl Physiol. 2006;101:644–654. doi: 10.1152/japplphysiol.00192.2006. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Lin D, Yuan Q, Zhu X, Tang X. Protein adducts generated from products of lipid oxidation: focus on HNE and one. Drug Metab Rev. 2006;38:651–675. doi: 10.1080/03602530600959508. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Ustinova EE. Capsaicin receptors mediate free radical-induced activation of cardiac afferent endings. Cardiovasc Res. 1998;38:348–355. doi: 10.1016/s0008-6363(98)00031-5. [DOI] [PubMed] [Google Scholar]
- Shibata T, Yamada T, Ishii T, Kumazawa S, Nakamura H, Masutani H, Yodoi J, Uchida K. Thioredoxin as a molecular target of cyclopentenone prostaglandins. J Biol Chem. 2003;278:26046–26054. doi: 10.1074/jbc.M303690200. [DOI] [PubMed] [Google Scholar]
- Soukhova GK, Ahmed M, Fletcher EC, Yu J. Hydrogen peroxide in the lung parenchyma stimulates vagally mediated phrenic activity. Chest. 1999;116:1365–1368. doi: 10.1378/chest.116.5.1365. [DOI] [PubMed] [Google Scholar]
- Sprong RC, Aarsman CJ, van Oirschot JF, van Asbeck BS. Dimethylthiourea protects rats against gram-negative sepsis and decreases tumor necrosis factor and nuclear factor κB activity. J Lab Clin Med. 1997;129:470–481. doi: 10.1016/s0022-2143(97)90081-0. [DOI] [PubMed] [Google Scholar]
- Stahl GL, Pan HL, Longhurst JC. Activation of ischemia- and reperfusion-sensitive abdominal visceral C fiber afferents. Role of hydrogen peroxide and hydroxyl radicals. Circ Res. 1993;72:1266–1275. doi: 10.1161/01.res.72.6.1266. [DOI] [PubMed] [Google Scholar]
- Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Schultz HD. Activation of cardiac vagal afferents in ischemia and reperfusion. Prostaglandins versus oxygen-derived free radicals. Circ Res. 1994a;74:904–911. doi: 10.1161/01.res.74.5.904. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Schultz HD. Activation of cardiac vagal afferents by oxygen-derived free radicals in rats. Circ Res. 1994b;74:895–903. doi: 10.1161/01.res.74.5.895. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]