Abstract
It is known that UV modulates the expression of paracrine factors that regulate melanocyte function in the skin. We have investigated the consequences of repetitive UV exposure of human skin in biopsies of 10 subjects with phototypes 2-3.5 taken 1-4 yr later. The expression of melanogenic factors (TYR, MART1, MITF), growth factors/receptors (SCF/KIT, bFGF/FGFR1, ET1/EDNRB, HGF, GM-CSF), adhesion molecules (β-catenin, E-cadherin, N-cadherin), cell cycle proteins (PCNA, cyclins D1, E2) as well as Bcl-2, DKK1 and DKK3, were analyzed by immunohistochemistry. Most of those markers showed no detectable changes at ≥1 yr after the repetitive UV irradiation. While increased expression of EDNRB protein was detected in 3 of 10 UV-irradiated subjects, there was no detectable change in the expression of ET1 protein or in EDNRB mRNA levels. In summary, only the expression of TYR, MART1 and/or EDNRB, and only in some subjects, was elevated at ≥1 yr post-UV irradiation. Thus the long-term effects of repetitive UV irradiation on human skin did not lead to significant changes in skin morphology and there is considerable subject-to-subject variation in responses. The possibility that changes in the expression and function of EDNRB triggers downstream activation of abnormal melanocyte proliferation and differentiation deserves further investigation.
Keywords: ultraviolet, skin, long-term changes, long-lasting pigmentation
INTRODUCTION
The incidence of melanoma has tripled over the past 3 decades probably as a result, at least in part, from lifestyle changes that have led to an increased exposure of the fair-skinned population to ultraviolet radiation (UV) [NCI Seer Statistics, http://seer.cancer.gov/]. Epidemiological and laboratory data provide strong evidence that solar exposure is a major causative factor in melanomagenesis (Gilchrest et al., 1999). Emerging evidence suggests that there is a clear link between tanning devices and malignant melanoma (Clough-Gorr et al., 2008; International Agency for Research on Cancer, 2007; Ting et al., 2007). Pathomechanisms for the development of non-melanocytic skin cancers have already been revealed (Matsumura and Ananthaswamy, 2002; Stary et al., 1997), however, the exact mechanism(s) of UV-induced melanomagenesis in the skin in situ remains unknown. Since UV-fingerprint mutations are rare in melanomas, it has been speculated that UV causes melanoma development by indirect effects, for example by dysregulation of growth factors in the skin, as proposed in a human skin graft model for UV-induced melanomas (Berking et al., 2004). We have recently shown that UV modulates the production (by keratinocytes and by fibroblasts in vitro) of growth factors regulating melanocyte function (Brenner et al., 2005). UV is presently the only known environmental carcinogen for melanomas, however it is still controversial which wavelengths are critical. UVA has been shown to promote melanoma in a hybrid fish melanoma model (Setlow et al., 1993), while UVB promotes melanoma in several transgenic mouse models (Noonan et al., 2003).
There is an urgent need to determine the specific mechanisms that are involved in photocarcinogenesis of UV-irradiated human skin in situ. Numerous studies have examined the effects of UV on skin cells in vitro, however that experimental setting does not allow one to explore inter-individual differences such as skin phototype or minimal erythema dose (MED). The examination of UV-induced changes in UV-irradiated skin in situ, with respect to different wavelengths and doses, provides a more suitable approach to characterize UV photocarcinogenesis in the skin, particularly with respect to early events in the malignant cascade.
Our group has previously reported several studies on the acute effects of UV radiation on human skin of varying skin pigmentation phenotypes. So far, we have shown that even a single, relatively low (1 MED) UV dose (60% UVA/40% UVB) causes significant damage to DNA in epidermal cells and stimulates the production of photoprotective melanin (Tadokoro et al., 2003). Most UV-induced DNA damage is removed relatively quickly (within days), however, some UV-induced molecular changes might persist and could initiate malignant transformation in the skin. Our earlier studies examined only the short-term effects of UV on human skin in situ. To extend our understanding of the long-lasting and potentially photocarcinogenic effects of UV, we examined biopsies from sites exposed to repetitive UV irradiation several years ago. In particular, we characterized the long-lasting effects of UV on melanocytes and on keratinocytes. We examined various potential morphologic and molecular markers of malignant transformation in 2 different UV-irradiated groups. One group of 6 subjects had been repeatedly irradiated over several weeks at 3 different cumulative doses with Sunlamps emitting a UV spectrum commonly used in tanning salons (≥95% UVA/≤5% UVB) while in another group, 4 different subjects had been repeatedly irradiated over a period of 2 weeks with a solar simulator (SS, ≥90% UVA/≤10% UVB). The molecular markers examined can be divided into 6 subgroups and were selected due to their possible involvement in melanocyte transformation and/or because many previously published studies have analyzed their responses in human skin within 1 month of UV exposure:
a) Melanin content and several melanocyte-specific proteins: microphthalmia transcription factor (MITF), melanoma antigen recognized by T-cells (MART1), tyrosinase (TYR).
b) growth factors and their receptors: stem cell factor (SCF)/KIT, basic fibroblast growth factor/fibroblast growth factor receptor 1 (bFGF/FGFR1), endothelin 1/endothelin B receptor (ET1/EDNRB), granulocyte macrophage colony stimulating factor (GM-CSF) and hepatocyte growth factor (HGF).
c) cell cycle proteins: proliferating cell nuclear antigen (PCNA), cyclins D1 and E2.
d) adhesion molecules: E- and N-cadherins, β-catenin.
e) the anti-apoptotic factor: B-cell lymphoma 2 (Bcl-2).
f) Wnt-pathway regulator dickkopf 1 (DKK1) and its homologue dickkopf 3 (DKK3).
RESULTS
Morphological changes in epidermal structure
Subjects (listed in Table 1) from 2 independent studies (Miller et al., 2008; Wolber et al., 2008) were invited to return for reexamination >1 yr after their initial UV exposures. Areas previously irradiated were identified using photos taken at the time of initial exposure, considering surface features and in some cases, pigmented areas that still remained.
Table 1.
Visible Long-lasting Pigmentation Following UV Irradiation
| Group 1 - Sunlamp |
Days between final UV Exposure and Biopsy |
Long-lasting Visible Pigmentation |
Group 2 - SS |
Days between final UV Exposure and Biopsy |
Long-lasting Visible Pigmentation |
|
|---|---|---|---|---|---|---|
| T11 | 1310 | No | B1 | 406 | No | |
| T16 | 1282 | No | B2 | 402 | No | |
| T32 | 751 | No | B6 | 402 | No | |
| T35 | 730 | No | B8 | 406 | No | |
| T47 | 427 | No | ||||
| T50 | 520 | Yes |
Group 1 as detailed in (Miller et al., 2008)
Group 2 as detailed in (Wolber et al., 2008)
Skin specimens from all subjects were stained with H&E and were carefully examined by light microscopy for morphological changes in the structure of the epidermis and/or the dermis. No morphological changes were seen in any UV-irradiated skin specimens or from the adjacent unirradiated skin specimens [Figure 1]. There were no signs that could be correlated to any clinical aspect of a localized sunburn reaction, such as a superficial inflammatory infiltrate in the dermis or the presence of sunburn cells in the epidermis. Special attention was paid to detect possible changes in the density, distribution and/or localization of melanocytes (e.g. suprabasal localization of melanocytes, formation of melanocyte cell nests). No such changes were detected, although there was a significant increase in melanocyte density in some subjects (see below).
Figure 1.
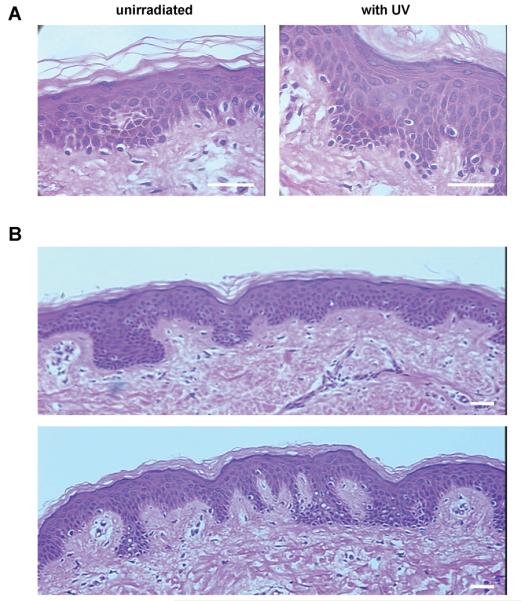
A) Histologically, no morphological changes were detectable in the UV-irradiated skin specimens (left) and the unirradiated controls (right); specimens from B6 are shown as an example. B) Inter-individual differences in skin morphology and melanin content were seen (subjects B2 and B6 are shown as examples, top and bottom, respectively).
Visible pigmentation, melanin content and expression of melanogenic proteins after UV irradiation
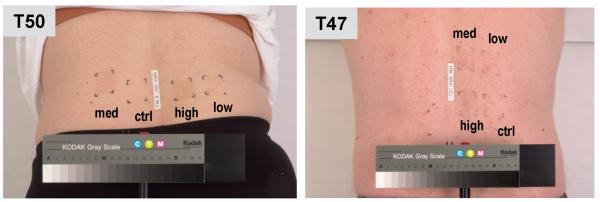
To evaluate long-lasting changes in melanin content, the UV-irradiated skin areas and the corresponding unirradiated control tissues were examined visually and were biopsied after >1 yr post-exposure. Repetitive UV irradiation with the Sunlamp or with the solar simulator produced good early tanning reactions in all subjects. However, only 1 subject (T50) in the Sunlamp-irradiated group showed long-lasting pigmentation (LLP) in the areas exposed to the intermediate or high cumulative UV doses (Miller et al., 2008). Figure 2 shows such pigmentation at 520 d post-irradiation. None of the subjects in the SS-irradiated group had visibly increased pigmentation in the irradiated areas at the time of biopsy [Table 1].
Figure 2.
Residual increased pigmentation was visible in only 1 subject (T50, photo at 520 d after exposure) after the repetitive Sunlamp-irradiation (in medium and high exposure areas). Subject T47 is shown as an example where no residual pigmentation was observed (photo taken at 427 d after exposure).
The melanin content in each skin specimen was determined by quantitative densitometry of the Fontana-Masson stained sections [Figure 3]. Only one subject (T50) in the Sunlamp-irradiated group and one subject (B6) in the SS-irradiated group showed significantly (p<0.05) increased total amounts of melanin [Table 2].
Figure 3.
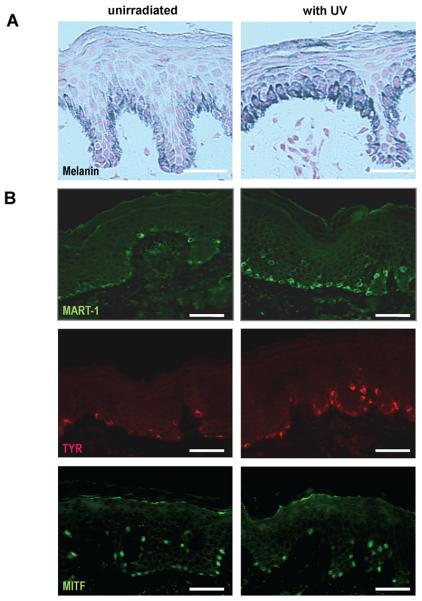
Significant increases were noted in melanin content in the skin of 4 of 10 subjects following UV-irradiation, while differences in the density of MART1-positive cells were seen in 2 of 10 subjects, in the density of TYR-positive cells in 4 of 10 subjects, but no differences were seen in the density of MITF-positive cells (B6 is shown as an example for all 4 markers) bars = 50 μm.
Table 2.
Characteristics of Skin Pigmentation Following UV Irradiation
| Differences in | Group 1 - Sunlamp-irradiated |
Group 2 - SS-irradiated |
|---|---|---|
| Melanin Content integrated density stain/area (mean) (control/UV) |
1 / 6 T11 (8.0/8.1) T16 (8.9/9.4) T32 (4.5/5.5) T35 (10.2/10.9) T47 (5.4/5.1) T50 (6.1/9.5)* |
1 / 4 B1 (6.2/7.1) B2 (16.3/15.1) B6 (13.0/18.2)* B8 (8.1/7.4) |
| Density of MITF-positive cells per mm (average) (control/UV) |
0 / 6 T11 (46/50) T16 (35/30) T32 (34/39) T35 (36/37) T47 (19/22) T50 (23/26) |
0 / 6 B1 (17/12) B2 (18/32) B6 (62/71) B8 (44/32) |
| Density of MART1-positive cells per mm (average) (control/UV) |
0 / 6 T11 (45/44) T16 (36/37) T32 (43/41) T35 (34/36) T47 (20/18) T50 (24/22) |
2 / 4 B1 (25/27) B2 (27/27) B6 (62/111)* B8 (18/41)* |
| Density of TYR-positive cells per mm (average) (control/UV) |
1 / 6 T11 (39/42) T16 (31/34) T31 (41/39) T35 (35/33) T47 (22/20) T50 (16/28)* |
3 / 4 B1 (34/35) B2 (23/36)* B6 (51/83)* B8 (21/43)* |
= p <0.05
There were no significant differences in the numbers of MITF-positive cells compared to the unirradiated controls in the UV-irradiated subjects. Although no difference could be found between the UV-irradiated and the control specimens in the number of MART1-positive cells in the Sunlamp-irradiated group, 2 subjects (B6 and B8) showed significantly higher (p<0.05) numbers of MART1-positive cells in the SS-irradiated group. In the Sunlamp-irradiated group, a significant increase in the number of TYR-positive cells compared to the unirradiated control was only found in subject T50, who was the only subject in either study group with visible pigmentation remaining in the previously UV-irradiated areas. In the SS-irradiated group, 3 out of 4 subjects (B2, B6 and B8) showed significant (p<.05) increases in the density of TYR-positive cells [Table 2, Figure 3], although no visibly increased pigmentation was noted in those previously UV-irradiated areas.
Expression patterns of markers in the skin after UV irradiation
Table 3 summarizes the molecular markers examined and their long-term responses to UV-irradiation and examples of the staining patterns are shown in Figure 4.
Table 3.
Summary of Staining Patterns of Various Markers after UV-irradiation
| Group 1 - Sunlamp |
control | Group 2 - SS |
control | |
|---|---|---|---|---|
| Ligands & Receptors | ||||
| SCF | + | + | + | + |
| KIT | ++ | ++ | ++ | ++ |
| bFGF | + | + | + | + |
| FGFR1 | + | + | + | + |
| ET1 | ++ | ++ | ++ | ++ |
| EDNRB | ++ (2/6) / + | ± | ++ (1/4) / + | ± |
| HGF | − | − | − | − |
| GM-CSF * | ++ * | ++ * | ++ * | ++ * |
| Adhesion Molecules | ||||
| β-catenin | +++ | +++ | +++ | +++ |
| E-cadherin | +++ | +++ | +++ | +++ |
| N-cadherin | − | − | − | − |
| Cell Cycle Proteins | ||||
| PCNA | ++ | ++ | +++ (B6) / ++ | ++ |
| Cyclin D1 | − | − | − | − |
| Cyclin E2 | − | − | − | − |
| Other Proteins | ||||
| Bcl-2 | + | + | + | + |
| DKK1 | − | − | − | − |
| DKK3 | ++ | ++ | ++ | ++ |
=irregular staining pattern
All tissue sections were read by the same investigator (MB). The Table shows an overall picture of the staining patterns with various markers after UV irradiation. Staining intensity and extent was graded as: − (no staining), + (weak staining intensity and small area stained), ++ (moderate staining intensity and middle-size area stained) or +++ (high staining intensity and large area stained). The cut-off to classify staining as positive was 1%, i.e. at least 1% of the epidermis stained positively as detected by immunohistochemistry.
Figure 4.
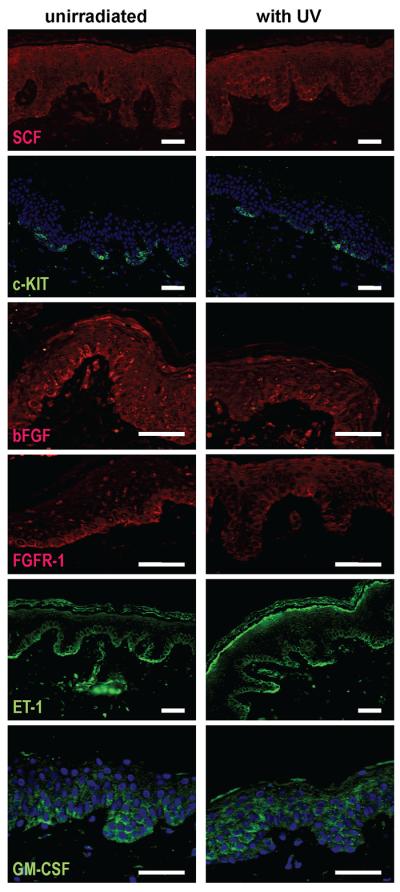
Staining intensity ranged from weak to moderate, but overall there were no marked differences in the expression of SCF, KIT, bFGF, FGFR1, ET1 or GM-CSF between UV-irradiated and unirradiated control specimens (B6 is shown as an example for all 6 markers) bars = 50 μm.
Growth factors and their receptors
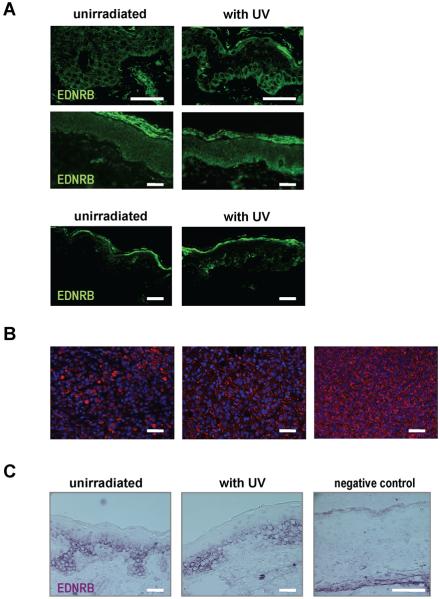
In the skin specimens examined, SCF, bFGF and ET1 were expressed in the basal epidermal layer. Between different samples, the staining intensity ranged from weak to moderate, but overall, no marked differences in expression could be detected between UV-irradiated and control specimens from either protocol. KIT expression was detected in all samples and showed a strong regular pattern of staining in the basal layers of the epidermis. FGFR1 staining was weak and was restricted to the basal epidermal layer. Expression of HGF could not be detected in the epidermis of any skin sections examined. GM-CSF expression in the skin showed irregular patterns, with some specimens showing basal expression and others showing dispersed expression throughout the whole epidermis [Figure 4]. Two of the 6 subjects in the Sunlamp-irradiated group (T35 and T47) and 1 of the 4 subjects in the SS-irradiated group (B6) showed marked differences in the expression of EDNRB in the basal epidermal layers compared to the unirradiated controls. We used a tissue array with 39 melanoma samples as a positive control for EDNRB [Figure 5].
Figure 5.
A) Differences in EDNRB expression were noted in 2 of 6 subjects (T35 and T47, top and middle, respectively) in the Sunlamp-irradiated group and in 1 of 4 subjects (B6, bottom) in the SS-irradiated group; B) Three different specimens on the melanoma tissue array were positive for EDNRB expression; C) Tissue-in situ hybridization for EDNRB mRNA showing basal expression but no differences between UV-irradiated and unirradiated control specimens (subject T35 shown as an example) bars = 50 μm.
Tissue-in situ hybridization for EDNRB mRNA in the Sunlamp- or SS- irradiated skin showed cytoplasmic staining mainly in the basal epidermal layers, but no marked difference in EDNRB mRNA expression between the UV-irradiated and control skin [Figure 5].
Adhesion molecules
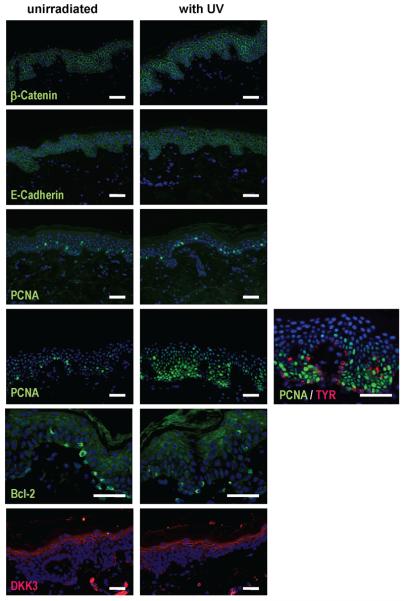
All skin specimens were examined for expression of various adhesion molecules (β-catenin, E- and N-cadherins). β-Catenin showed a regular strong cytoplasmic staining pattern throughout the whole epidermis with no changes in the expression between UV-irradiated and unirradiated skin. No nuclear staining pattern could be detected. In all skin specimens, E-cadherin was expressed in a regular strong cytoplasmic pattern throughout the epidermis and no marked differences between UV-irradiated and control specimens were found. N-cadherin expression could not be detected [Figure 6].
Figure 6.
Examples of staining patterns in UV-irradiated specimens (right) and in unirradiated controls (left). ß-Catenin (subject B6), E-cadherin (subject B6), PCNA (subject B1), PCNA (subject B6, double-stained for TYR in red on far right), Bcl-2 (subject T47) and DKK3 (subject B2) bars = 50 μm.
Cell cycle proteins
Skin specimens were analyzed for expression of the proliferative index of PCNA, cyclins D1 and E2. Neither cyclin D1 nor E2 showed a positive staining. PCNA expression showed no differences between UV-irradiated and control specimens, except for 1 subject (B6) in the SS-irradiated group. A clearly higher nuclear staining for PCNA was found in that specimen, however, double staining with TYR revealed that mainly keratinocytes, but not melanocytes, showed the increased expression of PCNA [Figure 6].
DKK1, DKK3 and Bcl-2
DKK1 could not be detected in any skin specimens examined, UV-irradiated or unirradiated. DKK3 expression showed a regular and strong expression pattern in the upper epidermal layers but did not exhibit any significant changes in response to UV irradiation. Bcl-2 immunoreactivity was found in melanocytes in the basal cell layer of the skin as expected, but no marked differences in the expression of Bcl-2 was noted between UV-irradiated and unirradiated specimens [Figure 6].
DISCUSSION
In the past, many in vitro and in vivo experiments have been conducted to characterize the carcinogenic effects of UV radiation on human skin cells. In vitro studies with human melanocytes (Bittner et al., 2000; Clark et al., 2000; Hipfel et al., 2000; Valery et al., 2001; Young et al., 1998b) and in vivo studies on human skin have examined the effects of short-term UV irradiation (Hachiya et al., 2004; Mass et al., 2003; Tadokoro et al., 2003; Tronnier et al., 1997; Young et al., 1998a) but so far there have been no studies about the long-lasting effects of UV. Some in vivo studies have examined molecular changes of atypical nevi (melanoma precursor lesions) (Wang et al., 1996), but it has been estimated that melanomas mainly arise in the skin de novo and in only 30% of melanocytic nevi (Wolff et al., 2005).
In our study, we tried to identify long-lasting molecular changes in human skin in situ many months after the initial repetitive UV irradiation. The analysis was mainly based on immunohistochemistry techniques and was completed by tissue in situ hybridization for the analysis of EDNRB mRNA. While this approach allowed the analysis of a considerable number of molecular factors it does not permit quantitative analysis of protein and/or RNA expression. We analyzed melanin content using Fontana-Masson staining and melanocyte density using antibodies to melanocyte-specific markers, such as MITF, MART1 and TYR. Earlier, we reported the results of 3 clinical studies measuring melanocyte density after UV irradiation that showed significant increases to levels about 3-fold higher than in unirradiated skin at up to 5 weeks after irradiation and that increase was consistent with the increased melanin content (Tadokoro et al., 2003; Tadokoro et al., 2005; Yamaguchi et al., 2006). In this study, a significant increase in MART1-positive melanocytes in 2 of 10 subjects and a significant increase in TYR-positive melanocytes in 4 of 10 subjects was found, although only 1 of those subjects still showed a visibly increased pigmentation in the UV-irradiated area. This indicates that the activation of melanocytes might persist much longer than the UV-induced pigmentation or the acute UV-induced changes in cell morphology.
As there is a wide variety of other possible genes of interest and their corresponding encoded proteins, we focused our analysis on representatives of 5 major groups of proteins where changes in expression could be involved in the transformation of melanocytes. The growth factors (and their cognate receptors) SCF/KIT, bFGF/FGFR1, ET1/EDNRB, GM-CSF/GM-CSFR and HGF/MET, are part of the paracrine network between melanocytes and keratinocytes/fibroblasts (Imokawa, 2004; Yamaguchi et al., 2007; Brenner and Hearing, 2008). SCF and ET1 are highly mitogenic for human melanocytes (Grichnik et al., 1995; 1998; Imokawa et al., 1996a). The expression of KIT, the cognate receptor for SCF, is down-regulated with progression in melanoma cell lines and tumors (Montone et al., 1997). Recently, it was shown that SCF and KIT are frequently expressed by melanomas and by dysplastic nevi suggesting an autocrine growth factor mechanism (Giehl et al., 2007). Also reported recently are studies showing that bFGF, SCF and ET3 may be involved in melanomagenesis (Berking et al., 2004). FGFR1 is the cognate receptor for bFGF and the co-expression of FGFR1 and bFGF in melanoma cells was reported to be associated with increased microvessel density (Straume and Akslen, 2002).
In contrast to KIT expression, which is gradually lost during the transformation of melanocytes to malignant melanoma, EDNRB expression is greatly enhanced and can serve as a marker of melanoma progression (Bittner et al., 2000; Demunter et al., 2001; Loftus et al., 1999). Those results and the finding that ET1 production by keratinocytes is increased following UV irradiation (Ahn et al., 1998) suggest that EDNRB as well as its ligand, ET1, contribute to melanomagenesis and progression (Demunter et al., 2001; Lahav, 2005). Increased EDNRB expression, probably coupled with epidermal hyper-secretion of ET1, could be a first step to generate local populations of melanocytes with the potential to escape from the epidermis if further changes occur (e. g. loss of E-cadherin). EDNRB has been recently reported to be up-regulated in solar lentigos (Aoki et al., 2007), which is consistent with the findings of our study, and suggests that over-expression of that receptor may result from chronic UV exposure and may be involved in the increased pigmentation of human skin following UV. One study in human skin in vivo showed an early increase in SCF and KIT mRNA expression and a subsequent increase in the expression of TYR, ET1 and EDNRB mRNA and protein 7 to 10 d after 2 MED UVB irradiation (Hachiya et al., 2004). HGF is a multifunctional cytokine, which, among other activities, acts as a growth factor for melanocytes. It has been shown that UVB irradiation leads to melanomas in neonatal HGF-transgenic mice (Noonan et al., 2001). HGF, which is expressed by fibroblasts and by melanoma cells, but not by normal melanocytes (Iyer et al., 1990; Li et al., 2001; Rosen et al., 1994) could not be detected in the epidermis of our skin specimens. GM-CSF has been shown to be secreted from keratinocytes after UVA (Imokawa et al., 1996b) or UVB irradiation (Hirobe et al., 2004) and might play an important role in regulating the proliferation and differentiation of human melanocytes after UV irradiation.
In our study, we could not detect any marked differences between the UV-irradiated skin and the unirradiated controls in long-term follow-up specimens regarding the expression of SCF, KIT, GM-CSF, bFGF, FGFR1 or ET1 in the epidermis. However, in 3 of 10 subjects, a clearly increased expression of EDNRB protein was detected in the irradiated skin; however, that was not detected at the mRNA level. Whether the increased expression of EDNRB in some of our subjects might predict an increased risk for skin cancer further in the future deserves a longer term follow-up study.
β-Catenin is a potent mediator of growth for melanoma cells and plays a dual role in cells, in cadherin-mediated cell adhesion (Steinberg and McNutt, 1999) and in the Wnt signaling pathway (Morin, 1999). E-cadherin, which is a tumor invasion suppressor, is down-regulated in most melanoma cells, and this renders them refractory to keratinocyte-mediated control and enhances their capability for invasion (Hsu et al., 2000). Staining for E-cadherin and for β-catenin resulted in strong staining patterns; however, no differences in their expression in UV-irradiated skin and in unirradiated controls were detected. Also, no expression of N-cadherin could be detected, which might indicate that changes in adhesion molecules occur in late stages of melanomagenesis and are signs of the invasive behavior of malignant skin cells.
Dysregulation of the cell cycle is a hallmark of tumor progression and increased proliferative activity of tumor cells is an important prognostic marker in many human cancers. Proliferation is regulated by the formation, activation and degradation of a series of cyclins. Widely-used immunohistochemical markers to assess cell proliferation include PCNA and Ki-67 (Linden et al., 1992). In 1 of our 10 UV-irradiated skin specimens, a strong increase in PCNA expression in keratinocytes, but not in melanocytes, was detected. We could not detect any changes in the expression of cyclin D1 or cyclin E2 in the UV-irradiated skin, which is possibly due to the fact that UV-induced changes in cell cycle proteins are early events that might already be removed at this late time-point (>1 yr).
DKK1, a secreted protein preferentially expressed by palmoplantar fibroblasts in the dermis inhibits melanocyte growth and differentiation through canonical Wnt signaling, β-catenin expression and MITF function (Yamaguchi et al., 2004). In contrast, DKK3, which is expressed at higher levels in fibroblasts in nonpalmoplantar dermis has no effect on melanocyte growth and function (Yamaguchi et al., 2005). The potential role(s) of DKK proteins in melanoma is totally unknown. An analysis of the expression profile of UVB responses in normal human melanocytes detected a >2-fold induction of DKK1 gene expression after UVB irradiation; however, in many melanoma cell lines a loss of DKK1 expression has been reported (Yang et al., 2006). A recent study found a strong reduction of DKK3 expression in human primary melanomas and metastases compared to normal skin (Kuphal et al., 2006). In our study, DKK1 could not be detected in the nonpalmoplantar skin that was UV-irradiated (confirming our earlier studies) while DKK3 expression showed a regular pattern in the upper epidermal layers and did not exhibit any significant changes in response to UV exposure.
We characterized the expression of Bcl-2 as it represents the prominent marker within the large Bcl-2 family and numerous studies have been published regarding the effects of UV irradiation on human skin with respect to Bcl-2 expression. In our study, Bcl-2 immunoreactivity was found predominantly in the basal cell layer of the skin specimens. One study that analyzed human skin in situ 6 h after a single SS-irradiation found a transient reduction of ∼40% in the expression of Bcl-2 (Isoherranen et al., 1999). We could not detect any significant differences in the expression of Bcl-2 between UV-irradiated and unirradiated skin. Regarding the expression pattern of Bcl-2 after UV exposure, the literature is contradictory. One in vitro study described an induction of Bcl-2 at the mRNA level, but not at the protein level after UVB (Bivik et al., 2005). In vitro studies on human melanocytes revealed no changes in expression of Bcl-2 after UVA or UVB irradiation (Kim et al., 2000; Zhang and Rosdahl, 2003), while other groups reported that Bcl-2 levels decrease after UV irradiation (Im et al., 1998; Isoherranen et al., 1999; Kadekaro et al., 2003).
In summary, among the 20 factors examined, only MART1, TYR and EDNRB protein expression, and only in some subjects, were elevated at ≥1 yr post-UV irradiation. These findings suggest that the long-term effects of repetitive UV irradiation on human skin in these studies do not lead to significant changes in skin morphology and there is considerable subject-to-subject variation in effects on skin pigmentation. The possibility that changes in the expression of the EDNRB protein trigger downstream activation of abnormal melanocyte proliferation and, considering the key role of the melanocortin system in regulating human skin pigmentation, the long-lasting UV-induced molecular changes in the expression of factors such as proopiomelanocortin (POMC), POMC-derived peptides and melanocortin 1 receptor (MC1R), also deserves further investigation.
MATERIALS AND METHODS
Study subjects, UV irradiation and dosimetry
This study involved volunteer subjects with skin phototypes 2-3.5, as shown in Table 1, and was approved by the Research Involving Human Subjects Committees of the U.S. Food and Drug Administration and Beiersdorf AG. Written informed consent was obtained from each donor. Each subject's MED was determined prior to the start of the experiment, as detailed in (Miller et al., 2006; 2008; Wolber et al., 2008; Yamaguchi et al., 2008b). Subjects did not receive significant UV exposure between the times of the initial experimental irradiation and the long-term biopsies.
Group 1 - Sunlamp
Six subjects (T11, T16, T32, T35, T47 and T50), from the study detailed in (Miller et al., 2008), were studied with long-term follow-up. For the repetitive Sunlamp-irradiation protocol, a 12-lamp tanning bed canopy (≥95%UVA, ≤5%UVB) was used and monitored as previously detailed (Miller et al., 2008). Three different repeated UV exposure regimens were used over the course of 3-4 weeks on the back of each subject. Cumulative doses were 1,900 J/m2, 2,900 J/m2 and 4,200 J/m2 for low, medium and high dose exposure regimens, respectively.
Group 2 - SS
Four subjects (B1, B2, B6 and B8, from (Wolber et al., 2008), were studied with long-term follow-up. For the repetitive SS-irradiation protocol, an Oriel 1600W SS was used and doses were monitored as previously detailed (Schlenz et al., 2005; Miyamura et al., 2007; Wolber et al., 2008). Irradiations were given a total of 10 times over 2 weeks on 5 consecutive days with cumulative doses that ranged between 333 and 1030 J/m2. Irradiation doses varied between individuals depending on each subject's MED to induce comparable tanning.
Biopsies and Immunohistochemical analysis
Skin biopsies were taken from the control and UV-exposed sites at various times after the last UV exposure. Each biopsy was placed dermis side down on a Millipore filter and was then fixed in 4% formaldehyde, embedded in paraffin, sectioned at 3 μm-thickness, mounted on silane-coated glass slides, and then stained using immunohistochemistry, as previously described (Yamaguchi et al., 2004; 2006). Briefly, specimens were deparaffinized twice with xylene for 5 min and were then dehydrated with a graduated series of ethanol, followed by antigen retrieval via boiling in antigen unmasking solution (Vector Laboratories, Inc. Burlingame, CA, USA) for 12 min. For antigens expressed in low abundance, 1 mM EDTA-heat antigen retrieval was used. Specimens were subsequently incubated with 10% goat, 10% horse or 10% donkey serum (Vector Laboratories) for 30 min at 37°C, as appropriate, and then with primary antibodies in 5% goat serum (5% horse or 5% donkey serum respectively) at 4°C overnight. Secondary antibodies were used as appropriate for the primary antibody, Alexa Fluor 488/594 anti-mouse, anti-rabbit or anti-goat IgG (H+L) (at 1:500 dilution, Molecular Probes Inc., Eugene, OR, USA). Staining was analyzed using a Leica DMRB/DMLD fluorescence microscope, and an internal control was used each time to control for reproducible antibody staining. Negative controls omitting the primary antibody were performed each time.
Protein expression using the following antibodies was measured: SCF antibody (at 1:10 dilution; R&D Systems, Minneapolis, MN, USA), SCF R/KIT antibody (at 1:100 dilution; R&D Systems), bFGF antibody (at 1:10 dilution; R&D Systems), FGFR1 antibody (at 1:100 dilution; Abcam Inc, Cambridge, MA, USA), ET-1 antibody (at 1:200 dilution; Abcam Inc), EDNRB antibody (at 1:100 dilution; Abcam Inc), GM-CSF antibody (at 1:20 dilution; R&D Systems), HGF antibody (at 1:10 dilution; R&D Systems), PCNA antibody (at 1:200 dilution; Dako Inc, Carpinteria, CA, USA), cyclin D1 antibody (at 1:20 dilution; Zymed Laboratories, South San Francisco, CA, USA), cyclin E2 antibody (at 1:20 dilution; Cell Signaling Technology, Beverly, MA, USA), E-cadherin antibody (at 1:100 dilution; Takara Bio Inc, Shiga, Japan), N-cadherin antibody (at 1:10 dilution; Dako Inc), β-catenin antibody (at 1:100 dilution; Cell Signaling Technology), Bcl-2 antibody (at 1:20 dilution; Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA), DKK-1 antibody (at 1:50 dilution; R&D Systems) and DKK-3 antibody (at 1: 100 dilution; R&D Systems).
Tissue in Situ Hybridization
Tissue in situ hybridization was performed as previously detailed (Passeron et al., 2007a; 2007b; Valencia et al., 2006). Oligonucleotide probes specific for human EDNRB were designed, and target sites were selected based on the analysis of sequence matches and mismatches BLAST (GenBank). Probes showed no evidence of cross-reaction with sequences of other genes including other ETR family genes. Optimal results were obtained with the following probes: sense primer 5′- CATACGATTTAGGTGACACTATAGgccatttggagctgagatgt -3′; antisense primer 5′-GCGCGTAATACGACTCACTATAGGGgacaaggaccaggcaaaaga -3′. The probes were 3′ tailed with digoxigenin-11-dUTP with a DIG RNA labeling kit (Roche, Basel, Switzerland), according to recommendations of the manufacturer. Briefly, after deparaffinization and rehydration, skin sections were immersed in antigen retrieval solution and heated in a microwave for 12 min then cooled for 20 min. Slides were then washed in glycine solution (2 mg/ml in PBS) for 10 min, washed twice in PBS, and then placed in 200 ml acetylation buffer (0.1 M triethylamine, pH 8.0, containing 0.25% acetic anhydride) for 15 min. After further washing in 4X SSC for 10 min, samples were incubated in prehybridization solution (2X SSC, 50% deionized formamide) for 1 h at 47°C. After overnight hybridization at 47°C, samples were placed in hybridization solution (Mutsuga et al., 2004) containing 10 μl purified DIG-labeled antisense riboprobe. Samples then were incubated in 10 mM Tris-HCl, 0.5 M NaCl, and 0.25 mM EDTA (TNE) buffer, treated with RNaseA for 30 min, and returned to TNE buffer for 3 min, all at 37°C. After washing in 0.1X SSC for 15 min at 47°C, samples were blocked for 30 min and incubated with anti-DIG/HRP conjugate (DAKO) for 40 min at room temperature. The tyramide signal amplification system (GenePoint kit, DAKO) was used with VIP solution (Vector Laboratories) according to the manufacturers' instructions. Samples were observed and photographed in a Leica DMRB microscope.
Melanocyte Density and Melanin Content
Melanocytes were counted following staining for tyrosinase, MART1 and MITF, and their density along the epidermal:dermal border was determined as cells/mm. Primary antibodies used were αPEP7h (at 1:750 dilution) to detect tyrosinase (Yamaguchi et al., 2004) and Ab3 (at 1:100 dilution, NeoMarkers, Fremont, CA, USA) to detect MART1. Fluorescence intensities for antibodies detecting melanocyte-specific markers were normalized against DAPI staining.
For measurements of melanin content, specimens were stained by the Fontana-Masson method (Bancroft and Stevens, 1982). Transmitted light intensity was measured by the Leica DMRB[unk]DMLD microscope and ScionImage software was used to analyze melanin quantity from integrated density in the skin sections, as previously described (Yamaguchi et al., 2006; 2008a). Five randomly selected areas of each specimen were photographed and quantitated.
Statistical analyses
Statistical analysis was performed using Microsoft Office Excel Analysis Toolpak. Statistical differences between irradiated specimens and controls were determined by Student's t-test with a two-tailed distribution (two-sample unequal variance). A p< 0.05 is defined as significant.
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the National Cancer Institute at NIH, and by the Office of Science, Office of Women's Health and the Center for Devices and Radiological Health, Food and Drug Administration (FDA). The authors wish to thank Drs. Thierry Passeron and Itaru Suzuki for their advice and help on the TISH analysis.
Abbreviations
- Bcl-2
B-cell lymphoma 2
- bFGF
basic fibroblast growth factor
- DKK
dickkopf
- EDNRB
endothelin receptor B
- ET
endothelin
- GM-CSF
granulocyte macrophage colony stimulating factor
- FGFR
fibroblast growth factor receptor
- HGF
hepatocyte growth factor
- MART1
melanoma antigen recognized by T-cells
- MED
minimal erythema dose
- MITF
microphthalmia transcription factor
- PCNA
proliferating cell nuclear antigen
- SCF
stem cell factor
- SS
solar simulator
- TYR
tyrosinase
- UV
ultraviolet radiation
- LLP
long-lasting pigmentation
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Ahn GY, Butt KI, Jindo T, Yaguchi H, Tsuboi R, Ogawa H. The expression of endothelin-1 and its binding sites in mouse skin increased after ultraviolet B irradiation or local injection of tumor necrosis factor alpha. J Dermatol. 1998;25:78–84. doi: 10.1111/j.1346-8138.1998.tb02354.x. [DOI] [PubMed] [Google Scholar]
- Aoki H, Moro O, Tagami H, Kishimoto J. Gene expression profiling analysis of solar lentigo in relation to immunohistochemical characteristics. Br J Dermatol. 2007;156:1214–1223. doi: 10.1111/j.1365-2133.2007.07830.x. [DOI] [PubMed] [Google Scholar]
- Bancroft JD, Stevens A. Theory and Practice of Histological Techniques. Churchill Livingstone; New York: 1982. [Google Scholar]
- Berking C, Takemoto R, Satyamoorthy K, Shirakawa T, Eskandarpour M, Hansson J, VanBelle PA, Elder DE, Herlyn M. Induction of melanoma phenotypes in human skin by growth factors and ultraviolet B. Cancer Res. 2004;64:807–811. doi: 10.1158/0008-5472.can-03-3438. [DOI] [PubMed] [Google Scholar]
- Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, Sampas N, Dougherty E, Wang E, Marincola F, Gooden C, Lueders J, Glatfelter A, Pollock P, Carpten J, Gillanders E, Leja D, Dietrich K, Beaudry C, Berens M, Alberts D, Sondak V. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- Bivik CA, Anderssn EB, Rosdahl IK. Wavelength-specific effects on UVB-induced apoptosis in melanocytes. A study of Bcl-2/Bax expression and keratinocyte rescue effects. Melanoma Res. 2005;15:7–13. doi: 10.1097/00008390-200502000-00003. [DOI] [PubMed] [Google Scholar]
- Brenner M, Degitz K, Besch R, Berking C. Differential expression of melanoma-associated growth factors in keratinocytes and fibroblasts by ultraviolet A and ultraviolet B radiation. Brit J Dermatol. 2005;153:733–739. doi: 10.1111/j.1365-2133.2005.06780.x. [DOI] [PubMed] [Google Scholar]
- Brenner M, Hearing VJ. Modifying skin pigmentation - approaches through intrinsic biochemistry and exogenous agents. Drug Discovery Today - Disease Mechanisms. 2008;5 doi: 10.1016/j.ddmec.2008.02.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- Clough-Gorr KM, Titus-Ernstoff L, Perry AE, Spencer SK, Ernstoff MS. Exposure to sunlamps, tanning beds, and melanoma risk. Cancer Causes Control. 2008 doi: 10.1007/s10552-008-9129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demunter A, De Wolf-Peeters C, Degreef H, Stas M, van den Oord JJ. Expression of the endothelin-B receptor in pigment cell lesions of the skin. Evidence for its role as tumor progression marker in malignant melanoma. Virchows Arch. 2001;438:485–491. doi: 10.1007/s004280000362. [DOI] [PubMed] [Google Scholar]
- Giehl KA, Nagele U, Volkenandt M, Berking C. Protein expression of melanocyte growth factors (bFGF, SCF) and their receptors (FGFR-1, c-kit) in nevi and melanoma. J Cutan Pathol. 2007;34:7–14. doi: 10.1111/j.1600-0560.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. New Eng J Med. 1999;340:1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- Grichnik JM, Burch JA, Burchette J, Shea CR. The SCF/KIT pathway plays a critical role in the control of normal human melanocyte homeostasis. J Invest Dermatol. 1998;111:233–238. doi: 10.1046/j.1523-1747.1998.00272.x. [DOI] [PubMed] [Google Scholar]
- Grichnik JM, Crawford J, Jimenez F, Kurtzberg J, Buchanan M, Blackwell S, Clark RE, Hitchcock MG. Human recombinant stem-cell factor induces melanocytic hyperplasia in susceptible patients. J Am Acad Dermatol. 1995;33:577–583. doi: 10.1016/0190-9622(95)91274-6. [DOI] [PubMed] [Google Scholar]
- Hachiya A, Kobayashi A, Yoshida Y, Kitahara T, Takema Y, Imokawa G. Biphasic expression of two paracrine melanogenic cytokines, stem cell factor and endothelin-1, in ultraviolet B-induced human melanogenesis. Am J Pathol. 2004;165:2099–2109. doi: 10.1016/S0002-9440(10)63260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipfel R, Schittek B, Bodingbauer Y, Garbe C. Specifically regulated genes in malignant melanoma tissues identified by subtractive hybridization. Br J Cancer. 2000;82:1149–1157. doi: 10.1054/bjoc.1999.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirobe T, Furuya R, Hara E, Horii I, Tsunenaga M, Ifuku O. Granulocyte-macrophage colony-stimulating factor (GM-CSF) controls the proliferation and differentiation of mouse epidermal melanocytes from pigmented spots induced by ultraviolet radiation. B. Pigment Cell Res. 2004;17:230–240. doi: 10.1111/j.1600-0749.2004.00132.x. [DOI] [PubMed] [Google Scholar]
- Hsu MY, Meier FE, Nesbit M, Hsu J-Y, Van Belle P, Elder DE, Herlyn M. E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Amer J Pathol. 2000;156:1515–1525. doi: 10.1016/S0002-9440(10)65023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S, Moro M, Peng F, Medrano EE, Cornelius J, Babcock G, Nordlund JJ, Abdel-Malek ZA. Activation of the cyclic AMP pathway by a-melanotropin mediates the response of human melanocytes to ultraviolet B radiation. Cancer Res. 1998;58:47–54. [PubMed] [Google Scholar]
- Imokawa G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 2004;17:96–110. doi: 10.1111/j.1600-0749.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Kimura M. Signalling mechanisms of endothelin-induced mitogenesis and melanogenesis in human melanocytes. Biochem J. 1996a;314:305–312. doi: 10.1042/bj3140305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Kimura M, Morisaki N. Granulocyte/macrophage colony-stimulating factor is an intrinsic keratinocyte-derived growth factor for human melanocytes in UVA-induced melanosis. Biochem J. 1996b;313(Pt 2):625–631. doi: 10.1042/bj3130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. Int J Cancer. 2007;120:1116–1122. doi: 10.1002/ijc.22453. [DOI] [PubMed] [Google Scholar]
- Isoherranen K, Sauroja I, Jansen C, Punnonen K. UV irradiation induces downregulation of bcl-2 expression in vitro and in vivo. Arch Dermatol Res. 1999;291:212–216. doi: 10.1007/s004030050396. [DOI] [PubMed] [Google Scholar]
- Iyer A, Kmiecik TE, Park M, Daar I, Blair D, Dunn KJ, Sutrave P, Ihle JN, Bodescot M, Vande Woude GF. Structure, tissue-specific expression, and transforming activity of the mouse met protooncogene. Cell Growth Differ. 1990;1:87–95. [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Wakamatsu K, Ito S, Pipitone MA, Abdel-Malek ZA. Cutaneous photobiology. The melanocyte versus the sun: who will win the final round? Pigment Cell Res. 2003;16:434–447. doi: 10.1034/j.1600-0749.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- Kim YG, Kim HJ, Kim DS, Kim SD, Han WS, Kim KH, Chung JH, Park KC. Up-regulation and redistribution of Bax in ultraviolet B-irradiated melanocytes. 13 ed. 2000. pp. 352–357. [DOI] [PubMed] [Google Scholar]
- Kuphal S, Lodermeyer S, Bataille F, Schuierer M, Hoang BH, Bosserhoff AK. Expression of Dickkopf genes is strongly reduced in malignant melanoma. Oncogene. 2006;25:5027–5036. doi: 10.1038/sj.onc.1209508. [DOI] [PubMed] [Google Scholar]
- Lahav R. Endothelin receptor B is required for the expansion of melanocyte precursors and malignant melanoma. Int J Dev Biol. 2005;49:173–180. doi: 10.1387/ijdb.041951rl. [DOI] [PubMed] [Google Scholar]
- Li G, Schaider H, Satyamoorthy K, Hanakawa Y, Hashimoto K, Herlyn M. Downregulation of E-cadherin and Desmoglein 1 by autocrine hepatocyte growth factor during melanoma development. Oncogene. 2001;20:8125–8135. doi: 10.1038/sj.onc.1205034. [DOI] [PubMed] [Google Scholar]
- Linden MD, Torres FX, Kubus J, Zarbo RJ. Clinical application of morphologic and immunocytochemical assessments of cell proliferation. Am J Clin Pathol. 1992;97:S4–13. [PubMed] [Google Scholar]
- Loftus SK, Chen Y, Gooden G, Ryan JF, Birznieks G, Hilliard M, Baxevanis AD, Bittner M, Meltzer P, Trent JM, Pavan WJ. Informatic selection of a neural crest-melanaocyte cDNA set for microarray analysis. Proc Natl Acad Sci USA. 1999;96:9277–9280. doi: 10.1073/pnas.96.16.9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass P, Hoffmann K, Gambichler T, Altmeyer P, Mannherz HG. Premature keratinocyte death and expression of marker proteins of apoptosis in human skin after UVB exposure. Arch Dermatol Res. 2003;295:71–79. doi: 10.1007/s00403-003-0403-x. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Ananthaswamy HN. Short-term and long-term cellular and molecular events following UV irradiation of skin: implications for molecular medicine. Expert Rev Mol Med. 2002;4:1–22. doi: 10.1017/S146239940200532X. [DOI] [PubMed] [Google Scholar]
- Miller SA, Coelho SG, Zmudzka BZ, Beer JZ. Reduction of the UV burden to indoor tanners through new exposure schedules: a pilot study. Photodermatol Photoimmunol Photomed. 2006;22:59–66. doi: 10.1111/j.1600-0781.2006.00206.x. [DOI] [PubMed] [Google Scholar]
- Miller SA, Coelho SG, Zmudzka BZ, Bushar HF, Yamaguchi Y, Hearing VJ, Beer JZ. Dynamics of pigmentation induction by repeated UV exposures: dose, dose interval and UV spectrum dependence. Brit J Dermatol. 2008 doi: 10.1111/j.1365-2133.2008.08708.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura Y, Coelho SG, Wolber R, Miller SA, Wakamatsu K, Zmudzka BZ, Ito S, Smuda C, Passeron T, Choi W, Batzer J, Yamaguchi Y, Beer JZ, Hearing VJ. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007;20:2–13. doi: 10.1111/j.1600-0749.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- Montone KT, van BP, Elenitsas R, Elder DE. Proto-oncogene c-kit expression in malignant melanoma: protein loss with tumor progression. Mod Pathol. 1997;10:939–944. [PubMed] [Google Scholar]
- Morin PJ. b-Catenin signaling and cancer. Bioessays. 1999;21:1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Mutsuga N, Shahar T, Verbalis JG, Brownstein MJ, Xiang CC, Bonner RF, Gainer H. Selective gene expression in magnocellular neurons in rat supraoptic nucleus. J Neurosci. 2004;24:7174–7185. doi: 10.1523/JNEUROSCI.2022-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan FP, Dudek J, Merlino G, De Fabo EC. Animal models of melanoma: an HGF/SF transgenic mouse model may facilitate experimental access to UV initiating events. Pigment Cell Res. 2003;16:16–25. doi: 10.1034/j.1600-0749.2003.00014.x. [DOI] [PubMed] [Google Scholar]
- Noonan FP, Recio JA, Takayama H, Duray P, Anver MR, Rush WL, De Fabo EC, Merlino G. Neonatal sunburn and melanoma in mice. Nature. 2001;413:271–272. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- Passeron T, Coelho SG, Miyamura Y, Takahashi K, Hearing VJ. Immunohistochemistry and tissue in situ hybridization in the study of human skin melanocytes. Exp Dermatol. 2007a;16:162–170. doi: 10.1111/j.1600-0625.2006.00538.x. [DOI] [PubMed] [Google Scholar]
- Passeron T, Valencia JC, Bertolotto C, Hoashi T, Takahashi K, Le Pape E, Ballotti R, Hearing VJ. SOX9 is a key player in UVB-induced melanocyte differentiation and pigmentation. Proc Natl Acad Sci USA. 2007b;104:13984–13989. doi: 10.1073/pnas.0705117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen EM, Nigam SK, Goldberg ID. Scatter factor and the c-met receptor: a paradigm for mesenchymal/epithelial interaction. J Cell Biol. 1994;127:1783–1787. doi: 10.1083/jcb.127.6.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenz K, Smuda C, Batzer J, Stab F, Wenck H, Elsaesser HP, Wolber R. Pigmentation mechanisms induced by different wavelengths of UV light. Pigment Cell Res. 2005;18:S33. [Google Scholar]
- Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanoma. Proc Natl Acad Sci USA. 1993;90:6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary A, Robert C, Sarasin A. Deleterious effects of ultraviolet A radiation in human cells. Mutat Res. 1997;383:1–8. doi: 10.1016/s0921-8777(96)00041-9. [DOI] [PubMed] [Google Scholar]
- Steinberg MS, McNutt PM. Cadherins and their connections: adhesion junctions have broader functions. Curr Opin Cell Biol. 1999;11:554–560. doi: 10.1016/s0955-0674(99)00027-7. [DOI] [PubMed] [Google Scholar]
- Straume O, Akslen LA. Importance of vascular phenotype by basic fibroblast growth factor, and influence of the angiogenic factors basic fibroblast growth factor/fibroblast growth factor receptor-1 and ephrin-A1/EphA2 on melanoma progression. Am J Pathol. 2002;160:1009–1019. doi: 10.1016/S0002-9440(10)64922-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, Korossy KS, Miller SA, Beer JZ, Hearing VJ. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin and photosensitivity. FASEB J. 2003;17:1177–1179. doi: 10.1096/fj.02-0865fje. [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Yamaguchi Y, Batzer J, Coelho SG, Zmudzka BZ, Miller SA, Wolber R, Beer JZ, Hearing VJ. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Invest Dermatol. 2005;124:1326–1332. doi: 10.1111/j.0022-202X.2005.23760.x. [DOI] [PubMed] [Google Scholar]
- Ting W, Schultz K, Cac NN, Peterson M, Walling HW. Tanning bed exposure increases the risk of malignant melanoma. Int J Dermatol. 2007;46:1253–1257. doi: 10.1111/j.1365-4632.2007.03408.x. [DOI] [PubMed] [Google Scholar]
- Tronnier M, Alexander M, Wolff HH. Adhesion molecule expression in normal skin and melanocytic lesions. Role of UV-irradiation and architectural characteristics in nevi. J Cutan Pathol. 1997;24:278–285. doi: 10.1111/j.1600-0560.1997.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Valencia JC, Watabe H, Chi A, Rouzaud F, Chen KG, Vieira WD, Takahashi K, Yamaguchi Y, Berens W, Nagashima K, Shabanowitz J, Hunt DF, Appella E, Hearing VJ. Sorting of Pmel17 to melanocytes through the plasma membrane by AP1 and AP2: evidence for the polarized nature of melanocytes. J Cell Sci. 2006;119:1080–1091. doi: 10.1242/jcs.02804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valery C, Grob JJ, Verrando P. Identification by cDNA microarray technology of genes modulated by artificial ultraviolet radiation in normal human melanocytes: relation to melanocarcinogenesis. J Invest Dermatol. 2001;117:1471–1482. doi: 10.1046/j.0022-202x.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rao U, Mascari R, Richards TJ, Panson AJ, Edington HD, Shipe-Spotloe JM, Donnelly SS, Kirkwood JM, Becker D. Molecular analysis of melanoma precursor lesions. Cell Growth Differ. 1996;7:1733–1740. [PubMed] [Google Scholar]
- Wolber R, Schlenz K, Wakamatsu K, Smuda C, Nakanishi Y, Hearing VJ, Ito S. Pigmentation effects of solar simulated radiation as compared with UVA and UVB radiation. Pigment Cell Res. 2008 doi: 10.1111/j.1755-148X.2008.00470.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff K, Johnson RA, Suurmond D. Fitzpatrick's Color Atlas and Synopsis of Clinical Dermatology. McGraw-Hill; New York: 2005. [Google Scholar]
- Yamaguchi Y, Beer JZ, Hearing VJ. Melanin mediated apoptosis of epidermal cells damaged by ultraviolet radiation: factors influencing the incidence of skin cancer. Arch Dermatol Res. 2008a;300(Suppl 1):S43–S50. doi: 10.1007/s00403-007-0807-0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007;282:27557–27561. doi: 10.1074/jbc.R700026200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Coelho SG, Zmudzka BZ, Takahashi K, Beer JZ, Hearing VJ, Miller SA. Cyclobutane pyrimidine dimer formation and p53 production in human skin after repeated UV irradiation. Exp Dermatol. 2008b;17 doi: 10.1111/j.1600-0625.2008.00722.x. in press. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Hearing VJ, Itami S, Yoshikawa K, Katayama I. Mesenchymal-epithelial interactions in the skin: aiming for site-specific tissue regeneration. J Dermatol Sci. 2005;40:1–9. doi: 10.1016/j.jdermsci.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Itami S, Watabe H, Yasumoto K, Abdel-Malek ZA, Kubo T, Rouzaud F, Tanemura A, Yoshikawa K, Hearing VJ. Mesenchymal-epithelial interactions in the skin: Increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J Cell Biol. 2004;165:275–285. doi: 10.1083/jcb.200311122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, Berens W, Beer JZ, Hearing VJ. Human skin responses to UV radiation: Pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006;20:1486–1488. doi: 10.1096/fj.06-5725fje. [DOI] [PubMed] [Google Scholar]
- Yang G, Zhang G, Pittelkow MR, Ramoni M, Tsao H. Expression profiling of UVB response in melanocytes identifies a set of p53-target genes. J Invest Dermatol. 2006;126:2490–2506. doi: 10.1038/sj.jid.5700470. [DOI] [PubMed] [Google Scholar]
- Young AR, Chadwick CA, Harrison GI, Nikaido O, Ramsden J, Potten CS. The similarity of action spectra for thymine dimers in human epidermis and erythema suggests that DNA is the chromophore for erythema. J Invest Dermatol. 1998a;111:982–988. doi: 10.1046/j.1523-1747.1998.00436.x. [DOI] [PubMed] [Google Scholar]
- Young AR, Potten CS, Nikaido O, Parsons PG, Boenders J, Ramsden JM, Chadwick CA. Human melanocytes and keratinocytes exposed to UVB or UVA in vivo show comparable levels of thymine dimers. J Invest Dermatol. 1998b;111:936–940. doi: 10.1046/j.1523-1747.1998.00435.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Rosdahl I. Ultraviolet A and B differently induce intracellular protein expression in human skin melanocytes - a speculation of separate pathways in initiation of melanoma. Carcinogenesis. 2003;24:1929–1934. doi: 10.1093/carcin/bgg171. [DOI] [PubMed] [Google Scholar]