Abstract
The functional consequences of changes in membrane lipid composition that coincide with malignant growth are poorly understood. Sufficient data have been acquired from studies of lipid binding proteins, post-translational modifications of signaling proteins, and biochemical inhibition of lipidogenic pathways to indicate that growth and survival pathways might be substantially re-directed by alterations in the lipid content of membranes. Cholesterol and glycosphingolipids segregate into membrane patches that exhibit a liquid-ordered state in comparison to membrane domains containing relatively lower amounts of these classes of lipids. These “lipid raft” structures, which may vary in size and stability in different cell types, both accumulate and exclude signaling proteins and have been implicated in signal transduction through a number of cancer-relevant pathways. In prostate cancer cells, signaling from epidermal growth factor receptor (EGFR) to the serine-threonine kinase Akt1, as well as from IL-6 to STAT3, have been demonstrated to be influenced by experimental interventions that target cholesterol homeostasis. The recent finding that classical steroid hormone receptors also reside in these microdomains, and thus may function within these structures in a signaling capacity independent of their role as nuclear factors, suggests a novel means of cross-talk between receptor tyrosine kinase-derived and steroidogenic signals. Potential points of intersection between components of the EGFR family of receptor tyrosine kinases and androgen receptor signaling pathways, which may be sensitive to disruptions in cholesterol metabolism, are discussed. Understanding the manner in which these pathways converge within cholesterol-rich membranes may present new avenues for therapeutic intervention in hormone-dependent cancers.
Cholesterol and Cancer
Extracellular signals influencing a tumor cell must cross a lipid barrier that is still poorly understood in terms of structure and regulatory function. This brief review focuses on the hypothesis that membrane cholesterol, through its capacity to mediate changes in lipid membrane structure, influences signal transduction mechanisms related to oncogenesis. Here, we specifically explore the concept that membrane cholesterol is a facilitator of cross-talk between members of the EGFR family of receptor tyrosine kinases and members of the nuclear hormone receptor family. Among other findings in a rapidly developing literature, this idea is based on recent evidence that nuclear receptors may signal independently from their transcription function and that they appear to inhabit cholesterol-rich membrane microdomains, generally referred to as “lipid rafts”[1, 2].
Because tumor cell biologists have traditionally focused on proteins as mediators of oncogenesis, the role of lipids in tumor development and spread has received relatively limited attention. The importance of alterations in lipid metabolism to tumor development is clear, however. Many lipid modifying enzymes, including proteins that hydrolyze lipids, attach them covalently to other proteins, and translocate them across membranes, have been shown to play important roles in signaling mechanisms related to tumor formation. Certain lipid synthetic enzymes, such as fatty acid synthase[3], which is overexpressed in breast and prostate cancer, can be considered to be metabolic oncogenes. A wide variety of lipid binding motifs, some displaying a very high level of biochemical specificity, are components of proteins involved in signal transduction[4]. A role for disruption of lipid metabolism in cancer is also suggested by epidemiologic studies linking rates of certain types of cancer to Western and/or high-fat diets[5, 6].
Cholesterol is a neutral lipid that plays an important role in the formation and stabilization of membrane topology. Together with sphingolipids and saturated phospholipids, cholesterol participates in the formation of “liquid-ordered” microdomains in membranes that can be distinguished from the less structured matrix of the bulk plasma membrane. These cholesterol-rich membrane patches are most commonly referred to as “lipid rafts” and can be envisioned as discrete membrane elements residing within a larger lipid “sea”[1, 2]. Lipid rafts arise from a non-random lipid sorting mechanism, and may be either stable or dynamic depending on the subcellular context. The size of individual raft domains is also likely to vary widely in size (6-100 nm). Caveolae, which are invaginated membrane structures and cytosolic vesicles are a relatively large subtype of lipid raft (ca. 80 nm)[7]. Caveolae can be visualized by electron microscopy and their size determined by direct measurement. In contrast, non-caveolar rafts, which do not harbor the membrane deforming proteins, caveolin-1 or -3, and thus do not exhibit the distinct caveolar shape, are believed to be substantially more mobile than caveolar rafts[8, 9]. These “flat” rafts are estimated to be much smaller (6-20 nm), more mobile and less stable than caveolae. The small size of the non-caveolar, flat type of lipid raft has made them more difficult to study and this has led to considerable controversy about the nature of these microdomains in the literature, even causing some to question their existence[10]. Concerns about whether raft-like structures actually exist in membranes, based on difficulties visualizing the non-caveolar form, were recently addressed in an excellent review[9].
In addition to its role in membrane structure and fluidity, cholesterol is able to regulate transcription and other signaling processes through interaction with sterol-sensing domains in multiple proteins. Caveolin-1, which is a major mediator of cholesterol transport to the plasma membrane, is negatively regulated by cholesterol binding to the sterol-sensing domain of sterol-regulatory element binding protein (SREBP)-cleavage activating protein (SCAP)[11]. SCAP activates the transcriptional regulatory function of SREBP, which inhibits cav-1 gene expression at the chromatin level, while causing the upregulation of many genes involved in cholesterol synthesis and uptake. Cholesterol is also an essential component of the mechanism of protein trafficking to discrete subcellular regions and therefore is likely to affect subcellular protein distribution[12].
Over the past decade, extensive evidence has accumulated, particularly from biochemical studies of caveolin-containing membrane preparations, that lipid rafts sequester and exclude signaling proteins, and harbor pre-formed signal transduction complexes, suggesting that they play an important role in cell signaling. Analysis of lipid raft preparations using biochemical methods indicates that raft-like structures are present on both the inner and outer leaflets of the plasma membrane[13]. Outer leaflet rafts sequester GPI-liked proteins, while inner leaflet rafts are enriched in acylated proteins (e.g. heterotrimeric G protein alpha subunits, and Src family tyrosine kinases). Recent molecular modeling studies have indicated that the smaller, non-caveolar rafts, which may actually be highly dynamic, transient structures that form and dissociate rapidly (in comparison to caveolae or other types of membrane domains), may be particularly effective at promoting intermolecular associations between proteins that migrate into them[14]. Rafts can capture proteins by encompassing a region of slower lateral diffusion than the liquid disordered component of the membrane, and also as a result of structural features on proteins, such as lipid modifications, that enhance protein affinities for rafts. Together with the regulated recruitment of signaling proteins to the plasma membrane, selective admission (and exclusion) of specific signaling components to the lipid raft platform(s) adds a separate layer of control to signaling events within the plasma membrane. An opportune area for investigation is experiments directed toward the elucidation of the nature of the lipid raft microdomain(s), the manner in which proteins transit into and out of these regions, and the characterization of the types of signals that pass through them. Current estimates indicate that proteins that can move laterally in the membrane (i.e., are not tethered to a larger structure) will reside within rafts for 25-60μs, depending on the size of the raft domain[14]. This view of raft organization and stability suggests that rafts that are not assembled into cytoskeletal or intercellular junctional complexes, form a platform where collision rates between cognate signaling components may be facilitated, thereby amplifying signal transduction through select pathways.
It was reported about a hundred years ago[15], and many times since in the biomedical literature[16, 17], that cholesterol and other fatty deposits accumulate in solid tumors. Animal studies that have modeled the effect of increases in circulating cholesterol have shown that the growth of human tumor xenografts is stimulated in the high cholesterol environment, and that this effect coincides with changes in signal transduction mechanisms consistent with the promotion of an oncogenic state [18]. Increases in circulating cholesterol also increase the cholesterol content of lipid raft membranes, as demonstrated by direct biochemical analysis of tumors carried in rodent hosts[18]. Epidemiologic studies have suggested that prostate cancer may be one of several malignancies that demonstrates a sensitivity to circulating cholesterol levels, with higher levels promoting the disease[19] and lower levels, caused by pharmacologic manipulation, to be protective[20].
The lipid raft model of membrane organization is useful in conceptualizing the effect of increased levels of membrane cholesterol on oncogenic signaling. Expansion of the cholesterol-rich membrane compartment, within certain limits, is likely to promote the formation of raft-resident signaling complexes that promote cell proliferation and cell survival[18, 21]. Expansion of the lipid raft compartment may also stimulate the production of tumor-derived microvesicles, which have been shown to contain a lipid composition similar to lipid rafts. These structures may transfer signaling complexes and other bioactive molecules between tumor cells and from tumor cells and inflammatory cells to various cell types in the tumor microenvironment. Experiments with isolated microvesicles indicate that these structures have a capacity to alter the behavior of tumor cells in a paracrine manner, and to alter the tumor microenvironment in ways relevant to cancer progression, including the stimulation of processes that promote angiogenesis and metastasis[22]. Consequently, increases in the extent of cholesterol-rich membranes may promote autocrine as well as paracrine mechanisms of signal transduction that affect not only tumor cell behavior but that can alter the tumor microenvironment and the capacity of tumor cells to metastasize to distant sites.
Our group has been studying the potential effect of cholesterol as a mediator of signal transduction using prostate cancer models. We have reported that EGFR signaling to the pro-survival serine-threonine kinase, Akt1[18, 23], and from IL-6 to STAT3[24], are cholesterol-sensitive in human prostate cancer cells and that these signaling mechanisms can be disrupted by cholesterol targeting approaches. The cholesterol-sensitivity of EGFR/ErbB-dependent pathways is potentially provocative in the case of prostate cancer because a number of studies have linked the EGFR family of receptor tyrosine kinases to mechanisms of androgenic regulation, including progression to androgen independence[25-27].
The EGFR and lipid raft microdomains
The EGFR/ErbB1 is a receptor tyrosine kinase that signals by ligand-dependent and – independent dimerization with itself or with several paralogs within the EGFR family[28, 29]. Within this family the proto-oncoprotein, HER2/ErbB2, is the preferred dimerization partner. Signaling through HER2 was recently implicated in androgen-independent activation of the androgen receptor (AR) in prostate cancer[26]. Studies from our group have suggested that signaling from EGFR may actually functionally inhibit the AR[30, 31], suggesting that even at the level of the plasma membrane, signals originating from the EGFR and HER2 may be directed downstream through different pathways[25].
Both EGFR and HER2 have been found from biochemical studies to be associated with lipid rafts. Several groups have shown that depletion of plasma membrane cholesterol can result in EGFR activation[32-34], suggesting that rafts may serve to sequester the EGFR in a manner that allows rapid activation from upstream events, such as ligand binding. Tyrosine phosphorylation sites on the cytoplasmic domain of the EGFR are differentially sensitive to absorption of cholesterol from cell membranes, indicating that some signaling pathways affected by the EGFR are cholesterol-sensitive, while others may not be[34]. Phosphorylation on Tyr845 on the EGFR, a site within the activation loop of the kinase domain, is phosphorylated by pp60Src. Phosphorylation at this site, which correlates with increased kinase activity, is enhanced by cholesterol depletion[34]. The finding that the Src phosphorylation site on EGFR is cholesterol-sensitive potentially links the EGFR to the actin cytoskeleton, and other sites of action of Src, through a cholesterol-regulated pathway. In contrast to the Tyr845 site, the Tyr1045 phosphosite, which binds the c-Cbl ubiquitin ligase involved in down-regulation of the receptor, was not found to be cholesterol-sensitive[34]. These data suggest that proteins that reside within rafts can still coordinate signals from both cholesterol-sensitive and –insensitive pathways. The manner in which this occurs is not understood.
A recent study using single-particle tracking has identified cholesterol as a mediator of lateral mobility of EGFR and HER2 in living cells[35]. In that study, cholesterol depletion resulted in the restriction of mobility and confinement of EGFR and HER2 into nanoscale membrane domains, while cholesterol repletion restored lateral movement of both receptors. Notably, HER2 was more mobile that EGFR, a distinction that is potentially attributable to differences in the relative ability of the two receptors to interact with the cortical cytoskeleton. These findings are consistent with the view that signals from the two receptors diverge at the level of the plasma membrane. Applied to a cancer cell that accumulates cholesterol relative to corresponding normal cell types, the implications of these results are (1) that lateral diffusion of EGFR family members is increased with malignancy and (2) that cholesterol may alter the rate of formation and dissociation of receptor-associated signaling complexes. They also imply that different receptor tyrosine kinases may respond differently to changes in the cholesterol content of cell membranes.
EGFR activation may also change the raft environment by promoting the formation of oligomeric caveolin at the plasma membrane[36]. This would have the effect of drastically increasing the potential binding sites for caveolin-associated proteins, with likely dramatic effects on downstream signaling. The EGFR appears to redirect cytosolic caveolin to the plasma membrane by a mechanism that involves phosphorylation of caveolin-1 on tyrosine 14 by Src[36], a well-known EGFR effector.
Nuclear receptors and lipid rafts
Evidence has accumulated that members of the classical nuclear receptor family of hormonal mediator proteins can mediate rapid responses to steroid hormones in a manner that does not directly involve their transcriptional regulatory function.
1,25 dihydroxy vitamin D3, (1,25-(OH)2D3; calcitriol) is the active form of vitamin D. 1,25-(OH)2D3 exerts its biological effects by binding to the Vitamin D receptor (VDR) which, as with the other nuclear hormone receptors, was thought to function exclusively as a nuclear receptor, expressed in a wide variety of tissues including muscle, adipose tissue, and bone. In the mid 1980’s reports began to surface describing rapid responses (occurring in minutes to hours) to 1,25-(OH)2D3 that were too quick to be caused by transcriptional upregulation (reviewed in [37]). Recent evidence using direct binding analysis and VDR knockout mice have revealed that the classical VDR is the likely mediator of at least some of these rapid effects [38, 39]. In addition, at least one other membrane-associated vitamin D binding protein has been described that mediates rapid cytosolic events: the 1,25D3-membrane associated rapid response steroid binding protein (1,25D3-MARRS) [40]. It is now clear that a fraction of the classical VDR is found in the plasma membrane of 1,25-(OH)2D3 cell targets and that it is concentrated in caveolae-enriched membrane fractions[39].
Rapid responses to estrogens that are not affected by translational or transcriptional inhibitors have also been reported in a wide variety of hormone sensitive tissue cells [41-44]. Thus began a search for the membrane localized estrogen receptor (ER). Here again there has been uncertainty as to whether the classical ERs were responsible for these effects or if another class of ER (e.g. G protein-coupled receptor (GPR)30) was involved[45-47]. Recent evidence using knockout mice, mass spectrometry and RNA interference has provided some clarity by demonstrating that in the tested cells and tissues only the classical ERs bind estradiol (E2) and signal in response to E2 stimulation, suggesting that nuclear and plasma membrane ERs are the same proteins [48].
Recent reports from several groups have shown ERα to be associated with lipid raft and/or caveolar fractions [49, 50]. Razandi et al. (2002) demonstrated that ERα colocalized with caveolin in membranes isolated by a unique silica-coating technique performed on rat lung microvessels in situ, that ERα and caveolin-1 coimmunoprecipiated, and that caveolin-1 and ERα co-localized at membranes as assessed by immunofluorescence microscopy[51]. Interestingly, this report also demonstrates that E2 can regulate caveolin synthesis, and that caveolin-1 assists in the translocation of the ER to the plasma membrane, suggesting that estrogen signaling results in greater ER plasma membrane expression (i.e. a positive feedback loop). A study in which E2 was administered in vivo to newborn and middle-aged rats (13-14 months) demonstrated that caveolae and caveolin-1 expression was increased in the bladder sarcolemma, supporting the type of positive regulation of caveolin expression by E2 previously noted only in tissue culture[52].
Additional reports have provided insight into the apparent caveolin/ERα interaction. Mutation of serine 522 to alanine (S522A) reduced ERα membrane localization and colocalization with caveolin-1, but other S to A mutations affected neither[53]. Other analyses suggest that plasma membrane localization of the ERα is palmitoylation dependent. One study on the 46 kD isoform of the ER (this protein lacks the N terminus but is otherwise identical to ERα) first revealed an enrichment in caveolar fractions and colocalization with caveolin-1, as well as evidence that the protein is palmitoylated[54]. Interestingly, two subsequent reports[55, 56], applying mutational approaches to the study of the full length ERα, establish that mutation of cysteine 447 diminishes ERα palmitoylation, membrane localization and response to E2. Given the likely importance of palmitoylation for association with raft membranes, regulated S-acylation of cysteine residues in the ER might provide the basis for its nuclear vs. raft localization.
Mapping studies have indicated that residues 82-101 of caveolin-1 (the caveolin interaction domain) and residues 1-282 of ERα mediate the direct interaction between the two proteins[57], while serine 522 of ERα appears to be important for membrane translocation[58]. In comparison to murine ERαWT, the ERαS522A mutant resulted in 62% fewer receptors expressed at the plasma membrane, with little influence on receptor affinity for ligand, and no effect on the ERα nuclear function. Additionally, the 522 mutant was shown to be much less effective at supporting E2-mediated ERK activation, cAMP generation, and stimulation of IP3. Other S to A substitutions (at amino acid residues 10 and 582) had no effect on either E2 binding to the membrane or signaling by E2, in comparison to ERαWT[58].
In contrast to the extensive reports describing the nongenomic function of ERα, the nongenomic signaling function of ERβ is not well described, and only a single report describes the localization of this protein to caveolae[59]. However, ERβ, along with the AR and Src, has been shown to rapidly assemble into a cytoplasmic complex in LNCaP cells following treatment with androgens or E2 and this complex appears to be involved in the control of cell proliferation in response to the hormonal signal[60].
The consequences of the ER localizing to, and functioning within, cholesterol-rich membrane microdomains may indeed explain some of the reported nongenomic signaling induced by E2. For example, an extensive literature describes the ability of E2 to activate eNOS[61, 62], with evidence indicating roles for signaling intermediates such as Src family tyrosine kinases, MAP kinases, PI3 kinase and Akt[63-67]. There is also evidence that the ERα to eNOS signals are mediated by heterotrimeric G protein Giα subunits (i.e. pertussis toxin sensitive) [58, 68, 69]. An important observation is that eNOS[70, 71] and heterotrimeric G protein α subunits[72-74] are known to preferentially localize to caveolae (and lipid rafts in general), thus providing a localization-based mechanism that might explain how E2 can rapidly promote NO production.
The literature on potential non-genomic actions of the androgen receptor (AR) is less robust than that of the ER; however, compelling evidence of similar non-genomic mechanisms of AR activation has been reported by several groups. Treatment of the LNCaP human prostate cancer cell line, or the MCF7 or T47D breast cancer cell lines, with either E2 or androgen results in the rapid formation of a cytosolic signaling complex containing ERα/ERβ the AR and the tyrosine kinase Src[60]. This multi-protein complex appears to be involved in the regulation of cell proliferation by these hormones. Src, through a physical interaction with the proline-rich region of the AR, seems to play an important role in this mechanism. Recently, the scaffolding protein, modulator of nongenomic activity of estrogen receptor (MNAR), was also identified as a component of this complex[75]. A recent report suggests that a nongenomic signaling pathway involving AR, Src and MNAR is upregulated constitutively in LNCaP variants that are androgen-independent, suggesting that this complex may play a role in progression to hormone-insensitive disease[76].
Like other nuclear receptors, the AR has been identified in caveolar/lipid raft biochemical fractions and AR has been shown to interact with caveolin-1. Using a mammalian two-hybrid system, AR domains required for interaction with caveolin-1 were mapped; they include the N-terminal AF1 domain and the ligand-binding domain[77]. AR contains a potential caveolin scaffolding domain interaction sequence (YSWMGLMVFAMGWRSF), however the potential role of this region in AR interactions with caveolins has not yet been studied in detail.
Caveolin-1 has been identified as a tumor progression marker in prostate cancer and has been shown in animal models to be a potentially direct mediator of aggressive disease[78-80]. This situation appears to be different from the role of caveolin in the mammary gland, where it functions to inhibit oncogenic signaling[81]. A signaling function that has been identified for caveolin-1 in prostate cancer cells involves its ability to facilitate activation of the pro-survival kinase, Akt1, by interaction with, and inhibition of, the phosphatases PP1 and PP2A[79]. This study also demonstrated that overexpression of caveolin-1 augments androgenic signaling by facilitating translocation of phosphorylated AR to the nucleus.
Intersection of AR and EGFR signaling within lipid raft microdomains
Akt1 has been reported by several groups to be sensitive to manipulations in cholesterol level. Direct biochemical analysis has verified that a subpopulation of Akt1 molecules resides within lipid raft microdomains[18, 82]. This is shown in Figure 1, where about 10% of endogenous Akt1 in the non-nuclear space (in the figure, cytoplasm + non-raft membrane (C+M) and lipid raft membrane (Raft) in LNCaP cells is shown to be present in the raft compartment. Rapid effects of androgen (R1881) on Akt1 phosphorylation can be seen from these data. In addition to interesting steady-state differences in immuno-reactivity with phosphosite-specific antibodies seen when the C+M and lipid raft fractions are compared, changes in phosphorylation state of Akt1 in response to androgen occur in the raft compartment within 15 min, and changes in the non-raft compartment are seen within 15-30 min. Interestingly, these changes are distinct and the results suggest that Akt1 in the raft and non-raft compartments are processing dissimilar signals. This pattern is in marked contrast to changes in phosphorylation state of Akt1 in response to EGFR activation with EGF. Although Akt1 phosphorylation changes are more rapid than those induced by R1881, the raft and non-raft compartments again are distinct from each other. Notably, the distinction between the pattern of phosphorylation seen with androgen and EGF is most evident when Akt1 is analyzed in the raft fraction. Another interesting feature of these results is that LNCaP cells do not express caveolin-1, and therefore the phenomena observed in the experiment shown in Figure 1 are independent of any influences from caveolin or caveolar components that are also not present in the flat form of lipid raft. We have verified that the AR is found in lipid raft membranes and can even be stabilized there in the presence of androgen (Cinar et al. in preparation). Studies on the functional role of lipid raft-resident AR are ongoing.
Figure 1. Androgen and EGF stimulate distinct changes in Akt phosphorylation state in lipid rafts isolated from LNCaP cells.
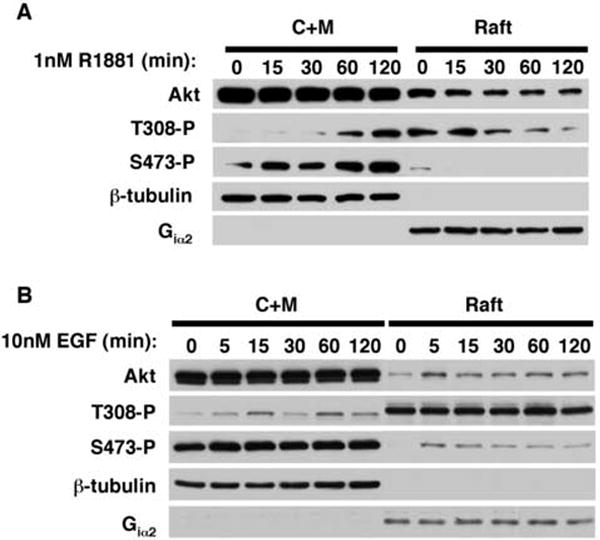
A) Serum-depleted LNCaP cells were treated with 1nM R1881 or B) 10nM EGF for the indicated times and fractionated into cytoplasmic/Triton X100-soluble membrane (C+M) and raft fractions. Equal amounts of C+M and raft fractions were resolved by SDS-PAGE and immunoblotted with antibodies to total Akt1, Thr308-phosphorylated Akt1 (T308-P), and Ser473-phosphorylated Akt1 (S473-P). The fidelity of fractionation was confirmed by blotting for β-tubulin (cytosolic marker) and Giα2 (raft marker).
These data suggest the following interesting hypothesis: Non-genomic signals from androgen, and from EGFR activation, traverse the cholesterol-rich membrane subcellular compartments and, further, that signals elicited from the steroidal and peptide hormones within the raft compartment are distinct. Because both types of signals are believed to converge in prostate cancer cells to regulate proliferation and survival, the implication of this hypothesis is that the lipid raft membranes process both signals within a similar time frame. The data in Figure 1 also show that a prominent mediator of cell growth and survival signaling, the serine-threonine kinase Akt1, is a point of convergence of the two hormonal stimuli. We suggest that a potentially fruitful avenue of investigation to develop this hypothesis further is an analysis, by proteomic methods, of signaling complexes within lipid rafts that include Akt1 as a component.
Conclusions
Many studies over a long period have provided evidence that cholesterol, in association with sphingolipids and saturated phospholipids, participates in the formation of liquid-ordered membrane microdomains that are physically distinct from the larger liquid-disordered membrane. The metaphor of a “raft” is useful in helping to imagine the coherence of these structures, and their potential for mobility, within the larger “lipid sea”. Because the bulk of intracellular cholesterol accumulates in the plasma membrane, lipid rafts may be a prominent type of microdomain at the interface between tumor cells and the tumor microenvironment. However, intracellular membranes also contain cholesterol and may similarly contain raft-like membrane microdomains that play important roles in signal transduction.
Biochemical analysis of lipid rafts, along with imaging studies, have provided evidence that these microdomains accumulate, as well as exclude, certain classes of proteins. The observations that EGFR family proteins are sensitive to disruptions in cholesterol synthesis and homeostasis, and that hormone receptors, such as the VDR, ERα/β, and AR can transit and potentially signal from these cholesterol-rich domains, provides an important new avenue for investigation about how hormone-sensitive cancers are regulated. These observations may also help us to understand the mechanisms behind diet-mediated effects on cancer progression. We believe that new opportunities for therapeutic intervention are likely to emerge from focused studies of signal transduction from the lipid raft signaling platform in prostate cancer and other malignancies.
Acknowledgments
This work was supported by NIDDK R3747556, P50 DK65298 and NCI R01 CA112303 to M.R.F. and NCI R01 CA101046 to K.R.S.
Abbreviations
- 1,25D3-MARRS
1,25D3-membrane associated rapid response steroid binding protein
- Akt1
serine threonine kinase (protein kinase Bα)
- AR
androgen receptor
- E2
estradiol
- EGFR
epidermal growth factor receptor
- eNOS
endothelial nitric oxide synthase
- ER
estrogen receptor
- ERK
extracellular-signal regulated kinase
- GPI
glycosylphosphatidylinositol
- GPR
G protein-coupled receptor
- HER2/ErbB2
EGFR paralog receptor tyrosine kinase
- IL-6
interleukin-6
- IP3
inositol trisphosphate
- MNAR
modulator of nongenomic activity of estrogen receptor
- PI3-kinase
phosphoinositide 3-kinase
- PP1 and PP2A
protein phosphatases 1 and 2A
- SCAP
SREBP-cleavage activating protein
- Src
non-receptor tyrosine kinase
- SREBP
sterol-regulatory element binding protein
- STAT3
signal transducer and activator of transcription-3
- VDR
vitamin D receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 3.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 4.DiNitto JP, Cronin TC, Lambright DG. Membrane recognition and targeting by lipid-binding domains. Sci STKE. 2003;2003:re16. doi: 10.1126/stke.2132003re16. [DOI] [PubMed] [Google Scholar]
- 5.Horn-Ross PL, Morrow M, Ljung BM. Diet and the risk of salivary gland cancer. Am J Epidemiol. 1997;146:171–176. doi: 10.1093/oxfordjournals.aje.a009248. [DOI] [PubMed] [Google Scholar]
- 6.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol. 2005;23:8152–8160. doi: 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 7.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 8.van Deurs B, Roepstorff K, Hommelgaard AM, Sandvig K. Caveolae: anchored, multifunctional platforms in the lipid ocean. Trends Cell Biol. 2003;13:92–100. doi: 10.1016/s0962-8924(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 9.Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 11.Bist A, Fielding PE, Fielding CJ. Two sterol regulatory element-like sequences mediate up-regulation of caveolin gene transcription in response to low density lipoprotein free cholesterol. Proc Natl Acad Sci U S A. 1997;94:10693–10698. doi: 10.1073/pnas.94.20.10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helms JB, Zurzolo C. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic. 2004;5:247–254. doi: 10.1111/j.1600-0854.2004.0181.x. [DOI] [PubMed] [Google Scholar]
- 13.Pike LJ, Han X, Gross RW. Epidermal growth factor receptors are localized to lipid rafts that contain a balance of inner and outer leaflet lipids: a shotgun lipidomics study. J Biol Chem. 2005;280:26796–26804. doi: 10.1074/jbc.M503805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolau DV, Jr, Burrage K, Parton RG, Hancock JF. Identifying optimal lipid raft characteristics required to promote nanoscale protein-protein interactions on the plasma membrane. Mol Cell Biol. 2006;26:313–323. doi: 10.1128/MCB.26.1.313-323.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White RM. On the occurrence of crystals in tumours. J Pathol Bacteriol. 1909;13:3–10. [Google Scholar]
- 16.Swyer G. The cholesterol content of normal and enlarged prostates. Cancer Res. 1942;2:372–375. [Google Scholar]
- 17.Schaffner CP. The Prostatic Cell: Structure and Function. Alan R. Liss, Inc.; 150 Fifth Ave., New York, NY 10011: 1981. Prostatic cholesterol metabolism: regulation and alteration; pp. 10279–10324. [Google Scholar]
- 18.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bravi F, Scotti L, Bosetti C, Talamini R, Negri E, Montella M, Franceschi S, La Vecchia C. Self-reported history of hypercholesterolaemia and gallstones and the risk of prostate cancer. Ann Oncol. 2006;17:1014–1017. doi: 10.1093/annonc/mdl080. [DOI] [PubMed] [Google Scholar]
- 20.Graaf MR, Beiderbeck AB, Egberts ACG, Richel DJ, Guchelaar H-J. The risk of cancer in users of statins. J Clin Oncol. 2004;15:2388–2394. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168:1107–1118. doi: 10.2353/ajpath.2006.050959. quiz 1404-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 23.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–2231. [PubMed] [Google Scholar]
- 24.Kim J, Adam RM, Solomon KR, Freeman MR. Involvement of cholesterol-rich lipid rafts in interleukin-6-induced neuroendocrine differentiation of LNCaP prostate cancer cells. Endocrinology. 2004;145:613–619. doi: 10.1210/en.2003-0772. [DOI] [PubMed] [Google Scholar]
- 25.Freeman MR. HER2/HER3 heterodimers in prostate cancer: Whither HER1/EGFR? Cancer Cell. 2004;6:427–428. doi: 10.1016/j.ccr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 27.Gross ME, Jo S, Agus DB. Update on HER-Kinase-Directed Therapy in Prostate Cancer. Clin Adv Hematol Oncol. 2004;2:53–64. [PubMed] [Google Scholar]
- 28.Carpenter G. The EGF receptor: a nexus for trafficking and signaling. Bioessays. 2000;22:697–707. doi: 10.1002/1521-1878(200008)22:8<697::AID-BIES3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res. 2003;284:99–110. doi: 10.1016/s0014-4827(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 30.Cinar B, De Benedetti A, Freeman MR. Post-transcriptional regulation of the androgen receptor by Mammalian target of rapamycin. Cancer Res. 2005;65:2547–2553. doi: 10.1158/0008-5472.CAN-04-3411. [DOI] [PubMed] [Google Scholar]
- 31.Adam RM, Kim J, Lin J, Orsola A, Zhuang L, Rice DC, Freeman MR. Heparin-binding epidermal growth factor-like growth factor stimulates androgen-independent prostate tumor growth and antagonizes androgen receptor function. Endocrinology. 2002;143:4599–4608. doi: 10.1210/en.2002-220561. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Resh MD. Cholesterol depletion from the plasma membrane triggers ligand-independent activation of the epidermal growth factor receptor. J Biol Chem. 2002;277:49631–49637. doi: 10.1074/jbc.M208327200. [DOI] [PubMed] [Google Scholar]
- 33.Ringerike T, Blystad FD, Levy FO, Madshus IH, Stang E. Cholesterol is important in control of EGF receptor kinase activity but EGF receptors are not concentrated in caveolae. J Cell Sci. 2002;115:1331–1340. doi: 10.1242/jcs.115.6.1331. [DOI] [PubMed] [Google Scholar]
- 34.Westover EJ, Covey DF, Brockman HL, Brown RE, Pike LJ. Cholesterol depletion results in site-specific increases in epidermal growth factor receptor phosphorylation due to membrane level effects. Studies with cholesterol enantiomers. J Biol Chem. 2003;278:51125–51133. doi: 10.1074/jbc.M304332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orr G, Hu D, Ozcelik S, Opresko LK, Wiley HS, Colson SD. Cholesterol dictates the freedom of EGF receptors and HER2 in the plane of the membrane. Biophys J. 2005;89:1362–1373. doi: 10.1529/biophysj.104.056192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlichenko L, Huang B, Krueger E, McNiven MA. Epithelial growth factor-induced phosphorylation of caveolin 1 at tyrosine 14 stimulates caveolae formation in epithelial cells. J Biol Chem. 2006;281:4570–4579. doi: 10.1074/jbc.M512088200. [DOI] [PubMed] [Google Scholar]
- 37.Norman AW. Vitamin D Receptor (VDR): New assignments for an already busy receptor. Endocrinology. 2006 doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 38.Bula CM, Huhtakangas J, Olivera C, Bishop JE, Norman AW, Henry HL. Presence of a truncated form of the vitamin D receptor (VDR) in a strain of VDR-knockout mice. Endocrinology. 2005;146:5581–5586. doi: 10.1210/en.2005-0806. [DOI] [PubMed] [Google Scholar]
- 39.Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrinol. 2004;18:2660–2671. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- 40.Nemere I, Farach-Carson MC, Rohe B, Sterling TM, Norman AW, Boyan BD, Safford SE. Ribozyme knockdown functionally links a 1,25(OH)2D3 membrane binding protein (1,25D3-MARRS) and phosphate uptake in intestinal cells. Proc Natl Acad Sci U S A. 2004;101:7392–7397. doi: 10.1073/pnas.0402207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12:152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- 42.Levin ER. Cellular Functions of the Plasma Membrane Estrogen Receptor. Trends Endocrinol Metab. 1999;10:374–377. doi: 10.1016/s1043-2760(99)00192-7. [DOI] [PubMed] [Google Scholar]
- 43.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 44.Pietras RJ, Nemere I, Szego CM. Steroid hormone receptors in target cell membranes. Endocrine. 2001;14:417–427. doi: 10.1385/ENDO:14:3:417. [DOI] [PubMed] [Google Scholar]
- 45.Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- 46.Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol. 2002;80:231–238. doi: 10.1016/s0960-0760(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 47.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 48.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 49.Kim HP, Lee JY, Jeong JK, Bae SW, Lee HK, Jo I. Nongenomic stimulation of nitric oxide release by estrogen is mediated by estrogen receptor alpha localized in caveolae. Biochem Biophys Res Commun. 1999;263:257–262. doi: 10.1006/bbrc.1999.1348. [DOI] [PubMed] [Google Scholar]
- 50.Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44–52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 51.Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 52.Zhu Q, Resnick NM, Elbadawi A, Kuchel GA. Estrogen and postnatal maturation increase caveolar number and caveolin-1 protein in bladder smooth muscle cells. J Urol. 2004;171:467–471. doi: 10.1097/01.ju.0000099480.18735.49. [DOI] [PubMed] [Google Scholar]
- 53.Pietras RJ, Marquez DC, Chen HW, Tsai E, Weinberg O, Fishbein M. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids. 2005;70:372–381. doi: 10.1016/j.steroids.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Acconcia F, Ascenzi P, Fabozzi G, Visca P, Marino M. S-palmitoylation modulates human estrogen receptor-alpha functions. Biochem Biophys Res Commun. 2004;316:878–883. doi: 10.1016/j.bbrc.2004.02.129. [DOI] [PubMed] [Google Scholar]
- 57.Schlegel A, Wang C, Pestell RG, Lisanti MP. Ligand-independent activation of oestrogen receptor alpha by caveolin-1. Biochem J. 2001;359:203–210. doi: 10.1042/0264-6021:3590203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 59.Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- 60.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. Embo J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caulin-Glaser T, Garcia-Cardena G, Sarrel P, Sessa WC, Bender JR. 17 beta-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ Res. 1997;81:885–892. doi: 10.1161/01.res.81.5.885. [DOI] [PubMed] [Google Scholar]
- 62.Kim KH, Bender JR. Rapid, estrogen receptor-mediated signaling: why is the endothelium so special? Sci STKE. 2005;2005:pe28. doi: 10.1126/stke.2882005pe28. [DOI] [PubMed] [Google Scholar]
- 63.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, Baron R, Collinge M, Sessa WC, Bender JR. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J Biol Chem. 2003;278:2118–2123. doi: 10.1074/jbc.M210828200. [DOI] [PubMed] [Google Scholar]
- 65.Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- 66.Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, Taniguchi N, Murata Y. Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2001;276:3459–3467. doi: 10.1074/jbc.M005036200. [DOI] [PubMed] [Google Scholar]
- 67.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal- regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein- and protein kinase A-dependent mechanism and intracellular activation of protein phosphatase 2A. Endocrinology. 2005;146:5397–5406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- 69.Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ. Regulation of cyclic adenosine 3’,5’- monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol Endocrinol. 2003;17:1792–1804. doi: 10.1210/me.2003-0040. [DOI] [PubMed] [Google Scholar]
- 70.Liu J, Garcia-Cardena G, Sessa WC. Palmitoylation of endothelial nitric oxide synthase is necessary for optimal stimulated release of nitric oxide: implications for caveolae localization. Biochemistry. 1996;35:13277–13281. doi: 10.1021/bi961720e. [DOI] [PubMed] [Google Scholar]
- 71.Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 72.Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995;270:15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- 73.Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol. 2004;143:235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moffett S, Brown DA, Linder ME. Lipid-dependent targeting of G proteins into rafts. J Biol Chem. 2000;275:2191–2198. doi: 10.1074/jbc.275.3.2191. [DOI] [PubMed] [Google Scholar]
- 75.Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci U S A. 2002;99:14783–14788. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, Marcelli M. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64:7156–7168. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- 77.Lu ML, Schneider MC, Zheng Y, Zhang X, Richie JP. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J Biol Chem. 2001;276:13442–13451. doi: 10.1074/jbc.M006598200. [DOI] [PubMed] [Google Scholar]
- 78.Yang G, Truong LD, Wheeler TM, Thompson TC. Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. Cancer Res. 1999;59:5719–5723. [PubMed] [Google Scholar]
- 79.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol. 2003;23:9389–9404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams TM, Hassan GS, Li J, Cohen AW, Medina F, Frank PG, Pestell RG, Di Vizio D, Loda M, Lisanti MP. Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer: genetic ablation of Cav-1 delays advanced prostate tumor development in tramp mice. J Biol Chem. 2005;280:25134–25145. doi: 10.1074/jbc.M501186200. [DOI] [PubMed] [Google Scholar]
- 81.Sotgia F, Williams TM, Schubert W, Medina F, Minetti C, Pestell RG, Lisanti MP. Caveolin-1 deficiency (-/-) conveys premalignant alterations in mammary epithelia, with abnormal lumen formation, growth factor independence, and cell invasiveness. Am J Pathol. 2006;168:292–309. doi: 10.2353/ajpath.2006.050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bauer B, Jenny M, Fresser F, Uberall F, Baier G. AKT1/PKBalpha is recruited to lipid rafts and activated downstream of PKC isotypes in CD3-induced T cell signaling. FEBS Lett. 2003;541:155–162. doi: 10.1016/s0014-5793(03)00287-4. [DOI] [PubMed] [Google Scholar]


