Evidence suggesting the use of adult stem cells as a viable treatment for myocardial infarction continues to mount. In this issue of the Journal of Molecular and Cellular Cardiology, Mills et al present compelling evidence that suggests mesenchymal stem cells (MSCs), when used to treat myocardial infarction, are not pro-arrhythmic and may actually help sustain the action potentials in myocytes surviving in the border zone and infarcted region. These findings stand in stark contrast to the failure of skeletal myoblasts (SKMBs) to propagate an action potential to those same regions, although both cell types appear to confer similar recovery of mechanical function in a rat myocardial infarction model. The main conclusions of the study are that (1) both approaches successfully improve mechanical function as compared to saline injection, (2) SKMBs increase the susceptibility to and incidence of ventricular tachycardia or fibrillation while the MSCs may decrease these serious electrical outcomes, (3) that the SKMBs exist as isolated islands with no gap junctional coupling to adjacent cells, while the MSCs effectively integrate into the cardiac syncytium, and (4) the amplitude of the action potential (AP) recorded by optical techniques in the presence of SKMBs shows a decrement similar to that observed in the presence of saline, while MSCs reduce this decrement in AP amplitude.
Some of these results are not surprising. Both in vitro studies[7] and human trials[8] have shown the propensity of SKMBs to induce arrhythmias while exerting mechanical benefit. Further, preliminary in vivo studies in animals and humans have shown that MSCs also can be mechanically beneficial[9-11]. However, whether this restoration of pump function is due to a change in the passive or contractile properties of the infarct is currently unknown. As SKMBs do not make electrical connections with the native myocardium, it is unlikely that they contract in synchrony with the rest of the heart. Thus, the improved mechanical function induced by SKMBs is likely due to a change in passive mechanical properties of the infarct leading to improved diastolic function. The same may be true with MSCs. However, the action potential recordings in Figure 4 of Mills et al., suggest the presence of cells with excitable membranes (as documented by the rapid upstroke in the MSC-treated group) in the infarct region. Therefore, MSCs may contribute to both passive and active contractile improvements in function. Further studies using techniques to determine regional diastolic and systolic function in the infarct region are needed to answer this question.
One of the key findings from Mills et al. is that the MSCs, unlike the SKMBs, do not increase the susceptibility to arrhythmias and may even exert a protective effect. It is the purpose of this editorial to first discuss the possible mechanisms of electrical protection exerted by the MSCs, proceed to consider the electrical properties of the cellular substrates currently under investigation for myocardial regeneration with an eye towards their electrical safety and finally discuss where future improvements and opportunities may lie.
Why do MSCs preserve electrical function more effectively than SKMBs? Some clues are provided in Figure 4 of Mills et al. In this figure they look at propagation from the normal heart (location 1) through the border zone (locations 2 and 3) and into the infarcted tissue (location 4). In the normal heart the optical recordings at all four locations show APs of similar size and shape. In the saline treated infarct, the optical APs decline in amplitude and slow in rise time in the border zone and virtually disappear in the infarct region strongly suggesting that the recordings at locations 3 and 4 are the product of passive spread of current and not active propagation. Similar recordings are made in the presence of SKMBs, again suggesting that active propagation fails in the border zone and electrotonus is responsible for the optical recordings both at the border and within the infarct zone. However, the situation is markedly different with MSC treatment; in this case the rise times of the optical APs are preserved throughout the border zone and are only minimally slowed within the infarct. The amplitude decrement is also much less severe. This suggests that active propagation rather than electrotonus may be responsible. Mills et al. use immunohistochemistry to demonstrate that all three common cardiac connexins (40, 43 and 45) are expressed by the delivered MSCs in vivo. As we have previously reported that human mesenchymal stem cells (hMSCs) make connexins and can form functional gap junctions with myocytes[12], it seems reasonable to suggest that the MSCs effectively interdigitate between islands of surviving myocytes providing a pathway for current flow (see Figure 1). But do MSCs serve only as a passive conduit of current between surviving myocytes? Although not examined in this study, a second advantage of the MSCs might be their ability to enhance the number of myocytes in both the border zone and within the infarct by facilitating survival of existing myocytes, or inducing proliferation of native myocytes or transdifferentation of native stem cells. MSCs are known to release paracrine factors that promote angiogenesis[13] and all of these processes may be enhanced by such factors.
Figure 1.
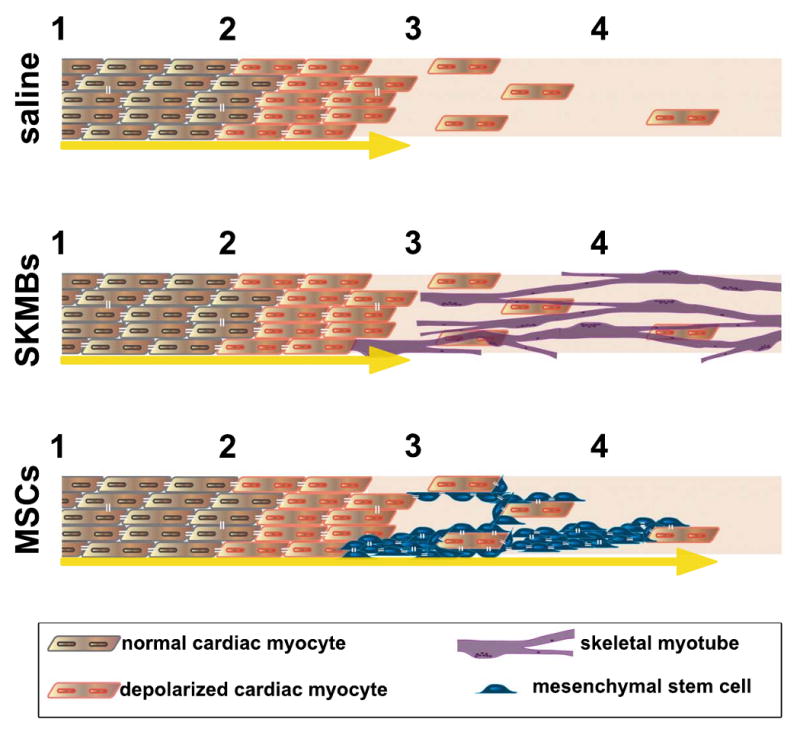
(A) Following the infarct, cells in the border zone (between 2 and 3) are depolarized, and the few surviving myocytes in the infarct zone (between 3 and 4) are poorly coupled to the border, prohibiting electric current flow into this region (yellow arrow depicts current flow terminating at interface between border zone and infarct). (B) When skeletal myoblasts (SKMBs) are added to the infarct zone they do not electrically couple, and thus do not promote electrical function in the infarct. (C) Mesenchymal stem cells (MSCs) couple to the surviving myocytes in the border and infarct zones, permitting electrical current spread into this region (depicted by yellow arrow extending into infarct zone). They may also enhance survival via angiogenesis or promote myocyte proliferation in the infarct by secretion of paracrine factors. The cartoon describes a localized region and is intended only to illustrate the basis of enhanced electrical conduction, not the diffuse (MSC) or punctuate (SKMB) distribution of the delivered cells as proposed by Mills et al.
Whatever the mechanism, Mills et al.'s results clearly indicate an advantage of MSCs over SKMBs in the setting of myocardial infarction because of their ability to more effectively preserve native electrical properties while maintaining similar benefits in mechanical function. It therefore seems reasonable to ask: is the best we can do? Or are there alternative cell therapies that might provide similar mechanical advantages and even additional advantages electrically?
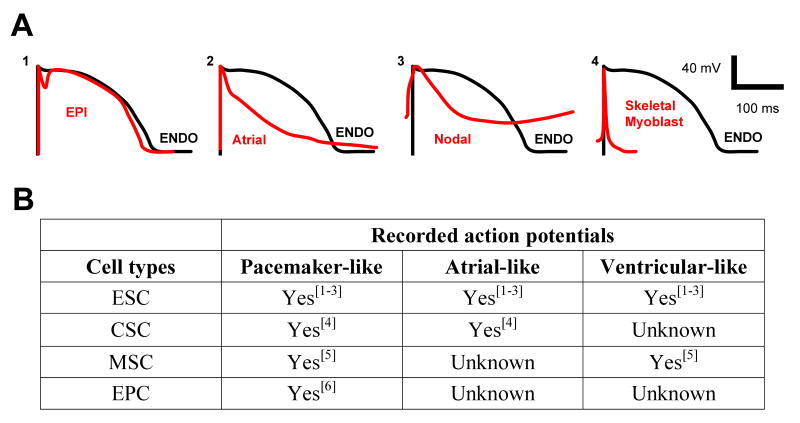
To answer this question, one can conceptualize the properties of an optimal substrate. The results of the present study suggest that gap junctional coupling is a key element to successful electrical recovery. In its absence, the SKMBs—a successful mechanical substrate—are arrhythmogenic. But would genetically engineering the SKMBs to express connexins and thus allowing them to integrate into the syncytium be sufficient? In vitro studies show that SKMBs can be made to express connexins and these connexin-containing cells are less prone to induce reentrant excitation[7, 14]. However, these studies were performed with neonatal rat ventricular myocytes where action potential duration is much shorter than in larger animals and closer in duration to that of the SKMBs. Thus this model might not provide the most relevant test. It is well known that increased dispersion of repolarization across the ventricular wall leads to increased risk of arrhythmias[15, 16]. It would therefore be optimal if the lost myocytes were replaced with mechanically active cells whose electrical phenotypes were identical to the native myocytes. Figure 2A illustrates the normal transmural dispersion of repolarization by comparing ventricular endocardial and epicardial action potentials. It also shows how much more profound this effect would be if native cells were replaced with SKMBs (even those expressing connexins) or indeed atrial or nodal myocytes. The electrical properties of each of the current substrates (embryonic stem cells, mesenchymal stem cells, endothelial progenitor cells, and cardiac stem cells) for myocardial regeneration are provided in Figure 2B. In vitro differentiation of these cells results in a heterogeneous mix of cardiac myocytes, including cells with persistent pacemaker activity. Although exogenous pacemaker cells have the potential to take over pacing function in circumstances of sick sinus syndrome or A-V block[17], spontaneous activity is not desirable in working ventricular muscle in a heart with an intact pacemaker and conducting system. The message from in vitro differentiation of stem cells is clear: although each of the substrates can form contracting myocytes, their electrical properties are at best heterogeneous and at worst potentially arrhythmogenic.
Figure 2.
(A) Differences in times of repolarization will result in electrical dispersion. (1). The differences in the action potentials (APs) across the ventricular wall—between epicardial (EPI) and endocardial myocytes (ENDO)—result in normal ventricular dispersion. (2) If an atrial myocyte is implanted into the ventricle, its action potential creates enhanced dispersion of repolarization compared with ventricular endocardial myocytes. (3) Enhanced dispersion of repolarization will occur when cells expressing nodal potentials are implanted into ventricle. (4) Even if skeletal myoblast are engineered to express connexins, the dispersion of repolarization between these cells and ventricular myocytes would be greatest of all the cell types shown. Schematics of action potentials adapted from the following references: Nodal AP from [23]; EPI and ENDO from [24]; Atrial AP from [25]; Skeletal myoblast from [26]. (B) When induced to differentiate, all of the cell types currently under investigation for cardiac repair yield heterogenous populations of cardiac myocytes. Delivery of such mixtures of cells could result in the type of electrical dispersion described above and induce fatal arrhythmias. (ESC = embryonic stem cell; CSC = cardiac stem cell; MSC = mesenchymal stem cell; EPC = endothelial progenitor cell).
Given this electrical heterogeneity, is there any possibility of delivering a cellular substrate to improve contractile function without potentially dangerous electrical consequences?
There are two ways in which improved safety may be possible. First, fully differentiated myocytes might be selected for homogeneous electrical properties. Such selection would likely use an external membrane epitope to maintain cell viability. Immediately, ion channels and proteins come to mind and some (i.e. Kv1.5, Cx 40) are much more highly expressed in the atrium[18, 19] while others (e.g. HCN4) are predominantly expressed in the SA node[20]. Negative selection criteria might remove SA nodal and atrial phenotypes leaving a population with predominantly ventricular properties. However, there is substantial electrical heterogeneity within the ventricle itself and this heterogeneity can still be arrhythmic if epicardial cells with shorter action potentials are placed in an endocardial location (see Figure 2A). On the other hand, this concern assumes an absence of electrical plasticity responsive to local environmental cues. Our own laboratory has shown that a ventricular transmural gradient in angiotensin II leads to the final phenotypic differences in transient outward current and Na/K pump current between endocardium and epicardium[21, 22]. Together, this suggests that a partially differentiated cardiogenic cell might be optimal since it would have the capacity to differentiate in a manner consistent with its local environment.
In conclusion, when myocardial infarction results in significant myocyte loss, pump function is compromised. Any of a number of cellular substrates can partially restore mechanical function. However, recovery of mechanical function without consideration of electrical consequences can predispose patients to life threatening consequences. Mechanical without electrical recovery will not suffice.
Acknowledgments
This work was supported by NIH grants HL28958 and HL67101 (ISC) and a Scientist Development Grant from the AHA (GRG)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kolossov E, Lu Z, Drobinskaya I, et al. Identification and characterization of embryonic stem cell-derived pacemaker and atrial cardiomyocytes. Faseb J. 2005;19(6):577–9. doi: 10.1096/fj.03-1451fje. [DOI] [PubMed] [Google Scholar]
- 2.He JQ, Ma Y, Lee Y, et al. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93(1):32–9. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 3.Maltsev VA, Rohwedel J, Hescheler J, et al. Embryonic stem cells differentiate in vitro into cardiomyocytes representing sinusnodal, atrial and ventricular cell types. Mech Dev. 1993;44(1):41–50. doi: 10.1016/0925-4773(93)90015-p. [DOI] [PubMed] [Google Scholar]
- 4.Laugwitz KL, Moretti A, Lam J, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433(7026):647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukuda K. Use of adult marrow mesenchymal stem cells for regeneration of cardiomyocytes. Bone Marrow Transplant. 2003;32 1:S25–7. doi: 10.1038/sj.bmt.1703940. [DOI] [PubMed] [Google Scholar]
- 6.Shmelkov SV, Meeus S, Moussazadeh N, et al. Cytokine preconditioning promotes codifferentiation of human fetal liver CD133+ stem cells into angiomyogenic tissue. Circulation. 2005;111(9):1175–83. doi: 10.1161/01.CIR.0000157155.44008.0F. [DOI] [PubMed] [Google Scholar]
- 7.Abraham MR, Henrikson CA, Tung L, et al. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res. 2005;97(2):159–67. doi: 10.1161/01.RES.0000174794.22491.a0. [DOI] [PubMed] [Google Scholar]
- 8.Hagege AA, Marolleau JP, Vilquin JT, et al. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients. Circulation. 2006;114(1 Suppl):I108–13. doi: 10.1161/CIRCULATIONAHA.105.000521. [DOI] [PubMed] [Google Scholar]
- 9.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102(32):11474–9. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon YS, Wecker A, Heyd L, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115(2):326–38. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen SL, Fang WW, Qian J, et al. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J (Engl) 2004;117(10):1443–8. [PubMed] [Google Scholar]
- 12.Valiunas V, Doronin S, Valiuniene L, et al. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J Physiol. 2004;555(Pt 3):617–26. doi: 10.1113/jphysiol.2003.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang YL, Zhao Q, Qin X, et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80(1):229–36. doi: 10.1016/j.athoracsur.2005.02.072. discussion 236-7. [DOI] [PubMed] [Google Scholar]
- 14.Tolmachov O, Ma YL, Themis M, et al. Overexpression of connexin 43 using a retroviral vector improves electrical coupling of skeletal myoblasts with cardiac myocytes in vitro. BMC Cardiovasc Disord. 2006;6:25. doi: 10.1186/1471-2261-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akar FG, Laurita KR, Rosenbaum DS. Cellular basis for dispersion of repolarization underlying reentrant arrhythmias. J Electrocardiol. 2000;33 Suppl:23–31. doi: 10.1054/jelc.2000.20313. [DOI] [PubMed] [Google Scholar]
- 16.Antzelevitch C, Yan GX, Shimizu W, Burashnikov A. In: Electrical Heterogeneity, the ECG, and Cardiac Arrhythmias, in Cardiac Electrophysiology: from Cell to Bedside. Zipes DP, Jalife J, editors. W.B. Saunders Co; Philadelphia: 2000. pp. 222–238. [Google Scholar]
- 17.Kehat I, Khimovich L, Caspi O, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22(10):1282–9. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 18.Vozzi C, Dupont E, Coppen SR, et al. Chamber-related differences in connexin expression in the human heart. J Mol Cell Cardiol. 1999;31(5):991–1003. doi: 10.1006/jmcc.1999.0937. [DOI] [PubMed] [Google Scholar]
- 19.Li GR, Feng J, Yue L, et al. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res. 1996;78(4):689–96. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- 20.Shi W, Wymore R, Yu H, et al. Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ Res. 1999;85(1):e1–6. doi: 10.1161/01.res.85.1.e1. [DOI] [PubMed] [Google Scholar]
- 21.Yu H, Gao J, Wang H, et al. Effects of the renin-angiotensin system on the current I(to) in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res. 2000;86(10):1062–8. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]
- 22.Gao J, Kim J, Sun X, Cohen IS, Mathias RT. Gradients in Na/K Pump Current and Angiotensin II across the Canine Ventricular Wall. 51st Annual Meeting of the Biophysical Society; Baltimore, MD. 2006. [Google Scholar]
- 23.McGuire MA, de Bakker JM, Vermeulen JT, et al. Atrioventricular junctional tissue. Discrepancy between histological and electrophysiological characteristics. Circulation. 1996;94(3):571–7. doi: 10.1161/01.cir.94.3.571. [DOI] [PubMed] [Google Scholar]
- 24.Rosen MR, Cohen IS. Molecular/genetic determinants of repolarization and their modification by environmental stress. J Intern Med. 2006;259(1):7–23. doi: 10.1111/j.1365-2796.2005.01592.x. [DOI] [PubMed] [Google Scholar]
- 25.Anyukhovsky EP, Sosunov EA, Chandra P, et al. Age-associated changes in electrophysiologic remodeling: a potential contributor to initiation of atrial fibrillation. Cardiovasc Res. 2005;66(2):353–63. doi: 10.1016/j.cardiores.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 26.Leobon B, Garcin I, Menasche P, et al. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci U S A. 2003;100(13):7808–11. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]