Abstract
Purpose
In planar 123I-metaiodobenzylguanidine (123I-MIBG) myocardial imaging mediastinum (M) activity is often used as a background correction in calculating “washout” (WO). However, the most likely sources for counts that might produce errors in estimating myocardial (Myo) activity are lung (Lu) and liver (Li), which typically have higher counts/pixel (cpp) than M. The present study investigated the relationship between changes in Lu, Li and Myo activity between early and late planar 123I-MIBG images, with comparison to M as the best estimator of non-specific background activity.
Methods
Studies on 98 subjects with both early (e) and late (l) planar 123I-MIBG images were analysed. There were 68 subjects with chronic heart failure (CHF), 14 with hypertension (HTN) but no known heart disease and 16 controls (C). For each image, regions of interest (ROIs) were drawn: an irregular whole Myo, Lu, upper M and Li. For each ROI, WO was calculated as [(cpp(e)-cpp(l:decay corrected))/cpp(e)]×100%.
Results
Multivariable forward stepwise regression analysis showed that overall a significant proportion of the variation in Myo WO could be explained by a model containing M WO and Lu WO (37%, p < 0.001). Only in controls was M WO the sole variable explaining a significant proportion of the variation in Myo WO (27%, p = 0.023).
Conclusion
Although increased Myo WO in CHF subjects reflects disease severity, part of the count differences measured on planar 123I-MIBG myocardial images likely reflects changes in the adjacent and surrounding Lu tissue. The results for the controls suggest that this is the only group where a mediastinum correction alone may be appropriate for cardiac WO calculations.
Keywords: Heart failure, Quantification, MIBG
Introduction
Radiolabelled metaiodobenzylguanidine (123I-MIBG), an analogue of the false neurotransmitter guanethidine, localizes in adrenergic nerve terminals primarily via the norepinephrine (NE) transporter (uptake-1) [1, 2]. In the past two decades, a large number of investigators have demonstrated decreased myocardial 123I-MIBG uptake in patients with chronic heart failure (CHF) and have shown that those with the lowest uptake tend to have the poorest prognosis [3–13]. There have also been findings suggesting that abnormalities of myocardial 123I-MIBG uptake may be predictive of increased risk for ventricular arrhythmia and sudden cardiac death [14–16].
Cardiac uptake of 123I-MIBG is rapid, reaching a relative plateau within a few minutes post-injection [2, 17, 18]. Over the next few hours post-injection, myocardial 123I-MIBG activity typically decreases; the magnitude of this cardiac washout is usually interpreted as a reflection of neuronal integrity (ability to retain NE in presynaptic granules). In normal myocardium, change in myocardial 123I-MIBG activity during the first 2–4 h post-injection is relatively small, with estimated means of 6–8% between 15 and 85 min by single photon emission computed tomography (SPECT) [19] and 7–10% between 15 min and 2.5–3 h from planar images [20, 21]. In patients with heart disease, particularly those with CHF, myocardial 123I-MIBG activity decreases more rapidly. This increased myocardial washout has been associated with adverse prognosis [5, 9, 12].
In most early reports which examined the significance of increased 123I-MIBG myocardial washout, this parameter was calculated as the simple percentage change in region of interest (ROI) counts or counts/pixel (cpp) between early (e: typically 15–30 min) and late (l: 3–4 h) planar or SPECT images [19, 22, 23]. Although background correction is often utilized in quantitative nuclear imaging (e.g. cardiac gated blood pool and renal scintigraphy), such correction is more challenging with 123I-MIBG studies because of the frequently high lung activity in patients with heart failure [19]. As a consequence, investigators have tended to use the mediastinum as a more consistent background correction for calculation of the parameter of myocardial washout. Typical 123I-MIBG myocardial washout data published in the past 20 years are based upon mediastinum-corrected calculations [13, 24–26], even though the most likely sources for non-cardiac counts in the myocardial ROI are the lungs and liver, which typically have higher cpp than mediastinum. In addition to these confounding factors, the precise relationship between calculated values of myocardial 123I-MIBG washout and the presynaptic kinetics of NE are not completely understood [27].
The objective of the present study was to investigate the degree to which changes in lung and liver activity contribute to changes in myocardial activity between early and late planar 123I-MIBG images. Comparisons were also made to mediastinum as the best estimator of non-specific background activity.
Methods
Study design
Data from myocardial 123I-MIBG scintigrams collected as part of a previously described trial were utilized in the present analyses [28]. The primary focus of the previous publication was the prognostic significance of the late heart to mediastinal ratio (H/M ratio) in 290 subjects with CHF. In only 68 patients with CHF from the former study population were both early and late 123I-MIBG scintigrams available. For the present analyses these 68 CHF subjects were examined. Data from 14 subjects with hypertension (HTN) but no known heart disease and 16 normal control subjects, collected as part of the trial but not previously described, were also analysed. In all 98 subjects both early and late 123I-MIBG scintigrams were available.
Image acquisition and analysis
All images had been acquired in a 128 × 128 matrix. Early (e) images were acquired 15–30 min post-injection, while the late (l) images were acquired a mean of 3.6 h later (range: 1.2–5.1 h).
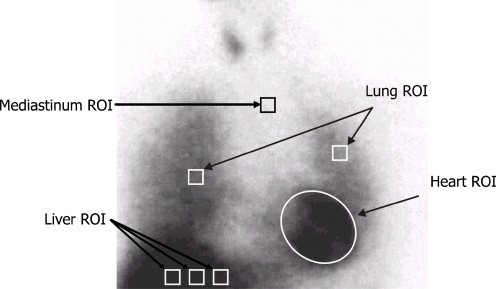
An experienced nuclear medicine technologist processed all images for this study. For each image (e and l), seven regions of interest (ROIs) were drawn (Fig. 1). The myocardial ROI was drawn manually to include all visible ventricular activity. All other ROIs were square with dimensions of 7 × 7 pixels. The mediastinal ROI was drawn in the upper mediastinum, using the apices of the lungs as anatomical landmarks. One ROI was drawn in the midline of each lung, with the left lung ROI positioned more superiorly to minimize proximity to the heart. Three adjacent liver ROIs were drawn at the same horizontal level, one in the right lobe, one in the left lobe and the third halfway between the other two. The cpp in each ROI was used in all calculations.
Fig. 1.
Example of processing procedure for late planar 123I-MIBG images. The positioning of the mediastinal ROI was standardized in relation to the lung apex, the lower boundary of the upper mediastinum and the midline between the lungs
Washout in each ROI was calculated as: [(cpp(e)-cpp(l:decay corrected))/cpp(e)]×100%. As there were no differences in the results for the two lung or the three liver ROIs, the respective data were combined to produce aggregate lung (Lu) and liver (Li) washout data for further analysis.
Statistical analysis
Data are presented as mean ± standard deviation, unless indicated otherwise. Differences between groups for continuous data were compared using analysis of variance (ANOVA) with a post hoc Bonferroni. Categorical data were compared using Fisher’s exact test. A forward stepwise multivariable regression analysis was performed to determine independent predictors of 123I-MIBG myocardial washout. Changes in mediastinal, lung and liver activity between early and late 123I-MIBG images were used as explanatory variables. The overall goodness-of-fit for each model was expressed as the adjusted R2. The F test was used to assess whether a model explained a significant proportion of the variability. A p value < 0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed with SPSS (SPSS for Windows, version 16.0.2, SPSS Inc, Chicago, IL, USA).
Results
Table 1 shows the baseline characteristics of all subjects and for the three different subject groups. CHF subjects were more often male compared to hypertension subjects but not compared to controls. Control and hypertension subjects were younger than those with CHF. Among CHF subjects, the aetiology of heart failure was ischaemic in 27 (40%) and non-ischaemic in 41 (60%). The mean left ventricular ejection fraction for the CHF subjects was 26.5% (range: 7–47%).
Table 1.
Patient parameters for all subjects and subdivided by patient subgroup
| Patient characteristics | All (n = 98) | Patient subgroup | ||
|---|---|---|---|---|
| CHF | HTN | Controls | ||
| (n = 68) | (n = 14) | (n = 16) | ||
| Male/femalea | 74/24 | 57/11 | 7/7 | 10/6 |
| Age (years)b | 52.8 ± 13.0 (range 22–83) | 56.6 ± 11.1 | 42.9 ± 13.9 | 44.9 ± 12.3 |
Data are presented as mean ± standard deviation.
CHF subjects with chronic heart failure, HTN subjects with hypertension
aGender: p = 0.02 for CHF vs HTN; p = 0.205 for CHF vs controls; p = 0.826 for HTN vs controls
bAge: p < 0.001 for CHF vs HTN; p = 0.002 for CHF vs controls; p = 0.735 for HTN vs controls
Myocardial washout was significantly higher in CHF subjects than in subjects with HTN or in controls (Table 2, p < 0.001 and p = 0.005, respectively). There were no differences in washout for lung and mediastinum across the three populations. Liver washout was significantly higher in controls than in CHF subjects (p = 0.007).
Table 2.
Myocardial, mediastinal, lung and liver 123I-MIBG washout
| n | Myocardial washouta | Mediastinal washout | Lung washout | Liver washoutb | |
|---|---|---|---|---|---|
| Overall | 98 | 22.50 (9.35) | 17.10 (9.31) | 29.20 (10.78) | 9.09 (21.49) |
| CHF | 68 | 25.20 (8.24) | 16.50 (10.00) | 28.74 (11.65) | 5.56 (22.30) |
| HTN | 14 | 14.92 (7.08) | 18.27 (8.25) | 27.44 (6.88) | 9.73 (13.58) |
| Controls | 16 | 17.65 (10.34) | 18.64 (7.10) | 32.73 (9.33) | 23.56 (18.02) |
aMyocardial washout: p = 0.005 for CHF vs controls and p < 0.001 for CHF vs HTN
bLiver washout: p = 0.007 for CHF vs controls
Table 3 shows the results of the stepwise multivariable regression analysis for all subjects. Mediastinal and lung washout were independent predictors of myocardial washout. This model predicted approximately 37% of the variation in myocardial washout (p < 0.001).
Table 3.
Multivariable analysis of mediastinal, lung and liver 123I-MIBG washout as predictors of 123I-MIBG myocardial washout for all subjects (n = 98)
| Variables | Coefficient b | Standard error b | p value |
|---|---|---|---|
| Constant | 7.546 | 2.171 | |
| Mediastinal washout | 0.255 | 0.111 | 0.023 |
| Lung washout | 0.531 | 0.087 | < 0.001 |
| Goodness-of-fit of the model | Adjusted R2 | p value | |
| 0.372 | < 0.001 |
In subjects with CHF, stepwise multivariable regression analysis showed mediastinal and lung washout to be independent predictors of myocardial washout (Table 4). This model predicted approximately 60% of myocardial washout (p < 0.001). In subjects with HTN, stepwise multivariable regression analysis showed that liver and lung washout were independent predictors of myocardial washout (Table 5). This model predicted approximately 76% of myocardial washout (p < 0.001). In controls, mediastinal washout was the sole independent predictor of myocardial washout (Table 6). Although mediastinal washout explained a statistically significant proportion of variability (p = 0.023), only 27% of the variation in myocardial washout was predicted by mediastinal washout.
Table 4.
Multivariable analysis of mediastinal, lung and liver 123I-MIBG washout as predictors of 123I-MIBG myocardial washout for chronic heart failure subjects (n = 68)
| Variables | Coefficient b | Standard error b | p value |
|---|---|---|---|
| Constant | 10.416 | 1.710 | |
| Mediastinal washout | 0.263 | 0.095 | 0.007 |
| Lung washout | 0.364 | 0.081 | < 0.001 |
| Goodness-of-fit of the model | Adjusted R2 | p value | |
| 0.595 | < 0.001 |
Table 5.
Multivariable analysis of mediastinal, lung and liver 123I-MIBG washout as predictors of 123I-MIBG myocardial washout in subjects with hypertension (n = 14)
| Variables | Coefficient b | Standard error b | p value |
|---|---|---|---|
| Constant | −3.743 | 3.922 | |
| Liver washout | 0.296 | 0.073 | 0.002 |
| Lung washout | 0.575 | 0.143 | 0.002 |
| Goodness-of-fit of the model | Adjusted R2 | p value | |
| 0.764 | < 0.001 |
Table 6.
Multivariable analysis of mediastinal, lung and liver 123I-MIBG washout as predictors of 123I-MIBG myocardial washout for controls (n = 16)
| Variables | Coefficient b | Standard error b | p value |
|---|---|---|---|
| Constant | 2.334 | 6.380 | |
| Mediastinal washout | 0.822 | 0.321 | 0.023 |
| Goodness-of-fit of the model | Adjusted R2 | p value | |
| 0.270 | 0.023 |
Discussion
The most important determinants of cardiac uptake and retention of 123I-MIBG are the functional status of the NE transporter (NET) and the vesicular monoamine transporter (VMAT) in presynaptic adrenergic neurons [3–11]. Impairment of either or both transporters can result in reduced neuronal uptake and increased rate of loss of NE and its analogue 123I-MIBG.
Given the physical and physiological characteristics of MIBG and the isotope 123I, it is not surprising that there can be considerable variation in myocardial washout assessments based on planar 123I-MIBG images. The key assumptions that underlie the simple methods used to calculate myocardial washout, namely that the counts in the myocardial ROI reflect only specific and non-specific neuronal uptake of 123I-MIBG and that the mediastinum ROI represents the non-specific/background cpp for the myocardium, have never been carefully validated. The findings of the present study indicate that the latter assumption is at best only partially correct in that a proportion of the variation in the myocardial 123I-MIBG washout for CHF and HTN patients can also be explained by washout of 123I-MIBG in surrounding lung and liver tissue. Only in controls does the use of a mediastinum correction alone appear appropriate, and even in this group, the proportion of the variation of calculated myocardial washout associated with mediastinum washout is relatively small.
Change in organ uptake over time has long been utilized as an adjunctive physiological assessment in nuclear imaging. For example, change in 201Tl uptake between early and late images has been used as both a primary indicator of stress-induced ischaemic (“redistribution”) and a secondary indicator of coronary artery disease (CAD) severity (“washout”) [29]. The frequent association of increased cardiac washout of 123I-MIBG and heart failure and other heart diseases was noted within a few years of the initial reports of 123I-MIBG imaging results [19, 22, 23]. This phenomenon has now been extensively studied for more than 20 years. While the physiological processes associated with neuronal uptake and loss of 123I-MIBG are clearly important, the present results suggest that methodological issues must also be considered, particularly for analysis of planar images. As a quantitative parameter, “washout” should reflect only changes in the organ under investigation, a condition that is clearly not met for the myocardial ROI in planar 123I-MIBG imaging. In addition, successful background correction in nuclear imaging usually requires a high count target organ surrounded by tissues with relatively homogeneous lower activity. However, for 123I-MIBG myocardial imaging in CHF, lung cpp is often greater than myocardial cpp, and the contribution of liver counts to the myocardial cpp is greatly dependent on the location and size of the left lobe. In most instances, use of the mediastinum cpp as background correction is unlikely to produce a reliable estimate of the net activity in the myocardium.
Despite its methodological deficiencies, the strong prognostic value of mediastinal background-corrected myocardial 123I-MIBG washout has been repeatedly demonstrated [24, 25]. While such calculated washout rates are probably not a true reflection of neuronal NET and VMAT status [30], they are still clearly related to the severity of neuronal dysfunction associated with CHF. The contribution of changes in lung activity to the calculated washout in CHF patients may enhance the prognostic significance of this parameter, possibly incorporating elements of abnormal sympathetic drive and preload conditions affecting the lung as a consequence of myocardial dysfunction. The findings of the present study suggest that especially in CHF patients a better understanding of the significance of changes in lung activity might improve the validity and value of the calculation of myocardial washout.
In CHF patients, sympathetic activity is initially increased as a compensatory mechanism. However, chronically elevated stimulation of the adrenergic system is associated with sustaining the process of remodelling. Abnormalities in calculated myocardial 123I-MIBG washout have been demonstrated in patients with most common cardiac pathological conditions such as CAD, CHF, and non-ischaemic cardiomyopathy [5, 9, 12, 14, 31, 32]. In fact, most studies have shown that both late H/M and 123I-MIBG myocardial washout are abnormal in these cardiac disease populations. Multivariable models have variously shown late H/M and 123I-MIBG myocardial washout as the most powerful predictors of outcome [7, 9, 12, 16]. The problem with most of these analyses is that they treated washout and H/M as independent variables, when in fact they are related, employing the same ROI cpp data in the calculations. As shown in the present study, the degree of relationship between these variables may depend on the population being studied.
The differences between the three groups included in this study provide insight into the physiology of 123I-MIBG and the physical attributes of planar 123I-MIBG imaging. A consistent feature of 123I-MIBG imaging in all individuals is high liver uptake, but only in HTN subjects did this organ contribute to variability in calculated myocardial washout. Whether this is a reflection of a change in sympathetic tone in the liver as a result of chronic hypertension is uncertain but may warrant further investigation. The finding that lung activity significantly contributes to myocardial activity in CHF subjects is consistent with the frequent visualization of prolonged lung uptake on delayed 123I-MIBG images. This suggests that some allowance for this phenomenon is needed for an accurate assessment of change in specific myocardial uptake over time in these subjects.
There was significantly higher myocardial washout in CHF than HTN or control subjects, which is in keeping with previous observations. In addition, washout from lungs was of a magnitude similar to that from myocardium. These findings suggest that there is a component of the change in myocardial cpp common to that of the lungs [e.g. clearance of blood pool activity, release of non-specific tracer accumulation (uptake-2)]. There is little doubt that changes in counts detected in the myocardium reflect a component of counts that originated from 123I emissions in the lungs. This is also consistent with the known effect of septal penetration of high-energy 123I photons on quantitative reliability of measured image counts [33, 34].
The primary limitation of the present study is the small number of subjects included, particularly in the hypertension and control subgroups. In addition, as the largest influence on the 123I-MIBG myocardial washout is myocardial disease severity, the retrospective nature of this study made it impossible to control for the effects of subject selection and image acquisition variables that might have had undetected impact on the calculated 123I-MIBG myocardial washout.
In conclusion, myocardial washout of 123I-MIBG in CHF subjects is likely to reflect in part changes in the adjacent and surrounding tissue. The results for the controls suggest that this is the only group where a mediastinum correction alone may be appropriate for myocardial washout calculations. Therefore, there remains the need to better characterize the significance of myocardial washout determined from early and late planar 123I-MIBG images. While an absolute determination of myocardial washout of 123I-MIBG would undoubtedly be a reflection of the capacity of sympathetic neurons to retain the neurotransmitter NE, the accuracy of the calculation method in common use for quantifying this phenomenon is uncertain. Particularly in CHF patients, a means to incorporate changes in lung activity in the calculation of absolute myocardial washout would appear to warrant further investigation.
Acknowledgments
Conflicts of interest Arnold F. Jacobson is employed by GE Healthcare. None of the other authors report any relationships to disclose.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Sisson JC, Shapiro B, Meyers L, Mallette S, Mangner TJ, Wieland DM, et al. Metaiodobenzylguanidine to map scintigraphically the adrenergic nervous system in man. J Nucl Med 1987;28:1625–36. [PubMed]
- 2.Kline RC, Swanson DP, Wieland DM, Thrall JH, Gross MD, Pitt B, et al. Myocardial imaging in man with I-123 meta-iodobenzylguanidine. J Nucl Med 1981;22:129–32. [PubMed]
- 3.Merlet P, Valette H, Dubois-Randé JL, Moyse D, Duboc D, Dove P, et al. Prognostic value of cardiac metaiodobenzylguanidine imaging in patients with heart failure. J Nucl Med 1992;33:471–7. [PubMed]
- 4.Merlet P, Benvenuti C, Moyse D, Pouillart F, Dubois-Randé JL, Duval AM, et al. Prognostic value of MIBG imaging in idiopathic dilated cardiomyopathy. J Nucl Med 1999;40:917–23. [PubMed]
- 5.Momose M, Kobayashi H, Iguchi N, Matsuda N, Sakomura Y, Kasanuki H, et al. Comparison of parameters of 123I-MIBG scintigraphy for predicting prognosis in patients with dilated cardiomyopathy. Nucl Med Commun 1999;20:529–35. doi:10.1097/00006231-199906000-00007. [DOI] [PubMed]
- 6.Cohen-Solal A, Esanu Y, Logeart D, Pessione F, Dubois C, Dreyfus G, et al. Cardiac metaiodobenzylguanidine uptake in patients with moderate chronic heart failure: relationship with peak oxygen uptake and prognosis. J Am Coll Cardiol 1999;33:759–66. doi:10.1016/S0735-1097(98)00608-1. [DOI] [PubMed]
- 7.Wakabayashi T, Nakata T, Hashimoto A, Yuda S, Tsuchihashi K, Travin MI, et al. Assessment of underlying etiology and cardiac sympathetic innervation to identify patients at high risk of cardiac death. J Nucl Med 2001;42:1757–67. [PubMed]
- 8.Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, et al. Spironolactone improves cardiac sympathetic nerve activity and symptoms in patients with congestive heart failure. J Nucl Med 2002;43:1279–85. [PubMed]
- 9.Yamada T, Shimonagata T, Fukunami M, Kumagai K, Ogita H, Hirata A, et al. Comparison of the prognostic value of cardiac iodine-123 metaiodobenzylguanidine imaging and heart rate variability in patients with chronic heart failure: a prospective study. J Am Coll Cardiol 2003;41:231–8. doi:10.1016/S0735-1097(02)02700-6. [DOI] [PubMed]
- 10.Kyuma M, Nakata T, Hashimoto A, Nagao K, Sasao H, Takahashi T, et al. Incremental prognostic implications of brain natriuretic peptide, cardiac sympathetic nerve innervation, and noncardiac disorders in patients with heart failure. J Nucl Med 2004;45:155–63. [PubMed]
- 11.Nakata T, Wakabayashi T, Kyuma M, Takahashi T, Tsuchihashi K, Shimamoto K. Cardiac metaiodobenzylguanidine activity can predict the long-term efficacy of angiotensin-converting enzyme inhibitors and/or beta-adrenoceptor blockers in patients with heart failure. Eur J Nucl Med Mol Imaging 2005;32:186–94. doi:10.1007/s00259-004-1624-8. [DOI] [PubMed]
- 12.Kasama S, Toyama T, Sumino H, Nakazawa M, Matsumoto N, Sato Y, et al. Prognostic value of serial cardiac 123I-MIBG imaging in patients with stabilized chronic heart failure and reduced left ventricular ejection fraction. J Nucl Med 2008;49:907–14. doi:10.2967/jnumed.107.047548. [DOI] [PubMed]
- 13.Verberne HJ, Brewster LM, Somsen GA, van Eck-Smit BL. Prognostic value of myocardial 123I-metaiodobenzylguanidine (MIBG) parameters in patients with heart failure: a systematic review. Eur Heart J 2008;29:1147–59. doi:10.1093/eurheartj/ehn113. [DOI] [PubMed]
- 14.Arora R, Ferrick KJ, Nakata T, Kaplan RC, Rozengarten M, Latif F, et al. I-123 MIBG imaging and heart rate variability analysis to predict the need for an implantable cardioverter defibrillator. J Nucl Cardiol 2003;10:121–31. doi:10.1067/mnc.2003.2. [DOI] [PubMed]
- 15.Paul M, Schäfers M, Kies P, Acil T, Schäfers K, Breithardt G, et al. Impact of sympathetic innervation on recurrent life-threatening arrhythmias in the follow-up of patients with idiopathic ventricular fibrillation. Eur J Nucl Med Mol Imaging 2006;33:866–70. doi:10.1007/s00259-005-0061-7. [DOI] [PubMed]
- 16.Kioka H, Yamada T, Mine T, Morita T, Tsukamoto Y, Tamaki S, et al. Prediction of sudden death in patients with mild-to-moderate chronic heart failure by using cardiac iodine-123 metaiodobenzylguanidine imaging. Heart 2007;93:1213–8. doi:10.1136/hrt.2006.094524. [DOI] [PMC free article] [PubMed]
- 17.Rabinovitch MA, Rose CP, Schwab AJ, Fitchett DH, Honos GN, Stewart JA, et al. A method of dynamic analysis of iodine-123-metaiodobenzylguanidine scintigrams in cardiac mechanical overload hypertrophy and failure. J Nucl Med 1993;34:589–600. [PubMed]
- 18.Arbab AS, Koizumi K, Toyama K, Arai T, Araki T. Dynamic SPET parameters of 123I-MIBG cardiac imaging. Nucl Med Commun 1999;20:617–22. doi:10.1097/00006231-199907000-00004. [DOI] [PubMed]
- 19.Henderson EB, Kahn JK, Corbett JR, Jansen DE, Pippin JJ, Kulkarni P, et al. Abnormal I-123 metaiodobenzylguanidine myocardial washout and distribution may reflect myocardial adrenergic derangement in patients with congestive cardiomyopathy. Circulation 1988;78:1192–9. [DOI] [PubMed]
- 20.Nakajima K, Taki J, Tonami N, Hisada K. Decreased 123I-MIBG uptake and increased clearance in various cardiac diseases. Nucl Med Commun 1994;15:317–23. doi:10.1097/00006231-199405000-00003. [DOI] [PubMed]
- 21.Kurata C, Wakabayashi Y, Shouda S, Okayama K, Yamamoto T, Ishikawa A, et al. Enhanced cardiac clearance of iodine-123-MIBG in chronic renal failure. J Nucl Med 1995;36:2037–43. [PubMed]
- 22.Glowniak JV, Turner FE, Gray LL, Palac RT, Lagunas-Solar MC, Woodward WR. Iodine-123 metaiodobenzylguanidine imaging of the heart in idiopathic congestive cardiomyopathy and cardiac transplants. J Nucl Med 1989;30:1182–91. [PubMed]
- 23.Taki J, Nakajima K, Bunko H, Simizu M, Muramori A, Hisada K. Whole-body distribution of iodine 123 metaiodobenzylguanidine in hypertrophic cardiomyopathy: significance of its washout from the heart. Eur J Nucl Med 1990;17:264–8. doi:10.1007/BF00812368. [DOI] [PubMed]
- 24.Carrió I. Cardiac neurotransmission imaging. J Nucl Med 2001;42(7):1062–76. [PubMed]
- 25.Yamashina S, Yamazaki J. Neuronal imaging using SPECT. Eur J Nucl Med Mol Imaging 2007;34 Suppl 1:S62–73. [DOI] [PubMed]
- 26.Agostini D, Carrio I, Verberne HJ. How to use myocardial (123)I-MIBG scintigraphy in chronic heart failure. Eur J Nucl Med Mol Imaging. 2008; In press. [DOI] [PubMed]
- 27.Somsen GA, Borm JJ, Dubois EA, Schook MB, Van der Wall EE, Van Royen EA. Cardiac 123I-MIBG uptake is affected by variable uptake in reference regions: implications for interpretation in clinical studies. Nucl Med Commun 1996;17:872–6. doi:10.1097/00006231-199610000-00008. [DOI] [PubMed]
- 28.Agostini D, Verberne HJ, Burchert W, Knuuti J, Povinec P, Sambuceti G, et al. I-123-mIBG myocardial imaging for assessment of risk for a major cardiac event in heart failure patients: insights from a retrospective European multicenter study. Eur J Nucl Med Mol Imaging 2008;35:535–46. doi:10.1007/s00259-007-0639-3. [DOI] [PubMed]
- 29.Watson DD, Campbell NP, Read EK, Gibson RS, Teates CD, Beller GA. Spatial and temporal quantitation of plane thallium myocardial images. J Nucl Med 1981;22:577–84. [PubMed]
- 30.Imamura Y, Fukuyama T. Prognostic value of myocardial MIBG scintigraphy findings in patients with cardiomyopathy–importance of background correction for quantification of MIBG activity. Ann Nucl Med 2002;16:387–93. doi:10.1007/BF02990076. [DOI] [PubMed]
- 31.Suwa M, Otake Y, Moriguchi A, Ito T, Hirota Y, Kawamura K, et al. Iodine-123 metaiodobenzylguanidine myocardial scintigraphy for prediction of response to beta-blocker therapy in patients with dilated cardiomyopathy. Am Heart J 1997;133:353–8. doi:10.1016/S0002-8703(97)70232-1. [DOI] [PubMed]
- 32.Parthenakis FI, Prassopoulos VK, Koukouraki SI, Zacharis EA, Diakakis GF, Karkavitsas NK, et al. Segmental pattern of myocardial sympathetic denervation in idiopathic dilated cardiomyopathy: relationship to regional wall motion and myocardial perfusion abnormalities. J Nucl Cardiol 2002;9:15–22. doi:10.1067/mnc.2002.118239. [DOI] [PubMed]
- 33.Verberne HJ, Feenstra C, de Jong WM, Somsen GA, van Eck-Smit BL, Busemann Sokole E. Influence of collimator choice and simulated clinical conditions on 123I-MIBG heart/mediastinum ratios: a phantom study. Eur J Nucl Med Mol Imaging 2005;32:1100–7. doi:10.1007/s00259-005-1810-3. [DOI] [PubMed]
- 34.Chen J, Garcia EV, Galt JR, Folks RD, Carrio I. Improved quantification in 123I cardiac SPECT imaging with deconvolution of septal penetration. Nucl Med Commun 2006;27:551–8. doi:10.1097/00006231-200607000-00002. [DOI] [PubMed]