Abstract
We re-engineered the immunoglobulin rearrangements from clonally expanded CSF B cells of three Multiple Sclerosis patients as Fab fragments, and used three methods to test for their Ag-specificity. Nine out of ten Fab fragments were reactive to Myelin Basic Protein (MBP). The one Fab that did not react to MBP was a product of receptor editing. Two of the nine MBP-reactive Fabs were also reactive to GFAP and CNPase, indicating that these clones were polyreactive. Targeting the mechanisms that allows these self-reactive B cells to reside in the CSF of MS patients may prove to be a potent immunotherapeutic strategy.
Keywords: Multiple Sclerosis, B lymphocytes, antigen specificity, antibodies, gene rearrangement
INTRODUCTION
Multiple Sclerosis (MS) is considered an inflammatory, demyelinating disease of the central nervous system (CNS). It is suspected that MS is an autoimmune disease involving immune responses directed against self-myelin associated antigens (Ag). There is substantial evidence that humoral immune components in the CNS may be involved in the development and perpetuation of MS (in at least a subset of patients)(reviewed in (Antel, 1999; Archelos et al., 2000; Cross et al., 2001; Martin Mdel and Monson, 2007; O’Connor et al., 2001)). More recently, several groups have demonstrated extensive clonal expansion of B cells in the CSF of MS patients (Blalock et al., 1999; Columbo et al., 2000; Owens et al., 2003; Qin et al., 1998; Ritchie et al., 2004). Our laboratory has also demonstrated that B cell clonal expansion and intraclonal diversity was evident in all 5 MS patients analyzed, even those that had been recently diagnosed with MS (Monson et al., 2005a). Taken together, these data strongly suggest that B cells in the CNS of MS patients may be contributing to the pathogenesis of MS by responding to antigens in that compartment. The focus of this study was to determine whether clonally expanded B cells in the CSF of MS patients are reactive to Ag components of the CNS, and are thus self-reactive.
Our laboratory has also demonstrated that several of the clonally expanded CSF B cells from these MS patient repertoires had undergone receptor editing. Typically, B cells in the bone marrow undergo receptor editing in an attempt to escape self-reactivity (Casellas et al., 2001; Edry and Melamed, 2004; Nemazee, 2000; Tiegs et al., 1993). This process can only occur when the recombination activating genes (RAGs) in B cells are re-expressed, allowing for re-initiation of immunoglobulin (Ig) gene recombination (Chen et al., 1997; Han et al., 1997; Kelsoe, 1996; Li et al., 2001; Papavasiliou et al., 1997; Prak and Weigert, 1995; Russell et al., 1991; Tiegs et al., 1993), resulting in substitution of either the original heavy or light chain of the antibody for a newly rearranged one. However, receptor editing does not guarantee that a productive rearrangement will occur or that self-reactivity will be abrogated. Thus, the second focus of this study was to determine whether receptor editing was a useful avenue to escape self-reactivity for these clonally expanded CSF B cells from MS patients.
To identify the antigen specificity of clonally expanded CSF B cells from MS patients and determine whether receptor editing successfully abrogated self-reactivity, we have taken the immunoglobulin rearrangements from clonally expanded CSF B cells of three patients with RRMS, expressed these rearrangements as Fab fragments, and tested for Ag-specificity by immunoprecipitation (IP), Western Blot (WB), ELISA, and DELFIA. Our results indicate that the majority of these Fabs generated from clonally expanded CSF B cells of MS patients are reactive to a prevalent protein of the CNS called myelin basic protein (MBP), and that this self-reactivity can occasionally be altered by receptor editing.
MATERIALS AND METHODS
Patient Description and selection criteria
Table 1 provides information pertaining to the patients utilized for this study. We chose patients who had been diagnosed with the relapsing remitting form of MS, but were otherwise discordant, in order to sample a broader spectrum of RRMS patients. From that list, we narrowed our candidates based primarily on molecular considerations using a combination of factors including the number of CSF B cell clones a patient had, the extent of mutation accumulation in that patient’s CSF B cell population, and whether any of the CSF B cell clones had evidence of receptor editing. For example, M125 was chosen because 1) this repertoire had a large number of clones that were often heavily mutated and 2) some of the clones had undergone receptor editing. M354 had only one CSF B cell clone that could be generated (there must be both a heavy and light chain to clone a Fab), but this B cell clone had undergone receptor editing, and thus became a priority to us. In addition, this B cell clone had also been detected in the peripheral blood of this patient by single cell PCR. M522 had two heavily mutated B cell clones, both of which utilized variable genes rarely detected in healthy donor peripheral blood, and thus became of interest as well. The studies have been reviewed and approved by UT Southwestern institutional review board.
Table 1.
Patient Summary1
| M1252 | M354 | M5223 | |
|---|---|---|---|
| Type of MS | Relapsing Remitting |
Relapsing Remitting |
Relapsing Remitting |
| Time since Diagnosis of MS | 4 months | 8 months | 3 years |
| Age/Sex | 32/F | 44/F | 35/F |
| Initial Events | Optic neuritis | Transverse Myelitis |
Transverse Myelitis |
| Exacerbation History | 1 additional | 2 additional | 0 additional |
| Treatment History Prior to LP | IV Steroids | None | None |
| MRI findings | + | + | + |
| Oligoclonal Banding (OCB)4 | Negative5 | Positive | Positive |
| Ig Index6 | normal | normal | high |
| Ig Synthesis Rate | not done | normal | not done |
All patients had CSF white blood cell (WBC) counts in the range of 1 ×103 to 1 ×104 per mL, typical of MS patients at UTSWMC (Stuve et al., 2006)
Patient M125 reported that 2 years prior to diagnosis, she had left leg weakness that resolved itself over several months which may have been attributable to MS
This patient summary has not been previously published
Positive OCB was defined as those samples with 2 or more bands
OCB positive on a subsequent lumbar puncture
Normal Ig Index is 0.00–0.85.
Cell preparation and cell sorting
CSF cells were isolated by centrifugation of the CSF, and CD19+ B cells sorted one cell per well into 96 well PCR plates as described (Yavuz et al., 2002).
Primer extension preamplification, amplification of VHDJH, VκJκ and VλJλ rearrangements and sequence analysis
These methods were carried out as previously described (Brezinschek et al., 1995; Farner et al., 1999; Foster et al., 1997).
Description of clones
Table 2 provides specific information on the immunoglobulin rearrangements from the clonally expanded B cells utilized for this study. Two unique clones of M354 were originally reported, although further analysis confirmed that these two clones were a single clone that had undergone receptor editing, which is defined as the replacement of either a heavy or light chain component of the antibody with a newly rearranged one. Therefore, original clone M354-B has been renamed M354-A1, and original clone M354-A has been renamed M354-A2. M354-A1 is the clone member that existed prior to receptor editing. M354-A2 is the clone member expressing the newly rearranged chain. The nomenclature is universal for all receptor edited clones such that the “1” designation at the end of the clone name indicates that this is the member of the clone that existed prior to receptor editing—the “non-edited” clone. The “2” designation at the end of the clone name indicates that this is the member of the clone expressing the newly rearranged chain—the “receptor edited” clone. Thus, clones that had undergone receptor editing were divided into two categories; non-edited and receptor edited clone members. Heavy chain receptor edited clone members (M125-A and –D) were further defined as those clone members that had the greatest mutation accumulation in their light chain rearrangement, whereas non-edited clone members had a lower mutation accumulation in their light chain rearrangement. Light chain edited clone members (M354-A) were defined as those clone members that had the lowest mutation accumulation in their light chain rearrangement, since this second rearrangement would have had less time to accumulate mutations compared to the non-edited (previously used) light chain rearrangement. However, since the heavy chain for both the receptor edited and non-edited clone members of M354-A were identical, there is a possibility that this order is not correct.
Table 2.
Summary of Immunoglobulin Rearrangements from CSF B cell Clones subsequently generated into Fabs
| Non-edited clone member1 |
Receptor edited clone member2 |
||||
|---|---|---|---|---|---|
| VH Rearrangement |
VL Rearrangement |
VH Rearrangement |
VL Rearrangement |
||
| M125 | |||||
| Clone A13 | 4–59:1–1:JH6 89.1%4 |
VκA27:Jκ2 95.9% |
Clone A2 | 4–30.4:5–12:JH6 93.8% |
VκA27:Jκ2 95.0% |
| Clone B | 3–30:2–2:JH6 95.6% |
Vλ3H:Jλ23 93.3% |
NA5 | ||
| Clone D1 | 4–39:6–25:JH2 100% |
Vλ2A2:Jλ1 98.7% |
Clone D2 | 3–64:1–26:JH4 97.6% |
Vλ2A2:Jλ1 97.4% |
| Clone G | 3–33:2–2:JH6 94.7% |
VκL2:Jκ1 95.4% |
NA5 | ||
| M354 | |||||
| Clone A1 | 3–48:3–16: JH3 95% |
Vλ1C: Jλ1 95.6% |
Clone A2 | 3–48:3–16: JH3 95% |
Vλ1G: Jλ7 96.9% |
| M5226 | |||||
| Clone A | 3–09:5–24:JH4 93.3% |
Vλ7B:Jλ3 96.9% |
NA5 | ||
| Clone B | 2–05:3–10:JH5 95.7% |
VκA27:Jκ2 95.1–98.6% |
NA5 | ||
Member of the clonal lineage prior to receptor editing
Member of the clonal lineage that had replaced either its heavy or light chain antibody component; a process called receptor editing
The clone order of M125-A1 and A2 was switched from the previous published order (Monson et al., 2005a)
Percent homology to germline V gene
These clones did not undergo receptor editing
The clonal populations of M522 have not been previously published
Description of the Vector Utilized to Construct Recombinant VHVL Fabs
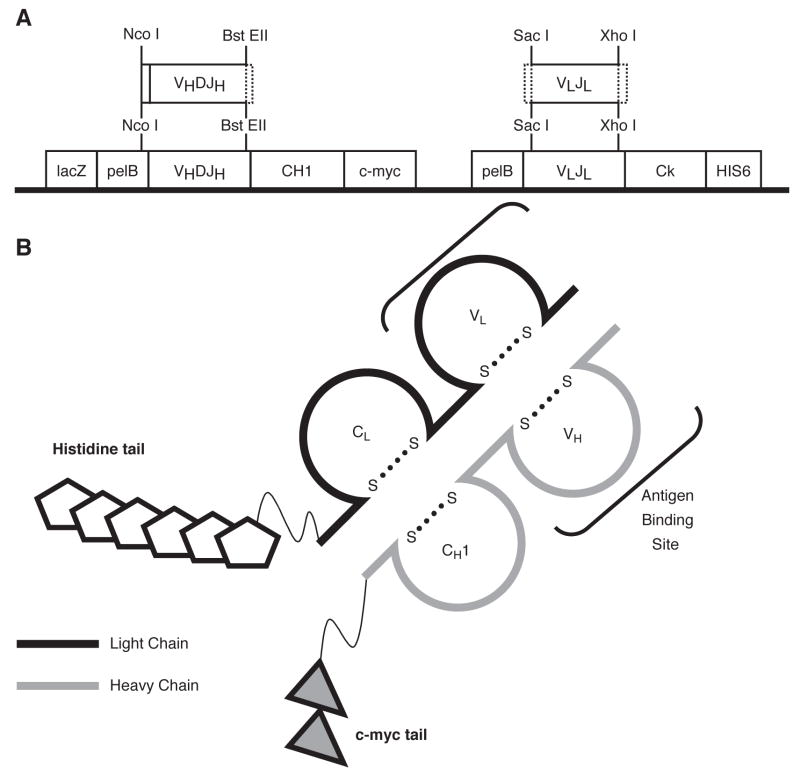
VH and VL products were cloned sequentially into the FabD1.3myc plasmid (Figure 1A) (Evan et al., 1985; Hoogenboom et al., 1991; Ruther et al., 1981) using conventional methods.
Figure 1. Description of the Vector Utilized to Construct Recombinant VHVL Fabs.
Panel A. Features of the D1.3v2 plasmid. The original FabD1.3mychis6 vector utilized the Pst I and Bst EII restriction digest enzymes to insert heavy chains into the heavy chain cassette. The Pst I site was converted to Nco I to prevent cutting within heavy chains, and is now called D1.3v2 Fab. Heavy and light chains are not linked by a disulfide bridge in this construct. Panel B. Illustration of a properly expressed Fab protein, including the features described.
Expression and Purification of Fab Proteins
This methodology has been described in detail elsewhere (Ward, 1992a; Ward, 1992b). The Fabs were isolated from E. coli supernatants using a nickel-NTA-agarose column (Amersham Biosciences, Upsala, Sweden), and collected in 2 mL fractions. The fractions are dialyzed in PBS, resolved by SDS-PAGE and stained with Coomassie Blue. Fractions containing a band at 25 kDa are pooled and assessed for antigen specificity. The amount of isolated Fab protein was highly variable and in the range of 1 to 12 μg/mL eluate. A schematic of the expressed Fabs is provided in Figure 1B. Supplemental Figure 1 provides documentation of how the Fab preparations were validated.
Antigens
Purified human MBP and bovine CNPase were purchased from Sigma (St. Louis, MO). Purified human GFAP was commercially available from Biodesign (Saco, ME). Adult human brain lysate (hBL) was obtained from Clontech (Mountain View, CA). MBP peptides were generated by C S Bio Co., Inc. (Menlo Park, CA). Mouse Brain Lysate was prepared from B10.PL mice bred in our colony. For the DELFIA experiments, MBP was purified from normal human white matter according to described methods (Deibler et al., 1972), lysozyme was isolated from human neutrophils obtained through a commercial supplier (Sigma) and histone H1 was also commercially available (Upstate, Charlottesville, VA). Recombinant MOG was a kind gift from Claude Genain (UCSF, San Francisco, CA).
Western Blot using Fabs as the primary antibody (Figures 2 and 4)
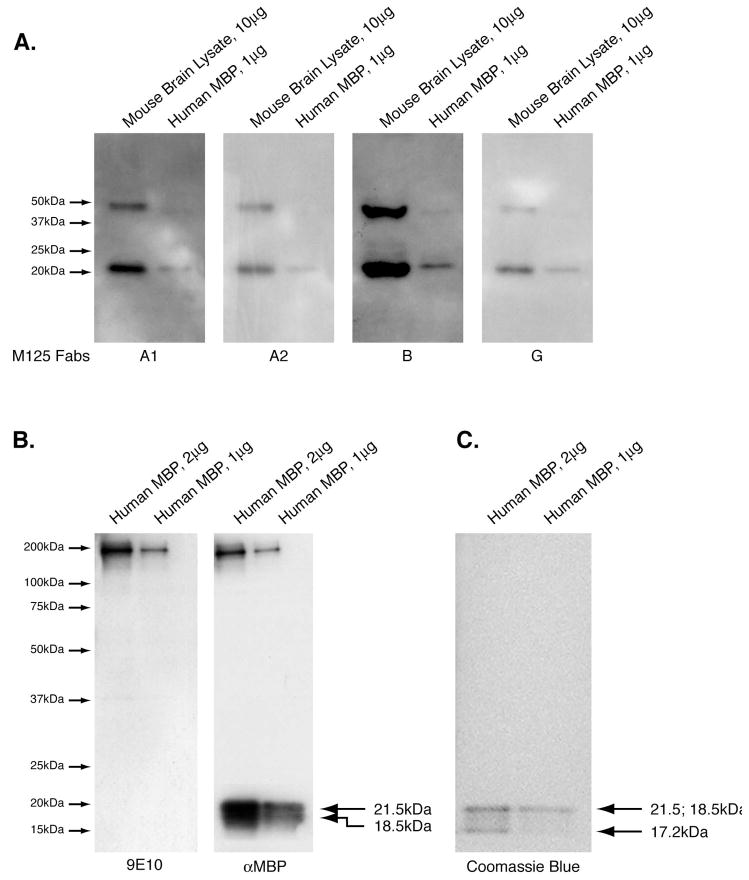
Figure 2. Fabs are reactive to MBP in preliminary screenings.
Panel A. Four sets of purified human MBP (1 ug/lane) and mouse Brain Lysate (10 ug/lane) were resolved by SDS-PAGE. Western blots were carried out as described in Materials and Methods using one of the four Fabs (M125-A1, -A2, -B or –G) as the primary Western blot antibody for one blot. All four of these Fabs are reactive to MBP. Panel B. Control blots showing reactivity of a commercially available anti-MBP antibody to human MBP (1 and 2 ug/lane), and the reactivity of the secondary 9E10, only. The bands at high molecular weight are due to incomplete reduction of the protein samples prior to electropheresis and correspond to the amount of protein loaded in the respective lanes. Panel C. Parallel SDS-PAGE of MBP (1 ug or 2 ug) resolved per lane then stained with coomassie blue to demonstrate the presence of MBP isoforms at 21.5 kDa, 18.5 kDa and 17.2 kDa.
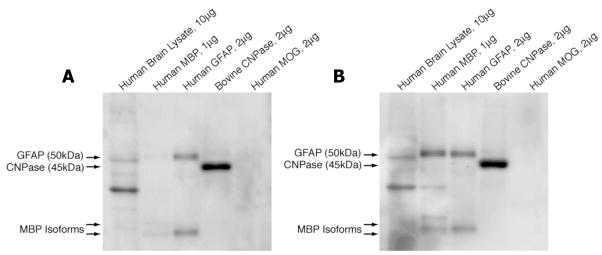
Figure 4. M125-D1 and -D2 react to CNPase.
Purified antigens as indicated were resolved by SDS-PAGE, transferred to membranes, and WBs using either M125-D1 (Panel A) or –D2 Fabs (Panel B) as the primary probe were performed as described in materials and methods. Both clone members of M125-D recognize hCNPase and GFAP by WB. M125-D2 also recognizes MBP. Both Fabs also reacted to an unidentified 30 kDa protein in hBL (Panels A and B). These experiments were performed 3 times with similar results.
Brain proteins were resolved by SDS-PAGE and transferred to PVDF membranes using a tank transfer system (Biorad, Hercules, CA). Membranes were washed in TBST and blocked for 1 hour in TBST/5% non-fat milk at room temperature. The membranes were probed with a 1:100 dilution (20 ng/ml) of the appropriate Fab in TBST/2% non-fat milk overnight on an orbital shaker at room temperature. Membranes were washed 3 times in TBST at room temperature and subsequently incubated with a 1:500 dilution of 9E10 (4 ng/ml). Use of GAM-HRP and ECL detection were performed as recommended by the manufacturer (Amersham-Pharmacia).
Immunoprecipitation (Figure 3)
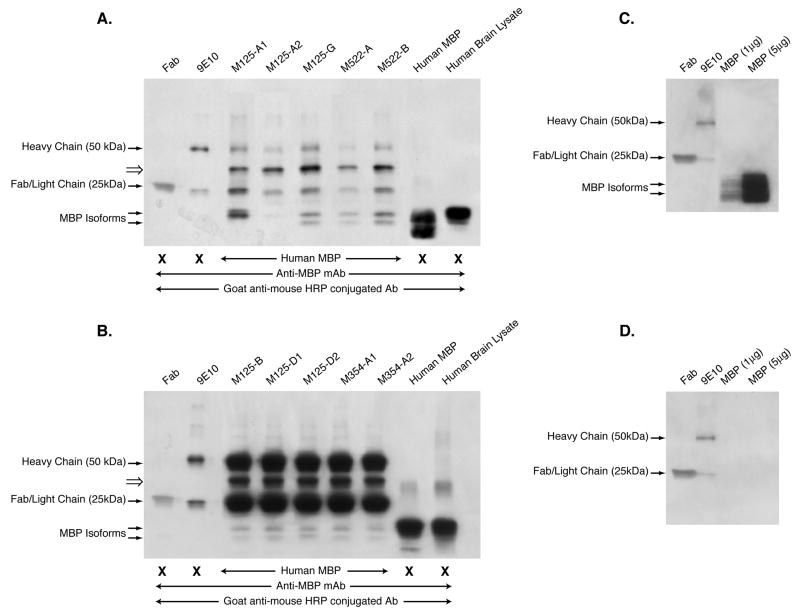
Figure 3. Fabs generated from clonally expanded CSF B cell antibody rearrangements are reactive to MBP.
Panels A and B. Fabs were used as capture antibodies to MBP, and the resultant eluates of the IP reactions resolved on 4–20% PAGE gradient gels, transferred to PVDF membranes and probed with the commercially available anti-MBP antibody. Bands at 25 kDa and 50 kDa correspond to the light and heavy chains respectively of the c-myc antibody used to precipitate the Fabs bound to MBP. Fragments corresponding to MBP were detected as a doublet at 20 kDa with the exception of the M125-A1 IP, which seems to contain only one fragment. Note that the data represented in Panels A and B were not performed in the same experiment. Panel C. Components of the IP reaction (Fab, 9E10, and purified hMBP), which were resolved by SDS-PAGE, transferred to PVDF, and probed with anti-MBP followed by GAM-HRP. Panel D. Same filter was probed with GAM-HRP only. These experiments were performed 4 times with similar results.
The Fabs (50 ng) were incubated overnight with 12.5 μg of purified human MBP (Sigma) in PBS (to maintain MBP conformational epitopes) with a proteinase inhibitor cocktail (Roche, Basel, Switzerland) in a volume of 300 μl on a rocker at 4°C. In parallel, 10 μg of the secondary antibody 9E10 was coupled to Protein A beads (100 μl, Pierce) also in PBS with a proteinase inhibitor cocktail (300 μl total volume) on a rocker at 4°C. The two fractions were combined and incubated for 4 hours on a rocker at 4°C. The washing and elution of antibodies and substrate from the beads was performed according to the recommendations of the manufacturer. The eluate volume was 50 μl.
Western Blot of Immunoprecipitated Samples (Figure 3)
Two μl of sample buffer (250 mM Tris/HCL, ph 6.8, 10% SDS, 0.5% Bromphenolblue, 500 mM β2-Mercaptoethanol, 50% Glycerol) was combined with 8 uL aliquots of the immunoprecipitate and denatured for 10 min at 100°C. The samples were resolved on 4–20% PAGE gradient gels, transferred to PVDF membranes as described above and probed with a commercially available anti-MBP antibody (Pharmingen, San Jose, CA). In order to confirm the identity of bands in the IPs, 8 ng of a Fab preparation, 12.5 ng of the anti-c-myc antibody, 0.5 μg of human MBP and 0.2 μg of a whole human brain lysate (Clontech) were resolved in separate lanes of the same gel. Following transfer to PVDF membranes as described above, membranes were washed in TBST and blocked for 1 hour in TBST/5% non-fat milk at room temperature, then incubated/probed a commercially available control anti-MBP monoclonal full length antibody (Clone Myelin BP, BD Pharmingen, San Diego, CA) at 5ng/mL for 2 hours at room temperature. Binding of the anti-MBP antibody was detected with a GAM-HRP antibody and visualized by ECL exposure as recommended by the manufacturer.
ELISA assay (Figure 5)
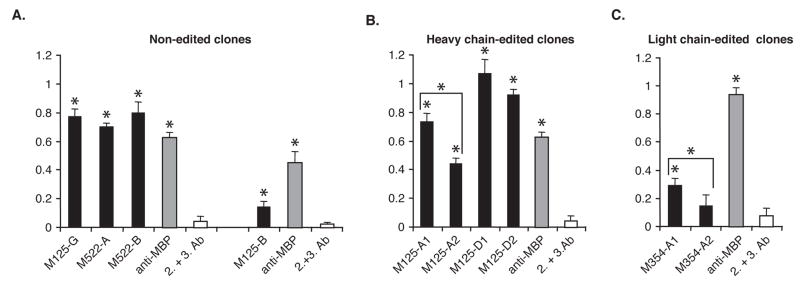
Figure 5. Receptor editing can diminish MBP binding as indicated by ELISA.
Panel A. MBP reactivity of the non-edited Fab clones (black bars) and a commercially available anti-MBP antibody (gray bars), or the secondary and tertiary antibodies alone (white bars). Panel B. MBP reactivity of the heavy chain edited Fab clones by ELISA. The receptor edited clone member M125-A2 had a significantly decreased reactivity to MBP compared to the non-edited clone member M125-A1. The non-edited and receptor edited clone members of M125-D had similar reactivity to MBP by ELISA. Panel C. MBP reactivity of the light chain edited clone M354-A2 had a significantly decreased reactivity to MBP in comparison to the non-edited clone member, M354-A1. In all assays, the ELISA plates were coated with whole human MBP (1 ug/mL) and ELISA was performed as described in Materials and Methods. Y-axis readout (color intensity) was determined at O.D. 405. Data shown represent the mean values for triplicate samples. Asterix (*) indicate Fabs that reacted to MBP statistically greater than background as assessed by student t-test (p≤0.05). This ELISA is representative of 6 independent experiments.
ELISA plates (Dynex Technologies, Inc., Chantilly, VA) were coated with the appropriate antigens (1 μg/ml) diluted in Blocking Buffer (1% BSA in PBS) overnight at 4°C. The wells were washed in PBS/Tween to remove unbound antigen, and blocked with PBS/1% BSA for 2 hours at room temperature. The plates were washed again in PBS/Tween and probed with Fabs (200 ng/ml) overnight at 4°C or the control anti-MBP antibody (0.5 ng/ml) diluted in Blocking Buffer for 2 hours at room temperature. The wells were washed extensively with PBS/Tween and probed with anti-his6 at a 1:200 dilution (2.5 ng/ml) in Blocking Buffer for 2 hours at room temperature. The wells were washed again, and probed a third time with GAM-HRP antibody (0.1 ng/ml) for 1 hour at room temperature. The wells were washed extensively and antibody binding detected by ABTS substrate (Sigma) color intensity readings at O.D.405. Samples were done in triplicate.
Analysis of antibody binding by solid phase DELFIA (Figure 6)
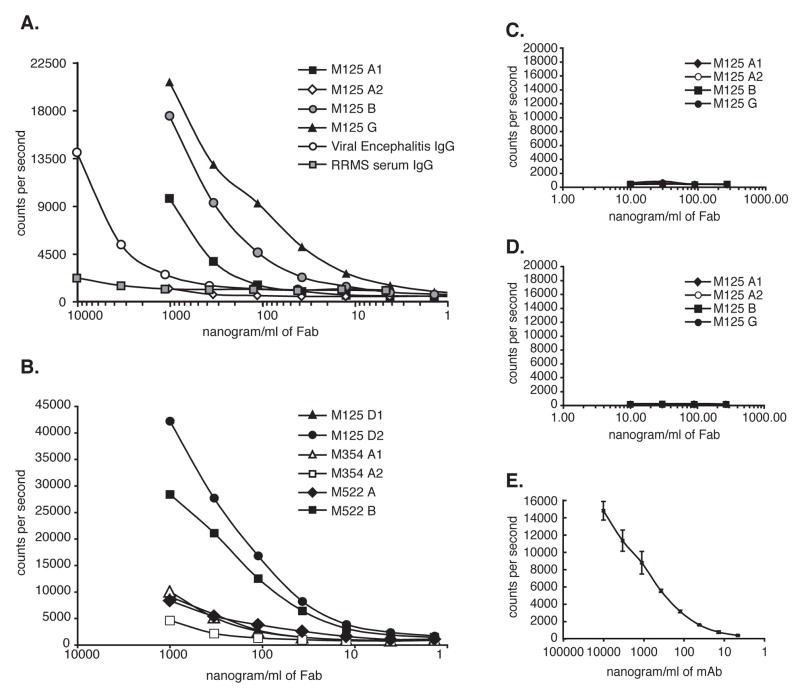
Figure 6. Fabs bind specifically to human MBP by DELFIA.
Plates were coated with either MBP (Panels A and B), Histone H1 (Panel C), or Lysozyme (Panel D) which are the control antigens with similar pI to MBP. Panel E. MBP binding of the anti-MBP antibody used throughout this study by DELFIA. A serum sample from a patient with confirmed Viral Encephalomyelitis (with high anti-MBP reactivity) and a serum sample from a patient with RRMS (with low anti-MBP reactivity) were included in the assay as internal controls and are shown in Panel A. M125-A1, B, D2, G and M522-B bind MBP with high titer, M125-D1, M522-A, M354-A1 and A2 bind MBP with low titer, and M125-A2 had negligible MBP reactivity.
To confirm the binding of the Fabs to MBP, we chose to use DELFIA (Butcher et al., 2003; Ogata et al., 1992). Our adaptation for detection of antibodies used a stringent assay buffer with 0.5% Triton X-100, which significantly reduced background and eliminated weak binding false positives. These conditions did not prevent the binding of physiologically relevant antibodies as we are routinely able to detect and titer robust antibody responses to hepatitis C and HIV antigens in the serum and CSF of individuals infected by these viruses (not shown).
Maxisorb low fluorescence 96 well plates (Perkin Elmer-Wallac, Boston, MA) were coated overnight at 4°C with 250 ng of antigen in 50 μl of PBS. After coating, wells were washed in 50 mM Tris pH 7.8, 150 mM NaCl containing 0.05% Tween-20, 20 μM EDTA and 0.5% Triton X-100, and blocked for 1 h at room temperature with DELFIA assay buffer (Perkin Elmer-Wallac) supplemented with 1% BSA in PBS and 0.5% Triton X-100. Purified Fabs were serially diluted as described in the appropriate figures, dilutions were made in blocking buffer and binding was allowed to proceed at 4°C overnight. Bound Fabs were detected by incubation with an anti-histidine antibody (Amersham-Pharmacia) followed by a biotin-conjugated secondary antibody (Zymed) each for 1 h at room temperature followed by incubation with strepavidin-labeled europium (Perkin Elmer-Wallac) for 1 h at room temperature. The time-resolved fluorescence signal was measured following addition of enhancement solution (Perkin Elmer-Wallac) and expressed as counts per second (cps).
RESULTS
Antigen specificity of M125-A1, -A2, -B and –G to MBP
In preliminary screening, we consistently observed reactivity of the Fabs generated from clonally expanded CSF B cells from MS patients to denatured proteins in human (h) brain lysate (BL) by ELISA or mouse (m) BL by western blot that migrate at approximately 20 and 50 kDa. The most prevalent isoform of MBP is 18.5 kDa (Harauz et al., 2004; Siegel et al., 1999), so we suspected that these Fabs were reactive to MBP. We repeated these experiments testing Fab reactivity to denatured BL in parallel with commercially available human MBP. Indeed, all 4 of the Fabs shown here as representative examples reacted to mBL at 50 and 20 kDa, as well as the commercially available hMBP at 20 kDa (Figure 2). Reactivity to the 50 kDa band in the purified MBP preparation is not as intense as was observed in mBL, suggesting that the more complex composition of the BL and formation of MBP aggregates may have resulted in some portion of the MBP in BL migrating at 50 kDa, which has been observed by others (Golds and Braun, 1978).
The MBP reactive Fabs precipitate MBP in solution
In order to verify this specificity for MBP, immunoprecipitations (IPs) were performed using the Fabs as capture antibodies and human MBP as the candidate antigen. The IPs were performed in PBS to maintain the native conformation of MBP. The IP components (9E10, Fab and captured antigen) were then resolved by SDS-PAGE, transferred to PVDF, and probed with a commercially available anti-MBP antibody. The primary and secondary antibodies used in the IP strategy (Fab and 9E10, respectively), as well as purified human MBP and human Brain Lysate (hBL) were resolved in parallel with the IPs to allow proper band identification in the WB. As indicated in Figure 3 (Panels 3A and 3B), two human MBP isoforms (21.5 and 18 kDa) were detectable by WB of the human MBP (Lane 8) and human brain lysate (hBL)(Lane 9) using the commercially available anti-MBP antibody. These same isoforms were recognized by 9 of the 10 Fabs under IP conditions that maintained conformational epitopes (Panels 3A and 3B, Lanes 3–7). Fab M125-A2 did not recognize MBP as strongly as the other 9 Fabs (Panel 3A, Lane 4). Interestingly, this particular Fab was generated from a CSF B cell clone that had undergone heavy chain editing (HCE) and at least under IP conditions, no longer recognized MBP as strongly as its non-edited progenitor, M125-A1 (Panel 3A, Lane 3). Bands detected at 50 kDa represent the heavy chain of 9E10, which was one of the IP components recognized by the WB secondary antibody, GAM-HRP. Bands detected at 25 kDa represent Fab or whole antibody light chain, also recognized by the WB secondary reagent, GAM-HRP.
There was also a band detected at approximately 30 kDa in all IP’s, which could not be identified by protein sequencing or mass spectrometry. In order to determine whether any of the IP components contained a contaminant at ~30 kDa, all components of the IP (Fab, 9E10, and purified hMBP) were resolved by SDS-PAGE and probed with either anti-MBP followed by GAM-HRP (Figure 3C), or GAM-HRP alone (Figure 3D). However, no additional bands (especially at 30 kDa) could be detected in the IP components resolved separately. Recently, another group has reported that CSF antibodies from some MS patients are reactive to triosephosphate isomerase (TPI), which is 27 kDa and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which is 37 kDa (Kolln et al., 2006). However, it is not possible that the identity of the ~30 kDa bands we observed in the immunoprecipitates could be TPI or GAPDH, because these proteins were not included in the IP components, and in the subsequent western blots, the primary antibody recognizes MBP specifically, and the secondary antibody recognizes antibody constant regions.
Polyreactivity of M125-D
Preliminary screening also led us to suspect that M125-D1 (the non-edited member of clone M125-D) was reactive to several denatured proteins in hBL that migrate at approximately 35–50 kDa. Since the three most prominent proteins in hBL that migrate at this size are 2, ′3′-cyclic nucleotide 3′-phosphodiesterase (CNPase), glial fibrillary acidic protein (GFAP), and myelin oligodendrocyte glycoprotein (MOG), we resolved purified samples of these three proteins, as well as hBL and hMBP by SDS-PAGE and probed the resulting blots with M125-D1 or M125-D2 (the receptor edited member of M125-D). The WB indicated that both M125-D1 (Figure 4A) and M125–D2 (Figure 4B) were reactive to denatured (bovine) CNPase, denatured hGFAP and denatured hMBP, but not to denatured hMOG. The polyreactivity of M125-D to CNPase, MBP, and GFAP in WB cannot be explained by similarities of these proteins to CNPase with regard to sequence, structure or isoelectric point (pI)(Sakamoto et al., 2005). Purified antigens used in this study were also not contaminated with other myelin components since the GFAP protein was free of MBP (and vice versa) when we tested them with the respective commercially available anti-MBP and anti-GFAP antibodies (data not shown).
The MBP reactivity confirmed by ELISA
As indicated in the IP experiments, 9 of the 10 Fabs were reactive to MBP. However, it was possible that the Fabs are able to nonspecifically bind to MBP because of the low stringency in the IP conditions. Thus, in order to confirm the anti-MBP reactivity of these Fabs under more stringent conditions, the 10 Fabs were tested for MBP reactivity by ELISA (Figure 5). The ELISA conditions would maintain MBP integrity, but perhaps not to the same degree as IP conditions since a small amount of detergent was used in the ELISA strategy. However, the addition of detergent in the strategy would also make this approach more stringent than the IPs. All 9 of the Fabs that reacted to MBP by IP also reacted to MBP by ELISA. M125-A2, which did not demonstrate considerable reactivity to MBP by IP, also had a significantly decreased reactivity to MBP by ELISA in comparison to M125-A1 (p=0.02)(Panel 5B). M125-D1 and D2, which both reacted to MBP by IP, also reacted to MBP by ELISA with no significant difference in their MBP reactivity when the two clone members were compared to each other (p=0.75)(Panel 5B). M354-A1 and –A2, which both reacted to MBP by IP, also reacted to MBP by ELISA (Panel 5C). Interestingly, the light chain edited member, M354-A2, had decreased reactivity to MBP in comparison to its non-edited clone member, M354-A1 (p=0.05). Thus, the ELISA approach (which has slightly higher stringency conditions) confirmed that these Fabs recognize MBP as documented by the IPs.
We also included reactivity to MBP by a commercially available full-length anti-MBP IgG antibody (clone myelin MBP) in all three panels as an internal control. However, caution should be used in comparing MBP reactivity of the Fabs to this control for two reasons. First, the Fab signal is amplified due to the combination of secondary and tertiary reagents, which are necessary for detection of Fab binding. Detection of the full-length anti-MBP IgG antibody required only a secondary reagent for detection. Second, the full-length anti-MBP IgG antibody would naturally have a greater avidity for MBP because it contains 2 binding domains per molecule whereas the Fabs have only 1 binding domain per molecule.
We also tested the reactivity of the Fabs by ELISA to the three main peptides of MBP that are strongly bound by human MHC class II (MBP83-106, MBP111-129, and MBP143-171)(Correale et al., 2000; Goodkin et al., 2000; Huh et al., 2004; Meinl and Hohlfeld, 2002; Muraro et al., 1997; Zhang et al., 1993; Zhang et al., 1995), one of which is the main B cell epitope (MBP82-106) (Warren et al., 1995). However, none of the Fabs reacted to any of the three MBP derived peptides (data not shown). This data indicated that either the MBP linear epitopes recognized by these clonally expanded CSF B cell clones from MS patients have yet to be described, or are more readily recognized in their native state, as is the case with MOG (von Budingen et al., 2002).
Confirmation of Fab binding by solid phase dissociation enhanced ianthanide fluorescence immunoassay (DELFIA)
The DELFIA system was used to confirm Ag-specificity to MBP by a third methodology that is even more specific and stringent than ELISA(Butcher et al., 2003; Ogata et al., 1992). Indeed, the DELFIA system confirmed that Fabs M125-A1, -B, D2, and –G, and M522-B bound to MBP under stringent conditions (Figure 6A and 6B and Table 3). Fabs M125-D1, M354-A1 and A2, as well as M522-A did not bind as well to MBP in the DELFIA system compared to the other Fabs. M125-A2, the heavy chain edited clone member of M125-A, had negligible MBP binding according to this assay. Serum IgG from a patient with RRMS that has very little MBP reactivity (O’Connor et al., 2003) and serum IgG from a patient with Viral Encephalomyelitis with known high affinity MBP reactivity (Panel 6A)(O’Connor et al., 2003) were included as internal controls. Comparing MBP reactivity of serum IgG to these engineered Fabs is invalid for the same reasons given when using the full-length anti-MBP IgG antibody in the ELISA assays (differences in detection strategy and avidity).
Table 3.
Summary of MBP reactivity of all Fabs
| Screening Approach | |||
|---|---|---|---|
| Clone Name | IP/WB1 | ELISA2 | DELFIA3 |
| M125 | |||
| Clone A1 | Yes | Yes | Yes |
| Clone A2 | Weak | Weak | No |
| Clone B | Yes | Weak | Yes |
| Clone D1 | Yes | Yes | Weak |
| Clone D2 | Yes | Yes | Yes |
| Clone G | Yes | Yes | Yes |
| M354 | |||
| Clone A1 | Yes | Weak | Weak |
| Clone A2 | Yes | Weak | Weak |
| M522 | |||
| Clone A | Yes | Yes | Weak |
| Clone B | Yes | Yes | Yes |
Summary of data in Figure 3. ”Yes” indicates strong reactivity to MBP by IP in PBS to maintain conformational epitopes, “Weak” indicates reactivity to MBP by IP in PBS not as apparent as the other Fab samples
Summary of data in Figure 5. ”Yes” indicates strong reactivity to MBP by ELISA, “Weak” indicates reactivity to MBP is not as not as apparent as the other Fab samples
Summary of data in Figure 6. ”Yes” indicates strong reactivity to MBP, “Weak” indicates reactivity to MBP not as apparent as the other Fab samples, and “No” indicates negligible MBP reactivity
MBP contains a disproportionate number of lysyl (K) and arginyl (R) residues giving it a highly basic character (pI ≈ 10.6)(Carnegie, 1971). Thus, antibodies that bind MBP may interact through a non-specific charge interaction. Like MBP, lysozyme and histone H1 are cationic at neutral pH and are thus ideal control Ag for MBP. Accordingly, we tested binding to lysozyme and histone H1 coated plates as a control for binding to MBP. None of the Fabs that were positive in the MBP assays were positive in these control assays (Panels 6C and 6D), indicating that the reactivity of these Fabs toward MBP was specific. DELFIA in Panel 6E illustrates the MBP reactivity of the commercially available anti-MBP antibody used throughout this study. Reactivity of all Fabs to MBP by these different approaches is summarized in Table 3.
DISCUSSION
Since the 1970’s when it was first demonstrated that antibodies in CSF samples from MS patients were oligoclonal rather than polyclonal, B cells and/or the antibodies they produce have been suspected to play a role in the immunopathogenesis of MS. However, it has historically been difficult to confirm their involvement, since most of these arguments for B cell contributions to MS are circular in nature. For example, several groups (including ours) have demonstrated that the B cells isolated from the CSF of MS patients are clonally expanded (Blalock et al., 1999; Columbo et al., 2000; Monson et al., 2005a; Owens et al., 2003; Qin et al., 1998; Ritchie et al., 2004). But clonal expansion of B cells in a particular compartment simply indicates that a B cell population is recognizing its Ag in that compartment, it does not show what the Ag specificity of a given B cell found in the CSF of an MS patient is, or subsequently, whether that Ag specificity of the B cell has any influence on the pathogenesis of the disease. In a recent publication about the specificity of recombinant antibodies cloned from the CSF fluid of two MS patients, several peptide sequences were identified, which specifically bound to the antibody clones. However, no homologies to known human or viral proteins were identified (Yu et al., 2006). The data summarized here mark our first analysis of the protein antigen specificity of clonally expanded CSF B cells isolated from patients with RRMS.
We used three different assays (IP with detection of MBP in the IP complex by WB, ELISA and DELFIA) to confirm Fab specificity since the integrity of antigens varies in different assay systems, depending on the stringency of the assay system. DELFIA uses a highly stringent wash and assay buffer, compared to the low stringent conditions used for IPs and WB. This stringency in the DELFIA system was required to reduce non-specific binding so that non-specific antibody interactions would be excluded. In contrast, WB washing and binding conditions are more lenient, and would allow for lower affinity (and perhaps non-specific) interactions to be detected. Of the ten Fabs that were generated for this study from clonally expanded CSF B cells of three patients diagnosed with RRMS, nine of them were reactive to MBP in these three different screening approaches, although to varying degrees (summarized in Table 3). We had predicted that at least some of the clonally expanding B cells from the CSF of MS patients would be reactive to MBP, given the large body of established data indicating that MBP is a central candidate antigen in the pathogenesis of MS. For example, anti-MBP antibodies can be readily detected in the spinal fluid and brain tissues of MS patients (Martino et al., 1991) at higher concentrations than what is observed in healthy donors and patients with other neurological diseases. Furthermore, anti-MBP antibodies (and anti-PLP antibodies) can be detected in areas of myelin damage within lesion sites of MS patients (Wucherpfennig et al., 1997). Others have demonstrated that the presence of anti-MBP and anti-MOG antibodies in the sera of patients is a reliable predictor of early conversion to clinically definite MS, suggesting their existence contributes to disease pathogenesis (Berger et al., 2003), although others have argued against this finding (Maguire, 2003). In addition, a clinical trial using an altered peptide ligand (APL) of MBP83-99 was terminated because some of the patients had adverse effects (including clinical flare-ups) related to a T cell response that was cross-reactive with the APL and MBP (Bielekova et al., 2000). Finally, EAE, the mouse model of MS, can be induced in naive mice by passive transfer of either MBP specific T cells (Ando et al., 1989; Baron et al., 1993; Racke et al., 1994; Zamvil and Steinman, 1990), MBP specific B cells (Lyons et al., 2002), and serum containing anti-MBP antibodies in combination with MBP reactive T cells (Lyons et al., 2002; Myers et al., 1992).
However, it has also been reported that anti-MBP antibodies are absent in the CSF of MS patients (Brokstad et al., 1994; Chou et al., 1983), and thus do not have a central role in the pathogenesis of MS. It has also been suggested that responses to MBP by B cells (or T cells) are likely not initiating events leading to MS pathology, since MBP is buried deep within the core of compact myelin sheaths and would not be exposed to B cells (and T cells) without some damage having occurred already (Harauz et al., 2004; Siegel et al., 1999). We are intrigued however, that prior to myelin folding, MBP is accessible as protein on the surface of oligodendrocytes (Siegel et al., 1999). In either case, the finding here that the majority of Fabs generated from clonally expanded CSF B cells from three different RRMS patients are reactive to MBP will not address their pathogenic potential, only their antigen specificity. However, several interesting findings regarding their reactivity as a result of these studies may reveal some important aspects regarding the instigation of clonal expansion these CSF B cells have undergone and their response to that driving force.
First, it should be noted that the Fabs we generated were from clone members that had accumulated the largest number of mutations. Mutation accumulation in combination with clonal expansion would indicate a robust antigen driven process, and thus provide the greatest probability of identifying antigen specificity. We had previously reported that the mutation accumulation in these CSF B cell clones was impressive (up to 9-fold what we observe in peripheral blood B cells), especially in patients M125 and M354, who had been diagnosed with RRMS only 4 and 8 months respectively prior to CSF sampling (Monson et al., 2005a). The CSF B cell clones we identified from these patients were all reactive to MBP, indicating that within months of diagnosis, the B cell response against self-antigen of the CNS is readily detectable at this level. If MBP responses are not initiating events in the pathogenesis of MS, as has been suggested by others, then our data would suggest that considerable damage must already have occurred prior to or within months of diagnosis.
However, it should be noted that affinity of these antibodies for MBP would likely influence the response of MBP reactive B cells. For example, if these B cells express antibodies with low affinity for MBP, it is possible that 1) they have escaped deletion by eliciting low to undetectable signals in response to MBP engagement, or 2) MBP was not the driving antigen, but only a coincidental cross-reactivity that we could readily detect. The concept that these self-reactive B cells were able to survive under selection is quite plausible given that a low frequency of self-reactive B cells have been demonstrated to persist even after germinal center selection in healthy donors (Wardemann et al., 2003). However, such self-reactive B cells would likely be anergic and thus unable to undergo clonal expansion. Since all of the MBP reactive B cells investigated here had undergone robust clonal expansion and high mutation accumulation, it is unlikely that they have low affinity for MBP, if MBP was the driving antigen. If MBP was not the driving antigen (as the second possible scenario), then it will be necessary to screen these antibodies against a wide range of candidate antigens to identify the driving antigen. Such investigations are currently underway in our laboratory.
We were particularly interested in the antigen specificity of the B cell clones that had undergone receptor editing (M125-A, M125-D and M354-A). Receptor editing is a mechanism by which a self-reactive B cell attempts to escape its autoreactivity by reinitiating Ig rearrangement and replacing either its light chain or heavy chain for a newly rearranged one (Chen et al., 1997; Han et al., 1997; Kelsoe, 1996; Li et al., 2001; Papavasiliou et al., 1997; Prak and Weigert, 1995; Russell et al., 1991; Tiegs et al., 1993). We had previously noted that receptor editing in these cases was not an early event, but had occurred much later in the clonal lineage (Monson et al, 2005). The data presented here do not address the events that led to this late receptor editing, but do address whether receptor editing was successful or not. For example, two of the clones (M125-A and –D) had undergone heavy chain editing. All three screening approaches indicated that M125-A had successfully receptor edited since MBP reactivity was weak or negligible in the receptor edited clone member (M125-A2) compared to the non-edited member (M125-A1)(Table 3). This is the first evidence in a suspected human autoimmune disease that a natural occurrence of receptor editing can successfully abrogate self-reactivity.
On the other hand, M125-D had not successfully receptor edited since MBP reactivity remained comparable for both M125-D1 (the non-edited clone member) and M125-D2 (the edited clone member). Furthermore, M125-D1 and –D2 appeared to have a broad range of reactivity to other myelin antigens besides MBP, including GFAP and CNPase. Most polyreactive antibodies have historically been of an IgM isotype. It is possible that M125-D was an IgM expressing B cell since germline homologies of both the heavy and light chains of M125-D are higher than the other clones analyzed here (97.4% to 100%), consistent with IgM expressing B cells. Interestingly, receptor editing was thought to occur in isotype switched B cells only, suggesting that M125-D could not be an IgM expressing B cell. However, it has been reported that IgM expressing B cells in fetal spleen can also undergo extensive receptor editing (Lee et al., 2000), and self-reactive IgM titers are as robust as self-reactive IgG titers in murine models designed to study receptor editing (Witsch et al., 2006).
We were also intrigued by the reactivity of M125-D to CNPase, since it is the third most prevalent myelin protein in the CNS, is readily detected on oligodendroglial membranes (Sprinkle, 1989; Tsukada and Kurihara, 1992), and human T cells respond to CNPase (Muraro et al., 2002). However, its polyclonal specificity for MBP, GFAP and CNPase in combination with a failure to successfully receptor edit marks this clone as a true enigma in current B cell dogma. In particular, how could this clonally expanded B cell which receptor edited late in its lineage continue to survive when it remains poly-reactive for several self-antigens found in the brain? The most likely scenario is that reactivity to these antigens is relatively low such that signaling above threshold does not occur, as discussed. A second possibility is that this B cell had undergone allelic inclusion, which is the co-expression of two different light chains; one that confers self-reactivity and a second that does not, and thus allows the B cell to survive antigen dependant selection (Doyle et al., 2006). It is possible that M125-D2 engaged allelic inclusion, but we did not detect the second (non self-reactive) light chain rearrangement. Nevertheless, this B cell would still have the potential to generate autoimmunity. Finally, although we have recently established that the overall B cell population in the CNS appears to have undergone normal germinal center selection by molecular analysis (Harp et al., 2007), at a clonal level, selection and regulation of B cells in this compartment may be more flexible than in the periphery, and thus harbor expansion of B cells with high affinity for self-antigens prevalent in the brain, such as MBP.
In summary, we show here for the first time that clonally expanded CSF B cells from three MS patients are reactive to MBP, and that receptor editing was only occasionally successful in preventing this self-reactivity. Taken together, these data provide the compelling suggestion that regulatory mechanisms are not being utilized efficiently to prevent self-reactive B cells to populate the CNS. Remarkably, these B cell activities were already robust in a patient who had only been diagnosed with MS 4 months prior to sampling. Targeting the mechanism(s) that allows these self-reactive B cells to reside in the CNS of recently diagnosed MS patients may prove to be a potent immunotherapeutic strategy. In fact, Rituximab, a B cell depleting monoclonal antibody therapy, has been reported to have efficacy in patients with MS (Monson et al., 2005b; Stuve et al., 2005)(www.medicalnewstoday.com/medicalnews.php?newsid=51006), which is likely due to a decreased frequency of potentially autoreactive B cells.
Supplementary Material
In order to test whether these CSF B cell clones from MS patients (Table 1) were reactive to Ag found in the CNS, we inserted the heavy and light chain polymerase chain reaction (PCR) products from ten clonally expanded CSF B cells (including three that were products of receptor editing, see Table 2 for details) into a vector that would allow us to re-express these pairs as Fab fragments (Figure 1)(Ward, 1992a; Ward, 1992b). Fabs were then resolved by SDS-PAGE and stained with coomassie blue to confirm the purity and size of each Fab (Supplemental Figure 1A). Each of the Fab preparations isolated from the CSF B cell clones contained one band at approximately 25 kDa, the estimated size of the Fab light and heavy chains (Supplemental Figure 1A). The bands with smaller sizes represent degradation products of the respective Fabs that can accumulate in preparations over time. In order to confirm that the 25 kDa proteins detected by coomassie blue staining were indeed Fab heavy and light chains, the Fab preparations were again resolved by SDS-PAGE, transferred to PVDF, and the resulting membrane probed with 9E10, an antibody that recognizes the c-myc tail of the Fab heavy chains generated through this particular vector (Figure 1). 9E10 recognized a single band at ~25 kDa, indicating that the purified heavy chain of each Fab was expressed (Supplemental Figure 1B). The light chain of the Fab, which contains a histidine tail (Figure 1), was also expressed since an anti-histidine antibody recognized a single band at ~25 kDa as well (Supplemental Figure 1C). The differences in intensity of the various Fab heavy chains in Figure 2B is likely due to cleavage of the c-myc epitope in the preparations.
Acknowledgments
The authors wish to thank the patients who consented to sampling for this study. We would also like to thank Drs. Olaf Stüve, Amy Lovett-Racke and Petra Cravens for critical review of this manuscript, our summer students Kelly Rula and Timothy Ahearne for their dedicated help in various Fab isolation processes, Alejandra Herrera for graphics support, and Gina Remington for organizing patient information.
FOOTNOTE TO TITLE
This study was supported by grants from the National Institutes of Health (NIH) to MKR (RO1 NS 37513, RO1 AI 47133, and K24 NS 44250) and NLM (RO1 NS 40993), the National Multiple Sclerosis Society (NMSS) to NLM (RG 3267-A-1) and the Yellow Rose Foundation (MKR and NLM). NLM is a Wadsworth Foundation Young Investigator. KCO is a career transition fellow of the NMSS.
Abbreviations in alphabetical order
- CSF
cerebrospinal fluid
- CNPase
2,′3′-cyclic nucleotide 3′-phosphodiesterase
- DELFIA
dissociation enhanced ianthanide fluorescence immunoassay
- GFAP
glial fibrillary acidic protein
- HCE
heavy chain editing
- IP
immunoprecipitation
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- RAG
recombination activating genes
- RRMS
relapsing remitting multiple sclerosis
- WB
western blot
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ando DG, Clayton J, Kono D, Urban JL, Sercarz EE. Encephalitogenic T cells in the B10.PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol. 1989;124:132–143. doi: 10.1016/0008-8749(89)90117-2. [DOI] [PubMed] [Google Scholar]
- Antel J. Multiple sclerosis - emerging concepts of disease pathogenesis. J Neuroimmunol. 1999;98:45–48. doi: 10.1016/s0165-5728(99)00080-6. [DOI] [PubMed] [Google Scholar]
- Archelos JJ, Storch MK, Hartung HP. The role of B cells and autoantibodies in multiple sclerosis. Annu Neurol. 2000;47:694–706. [PubMed] [Google Scholar]
- Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Rubner P, Schautzer F, Egg R, Ulmer H, Mayringer I, Dilitz E, Deisenhammer F, Reindl M. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med. 2003;349:139–145. doi: 10.1056/NEJMoa022328. [DOI] [PubMed] [Google Scholar]
- Bielekova B, Goodwin B, Richert N, Cortese I, Takayuki K, Ghazaleh A, Bruno G, Eaton J, Antel J, Frank J, McFarland H, Martin R. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- Blalock JE, Zhou SR, Maier CC, Galin FS, Whitaker JN. Highly related immunolgobulin light chain sequences in different multiple sclerosis patients. J Neuroimmunol. 1999;100:98–101. doi: 10.1016/s0165-5728(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Brezinschek H-P, Brezinschek RI, Lipsky PE. Analysis of the heavy chain repertoire of human peripheral B cells using single-cell polymerase chain reaction. J Immunol. 1995;155:190–202. [PubMed] [Google Scholar]
- Brokstad KA, Page M, Nyland H, Haaheim LR. Autoantibodies to myelin basic protein are not present in the serum and CSF of MS patients. Acta Neurol Scand. 1994;89:407–411. doi: 10.1111/j.1600-0404.1994.tb02657.x. [DOI] [PubMed] [Google Scholar]
- Butcher H, Kennette W, Collins O, Demoor J, Koropatnick J. A sensitive time-resolved fluorescent immunoassay for metallothionein protein. J Immunol Methods. 2003;272:247–256. doi: 10.1016/s0022-1759(02)00441-6. [DOI] [PubMed] [Google Scholar]
- Carnegie PR. Amino acid sequence of the encephalitogenic basic protein from human myelin. Biochem J. 1971;123:57–67. doi: 10.1042/bj1230057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casellas R, Shih T, Kleinewietfeld M, Rakonjac J, Nemazee D, Rajewsky K, Nussenzweig M. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–1542. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- Chen C, Prak EL, Weigert M. Editing disease-associated autoantibodies. Immunity. 1997;6:97–105. doi: 10.1016/s1074-7613(00)80673-1. [DOI] [PubMed] [Google Scholar]
- Chou CH, Tourtellotte WW, Kibler RF. Failure to detect antibodies to myelin basic protein or peptic fragments of myelin basic protein in CSF of patients with MS. Neurology. 1983;33:24–28. doi: 10.1212/wnl.33.1.24. [DOI] [PubMed] [Google Scholar]
- Columbo M, Dono M, Gazzola P, Roncella S, Valetto A, Chiorazzi N, Mancardi GL, Ferrarini M. Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J Immunol. 2000;164:2782–2789. doi: 10.4049/jimmunol.164.5.2782. [DOI] [PubMed] [Google Scholar]
- Correale J, Lund B, McMillan M, Ko DY, McCarthy K, Weiner LP. T cell vaccination in secondary progressive multiple sclerosis. J Neuroimmunol. 2000;107:130–139. doi: 10.1016/s0165-5728(00)00235-6. [DOI] [PubMed] [Google Scholar]
- Cross AH, Trotter JL, Lyons J. B cells and antibodies in CNS demyelinating disease. J Neuroimmunol. 2001;112:1–14. doi: 10.1016/s0165-5728(00)00409-4. [DOI] [PubMed] [Google Scholar]
- Deibler GE, Martenson RE, Kies MW. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2:139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Doyle CM, Han J, Weigert MG, Prak ET. Consequences of receptor editing at the lambda locus: multireactivity and light chain secretion. Proc Natl Acad Sci U S A. 2006;103:11264–11269. doi: 10.1073/pnas.0604053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edry E, Melamed D. Receptor editing in positive and negative selection of B lymphopoiesis. J Immunol. 2004;173:4265–4271. doi: 10.4049/jimmunol.173.7.4265. [DOI] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Bio. 1985;5:3610. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farner NL, Dorner T, Lipsky PE. Molecular mechanisms and selection influence the generation of the human VlJl repertoire. J Immunol. 1999;162:2137–2145. [PubMed] [Google Scholar]
- Foster SJ, Brezinschek HP, Brezinschek RI, Lipsky PE. Molecular mechanisms and selective influences that shape the kappa chain repertoire of IgM+ B cells. J Clin Invest. 1997;99:1614–1627. doi: 10.1172/JCI119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golds EE, Braun PE. Cross-linking studies on the conformation and dimerization of myelin basic protein in solution. J Biol Chem. 1978;253:8171–8177. [PubMed] [Google Scholar]
- Goodkin DE, Shulman M, Winkelhake J, Waubant E, Andersson P, Stewart T, Nelson S, Fischbein N, Coyle PK, Frohman E, Jacobs L, Holcenberg J, Lee M, Mocci S. A phase I trial of solubilized DR2:MBP84-102 (AG284) in multiple sclerosis. Neurology. 2000;54:1414–1420. doi: 10.1212/wnl.54.7.1414. [DOI] [PubMed] [Google Scholar]
- Han S, Dillon SR, Zheng B, Shimoda M, Schlissel MS, Kelsoe G. VDJ recombinase activity in a subset of germinal center B cells: a mechanism for altering antibody responses. Science. 1997;278:301–305. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- Harauz G, Ishiyama N, Hill CM, Bates IR, Libich DS, Fares C. Myelin basic protein-diverse conformational states of an intrinsically unstructured protein and its roles in myelin assembly and multiple sclerosis. Micron. 2004;35:503–542. doi: 10.1016/j.micron.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Harp C, Lee J, Lambracht-Washington D, Cameron E, Olsen G, Frohman E, Racke M, Monson N. Cerebrospinal fluid B cells from multiple sclerosis patients are subject to normal germinal center selection. J Neuroimmunol. 2007;183:189–199. doi: 10.1016/j.jneuroim.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboom HR, Griffins AD, Johnson KS, Chiswell DJ, Hudson P, Winter G. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Research. 1991;19:4133. doi: 10.1093/nar/19.15.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh J, Yao K, Quigley L, Ludwin SK, McFarland HF, Muraro PA, Martin R, Ito K. Limited repertoire of HLA-DRB1*0401-restricted MBP111-129-specific T cells in HLA-DRB1*0401 Tg mice and their pathogenic potential. J Neuroimmunol. 2004;151:94–102. doi: 10.1016/j.jneuroim.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Kelsoe G. Life and death in germinal centers. Immunity. 1996;4:107–111. doi: 10.1016/s1074-7613(00)80675-5. [DOI] [PubMed] [Google Scholar]
- Kolln J, Ren HM, Da RR, Zhang Y, Spillner E, Olek M, Hermanowicz N, Hilgenberg LG, Smith MA, van den Noort S, Qin Y. Triosephosphate isomerase- and glyceraldehyde-3-phosphate dehydrogenase-reactive autoantibodies in the cerebrospinal fluid of patients with multiple sclerosis. J Immunol. 2006;177:5652–5658. doi: 10.4049/jimmunol.177.8.5652. [DOI] [PubMed] [Google Scholar]
- Lee J, Monson N, Lipsky P. The VlJl repertoire in human fetal spleen: evidence for positive selection and extensive receptor editing. J Immunol. 2000;165:6322–6333. doi: 10.4049/jimmunol.165.11.6322. [DOI] [PubMed] [Google Scholar]
- Li H, Jiang Y, Prak EL, Radic M, Weigert M. Editors and editing of anti-DNA receptors. Immunity. 2001;15:947–957. doi: 10.1016/s1074-7613(01)00251-5. [DOI] [PubMed] [Google Scholar]
- Lyons JA, Ramsbottom MJ, Cross AH. Critical role of antigen-specific antibody in experimental autoimmune encephalomyelitis induced by recombinant myelin oligodendrocyte glycoprotein. Eur J Immunol. 2002;32:1905–1913. doi: 10.1002/1521-4141(200207)32:7<1905::AID-IMMU1905>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Maguire GA. Antimyelin antibodies in multiple sclerosis. N Engl J Med. 2003;349:2269–2271. doi: 10.1056/NEJM200312043492321. author reply 2269–2271. [DOI] [PubMed] [Google Scholar]
- Martin Mdel P, Monson NL. Potential role of humoral immunity in the pathogenesis of multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE) Front Biosci. 2007;12:2735–2749. doi: 10.2741/2268. [DOI] [PubMed] [Google Scholar]
- Martino G, Olsson T, Fredrikson S, Hojeberg B, Kostulas V, Grimaldi LM, Link H. Cells producing antibodies specific for myelin basic protein region 70–89 are predominant in cerebrospinal fluid from patients with multiple sclerosis. Eur J Immunol. 1991;21:2971–2976. doi: 10.1002/eji.1830211211. [DOI] [PubMed] [Google Scholar]
- Meinl E, Hohlfeld R. Immunopathogenesis of multiple sclerosis: MBP and beyond. Clin Exp Immunol. 2002;128:395–397. doi: 10.1046/j.1365-2249.2002.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson NL, Brezinschek HP, Brezinschek RI, Mobley A, Vaughan GK, Frohman EM, Racke MK, Lipsky PE. Receptor revision and atypical mutational characteristics in clonally expanded B cells from the cerebrospinal fluid of recently diagnosed multiple sclerosis patients. J Neuroimmunol. 2005a;158:170–181. doi: 10.1016/j.jneuroim.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Monson NL, Cravens PD, Frohman EM, Hawker K, Racke MK. Effect of rituximab on the peripheral blood and cerebrospinal fluid B cells in patients with primary progressive multiple sclerosis. Arch Neurol. 2005b;62:258–264. doi: 10.1001/archneur.62.2.258. [DOI] [PubMed] [Google Scholar]
- Muraro PA, Kalbus M, Afshar G, McFarland HF, Martin R. T cell response to 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) in multiple sclerosis patients. J Neuroimmunol. 2002;130:233–242. doi: 10.1016/s0165-5728(02)00229-1. [DOI] [PubMed] [Google Scholar]
- Muraro PA, Vergelli M, Kalbus M, Banks DE, Nagle JW, Tranquill LR, Nepom GT, Biddison WE, McFarland HF, Martin R. Immunodominance of a low-affinity major histocompatibility complex-binding myelin basic protein epitope (residues 111–129) in HLA-DR4 (B1*0401) subjects is associated with a restricted T cell receptor repertoire. J Clin Invest. 1997;100:339–349. doi: 10.1172/JCI119539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KJ, Sprent J, Dougherty JP, Ron Y. Synergy between encephalitogenic T cells and myelin basic protein-specific antibodies in the induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 1992;41:1–8. doi: 10.1016/0165-5728(92)90188-q. [DOI] [PubMed] [Google Scholar]
- Nemazee D. Receptor editing in B cells. Adv Immunol. 2000;74:89–126. doi: 10.1016/s0065-2776(08)60909-8. [DOI] [PubMed] [Google Scholar]
- O’Connor KC, Baror A, Hafler DA. The neuroimmunology of multiple sclerosis: possible roles of T and B lymphocytes in immunopathogenesis. J Clin Immunol. 2001;21:81–92. doi: 10.1023/a:1011064007686. [DOI] [PubMed] [Google Scholar]
- O’Connor KC, Chitnis T, Griffin DE, Piyasirisilp S, Bar-Or A, Khoury S, Wucherpfennig KW, Hafler DA. Myelin basic protein-reactive autoantibodies in the serum and cerebrospinal fluid of multiple sclerosis patients are characterized by low-affinity interactions. J Neuroimmunol. 2003;136:140–148. doi: 10.1016/s0165-5728(03)00002-x. [DOI] [PubMed] [Google Scholar]
- Ogata A, Tagoh H, Lee T, Kuritani T, Takahara Y, Shimamura T, Ikegami H, Kurimoto M, Yoshizaki K, Kishimoto T. A new highly sensitive immunoassay for cytokines by dissociation-enhanced lanthanide fluoroimmunoassay (DELFIA) J Immunol Methods. 1992;148:15–22. doi: 10.1016/0022-1759(92)90153-k. [DOI] [PubMed] [Google Scholar]
- Owens G, Ritchie A, Burgoon M, Williamson R, Corboy J, Gilden D. Single Cell Repertoire Analysis Demonstrates Clonal Expansion is Prominent Feature of the B cell Response in Multiple Sclerosis Spinal Fluid. J Immunol. 2003;171:2725–2733. doi: 10.4049/jimmunol.171.5.2725. [DOI] [PubMed] [Google Scholar]
- Papavasiliou F, Casellas R, Suh H, Zin XF, Besmer E, Pelanda R, Nemazee D, Rajewski K, Nussenzweig MC. VDJ recominbation in mature B cells: a mechanisms for altering antibody responses. Science. 1997;278:298–302. doi: 10.1126/science.278.5336.298. [DOI] [PubMed] [Google Scholar]
- Prak EL, Weigert M. Light chain replacement: a new model for antibody gene rearrangement. J Exp Med. 1995;182:541–548. doi: 10.1084/jem.182.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Duquette P, Zhang Y, Talbot P, Poole R, Antel J. Clonal expansion and somatic hypermutation of VH genes of B cells from cerebrospinal fluid in multiple sclerosis. J Clin Invest. 1998;102:1045–1050. doi: 10.1172/JCI3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Rocken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994;180:1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie AM, Gilden DH, Williamson RA, Burgoon MP, Yu X, Helm K, Corboy JR, Owens GP. Comparative analysis of the CD19+ and CD138+ cell antibody repertoires in the cerebrospinal fluid of patients with multiple sclerosis. J Immunol. 2004;173:649–656. doi: 10.4049/jimmunol.173.1.649. [DOI] [PubMed] [Google Scholar]
- Russell DM, Dembic Z, Morahan G, Miller JF, Burki K, Nemazee D. Peripheral deletion of self-reactive B cells. Nat. 1991;354:308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruther U, Koenen M, Otto K, Muller-Hill B. pUR322, a vector for cloning and rapid chemical sequencing of DNA. Nucleic Acids Research. 1981;9:4087. doi: 10.1093/nar/9.16.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y, Tanaka N, Ichimiya T, Kurihara T, Nakamura KT. Crystal structure of the catalytic fragment of human brain 2′,3′-cyclic-nucleotide 3′-phosphodiesterase. J Mol Biol. 2005;346:789–800. doi: 10.1016/j.jmb.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD. Basic Neurochemistry. Lippincott, Williams and Wilkins; 1999. [Google Scholar]
- Sprinkle TJ. 2′,3′-cyclic nucleotide 3′-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit Rev Neurobiol. 1989;4:235–301. [PubMed] [Google Scholar]
- Stuve O, Cepok S, Elias B, Saleh A, Hartung HP, Hemmer B, Kieseier BC. Clinical Stabilization and Effective B cell Depletion in the Cerebrospinal Fluid and Peripheral Blood in a Patient with Fulminant Relapsing Remitting Multiple Sclerosis. Arch Neurol. 2005 doi: 10.1001/archneur.62.10.1620. In Press. [DOI] [PubMed] [Google Scholar]
- Stuve O, Marra CM, Jerome KR, Cook L, Cravens PD, Cepok S, Frohman EM, Phillips JT, Arendt G, Hemmer B, Monson NL, Racke MK. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2006;59:743–747. doi: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada Y, Kurihara T. 2Åå, 3Åå-Cyclic nucleotide 3Åå-phosphodiesterase: molecular characterization and possible functional significance. In: Martenson RE, editor. Myelin: Biology and Chemistry. CRC Press; Boca Raton, FL: 1992. pp. 448–480. [Google Scholar]
- von Budingen HC, Hauser SL, Fuhrmann A, Nabavi CB, Lee JI, Genain CP. Molecular characterization of antibody specificities against myelin/oligodendrocyte glycoprotein in autoimmune demyelination. Proc Natl Acad Sci U S A. 2002;99:8207–8212. doi: 10.1073/pnas.122092499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. Secretion of T cell receptor fragments from recombinant Escherichia coli cells. J Mol Biol. 1992a;224:885–890. doi: 10.1016/0022-2836(92)90455-s. [DOI] [PubMed] [Google Scholar]
- Ward ES. Antibody engineering: the use of Escherichia coli as an expression host. FASEB Journal. 1992b;6:2422–2427. doi: 10.1096/fasebj.6.7.1563594. [DOI] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Warren KG, Catz I, Steinman L. Fine specificity of the antibody response to myelin basic protein in the central nervous system in multiple sclerosis: the minimal B cell epitope and a model of its features. Proceedings of the National Academy of Science, USA. 1995;92:11061–11065. doi: 10.1073/pnas.92.24.11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witsch EJ, Cao H, Fukuyama H, Weigert M. Light chain editing generates polyreactive antibodies in chronic graft-versus-host reaction. J Exp Med. 2006;203:1761–1772. doi: 10.1084/jem.20060075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig KW, Catz I, Hausmann S, Strominger JL, Steinman L, Warren KG. Recognition of the immunodominant myelin basic protein peptide by autoantibodies and HLA-DR2-restricted T cell clones from multiple sclerosis patients. Identity of key contact residues in the B-cell and T-cell epitopes. J Clin Invest. 1997;100:1114–1122. doi: 10.1172/JCI119622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuz AS, Monson NL, Yavuz S, Grammer AC, Longo N, Girschick HJ, Lipsky PE. Different patterns of bcl-6 and p53 gene mutations in tonsillar B cells indicate separate mutational mechanisms. Mol Immunol. 2002;39:485–493. doi: 10.1016/s0161-5890(02)00117-7. [DOI] [PubMed] [Google Scholar]
- Yu X, Gilden DH, Ritchie AM, Burgoon MP, Keays KM, Owens GP. Specificity of recombinant antibodies generated from multiple sclerosis cerebrospinal fluid probed with a random peptide library. J Neuroimmunol. 2006;172:121–131. doi: 10.1016/j.jneuroim.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- Zhang J, Medaer R, Stinissen P, Hafler D, Raus J. MHC-restricted depletion of human myelin basic protein-reactive T cells by T cell vaccination. Science. 1993;261:1451–1454. doi: 10.1126/science.7690157. [DOI] [PubMed] [Google Scholar]
- Zhang J, Vandevyver C, Stinissen P, Raus J. In vivo clonotypic regulation of human myelin basic protein-reactive T cells by T cell vaccination. J Immunol. 1995;155:5868–5877. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In order to test whether these CSF B cell clones from MS patients (Table 1) were reactive to Ag found in the CNS, we inserted the heavy and light chain polymerase chain reaction (PCR) products from ten clonally expanded CSF B cells (including three that were products of receptor editing, see Table 2 for details) into a vector that would allow us to re-express these pairs as Fab fragments (Figure 1)(Ward, 1992a; Ward, 1992b). Fabs were then resolved by SDS-PAGE and stained with coomassie blue to confirm the purity and size of each Fab (Supplemental Figure 1A). Each of the Fab preparations isolated from the CSF B cell clones contained one band at approximately 25 kDa, the estimated size of the Fab light and heavy chains (Supplemental Figure 1A). The bands with smaller sizes represent degradation products of the respective Fabs that can accumulate in preparations over time. In order to confirm that the 25 kDa proteins detected by coomassie blue staining were indeed Fab heavy and light chains, the Fab preparations were again resolved by SDS-PAGE, transferred to PVDF, and the resulting membrane probed with 9E10, an antibody that recognizes the c-myc tail of the Fab heavy chains generated through this particular vector (Figure 1). 9E10 recognized a single band at ~25 kDa, indicating that the purified heavy chain of each Fab was expressed (Supplemental Figure 1B). The light chain of the Fab, which contains a histidine tail (Figure 1), was also expressed since an anti-histidine antibody recognized a single band at ~25 kDa as well (Supplemental Figure 1C). The differences in intensity of the various Fab heavy chains in Figure 2B is likely due to cleavage of the c-myc epitope in the preparations.