Abstract
The discovery of host-microRNA (miRNA) targets in the genomes of many vertebrate viruses indicates that the corresponding miRNAs are a part of the host’s innate antiviral defense. However, given that viruses evolve much faster than host miRNAs, it is surprising that viral variants lacking these “antiviral” miRNA target sequences have not become established. We present an alternate view that miRNAs are among the host molecules that viruses co-opt to suppress their own replication to evade immune elimination and establish a persistent infection. The presence of host-miRNA targets in the genomes of rapidly evolving viruses probably reflects the adaptation of these viruses to the cellular miRNA milieu.
RNA silencing and immunity
RNA silencing (Figure 1) is a widely conserved mechanism of post-transcriptional gene regulation. RNA interference (RNAi) is also an integral part of the antiviral defense in plants and invertebrates, which can process virus-derived long double stranded RNAs (dsRNA) into small interfering RNAs (siRNAs), amplify siRNAs by RNA dependent RNA polymerases (RDRP) and induce systemic RNAi [1]. The presence of a viral counter-defense in the form of viral suppressors of RNA silencing (VSRs) in numerous plant and invertebrate viruses is strong supporting evidence for the antiviral nature of RNAi in plants and invertebrates (Box 1).
Figure 1.
RNA Silencing. The RNA silencing pathway in plants and animals. In general, small RNA duplexes are generated from long viral dsRNA (in the case of siRNAs) or from single stranded RNA with stem loop structures (in the case of miRNAs). In vertebrates, miRNAs are generated in two steps. A stem-loop structure in the primary transcript (pri-miRNA) is cleaved by an RNase, Drosha, into pre-miRNA, which is then trimmed by Dicer into a mature miRNA duplex. The vertebrate Dicer generates small RNA duplexes only from single stranded RNA precursors with specific types of RNA secondary structure (stem-loops) and lacks the ability to process long dsRNA substrates. Long dsRNA molecules are potent triggers of the IFN response in vertebrates. Plants and invertebrates have multiple Dicer proteins, some of which can process long dsRNA substrates into siRNA. In many plants and invertebrates, the RNAi response is also amplified by the generation of secondary siRNAs by RDRPs from the RNAi degradation products and the spread of siRNA between cells (systemic RNAi). After the mature small RNA duplex is generated, the guide strand is loaded onto an Argonaute (AGO) family protein in the RNA-induced silencing complex (RISC). One of the strands of the RNA duplex, called a guide strand, is loaded onto a member of the AGO family protein in the RISC. The guide strand directs RISC to complementary targets in mRNA (indicated in red). Some AGO family proteins have slicer activity and can catalytically cleave the target mRNA. This also requires an extensive match of the guide strand with the target. AGO family members lacking slicer activity induce translational suppression of the target mRNA. In vertebrates, this does not require perfect complementarity of the guide strand with the target, and the ability of the 5′ end of the guide strand (seed region) to base-pair with the target mRNA is crucial in determining its specificity. Most organisms have both types of AGO family proteins.
Box 1. VSRs
Many viruses encode VSRs that can interfere with various components of the RNAi machinery [32]. VSRs are particularly common in plant and invertebrate viruses in which they primarily act as a counter-defense against the antiviral RNAi pathway [32]. Some vertebrate viruses also encode VSRs, which have been suggested to function as a counter-defense against putatively antiviral miRNAs. Many viruses generate dsRNA during replication or because of convergent transcription from compact genomes [33]. Some VSRs, such as influenza NS1 protein and vaccinia E3L protein, are dsRNA-binding proteins [34]. By sequestering dsRNA, they can outcompete the innate immune receptors, which recognize dsRNA of viral origin, and thereby dampen the IFN response. It is possible that dsRNA-binding VSRs are a counter-defense against primarily the IFN response rather than the RNAi pathway. Other VSRs, such as adenovirus VA1 RNA, are decoy non-coding RNAs that competitively inhibit cellular miRNAs processing enzymes [35]. HIV Tat and PFV Tas are viral proteins that interfere with the miRNA processing enzymes [19,20]. Such VSRs would affect the entire cellular miRNA milieu rather than just the host “antiviral” miRNAs. Furthermore, recent work has raised doubts about the potential of VSRs of primate retroviruses to suppress human RNAi machinery under physiological conditions [36]. Interfering with the miRNA processing pathway might enable the virus to favorably alter the miRNA profiles of the cell by affecting the host transcriptome. Because they tend to modulate global gene expression in the host, VSRs of vertebrate-infecting viruses might be more appropriately called virulence factors rather than counter-defense measures against the host miRNAs.
However, in vertebrates, the interferon (IFN) response is the primary form of innate antiviral defense. It is triggered by innate immune recognition of various viral intermediates and products, such as 5′-triphosphate on viral RNA, cytosolic DNA and dsRNA. Binding of IFNs to their receptors initiates signaling that leads to a global shutdown in protein translation, cellular RNA degradation and deamination and often the death of virus-infected cells [2]. As with RNAi in plants and invertebrates, numerous vertebrate viruses have evolved mechanisms to evade the IFN response [3].
It is not yet established whether small RNAs encoded in the vertebrate genome have a direct role in innate antiviral responses. Synthetic siRNAs have been shown to be able to inhibit the replication of a wide range of pathogenic viruses when introduced into mammalian cells and animal models [4,5]. In addition, several endogenous host microRNAs (miRNAs) have been found to target viral pathogens. For instance, one study analyzed the presence of human miRNA target sequences in many viral genomes and found that human-infecting viruses, especially single-stranded RNA viruses, are enriched for target sequences of human miRNAs [6]. Another study showed that in humans, the antiviral IFN response induces the expression of several endogenous miRNAs (i.e. miR-196, miR-296, miR-351, miR-431 and miR-448), which can inhibit the multiplication of hepatitis C virus (HCV) replicons, and some of them (i.e. miR-196 and miR-448) can inhibit the replication of HCV viruses in vitro [7]. The targets of these miRNAs are remarkably conserved across multiple HCV genotypes [7]. Finally, putative VSRs have been found in vertebrate viruses (Box 1) and their presence is taken as further evidence for miRNA-based antiviral immunity. Based on these observations, the previous studies conclude that miRNAs extensively mediate antiviral defenses in humans.
Although a role for IFN-induced miRNAs in antiviral defense seems to make sense, a critical examination of this issue indicates otherwise. From an evolutionary perspective on host-pathogen interactions, it becomes clear that seemingly antiviral host-encoded miRNAs, which evolve much slower than the virus they target, are unlikely to constitute a bona fide innate antiviral immune defense. It is more likely that rapidly evolving viruses take advantage of slowly evolving host miRNAs to increase their survival or persistence in the host, analogous to the manner in which viruses take advantage of other cellular processes. This seems to be particularly true for viruses that cause chronic infections. Here, we discuss how host-miRNA targets in viral genomes are beneficial viral adaptations that exploit the host-miRNA milieu. Although we have focused on vertebrate hosts and their well characterized viral pathogens, any rapidly evolving virus interacting with a relatively static host-miRNA milieu would be expected to develop such adaptations over time.
Host miRNAs and chronic HCV infection
HCV is unique among the hepatitis viruses in its striking predilection for inducing chronic infection, with more than three-quarters of HCV infections resulting in chronic hepatitis [8]. The impaired innate immune responses and the ability of HCV to rapidly evolve under constant immune pressure might both contribute to viral persistence [9]. Like other RNA viruses, HCV encodes its own RNA polymerase that lacks a proofreading activity. As a result, replication of HCV is highly error prone and the population of HCV virions in a single host has extensive sequence heterogeneity [9]. In contrast, host-miRNA seed regions evolve very slowly and are highly conserved. For example, consider the two miRNAs shown to inhibit HCV replication [7]: miR-196 is perfectly conserved in all vertebrates examined so far including humans, rats, mice and puffer fish and miR-448 is conserved in humans, rats and mice [10]. Yet studies have shown that a single mismatch, especially one that results in a G:U base pair wobble, in the miRNA seed region can greatly reduce miRNA-mediated repression [11]. Single point mutations in the miR-196 and miR-448 target regions in the HCV genome are indeed sufficient to make the virus resistant to miRNA-mediated suppression without affecting its replication [7]. Thus, based on the high mutation rate of HCV replication, and given that the putative miRNA target region in the virus is free to mutate without compromising HCV life cycle [7], HCV variants that lack the miRNA targets would probably have rapidly emerged if the host miRNAs were ‘antiviral’. Paradoxically, there seems to be a selective pressure on HCV to retain the miRNA target sequences in their genome.
There are six different genotypes of HCV that exhibit 31%–34% nucleotide sequence divergence. Based on the rate of evolution of conserved non-structural HCV proteins, it has been estimated that HCV originated as recently as 500 to 2000 years ago [10,12]. Yet the miRNA seed sequences with the most potent anti-HCV activity have remained unchanged since long before humans or HCV evolved [10]. Thus, it is unlikely that humans have evolved IFN-induced miRNAs to inhibit HCV, which did not emerge until much later. A claim that HCV-specific host miRNAs have acquired an antiviral function against HCV needs to be supported by showing that the miRNAs are specifically induced in HCV tropic tissues upon IFN stimulation. However, there are no reports to indicate that divergent miRNAs are induced in different cell types by type 1 IFNs. More likely, HCV itself has evolved IFN-inducible host-miRNA target sequences.
In general, the evolution of individual host miRNAs might be constrained by the fact they regulate multiple genes in their respective hosts, and host-miRNA seed sequences are highly conserved across insect, nematode and vertebrate hosts [13]. Although miRNA seed sequences and their complementary target regions in host genes are well-conserved, orthologous host miRNAs regulate varying sets of target genes in different organisms [14]. This indicates that host genes evolve miRNA targets that match the slowly evolving host-miRNA seed sequences. Similarly, rapidly evolving viral genes might also evolve targets that match host miRNAs.
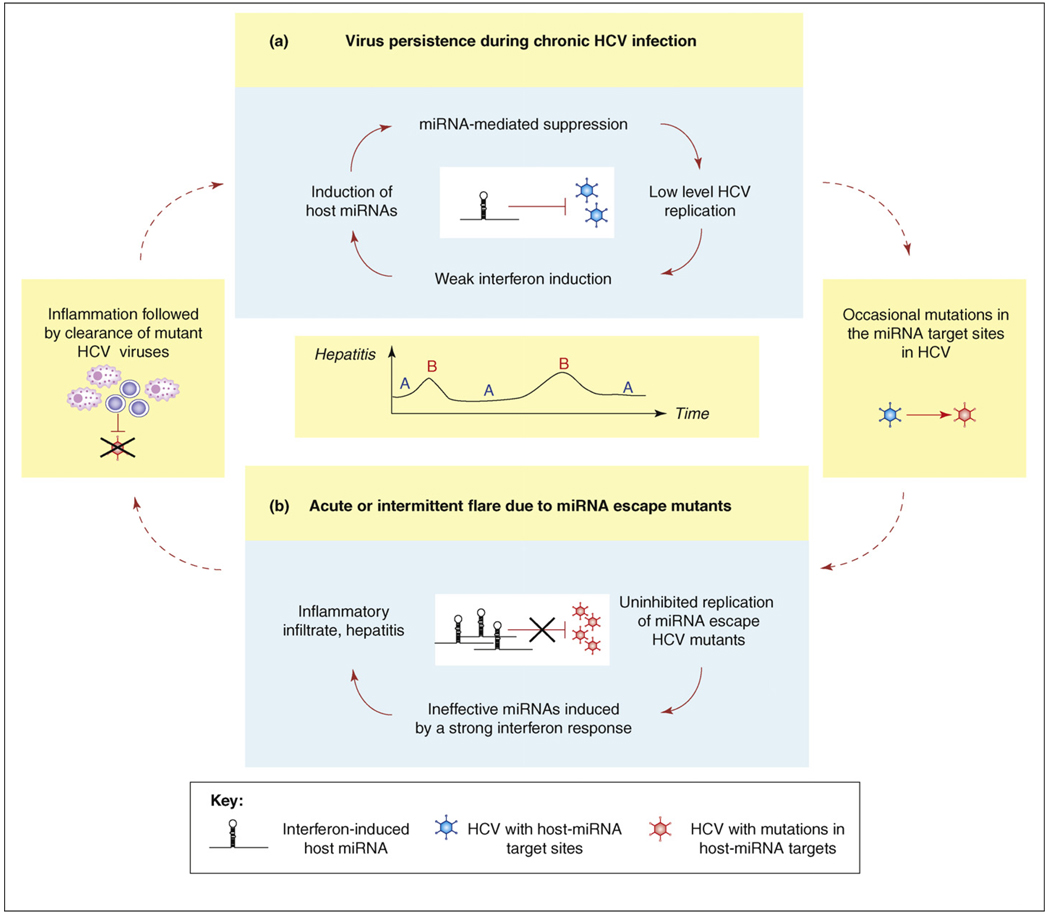
We propose that viral adaptation to pre-existing IFN-inducible miRNAs is one mechanism by which HCV avoids sterilizing immune responses and establishes persistent infection (Figure 2a). HCV is a potent inducer of the IFN response, and a strong IFN response often leads to the death of infected cells and the viruses in the cells. To avoid immediate elimination by the host, HCV seems to have evolved means to reduce its own replication and/or viral gene expression in an IFN-dependent manner. Because of the relatively modest effect on HCV gene expression, miRNA-mediated suppression would enable HCV to establish a low level of persistent infection without provoking a sterilizing immune response. Thus, HCV might exploit host miRNAs, which are induced by the innate immune response, to perpetuate a state of virus persistence. This view is reminiscent of the way another miRNA, miR-122, is utilized by HCV to aid its replication [15].
Figure 2.
Host miRNAs are central to HCV pathogenesis and viral persistence. This figure illustrates how the host miRNA and HCV interactions can explain the features of HCV pathogenesis such as the establishment of (a) virus persistence and (b) intermittent hepatitis. (a) Virus persistence during chronic HCV infection. HCV infection induces type 1 IFNs, which in turn induce expression of host miRNAs. These miRNAs then suppress the replication of HCV bearing their target sequences. This would maintain a low level of HCV replication and promote the persistence of HCV during chronic infection. (b) Acute or intermittent replication flare because of the appearance of miRNA escape mutants. Intermittent disruptions in the steady state of chronic HCV infection can arise from mutations in the miRNA target sequences in the HCV genome. Because miRNAs do not inhibit the replication of mutant HCV, a strong type 1 IFN response is triggered. The resulting inflammatory response could clear HCV viruses both with and without miRNA target sequences. The steady state of HCV persistence can be re-established once the mutant viruses are eliminated.
Our proposal is consistent with the evolution of IFN-inducible miRNAs long before the emergence of HCV, the absence of HCV variants that lack the miRNA target sites and the tendency of HCV to establish persistent infection. It also provides an explanation for the pathogenesis of both acute and chronic HCV infection. Many patients with HCV-associated chronic liver disease suffer from characteristic intermittent hepatitis with alternating quiescent periods [8]. As discussed, HCV RNA polymerase is error prone, and escape mutants from miRNA-mediated suppression are expected. These escape virions would not be able to regulate their own replication by using the IFN-induced miRNAs, and as a result, induce potent IFN responses (Figure 2b). This would result in the killing of a majority of hepatocytes harboring HCV viruses both with and without miRNA target sequences, causing inflammatory damage to the liver. However, miRNA-mediated suppression enables a population of sensitive virions to survive and persist until the next round of escape mutants arise. This would explain the intermittent flares of hepatitis seen in chronic HCV infection. If the escape mutants arise early in acute infection, they could trigger the occasional clearance of acute HCV infection observed in some patients. This notion is consistent with the observation that the combined therapy of exogenous IFN and the antiviral drug ribavirin can often cure chronic HCV hepatitis [16]. Thus, the adaptation of HCV to host IFN-inducible miRNAs could be central to the pathogenesis of HCV infection.
Based on the earlier discussion, if escape mutants are responsible for intermittent flares of hepatitis in chronic HCV infections, one would expect an increased number or frequency of miRNA escape-mutant viruses during ‘viral flares’. Sequencing of HCV isolates during and between flare-ups would enable the direct testing of these predictions. In addition, treatments that promote viral mutation, and thus miRNA escape, would cause increased inflammation and possibly complete elimination of the viruses.
Host miRNAs and other persistent virus infections
Co-option of host miRNAs by viruses to suppress their own gene expression could also contribute to persistent infection of other viruses. Retroviruses such as human immunodeficiency virus (HIV) are maintained in latent reservoirs in the host throughout the course of infection [17]. By shutting off replication, the latent reservoir of viruses can escape the immune system until future reactivation. HIV replication is suppressed by cellular miRNAs such as miR-28, miR-125b, miR-223 and miR-382 in resting CD4+ T cells, and it is speculated that these miRNAs help in maintaining viral latency [18]. A stem loop in the HIV transcript has also been shown to be processed into an ‘antiviral’ siRNA capable of degradation of the viral transcript [19]. An alternative explanation is that the auto-inhibitory siRNA in HIV might promote viral persistence and immune evasion. As latently infected cells are resistant to combination antiretroviral regimens, it has been suggested that interfering with the host miRNAs that promote HIV latency could render the virus susceptible to antiretroviral therapy [18].
The primate foamy retroviruses (PFVs) cause persistent infections in non-human primates and domestic animals. miR-32 was shown to inhibit the replication of PFV in human 293T cells [20]. The permissivity of PFV indifferent cell types is correlated with low expression of miR-32. Infected hematopoietic cells, in which miR-32 is highly expressed [21], support very little viral replication and are not killed, whereas non-hematopoietic cells, in which miR-32 is not abundant, are lysed and assume a foamy appearance because of severe cytopathic effects [22]. The low level of replication in hematopoietic cells, or other cell types that express high levels of miR-32, might well be the key to establishing a persistent PFV infection in susceptible hosts.
Sequence analyses have identified a plethora of miRNA targets in virus genomes [6,23]. Host-miRNA targets are particularly enriched in single-stranded RNA viruses and single-stranded DNA viruses, both of which are rapidly evolving viruses that lack proofreading activity in their polymerases. Among DNA viruses, the human papilloma-viruses are strikingly enriched for predicted host-miRNA targets [6], consistent with the ability of these viruses to establish chronic infections in epithelial tissues. Thus, adaptations of viruses to the host-miRNA milieu to avoid sterilizing immune responses and establish a persistent infection might be a widespread phenomenon.
Virus-host miRNA interactions in dead-end hosts
Two widely expressed miRNAs, miR-24 and miR-93, have been shown to target vesicular stomatitis virus (VSV) and protect mice against VSV infection [24]. VSV infects both insects and animals in the wild because of the broad tropism of the coat protein. Infected black flies can horizontally transmit VSVto uninfected flies when they co-feed on the same host [25]. Therefore, it has been suggested that livestock are just indicator hosts of a passive insect reservoir of VSV [26]. Although laboratory mice can be experimentally infected with VSV, they are dead-end hosts for VSV and in most cases do not transmit the virus even if infected and non-infected mice are housed in the same cage. Although this makes VSV-infected mice a very convenient model for studying mammalian immune responses to systemic viral infection, the mouse model of VSV infection cannot be used for analysis of host-virus co-evolution. Indeed, only the Indiana strain of VSV, which is commonly used in the laboratory, is inhibited by miR-24 and miR-93. The New Jersey strain, which represents the majority of the field isolates in the Americas [27], is resistant to these miRNAs because of differences in the miRNA target regions [27]. This highlights the limitation of host reliance on a miRNA-dependent antiviral response. The miRNA seed matches seen in VSV viral genomes might well be fortuitous matches, and their role in antiviral defense is hard to assess in the setting of an unnatural infection model.
New viruses constantly emerge, often by jumping across host species barriers as zoonoses. Cross-species viral transfers often result in dead-end infections in their new hosts. Viruses need to be able to adapt rapidly to establish efficient transmission and propagation within the new hosts. Consider a hypothetical virus, which has just crossed a species barrier into a new pool of hosts that is possibly only distantly related to its historic carrier. Given that a perfect 7-mer seed sequence match with its target is sufficient for miRNA activity [28,29], a complete 7-mer sequence match will be found in a random stretch of DNA or RNA every 47 (=16384) nucleotides on average, which is approximately the size of an RNA virus genome such as HCV or influenza. As per the Poisson distribution, two-thirds of vertebrate miRNAs are expected to have at least one hit in a virus with a genome of ~16 kb by random chance alone. Vertebrates encode several hundred miRNA families with distinct seed sequences [30]. So, a large number of host miRNAs can be expected to have fortuitous matches with viral genomes even in dead-end hosts which have not co-evolved with the virus and a significant number of these matches could be functional depending on their accessibility, location and energetics. Once efficient host to host transmission is established by the hypothetical virus, any antiviral miRNA target sequences in the virus can be expected to mutate rapidly to maximize mismatches and, thus, minimize the impact of ‘antiviral’ miRNAs. The retention of host-miRNA targets in multiple virus isolates from natural hosts would indicate that the targets of host miRNA in a viral genome have an adaptive advantage for the virus unless the miRNA target region is conserved for reasons other than maintaining complementarity to host miRNA (Figure 3).
Figure 3.
Co-evolution of the miRNA pathway in hosts and viruses. The interaction between hosts and viruses involving miRNAs is illustrated in this figure from the perspective of host-virus co-evolution. Being obligate cellular parasites, viruses exploit numerous cellular components for their replication and survival, including components of the host-miRNA pathway such as (a) miRNA genes, (b) miRNA targets and (c) regulators of RNAi machinery. (a) miRNA genes have been observed in host and viral genomes. The genetic interchange of miRNA genes between viruses and their hosts might have contributed to the origin of some viral miRNAs in addition to the diversification of host miRNAs. (b) Viruses have evolved targets for host miRNAs for the regulation of their own genes by host miRNAs. (c) The RNAi machinery is accompanied by several regulatory components in the host as it has a crucial role in host gene regulation. These regulatory components might have also been co-opted by viruses and given rise to VSRs.
Conclusions
We propose that IFN-inducible miRNAs are among the host molecules that HCV co-opts to suppress its own replication to avoid sterilizing immunity and establish chronic infection. By the same notion, we speculate that other rapidly evolving viruses, such as HIV and PFV, have also evolved mechanisms to utilize host miRNAs for immune evasion and persistent infection. The proposed host miRNA-dependent mechanism probably acts in concert with other host and viral factors, such as immune exhaustion and T regulatory cells, for establishing chronic infection [31]. Our proposal shifts away from the simplistic notion that host miRNAs with specific sequence complementarity to viruses are indicative of a bona fide innate antiviral immune mechanism to an established viral pathogen. However, host miRNAs might contribute to the species barrier and result in dead-end infections following recent zoonoses. Our alternative interpretation of recent findings better explains features of HCV infection and pathogenesis, in addition to providing a novel and general perspective on host-virus interactions involving small RNA. Furthermore, in an era in which exogenous siRNA therapeutics against viruses are increasingly being developed, the potential therapeutic effect of modulating host-miRNA levels is also worth considering. By boosting the level of the host virus-specific miRNAs, it might be possible to turn what was a desirable outcome for the virus into a desirable outcome for the patient. Under the current hypothesis, we would predict that decreasing the level of host virus-specific miRNAs would result in promotion of viral replication, leading to immunological recognition and clearance of the virus by the host immune system. However, manipulating the level of host miRNAs could have unintended consequences because the physiological functions of the miRNAs might be altered or viral pathology might be enhanced. Nevertheless, both of these potential interventions merit further evaluation.
Acknowledgements
We would like to thank Dr. Herman N. Eisen for a critical review of the manuscript, Dr. Patrick Stern, Dr. Ramya Rajagopalan and Dr. Ching-Hung Shen for helpful discussions. This work was supported in part by grants from National Institutes of Health (AI40146 and AI69208) and Singapore-MIT Alliance in Research and Technology (SMART) to J.C.
References
- 1.Ding SW, Voinnet O. Antiviral immunity directed by small RNAs. Cell. 2007;130:413–426. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Goodbourn S, et al. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 2000;81:2341–2364. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- 4.Gitlin L, Andino R. Nucleic acid-based immune system: the antiviral potential of mammalian RNA silencing. J. Virol. 2003;77:7159–7165. doi: 10.1128/JVI.77.13.7159-7165.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ge Q, et al. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8676–8681. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe Y, et al. Computational analysis of microRNA-mediated antiviral defense in humans. FEBS Lett. 2007;581:4603–4610. doi: 10.1016/j.febslet.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen IM, et al. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–922. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V, et al. Robbins and Cotran Pathologic Basis of Disease. 7th edition. Elsevier Saunders; 2005. Liver and bilary tract; pp. 894–895. [Google Scholar]
- 9.Dustin LB, Rice CM. Flying under the radar: the immunobiology of hepatitis C. Annu. Rev. Immunol. 2007;25:71–99. doi: 10.1146/annurev.immunol.25.022106.141602. [DOI] [PubMed] [Google Scholar]
- 10.John B, et al. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmonds P. The origin and evolution of hepatitis viruses in humans. J. Gen. Virol. 2001;82:693–712. doi: 10.1099/0022-1317-82-4-693. [DOI] [PubMed] [Google Scholar]
- 13.Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat. Rev. Genet. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- 14.Chen K, Rajewsky N. Deep conservation of microRNA-target relationships and 3′UTR motifs in vertebrates, flies, and nematodes. Cold Spring Harb. Symp. Quant. Biol. 2006;71:149–156. doi: 10.1101/sqb.2006.71.039. [DOI] [PubMed] [Google Scholar]
- 15.Jopling CL, et al. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 16.Manns MP, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 17.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat. Med. 2007;13:1241–1247. doi: 10.1038/nm1639. [DOI] [PubMed] [Google Scholar]
- 19.Bennasser Y, et al. Evidence that HIV-1 encodes an siRNA and a suppressor of RNA silencing. Immunity. 2005;22:607–619. doi: 10.1016/j.immuni.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Lecellier CH, et al. A cellular microRNA mediates antiviral defense in human cells. Science. 2005;308:557–560. doi: 10.1126/science.1108784. [DOI] [PubMed] [Google Scholar]
- 21.Landgraf P, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meiering CD, Linial ML. Historical perspective of foamy virus epidemiology and infection. Clin. Microbiol. Rev. 2001;14:165–176. doi: 10.1128/CMR.14.1.165-176.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu PW, et al. ViTa: prediction of host microRNAs targets on viruses. Nucleic Acids Res. 2007;35:D381–D385. doi: 10.1093/nar/gkl1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otsuka M, et al. Hypersusceptibility to vesicular stomatitis virus infection in Dicer1-deficient mice is due to impaired miR24 and miR93 expression. Immunity. 2007;27:123–134. doi: 10.1016/j.immuni.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Mead DG, et al. Transmission of vesicular stomatitis virus from infected to noninfected black flies co-feeding on nonviremic deer mice. Science. 2000;287:485–487. doi: 10.1126/science.287.5452.485. [DOI] [PubMed] [Google Scholar]
- 26.Pringle CR. Vesicular Stomatitis Virus, Encyclopedia of life sciences. Nature Publishing Group; 2002. [Google Scholar]
- 27.Muller S, Imler JL. Dicing with viruses: microRNAs as antiviral factors. Immunity. 2007;27:1–3. doi: 10.1016/j.immuni.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Brennecke J, et al. Principles of microRNA-target recognition. PLoS Biol. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis BP, et al. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths-Jones S, et al. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinic MM, von Herrath MG. Novel strategies to eliminate persistent viral infections. Trends Immunol. 2008;29:116–124. doi: 10.1016/j.it.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Li F, Ding SW. Virus counterdefense: diverse strategies for evading the RNA-silencing immunity. Annu. Rev. Microbiol. 2006;60:503–531. doi: 10.1146/annurev.micro.60.080805.142205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs BL, Langland JO. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology. 1996;219:339–349. doi: 10.1006/viro.1996.0259. [DOI] [PubMed] [Google Scholar]
- 34.Li WX, et al. Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1350–1355. doi: 10.1073/pnas.0308308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu S, Cullen BR. Adenovirus VA1 noncoding RNA can inhibit small interfering RNA and MicroRNA biogenesis. J. Virol. 2004;78:12868–12876. doi: 10.1128/JVI.78.23.12868-12876.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin J, Cullen BR. Analysis of the interaction of primate retroviruses with the human RNA interference machinery. J. Virol. 2007;81:12218–12226. doi: 10.1128/JVI.01390-07. [DOI] [PMC free article] [PubMed] [Google Scholar]