Abstract
Clostridium difficile is a pathogen with increasing severity for which host antibody responses provide protection from disease. DNA vaccination has several advantages compared to traditional vaccine methods, however no study has examined this platform against C. difficile toxins. A synthetic gene was created encoding the receptor-binding domain (RBD) of C. difficile toxin A, optimized for expression in human cells. Gene expression was examined in vitro. Mice were inoculated then challenged with parenteral toxin A. Vaccination provided high titer antibodies and protected mice from death. This represents the first report of DNA vaccine inducing neutralizing antibodies to Clostridium difficile toxin A.
Keywords: Vaccine, Clostridium difficile, DNA
1.0 Introduction
Clostridium difficile is a gram-positive, anaerobic, spore-forming bacterium recognized as a major cause of hospital-acquired diarrhea [1–6]. While as many as 3% of healthy outpatient adults may be colonized with the organism, this rate increases dramatically following hospitalization [7, 8]. Disease results from the production of one of two major cytotoxins, toxins A and B, whose clinical manifestations range from asymptomatic carriage to diarrhea, toxic megacolon, and death [3, 9–12]. Since 2000, extensive publications describe changes in the rates and severity of C. difficile disease generating renewed interest in novel approaches to disease treatment and prevention, including toxin-specific vaccines [4, 13–20].
It has been observed that toxin-specific, host antibodies influence the outcome of C. difficile colonization and infection [21]. Patients with anti-toxin A antibodies at the time of colonization with C. difficile spores are at lower risk of progression to active and severe disease [22]. Once infected, individuals who develop strong anti-toxin antibody responses clear their disease following antimicrobial treatment and remain disease free [23]. Such studies provide scientific rationale for development of a vaccine against C. difficile toxins. While numerous studies have presented candidate C. difficile vaccines [21, 24–28], to date, none has examined the DNA vaccine platform. DNA vaccination has a several advantages versus other modalities including established safety, ease of manufacturing, and the potential to include immunogenic coding sequences.
As proof of principle, we created a synthetic gene encoding the RBD of C. difficile toxin A, optimized for expression in human cells. The following data demonstrate that this gene is well expressed in vitro, is immunogenic in mice, and protects mice from a lethal toxin challenge. To our knowledge, this is the first report of a DNA vaccine targeting C. difficile toxins capable of inducing protective immune responses.
2.0 Materials and Methods
2.1 Plasmid design
The amino acid sequence corresponding to the receptor-binding domain of C. difficile toxin A (strain VPI 10463, Genebank Assession number CAA63564.1, residue positions 1839–2710) was identified. [3] The amino-acid sequence was back-translated in silico to provide a gene composed of those codons most commonly employed by human cells (http://www.entelechon.com/). Restriction sequences, a kozak sequence, and a methionine start site were incorporated as shown in Supplemental Figure 1 [29, 30]. Following commercial synthesis (BlueHeron Biotechnology, Seattle, WA) the gene was inserted into the commercial vector, pVAX (Invitrogen, Carlsbad, CA) with or without a tissue plasminogen activator (tPA) sequence as previously described [31]. TOP10 chemically competent E. coli (Invitrogen, Carlsbad, CA) were transformed and positive clones confirmed by restriction digestion and DNA sequencing (GeneWiz, North Brunswick, NJ.). The resulting two plasmids differ only in the presence or absence of a tPA leader sequence following the ATG start codon and are referred to as 1. TxA-RBD and 2. tPA-TxA-RBD (Figure 1).
Figure 1. A schematic description of C. difficile toxin A and the vaccine vectors.
A Linear depiction of the three major domains identified within C. difficile toxin A. (Modified from Voth and Ballard. Clin Micro Reviews, April 2005; p 247–263.) The region containing the vaccine sequence is indicated by the underline. B. Schematic depiction of the vaccine gene sequence as inserted into the eukaryotic expression vector, pVAX. These plasmids differ only in the presence or absence of a tissue plasminogen activator (tPA) signal peptide sequence.
2.2 Protein Expression
293T cells were split and plated in a 12-well dish at a concentration of 3–4 ×105 cells per well in DMEM with 10% inactivated FBS (v/v) (Invitrogen, Inc. Carlsbad, CA) and 1% penicillin-streptomycin (v/v) (Invitrogen, Carlsbad, CA). Twenty-four hours post-plating, cells were transfected with 2μg of TxA-RBD, tPA-TxA-RBD, or pVAX expressing green fluorescent protein as a negative control using lipofectamine 2000 (Invitrogen, Calsbad, CA) per the manufacturers instructions. Forty-eight hours post-transfection, supernatant and cell lysates were collected and stored at −20°C. Supernatant was clarified by centrifugation at 22,000 × g for 30 minutes prior to immunoblot.
Immunoblots were performed using the Invitrogen SureLock system according to the manufacturer’s recommendations. Briefly, 32.5μl of sample was added to 12μl NuPAGE LDS loading buffer and 5μl of reducing agent and heated to 70°C for 10 minutes. Samples were subjected to electrophoresis in a 10% BisTris gel (Invitrogen, Carlsbad, CA) at a constant voltage of 200V. Samples were transferred to PVDF membranes and blocked for two hours in blocking buffer (5% dry milk, 0.5% bovine albumin in PBS (Invitrogen, Carlsbad, CA)). Membranes were incubated with primary goat polyclonal anti-toxin A (List Biological Laboratories, Inc.) 1:2000 in blocking buffer overnight at 4°C. Membranes were then washed with wash buffer (PBS with 0.05% Tween, Sigma, Inc. St.Louis, MO) and HRPconjugated anti-goat secondary antibody (Sigma Inc., St.Louis, MO) 1:8000 in blocking buffer was added for 1 hour at room temperature. Membranes were washed as above and developed using the Amersham ECL development system (GE Healthcare, Piscataway, NJ). The procedure was repeated as described and samples analyzed using anti-β actin primary antibody (murine host) (Sigma Inc., St.Louis, MO) and anti-mouse IgG secondary antibody (Amersham Biosciences, Inc. Piscataway, NJ) 1:25,000 in blocking buffer to evaluate variations in loading volumes.
2.3 Animal Inoculations
6–8 week old BALB/c mice (6 mice per group) and CD-1 Swiss-Webster mice (5 mice per group) were obtained (Charles River Laboratories, Wilmington, MA) and housed in at the Laboratory Animal Research Center of The Rockefeller University. All procedures were carried out as described under protocols approved by the Institutional Animal Care and Use Committee of The Rockefeller University. Each experiment was repeated in two independent experiments.
Endotoxin free (<100IU) plasmid DNA for vaccination was obtained (Aldevron Inc., Fargo, ND). Five groups of BALB/c (6 per group) or CD-1 (5 per group) mice were vaccinated at weeks 0 and 2 with 50μg of plasmid DNA divided into two doses delivered by either standard syringe injection (IM) or in vivo electroporation (IM) into each of the rear limbs. Groups were inoculated with 1. pVAX alone; 2. tPA-TxA-RBD (IM); 3. TxA-RBD (IM); 4. tPA-TxA-RBD (EP); and 5. TxA-RBD (EP) as shown in Figure 3. Serum was obtained at week 6 post injection for immunologic evaluation prior to toxin challenge. Animals were sedated with ketamine and xylazine prior to the procedure. In vivo electroporation was performed using the TriGrid™ electroporation system for mice as recommended by the manufacturer, Ichor Medical Systems, Inc. (www.ICHORMS.com).
Figure 3. Schematic representation of the vaccination sequence.
AAnimal groups, vaccine doses, and vaccine delivery mechanism for subsequent immunological analysis for both BALB/c and CD-1 mice. B. Vaccine inoculation sequence, timing of blood harvest, and final toxin challenge for both BALB/c and CD-1 mice.
2.4 ELISA
96-well high protein-binding polystyrene EIA plates (Costar 9018, Corning Inc., Corning, NY.) were coated with 50 ng of purified, whole toxin A from C. difficile (List Biological Laboratory) in PBS overnight at 4°C. The following day, plates were blocked for 1.5 hours with blocking buffer (PBS-T, 5% dry milk w/v, 0.5% BSA w/v). Serum samples were added in duplicate following serial ten-fold dilution in blocking buffer and incubated for two hours at 37°C. Plates were washed five times with wash buffer (PBS-0.05% Tween 20) and incubated for 45 minutes with AKP-conjugated goat anti-mouse secondary antibody (1:10,000 in blocking buffer, BD Pharmingen, San Diego, CA). Plates were developed using the AMPAK ELISA development kit according to manufacturer’s specifications (DAKO Corporation, Carpinteria, CA). Optical density of plate wells was determined at 490 nm (Dynex Technologies, Chantilly, Va). The end-point antibody titers represent the reciprocal dilution of the last dilution providing an O.D. 2-fold higher than the O.D. of sera controls at the lowest performed dilution.
2.5 Toxin Challenge
Eight weeks post-vaccination, animals were challenged with 300ng of freshly reconstituted toxin A from C. difficile in 100μl of sterile saline delivered via intraperitoneal syringe injection (IP) [32]. Animals were examined twice daily for mortality up to 14 days post challenge.
2.6 Statistical Analysis
Comparison of group-specific ELISA titers was performed as follows. Groups were compared using Kruskal-Wallis non-parametric analysis for over-all significance. If positive (p<0.05), groups were compared pair-wise using one-way ANOVA with a Bonferroni correction for multiple comparisons. Significance was determined at p<0.05. Differences between median survival times post challenge were examined with pair-wise log-rank analysis. The Kaplan-Meyer survival curves and overall statistic were calculated and plotted using MedCalc™. Kruskal-Wallis, one way ANOVA, and Log-Rank pair wise comparisons were calculated using SPSS version 14.
3.0 Results
3.1 Gene Expression
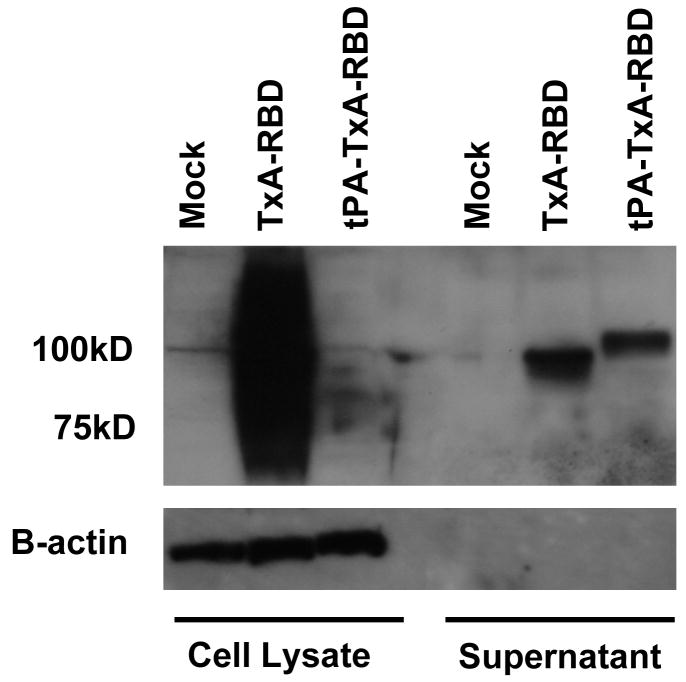
Cell lysates and supernatants were harvested forty-eight hours post transfection and examined for protein expression via immunoblot (Figure 2). Levels of protein present in cell lysates were significantly higher in the TxA-RBD sample as compared to tPA-TxA-RBD or the GFP plasmid control. Detection of β-actin provides a loading control and indicates an equivalent volume of sample in each well. Protein levels detectable in the clarified supernatant are comparable between the two vaccine vectors. The electrophoretic sizes of the observed proteins in the supernatant differ between the two vaccine vectors with tPA-TxA-RBD yielding a larger product than TxA-RBD.
Figure 2. Protein expression from vaccine vectors or control following transient transfection in vitro.
Immunoblot of 293T cell lysates and supernatants following transient transfection with pVAX encoding green-fluorescent protein (Mock), TxA-RBD in pVAX, or tPA-TxA-RBD in pVAX for detection of expressed protein products. Supernatant was clarified at 20,000 × g for 30 minutes prior to the procedure. The expected size of the expressed product is 100kD. Identical preparations were examined for the presence of human β-actin using a monoclonal antibody specific for β-actin as a volume loading control.
3.2 Immunogenicity
Antibody responses to the vaccine plasmids were first examined in BALB/c mice (Charles River), an H2d background strain commonly employed in vaccine studies [33–36]. Six to 8 week old female BALB/c mice were obtained and inoculated as described in Methods and in Figure 3. Serum was harvested 6 weeks post-injection and anti-toxin antibody responses evaluated by ELISA. The procedure was performed twice in independent experiments (6 animals per group in each experiment) and the results are presented in Figure 4.
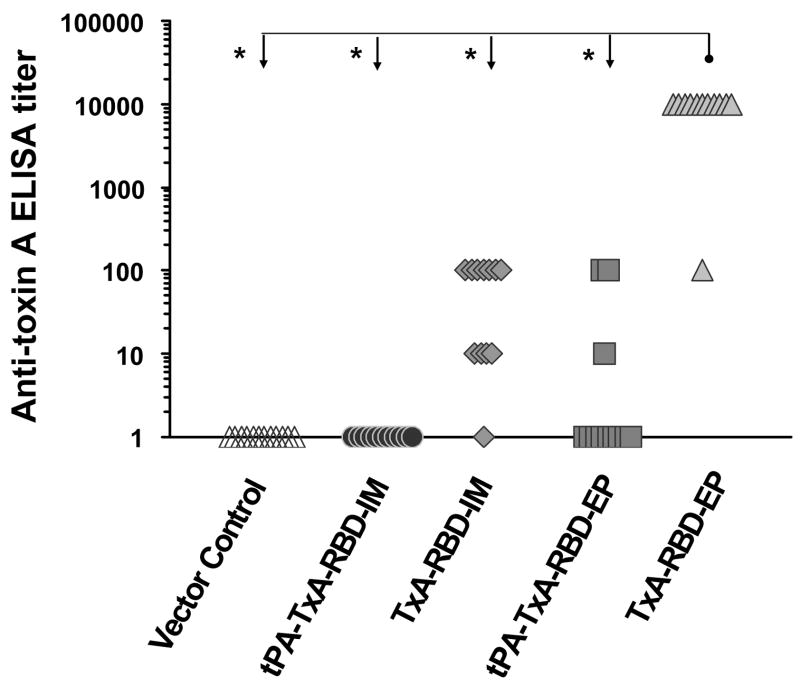
Figure 4. Toxin-specific antibody responses in BALB/c mice following the vaccination procedures.
Serum anti-toxin IgG ELISA responses in BALB/c mice. The data is the result of two independent experiments. Values are representative of two independent ELISA procedures. Group-specific titers were compared using Kruskal-Wallis test for overall significance (p< 0.0001). Asterisk (*) represents statistical significance between group titers at p < 0.05 following pair-wise one-way ANOVA with Bonferroni correction for multiple comparisons.
Inoculation with vector only (pVAX) yielded negligible background reactivity. Vaccination with plasmid DNA including a tPA leader sequence did not result in significant antibody responses and was not significantly improved by electroporation (Figure 4). In contrast, the vaccine vector lacking the tPA-signal peptide provided significant immunogenicity. Among animals receiving TxA-RBD via syringe injection, 11/12 mounted detectable anti-toxin antibody titers, a result not statistically different from those groups receiving tPA-TxA-RBD (p>0.05). Finally, all animals receiving TxA-RBD by electroporation mounted strong antitoxin antibody responses with most animals reaching a 1:10,000 titer, a result significantly different from all other groups (p<0.05).
To confirm these results, the protocol was repeated in a second mouse strain. Outbred CD-1 mice (5 animals per group per experiment) were vaccinated using a schedule identical to that employed in the BALB/c mice. The procedure was performed twice in independent experiments and the results are presented in Figure 5. Consistent with the BALB/c strain, antibody responses in animals receiving vector control were negligible. In contrast, both vaccine plasmids yielded antibody responses in nearly all animals regardless of the mode of delivery. Antibody titers in animals receiving TxA-RBD via electroporation were significantly higher than those of all other groups except those of animals who received tPA-TxA-RBD via electroporation. A single animal which received the TxA-RBD plasmid via electroporation did not have detectable anti-toxin antibodies. In contrast to results in BALB/c mice, electroporation significantly improved the mean antibody titers in animals receiving the tPA-TxA-RBD plasmid (p<0.05) as compared to titers in animals who received standard IM injection.
Figure 5. Toxin-specific antibody responses in CD-1 mice following the vaccination procedures.
Serum anti-toxin IgG ELISA responses in CD-1 mice representing data from of two independent experiments. Group-specific antibody titers were compared using a Kruskal-Wallis test for overall significance (p< 0.0001). Asterisk (*) represents statistical significance between group titers at p < 0.05 following pair-wise one-way ANOVA with Bonferroni correction for multiple comparisons. Symbol (§) indicates p> 0.05 for the indicated pairwise comparison.
3.3 Toxin A Challenge
Mice are sensitive to purified C. difficile toxin A with LD50 values of approximately 50ng following intraperitoneal (IP) challenge [37]. Thus, eight weeks post-vaccination, mice were challenged with 300ng (6 × LD50) of purified C. difficile toxin A via IP injection and examined every 12 hours for two weeks. Survival between groups was compared using pair-wise, log-rank analysis. Results are presented in Figure 6. Consistent with the sensitivity of mice to toxin A, 100% of control animals died within 60 hours of toxin challenge, with most succumbing within 24 hours. By contrast, animals which had received TxA-RBD via electroporation demonstrated 100% survival. Mice in other groups manifested intermediate survival phenotypes. Animals inoculated with tPA-TxA-RBD suffered significant mortality regardless of delivery modality that was not significantly different from controls (2/12). Animals which received TxA-RBD by IM injection showed intermediate survival (4/12), a result significantly different from animals which received the tPA-TxA-RBD plasmids. Detailed results of the pair-wise comparison of mean survival times are provided in Supplemental Table 1.
Figure 6. Survival in vaccinated BALB/c mice following challenge with purified C. difficile toxin A.
Kaplan Meier survival curve in vaccinated BALB/c mice following challenge with 300ng of freshly reconstituted toxin A from C. difficile in 100μl of sterile saline. The data is the result of two independent experiments. Statistical (p) value represents the likelihood of a significant difference between all groups following pair-wise log-rank analysis between groups.
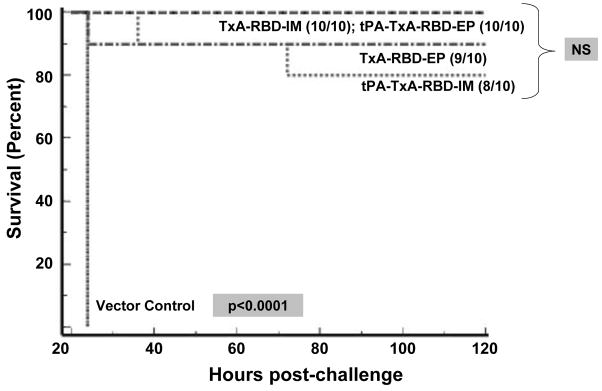
The procedure and analysis was repeated as above, now using CD-1 mice. Similar to the BALB/c mice, 100% of control animals were dead at 24 hours post-challenge. In contrast to BALB/c mice and consistent with the uniformly high antibody titers, most animals in all other groups survived. Ten of 10 animals inoculated with TxA-RBD IM and tPA-TxA-RBD-EP survived while 9/10 animals receiving TxA-RBD-EP survived (NS, p>0.05) and 8/10 animals inoculated with tPA-TxA-RBD-EP survived (NS, p>0.05). Of note, the single animal in the TxA-RBD-EP group non-responsive to the vaccine by ELISA accounted for the sole mortality in that group, while the two animals in the tPA-TxA-RBD-IM group which succumbed to challenge had titers of 1:1000. Detailed results of the pair-wise comparison of mean survival times are provided in Supplemental Table 2.
4.0 Discussion
This report presents the first description of a DNA vaccine targeting C. difficile toxin A. Over the past decade, a variety of vaccines and immunotherapeutics intended to treat or prevent C. difficile disease have been tested in murine and hamster animal models [21, 25–28, 38–42]. The inactivated toxoid and monoclonal antibody platforms are the furthest in clinical development [43, 44]. In fact, recently released phase II results of a monoclonal antibody product has provided the first evidence of the efficacy of an immunotherapeutic product in preventing recurrent diarrhea in affected human subjects [45]. While conceptually straight-forward, inactivated toxin strategies have certain disadvantages. Large proteins rely on the exogenous pathway of antigen presentation by antigen-presenting cells (APCs) and produce TH2 biased immune responses, particularly when administered with alum [46]. The lack of approved adjuvants (to date, only alum and MF-59) for co-formulation limits the ability to significantly alter this immunology [46]. Further, toxoids are based upon inactivation of whole, enzymatically active protein and carry the risk of toxicity due to inadequate inactivation procedures or difficulties in purification [47, 48]. Finally, the inactivation process itself may alter structural domains important for antibody recognition [49].
DNA vaccines on the other hand, have several advantages as a vaccine platform. First, DNA vaccination involves the injection of recombinant genes encoding a protein of interest and relies upon endogenous synthesis of antigen by the inoculated host to induce effector immune responses [50]. As such, the DNA platform is often compared to viral infection in its ability to provide both humoral and cell-mediated immunity [51]. This technique permits rational inactivation or removal of potentially toxic enzymatic domains as well as inclusion of species-specific molecular adjuvants in a single product [52]. Indeed, the synthesis of DNA plasmids by bacterial cells during manufacturing process ensures the presence of potentially immunogenic CpG motifs [53]. However, despite examples of successful DNA vaccination against bacterial and toxin-mediated diseases in animal models [54–58], a DNA vaccine against C. difficile toxin A presented several challenges.
Firstly, the toxin A gene is over 9 kilobases in length and encodes a protein product of over 300kD. Genes encoding protein products of such size are difficult to construct, may express poorly, and provide poor immunogenicity when delivered as DNA vaccines. Fortunately, neutralizing antibodies map to the carboxy-terminal receptor binding domain [59–63] permitting a significant reduction in size. Secondly, the genome of C. difficile contains significant A–T content. While not universally true, several DNA vaccines encoded by AT rich sequences have proven poorly immunogenic as a result of low protein expression [58, 64] or a paucity of endogenous CpG motifs [53, 58]. The DNA vaccine candidates employing AT-rich wild-type genes which have proven immunogenic often express well in vitro, despite their AT content [55, 65, 66]. For example, a study comparing codon-optimized versus non-optimized DNA vaccines against C. tetani toxin showed minimal expression of the non-optimized vaccine in vitro and no immunogenicity in vivo despite controlling for CpG content [58]. To avoid this issue, a synthetic C. difficile gene was created employing only the most common codons used in the human genome (http://www.entelechon.com).
The third hurdle in the design of this vaccine was whether to retain eukaryotic asparagine-linked glycan residues (N-linked glycans). Most published data describing DNA vaccines targeting other prokaryotic toxins reveals conservation of N-glycan signals. This bias may follow from their direct assembly from amplified genomic sequences rather than intentional design [54, 55, 57, 65–67]. One study directly examined the effect of elimination of N-glycan signals in a recombinant DNA vaccine against anthrax PA- and LF-toxins [55]. In both cases elimination of N-glycans dramatically reduced in vitro expression and subsequent immune responses in vaccinated mice [55]. With this work in mind, all N-glycan signals were retained in the synthetic C. difficile gene.
Following confirmation of in vitro expression, studies were undertaken to describe vaccine immunogenicity in mice. While often effective in mice, DNA vaccines suffer from lackluster performance in larger animals, possibly due to dose limitations and limited plasmid uptake by host cells [68, 69]. Methods which augment DNA uptake by host cells, whether natural (bacterial [70], viral [71]) or artificial (electroporation [72], gene-gun [73], biojector [74]) provide the means to improve DNA vaccine immunogenicity. This study employed in vivo electroporation (Ichor Medical Systems, Inc.), a method proven to logarithmically increase antigen expression and the resulting immune responses, in particular, humoral immune responses [75–77]. The results in the BALB/c strain of mice strongly support the utility of in vivo electroporation as a method to enhance immune responses to DNA vaccines. In this animal strain, electroporation significantly improved performance of the TxA-RBD vaccine such that all animals who received the vaccine via electroporation were protected from toxin challenge. While less obvious, antibody titers in CD-1 mice also segregated by delivery method with those animals receiving vaccine by electroporation showing significantly higher antibody titers.
Antibody titers observed in the BALB/c strain were higher in animals receiving the vector without a tPA leader sequence. The marked difference in protein production seen in the in vitro analysis is one likely explanation. Further, the obvious size difference in the protein products observed in the cell supernantant suggests that the tPA sequence was not cleaved from the corresponding protein. Thus, this tPA sequence may not function as expected in association with this protein sequence. Finally, potential differences in glycosylation between the protein products produced by the two vectors may have influenced subsequent immune responses.
The final question examined in this study was whether the vaccine-specific immune responses were able to protect animals from toxin challenge in vivo. Mice are not susceptible to gastric challenge with C. difficile spores but are very sensitive to parenteral delivery of active, purified C. difficile toxins [32, 37, 78]. While not recapitulating gastrointestinal disease, this method is a first step to examine the functionality of antibody responses to C. difficile toxins in vivo. In this study, survival corresponded best to groups with anti-toxin antibody titers ≥ 1:1000, regardless of strain. In BALB/c mice, 100% of animals who received TxA-RBD via electroporation were protected from toxin A challenge while 100% of animals inoculated with vector control succumbed to toxin. The other vaccine regimens produced intermediate survival phenotypes. Consistent with their high antibody titers, all CD-1 animal groups receiving vaccine experienced high survival rates following toxin challenge regardless of plasmid or delivery method. The survival rates demonstrated in the BALB/c mice receiving TxA-RBD via electroporation and in all CD-1 groups receiving vaccine compare favorably to published reports of the protection provided by inactivated toxoid and carrier conjugate vaccines [32, 78].
One unexpected observation made in the study was the mouse strain dependent difference in vaccine immunogenicity. Several similar reports specific to BALB/c and CD-1 mice have been published previously. First, Pirzadeh and colleagues published an examination of the immunogenicity of a DNA vaccine encoding the glycoprotein 5 (GP5) of porcine reproductive and respiratory syndrome virus (PRRSV) in BALB/c and CD1 mice [79]. Animals were inoculated with 50μg of a DNA vaccine encoding PRRSV-GP5 protein fused to glutathione S-transferase or control plasmid. By day 65 post-inoculation, CD-1 mice mounted binding antibody titers of >12800 (reciprocal of the highest dilution by ELISA) while titers in BALB/c mice reached only 2560. Interestingly, serum from CD-1 mice was not capable of neutralizing virus in an in vitro neutralization assay while serum from BALB/c mice demonstrated a viral neutralization titer of 102. Authors hypothesized strain dependent differences in the recognition of linear B-cell epitopes as a possible explanation. A study published by Toapanta and Ross examined the immune responses to a DNA-based HIV vaccine expressing gp120 fused to multiple copies of the complement component C3D in three inbred (BALB/c, C57BL/6, and C3H/He) and one outbred mouse strain (CD-1) [80]. Overall antibody titers at the end of study were similar, however, differences were observed between inbred and outbred strains in antibody isotype, antibody avidity, and in the profile of cytokines released by splenocytes following gp120 stimulation. Antibody responses in the inbred strains were of a predominantly IgG1 isotype and manifested low to moderate avidity. Splenocyte cytokine release from vaccinated inbred mice was exclusively IL-4. In contrast, CD-1 mice manifested a mixed IgG isotype response (IgG1 and IgG2a) with high avidity. Splenocyte cytokine release from vaccinated CD-1 mice demonstrated both IFN-γ and IL-4. Finally, data published in 2004 by Ito and colleagues specifically examined the differences in antibody responses to DNA and protein vaccination between outbred (ICR, ddY), H-2 congenic strains (B10.A, B10.BR, B10.D2), and inbred strains (C57BL/6, A/J, C3H/He, BALB/c, C57BL/10) of mice [81]. Using vectors encoding green fluorescent protein (GFP) or β-galactosidase (β-gal), authors found differences that varied by encoded protein and strain. In one example, no inbred or H-2 congenic strain of mouse responded to immunization with plasmid expressing GFP while both outbred strains responded with high antibody titers. No differences were found in RNA transcript levels within transfected muscle and the difference was overcome by GFP peptide inoculation indicating possible differences in protein expression beyond the level of transcription. Unfortunately, tissue was not examined for in vivo GFP or β-gal expression. In contrast, immunization with DNA encoding β-gal induced antibody responses in all strains. However, antibody titers among the inbred and congenic strains segregated by H-2 background. Authors concluded that antibody response to DNA vaccination in mice was influenced by both in vivo antigen expression as well as the haplotype background of the animals.
In conclusion, we have developed a DNA vaccine encoding the receptor binding domain of C. difficile toxin A. This vaccine is well expressed in vitro, is immunogenic in vivo, and protects mice from lethal toxin challenge. A similar vaccine approach specific for the toxin B of C. difficile is underway. We believe such a combination vaccine may warrant further testing in other animal models.
Supplementary Material
Figure 7. Survival in vaccinated CD-1 mice following challenge with purified C. difficile toxin A.
Kaplan Meyer survival curve in vaccinated CD-1 mice following challenge with 300ng of freshly reconstituted toxin A from C. difficile in 100μl of sterile saline. The data is the result of two independent experiments. Statistical (p) value represents the likelihood of a significant difference between all groups following pair-wise log-rank analysis between groups. “NS” indicates differences not significant p>0.05 following pair-wise log rank analysis.
Acknowledgments
Support
Dr. Gardiner was supported by NIH K08 5 K08 AI58747-04.
Dr. Gardiner would like to acknowledge David Lyerly for his advice while establishing the mouse challenge model.
Dr. Gardiner would like to thank Drew Hannaman at Ichor Medical Systems, Inc. for the material support of in vivo electroporation materials and advice on electroporation procedures.
Dr. Gardiner would like to thank Andre Dascal for his kind review of this manuscript, and his encouragement.
Footnotes
Disclosures
Dr Gardiner received material support (in vivo electroporation device) from Ichor Medical Systems, San Diego, CA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yannelli B, Gurevich I, Schoch PE, Cunha BA. Yield of stool cultures, ova and parasite tests, and Clostridium difficile determinations in nosocomial diarrheas. Am J Infect Control. 1988;16(6):246–9. doi: 10.1016/s0196-6553(88)80003-8. [DOI] [PubMed] [Google Scholar]
- 2.Siegel DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. Jama. 1990;263(7):979–82. [PubMed] [Google Scholar]
- 3.Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18(2):247–63. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giannasca PJ, Warny M. Active and passive immunization against Clostridium difficile diarrhea and colitis. Vaccine. 2004;22(7):848–56. doi: 10.1016/j.vaccine.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 5.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204–10. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 6.Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994;330(4):257–62. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 7.McFarland LV, Surawicz CM, Greenberg RN, Fekety R, Elmer GW, Moyer KA, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. Jama. 1994;271(24):1913–8. [PubMed] [Google Scholar]
- 8.Samore MH, DeGirolami PC, Tlucko A, Lichtenberg DA, Melvin ZA, Karchmer AW. Clostridium difficile colonization and diarrhea at a tertiary care hospital. Clin Infect Dis. 1994;18(2):181–7. doi: 10.1093/clinids/18.2.181. [DOI] [PubMed] [Google Scholar]
- 9.Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J., Jr Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459–77. doi: 10.1086/648363. [DOI] [PubMed] [Google Scholar]
- 10.McFarland LV, Stamm WE. Review of Clostridium difficile-associated diseases. Am J Infect Control. 1986;14(3):99–109. doi: 10.1016/0196-6553(86)90018-0. [DOI] [PubMed] [Google Scholar]
- 11.Gerding DN. Disease associated with Clostridium difficile infection. Ann Intern Med. 1989;110(4):255–7. doi: 10.7326/0003-4819-110-4-255. [DOI] [PubMed] [Google Scholar]
- 12.Lyerly DM, Krivan HC, Wilkins TD. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988;1(1):1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. Cmaj. 2005;173(9):1037–42. doi: 10.1503/cmaj.050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pepin J, Alary ME, Valiquette L, Raiche E, Ruel J, Fulop K, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40(11):1591–7. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]
- 15.Pepin J, Valiquette L, Alary ME, Villemure P, Pelletier A, Forget K, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. Cmaj. 2004;171(5):466–72. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valiquette L, Low DE, Pepin J, McGeer A. Clostridium difficile infection in hospitals: a brewing storm. Cmaj. 2004;171(1):27–9. doi: 10.1503/cmaj.1040957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23):2433–41. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 18.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in Patients Discharged from US Short-Stay Hospitals, 1996–2003. Emerg Infect Dis. 2006;12(3):409–16. doi: 10.3201/eid1203.051064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442–9. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 20.Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366(9491):1079–84. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 21.Giannasca PJ, Zhang ZX, Lei WD, Boden JA, Giel MA, Monath TP, et al. Serum antitoxin antibodies mediate systemic and mucosal protection from Clostridium difficile disease in hamsters. Infect Immun. 1999;67(2):527–38. doi: 10.1128/iai.67.2.527-538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342(6):390–7. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 23.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251):189–93. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 24.Libby JM, Wilkins TD. Production of antitoxins to two toxins of Clostridium difficile and immunological comparison of the toxins by cross-neutralization studies. Infect Immun. 1982;35(1):374–6. doi: 10.1128/iai.35.1.374-376.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyerly D, Johnson JL, Frey SM, Wilkins TD. Vaccination against lethal Clostridium difficile enterocolitis with a nontoxic recombinant peptide of toxin A. Curr Microbiol. 1990;21:29–32. [Google Scholar]
- 26.Kim PH, Iaconis JP, Rolfe RD. Immunization of adult hamsters against Clostridium difficile-associated ileocecitis and transfer of protection to infant hamsters. Infect Immun. 1987;55(12):2984–92. doi: 10.1128/iai.55.12.2984-2992.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernie DS, Thomson RO, Batty I, Walker PD. Active and passive immunization to protect against antibiotic associated caecitis in hamsters. Dev Biol Stand. 1983;53:325–32. [PubMed] [Google Scholar]
- 28.Ward SJ, Douce G, Figueiredo D, Dougan G, Wren BW. Immunogenicity of a Salmonella typhimurium aroA aroD vaccine expressing a nontoxic domain of Clostridium difficile toxin A. Infect Immun. 1999;67(5):2145–52. doi: 10.1128/iai.67.5.2145-2152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell JE. Molecular Cell Biology. 4. New York: W. H. Freeman & Co.; 1999. [Google Scholar]
- 30.Kumar S, Yan J, Muthumani K, Ramanathan MP, Yoon H, Pavlakis GN, et al. Immunogenicity testing of a novel engineered HIV-1 envelope gp140 DNA vaccine construct. DNA Cell Biol. 2006;25(7):383–92. doi: 10.1089/dna.2006.25.383. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Chen Z, Zhang W, Gurner D, Song Y, Gardiner DF, et al. Design, construction, and characterization of a dual-promoter multigenic DNA vaccine directed against an HIV-1 subtype C/B’ recombinant. J Acquir Immune Defic Syndr. 2008;47(4):403–11. doi: 10.1097/QAI.0b013e3181651b9d. [DOI] [PubMed] [Google Scholar]
- 32.Pavliakova D, Moncrief JS, Lyerly DM, Schiffman G, Bryla DA, Robbins JB, et al. Clostridium difficile recombinant toxin A repeating units as a carrier protein for conjugate vaccines: studies of pneumococcal type 14, Escherichia coli K1, and Shigella flexneri type 2a polysaccharides in mice. Infect Immun. 2000;68(4):2161–6. doi: 10.1128/iai.68.4.2161-2166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tonui WK, Titust RG. Leishmania major soluble exo-antigens (LmSEAgs) protect neonatal BALB/C mice from a subsequent challenge with L. major and stimulate cytokine production by Leishmania-naive human peripheral blood mononuclear cells. J Parasitol. 2006;92(5):971–6. doi: 10.1645/GE-782R.1. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Hinnebusch BJ, Trunkle T, Bosio CM, Suo Z, Tighe M, et al. Oral vaccination with salmonella simultaneously expressing Yersinia pestis F1 and V antigens protects against bubonic and pneumonic plague. J Immunol. 2007;178(2):1059–67. doi: 10.4049/jimmunol.178.2.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardiner DF, Huang Y, Basu S, Leung L, Song Y, Chen Z, et al. Multiple-site DNA vaccination enhances immune responses in mice. Vaccine. 2006;24(3):287–92. doi: 10.1016/j.vaccine.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 36.Chen JY, Li F. Development of hepatitis C virus vaccine using hepatitis B core antigen as immuno-carrier. World J Gastroenterol. 2006;12(48):7774–8. doi: 10.3748/wjg.v12.i48.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas W, Giannasca P, Zhang Z, Lei W, Monath T, et al. Active immunization against clostridium difficile disease. 2005 [Google Scholar]
- 38.Kink JA, Williams JA. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect Immun. 1998;66(5):2018–25. doi: 10.1128/iai.66.5.2018-2025.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allo M, Silva J, Jr, Fekety R, Rifkin GD, Waskin H. Prevention of clindamycin-induced colitis in hamsters by Clostridium sordellii antitoxin. Gastroenterology. 1979;76(2):351–5. [PubMed] [Google Scholar]
- 40.Kelly CP, Pothoulakis C, Vavva F, Castagliuolo I, Bostwick EF, O’Keane JC, et al. Anti-Clostridium difficile bovine immunoglobulin concentrate inhibits cytotoxicity and enterotoxicity of C. difficile toxins. Antimicrob Agents Chemother. 1996;40(2):373–9. doi: 10.1128/aac.40.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyerly DM, Bostwick EF, Binion SB, Wilkins TD. Passive immunization of hamsters against disease caused by Clostridium difficile by use of bovine immunoglobulin G concentrate. Infect Immun. 1991;59(6):2215–8. doi: 10.1128/iai.59.6.2215-2218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Libby JM, Jortner BS, Wilkins TD. Effects of the two toxins of Clostridium difficile in antibiotic-associated cecitis in hamsters. Infect Immun. 1982;36(2):822–9. doi: 10.1128/iai.36.2.822-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aboudola S, Kotloff KL, Kyne L, Warny M, Kelly EC, Sougioultzis S, et al. Clostridium difficile vaccine and serum immunoglobulin G antibody response to toxin A. Infect Immun. 2003;71(3):1608–10. doi: 10.1128/IAI.71.3.1608-1610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor CP, Tummala S, Molrine D, Davidson L, Farrell RJ, Lembo A, et al. Open-label, dose escalation phase I study in healthy volunteers to evaluate the safety and pharmacokinetics of a human monoclonal antibody to Clostridium difficile toxin A. Vaccine. 2008;26(27–28):3404–9. doi: 10.1016/j.vaccine.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.http://www.umassmed.edu/Content.aspx?id=62670. In.
- 46.Del Giudice G, Pizza M, Rappuoli R. Molecular basis of vaccination. Mol Aspects Med. 1998;19(1):1–70. doi: 10.1016/s0098-2997(98)00002-8. [DOI] [PubMed] [Google Scholar]
- 47.Relyveld E, Bizzini B, Huet M. Preparation of diphtheria vaccines using highly purified toxins. Vaccine. 1997;15(4):459–60. doi: 10.1016/s0264-410x(97)00240-5. [DOI] [PubMed] [Google Scholar]
- 48.Relyveld EH, Bizzini B, Gupta RK. Rational approaches to reduce adverse reactions in man to vaccines containing tetanus and diphtheria toxoids. Vaccine. 1998;16(9–10):1016–23. doi: 10.1016/s0264-410x(97)00288-0. [DOI] [PubMed] [Google Scholar]
- 49.Ibsen PH. The effect of formaldehyde, hydrogen peroxide and genetic detoxification of pertussis toxin on epitope recognition by murine monoclonal antibodies. Vaccine. 1996;14(5):359–68. doi: 10.1016/0264-410x(95)00230-x. [DOI] [PubMed] [Google Scholar]
- 50.Donnelly JJ, Liu MA, Ulmer JB. Antigen presentation and DNA vaccines. Am J Respir Crit Care Med. 2000;162(4 Pt 2):S190–3. doi: 10.1164/ajrccm.162.supplement_3.15tac10. [DOI] [PubMed] [Google Scholar]
- 51.Shedlock DJ, Weiner DB. DNA vaccination: antigen presentation and the induction of immunity. J Leukoc Biol. 2000;68(6):793–806. [PubMed] [Google Scholar]
- 52.Manoj S, Babiuk LA, van Drunen Littel-van den Hurk S. Approaches to enhance the efficacy of DNA vaccines. Crit Rev Clin Lab Sci. 2004;41(1):1–39. doi: 10.1080/10408360490269251. [DOI] [PubMed] [Google Scholar]
- 53.Krieg AM, Yi AK, Schorr J, Davis HL. The role of CpG dinucleotides in DNA vaccines. Trends Microbiol. 1998;6(1):23–7. doi: 10.1016/S0966-842X(97)01145-1. [DOI] [PubMed] [Google Scholar]
- 54.Galloway D, Liner A, Legutki J, Mateczun A, Barnewall R, Estep J. Genetic immunization against anthrax. Vaccine. 2004;22(13–14):1604–8. doi: 10.1016/j.vaccine.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 55.Hermanson G, Whitlow V, Parker S, Tonsky K, Rusalov D, Ferrari M, et al. A cationic lipid-formulated plasmid DNA vaccine confers sustained antibody-mediated protection against aerosolized anthrax spores. Proc Natl Acad Sci U S A. 2004;101(37):13601–6. doi: 10.1073/pnas.0405557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jathoul AP, Holley JL, Garmory HS. Efficacy of DNA vaccines expressing the type F botulinum toxin Hc fragment using different promoters. Vaccine. 2004;22(29–30):3942–6. doi: 10.1016/j.vaccine.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 57.Kamachi K, Arakawa Y. Expression of a C terminally truncated form of pertussis toxin S1 subunit effectively induces protection against pertussis toxin following DNA-based immunization. Infect Immun. 2004;72(7):4293–6. doi: 10.1128/IAI.72.7.4293-4296.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stratford R, Douce G, Zhang-Barber L, Fairweather N, Eskola J, Dougan G. Influence of codon usage on the immunogencity of a DNA vaccine against tetanus. Vaccine. 2001;19:810–15. doi: 10.1016/s0264-410x(00)00246-2. [DOI] [PubMed] [Google Scholar]
- 59.Lyerly DM, Phelps CJ, Wilkins TD. Monoclonal and specific polyclonal antibodies for immunoassay of Clostridium difficile toxin A. J Clin Microbiol. 1985;21(1):12–4. doi: 10.1128/jcm.21.1.12-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lyerly DM, Phelps CJ, Toth J, Wilkins TD. Characterization of toxins A and B of Clostridium difficile with monoclonal antibodies. Infect Immun. 1986;54(1):70–6. doi: 10.1128/iai.54.1.70-76.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Price SB, Phelps CJ, Wilkins TD, Johnson JL. Cloning of the Carbohydrate-binding portion of the Toxin A Gene of Clostridium difficile. Curr Microbiol. 1987;16:55–60. [Google Scholar]
- 62.Frey SM, Wilkins TD. Localization of two epitopes recognized by monoclonal antibody PCG-4 on Clostridium difficile toxin A. Infect Immun. 1992;60(6):2488–92. doi: 10.1128/iai.60.6.2488-2492.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Babcock GJ, Broering TJ, Hernandez HJ, Mandell RB, Donahue K, Boatright N, et al. Human Monoclonal Antibodies Directed against Toxins A and B Prevent Clostridium difficile-Induced Mortality in Hamsters. Infect Immun. 2006;74(11):6339–47. doi: 10.1128/IAI.00982-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andre S, Seed B, Eberle J, Schraut W, Bultmann A, Haas J. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J Virol. 1998;72(2):1497–503. doi: 10.1128/jvi.72.2.1497-1503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bennett AM, Perkins SD, Holley JL. DNA vaccination protects against botulinum neurotoxin type F. Vaccine. 2003;21(23):3110–7. doi: 10.1016/s0264-410x(03)00260-3. [DOI] [PubMed] [Google Scholar]
- 66.Kamachi K, Konda T, Arakawa Y. DNA vaccine encoding pertussis toxin S1 subunit induces protection against Bordetella pertussis in mice. Vaccine. 2003;21(31):4609–15. doi: 10.1016/s0264-410x(03)00441-9. [DOI] [PubMed] [Google Scholar]
- 67.Gu ML, Leppla SH, Klinman DM. Protection against anthrax toxin by vaccination with a DNA plasmid encoding anthrax protective antigen. Vaccine. 1999;17(4):340–4. doi: 10.1016/s0264-410x(98)00210-2. [DOI] [PubMed] [Google Scholar]
- 68.Babiuk S, Baca-Estrada ME, Foldvari M, Storms M, Rabussay D, Widera G, et al. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine. 2002;20(27–28):3399–408. doi: 10.1016/s0264-410x(02)00269-4. [DOI] [PubMed] [Google Scholar]
- 69.Babiuk S, Baca-Estrada ME, Foldvari M, Middleton DM, Rabussay D, Widera G, et al. Increased gene expression and inflammatory cell infiltration caused by electroporation are both important for improving the efficacy of DNA vaccines. J Biotechnol. 2004;110(1):1–10. doi: 10.1016/j.jbiotec.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 70.Daudel D, Weidinger G, Spreng S. Use of attenuated bacteria as delivery vectors for DNA vaccines. Expert Rev Vaccines. 2007;6(1):97–110. doi: 10.1586/14760584.6.1.97. [DOI] [PubMed] [Google Scholar]
- 71.Brave A, Ljungberg K, Wahren B, Liu MA. Vaccine delivery methods using viral vectors. Mol Pharm. 2007;4(1):18–32. doi: 10.1021/mp060098+. [DOI] [PubMed] [Google Scholar]
- 72.Babiuk S, van Drunen Littel-van den Hurk S, Babiuk LA. Delivery of DNA vaccines using electroporation. Methods Mol Med. 2006;127:73–82. doi: 10.1385/1-59745-168-1:73. [DOI] [PubMed] [Google Scholar]
- 73.Fuller DH, Loudon P, Schmaljohn C. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods. 2006;40(1):86–97. doi: 10.1016/j.ymeth.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 74.Baxter J, Mitragotri S. Needle-free liquid jet injections: mechanisms and applications. Expert Rev Med Devices. 2006;3(5):565–74. doi: 10.1586/17434440.3.5.565. [DOI] [PubMed] [Google Scholar]
- 75.Luxembourg A, Hannaman D, Ellefsen B, Nakamura G, Bernard R. Enhancement of immune responses to an HBV DNA vaccine by electroporation. Vaccine. 2006;24(21):4490–3. doi: 10.1016/j.vaccine.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 76.Otten GR, Schaefer M, Doe B, Liu H, Megede JZ, Donnelly J, et al. Potent immunogenicity of an HIV-1 gag-pol fusion DNA vaccine delivered by in vivo electroporation. Vaccine. 2006;24(21):4503–9. doi: 10.1016/j.vaccine.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 77.Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol. 2000;164(9):4635–40. doi: 10.4049/jimmunol.164.9.4635. [DOI] [PubMed] [Google Scholar]
- 78.Sauerborn M, Leukel P, von Eichel-Streiber C. The C-terminal ligand-binding domain of Clostridium difficile toxin A (TcdA) abrogates TcdA-specific binding to cells and prevents mouse lethality. FEMS Microbiol Lett. 1997;155(1):45–54. doi: 10.1111/j.1574-6968.1997.tb12684.x. [DOI] [PubMed] [Google Scholar]
- 79.Pirzadeh B, Dea S. Immune response in pigs vaccinated with plasmid DNA encoding ORF5 of porcine reproductive and respiratory syndrome virus. J Gen Virol. 1998;79 ( Pt 5):989–99. doi: 10.1099/0022-1317-79-5-989. [DOI] [PubMed] [Google Scholar]
- 80.Toapanta FR, Ross TM. Mouse strain-dependent differences in enhancement of immune responses by C3d. Vaccine. 2004;22(13–14):1773–81. doi: 10.1016/j.vaccine.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 81.Ito K, Takeuchi Y, Ito K, Kato S. Strain-dependent antibody response induced by DNA immunization. Immunol Lett. 2000;74(3):245–50. doi: 10.1016/s0165-2478(00)00266-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.