Abstract
γ-Aminobutyric acid type A (GABAA) receptors mediate the majority of fast synaptic inhibition in the mammalian brain, controlling activity both at the network and cellular level. The diverse functions of GABA in the central nervous system are matched not just by the heterogeneity of GABAA receptors, but also the complex trafficking mechanisms and protein-protein interactions that generate and maintain appropriate receptor cell surface localization. In this review, we discuss recent progress in our understanding of the dynamic regulation of GABAA receptor composition, trafficking to and from the neuronal surface, and lateral movement of receptors between synaptic and extrasynaptic locations. Finally, we highlight a number of neurological disorders, including epilepsy and schizophrenia, in which alterations in GABAA receptor trafficking occur.
1. Introduction
Synaptic inhibition in the brain is largely mediated via γ-aminobutyric acid (GABA). The fast inhibitory actions of GABA are mediated via the activation of GABAA receptors (GABAARs) in the brain1,2 and via GABAC receptors in the retina3, whereas the slow prolonged actions of this transmitter are mediated via metabotropic G-protein coupled GABAB receptors4,5. GABAARs are also clinically relevant drug targets for anti-convulsant, anxiolytic, and sedative-hypnotic agents. Moreover, deficits in the functional expression of GABAARs are critical in epilepsy, anxiety disorders, cognitive deficits, schizophrenia, depression, and substance abuse. Understandably, there has been considerable interest in comprehending the cellular mechanisms that regulate their accumulation on the neuronal plasma membrane.
Molecular studies have demonstrated that GABAARs are members of a ligand-gated ion channel superfamily, whose members include nicotinic acetylcholine, glycine and 5-hydroxy-tryptamine 3 (5-HT3) receptors6,7. Members of this superfamily are heteropentamers that are assembled from a range of homologous subunits that share a common structure: a large N-terminal extracellular domain and 4 transmembrane domains (TMs) with a large intracellular domain between TM 3 and 4 (Fig. 1A). To date, 18 GABAAR subunits have been identified. Based on sequence homology, these are divided into 7 subunit classes, each of which has multiple members; α(1–6), β(1–3), γ(1–3), δ, ε(1–3), θ, π. GABAAR structural diversity is further increased via the alterative splicing of some receptor mRNAs. However, most GABAARs are composed of 2α, 2β and 1γ (or 1δ) subunit2 (Fig. 1B). GABAARs with different subunit composition have different physiological and pharmacological properties, are differentially expressed throughout the brain, and targeted to different subcellular regions. For instance, receptors composed of α(1,2,3 or 5) subunits together with β and γ subunits form benzodiazepine-sensitive receptors that are largely synaptically located and mediate the majority of phasic inhibition in the brain2 (with the notable exception of extrasynaptically-localized α5-containing receptors8,9) (Fig. 1C). In contrast, those composed of α(4/6)βδ subunits form a specialized population of predominantly extrasynaptic receptor subtypes that mediate tonic inhibition and are insensitive to benzodiazepine modulation9. In addition, GABAARs at presynaptic sites also exist10.
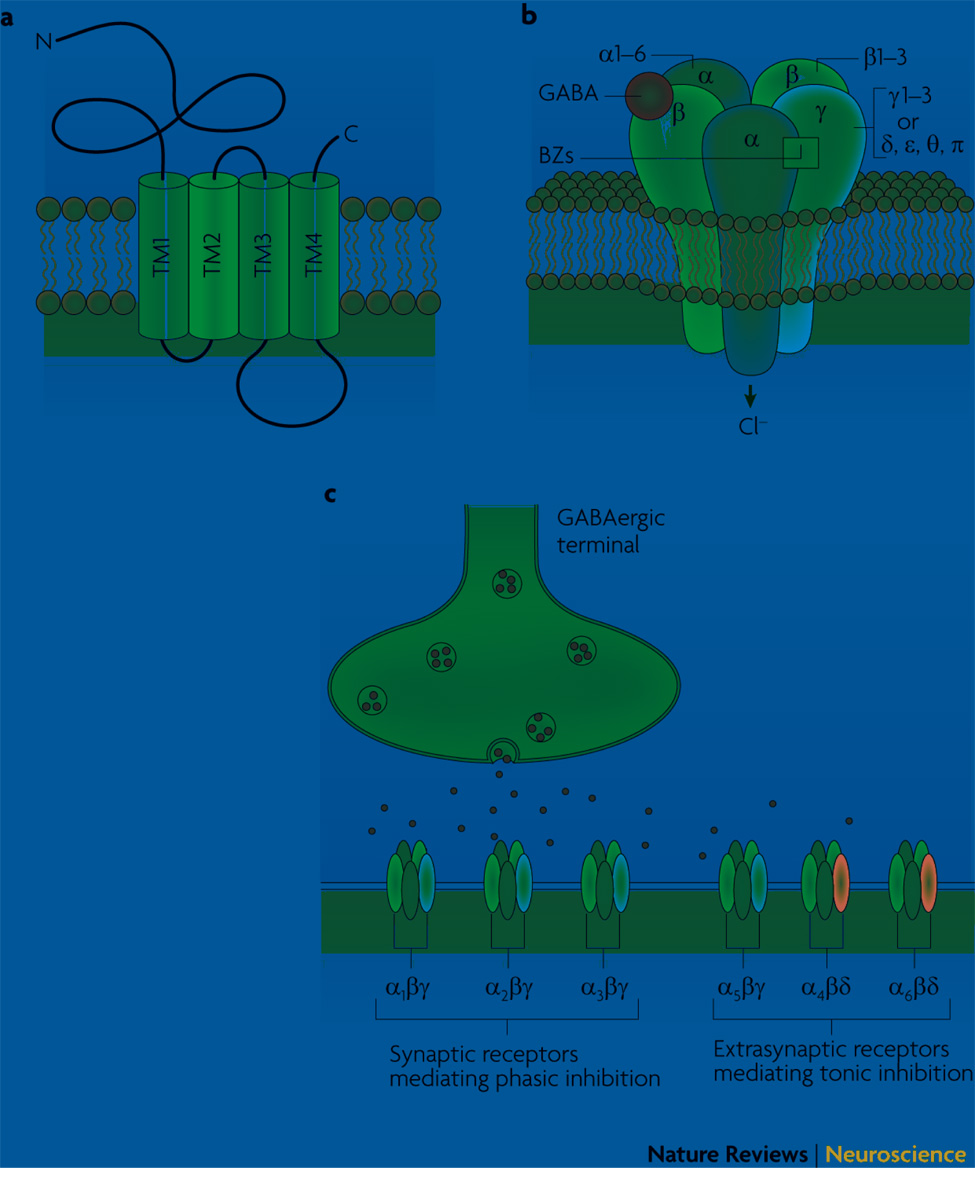
Figure 1. GABAA receptor structure and neuronal localization.
(A) GABAA receptors are members of the ligand-gated ion channel superfamily. Receptor subunits consist of four hydrophobic transmembrane (TM1–4) domains, where TM2 is believed to line the pore of the channel. The large extracellular N-terminus is the site for the binding of the neurotransmitter GABA, as well as containing binding sites for psychoactive drugs, such as benzodiazepines (BZ). Each receptor subunit also contains a large intracellular domain between TM3 and TM4, which is the site for various protein interactions as well as the site for various post-translational modifications that modulate receptor activity. (B) Five subunits from 7 subunit subfamilies (α,β,γ,δ,ε,θ,π) assemble to form a heteropentameric chloride-permeable channel. Despite the extensive heterogeneity of GABAA receptor subunits, the majority of GABAA receptors expressed in the brain consist of 2α, 2β, and 1γ subunit, where the γ subunit can be replaced by δ, ε or π. Binding of the neurotransmitter GABA occurs at the interface between the α and β subunits and triggers the opening of the channel, allowing the rapid influx of chloride ions. BZ-binding occurs at the interface between α(1,2,3 or 5) and γ subunits and potentiates GABA-induced chloride flux. (C) GABAA receptors composed of α(1–3) subunits together with β and γ subunits are thought to be primarily synaptically localized, whereas α5βγ receptors are located largely at extrasynaptic sites. Receptors composed of the aforementioned subunits are benzodiazepine-sensitive. In contrast, receptors composed of α(4,6)βδ are benzodiazepine-insensitive, and are localized at extrasynaptic sites.
Here we will address how neurons regulate the assembly, membrane trafficking, synaptic accumulation and function of these distinct GABAAR subtypes, and the relevance of these emerging regulatory processes for the efficacy of neuronal inhibition in both health and disease.
2. Controlling GABAAR assembly
GABAARs are assembled from their component subunits in the endoplasmic reticulum (ER). This process plays a critical role in determining the diversity of GABAARs that are expressed on the neuronal cell surface, because exit from the ER is dependent upon proteins reaching “conformation maturity” and misfolded proteins are retro-translocated from this organelle for degradation in the proteasome.
Limiting diversity via selective oligomerization
Many different subunit combinations are theoretically possible, however studies reveal that only a limited number of these combinations can actually exit the ER and access the neuronal cell surface. Most studies agree that most GABAARs expressed on the surface of neurons are composed of 2α, 2β, and 1γ subunit (the γ subunit can be replaced by δ, ε or π depending on the neuron type and subcellular localization of the receptor)2,11. Most homomeric subunits, and αγ and βγ heteromers, are retained in the ER and degraded (for a review, see12). Thus, the expression and assembly of these subunits must be carefully regulated in the ER, via mechanisms that involve classical ER-resident chaperones, such as heavy chain binding protein and calnexin13.
Sequences in the N-terminus of GABAAR subunits control receptor oligomerization, thus promoting the assembly of particular subunit combinations12. Oligomerization of individual GABAA receptor subunits into heteromers occurs within 5 minutes after translation14. However, it is inefficient, with less than 25% of translated subunits being assembled into heteromeric receptors14. GABAAR subunit-deficient mice have provided insights into the preferential assembly of select GABAARs in vivo. For example, a loss of the δ subunit from the plasma membrane of cerebellar granule cells is observed in α6 knock-out mice15. Similarly, there is a decrease in the α4 subunit levels in the forebrain of δ subunit-deficient mice, whereas the levels of α1 subunits remain unchanged16,17. This indicates that the δ subunit preferentially assembles with α4 and α6 subunits. There is also a compensatory increase in γ2 subunit levels in δ subunit-deficient mice16,17, suggesting that γ2 associates with the α4 subunit in the absence of the δ subunit. These findings suggest that subunits compete to find their preferential oligomerization partners in the ER. However, the details of these processes remain to be determined.
Activity-dependent GABAAR ubiquitination
The ER is responsible for the retention and degradation of misfolded or unassembled subunits and, accordingly, homomeric unassembled GABAARs subunits have been shown to be degraded in this organelle14,18. ER-associated degradation (ERAD) involves protein ubiquitination and degradation via the ubiquitin-proteasome system (UPS)19. GABAAR subunits have recently been shown to be ubiquitinated in an activity-dependent manner20. Chronic blockade of neuronal activity dramatically increased the levels of GABAAR ubiquitination within the ER, resulting in decreased insertion at the plasma membrane20. Correspondingly, increasing the level of neuronal activity resulted in a decrease in the level of GABAAR ubiquitination and an enhancement of receptor cell surface expression20. Thus, neuronal activity can regulate the ubiquitination of GABAARs in the ER, affecting their rate of degradation via the UPS. This may be one mechanism neurons use to homeostatically regulate synaptic inhibition.
The fate of ubiquitinated GABAARs is also likely to be modulated via their association with the ubiquitin-like proteins Plic-1 and Plic-218, which have been demonstrated to block the degradation of ubiquitinated substrates21. Plic-1 binds to the intracellular domain of α and β GABAAR subunits via its ubiquitin-associated (UBA) domain18. Plic-1 increases the half-life of GABAARs, resulting in an increase in the number of receptors available for insertion into the plasma membrane18. Plic-1 does not affect the rate of receptor endocytosis18, thus it appears to function solely in the secretory pathway by stabilizing and/or inhibiting the degradation of GABAARs by the UPS, thereby facilitating receptor accumulation at inhibitory synapses.
3. Facilitating GABAAR trafficking
After assembly in the ER, transport-competent GABAARs are trafficked to the Golgi apparatus and segregated into vesicles for transport to, and insertion into, the plasma membrane. Our understanding of these processes remains rudimentary, but it is becoming clear that they are facilitated by a number of receptor-associated proteins (Fig. 2), which are described in the following sections.
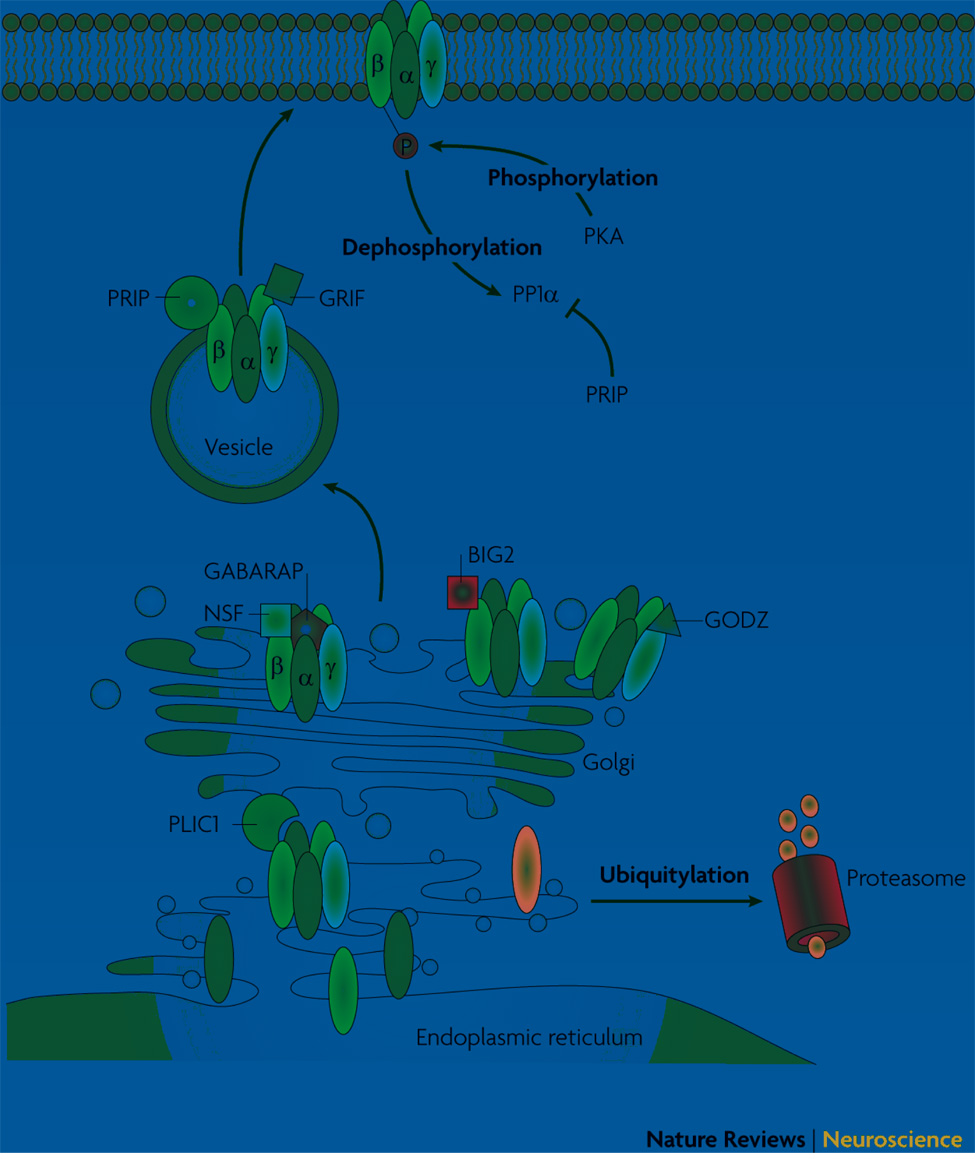
Figure 2. Trafficking of GABAA receptors.
GABAA receptor subunits are synthesized and assembled into pentameric structures in the endoplasmic reticulum (ER). This process is carefully regulated in the ER. The fate of GABAA receptor subunits can be modulated by ubiquitination and subsequent ER-associated degradation via the proteasome. Ubiquitinated GABAA receptor subunits can also be modulated via their association with Plic-1. Plic-1 facilitates GABAA receptor accumulation at the synapse by preventing the degradation of ubiquitinated GABAA receptors. Exit into the Golgi network and subsequent trafficking to the plasma membrane are also facilitiated by a number of GABAA receptor-associated proteins. GABARAP associates with the γ2 subunit of GABAA receptors and aids in the trafficking of receptors from the Golgi network to the plasma membrane. NSF and BIG2 are also localized to the Golgi network, where they bind to β subunits of GABAA receptors and modulate receptor trafficking. Palmitoylation of γ subunits occurs in the Golgi apparatus as a result of an association with the palmitoyltransferase, GODZ, and is a critical step in the delivery of GABAA receptors to the plasma membrane. GRIF proteins play a role in the trafficking of GABAA receptors to the membrane. PRIP proteins also play essential roles in the trafficking of GABAA receptors, as well as in modulating the phosphorylation state of GABAA receptors.
GABARAP and NSF
GABA receptor-associated protein (GABARAP) interacts with the intracellular domain of GABAAR γ subunits in vitro and in vivo22. It also binds to microtubules23 and to N-ethylmaleimide-sensitive factor (NSF)24, a protein involved in intracellular vesicular fusion events25. GABARAP is concentrated in the Golgi apparatus and in intracellular vesicles, but is not present at GABAergic synapses22,24,26, suggesting that its main role is in the intracellular transport of GABAARs. Overexpressing GABARAP with GABAARs results in increased cell surface receptor expression, possibly as a result of enhanced intracellular receptor trafficking27,28,29. This effect can be abolished by a mutation that disrupts the addition of phospholipids to GABARAP30, which apparently increases its membrane association, and is thus critical for GABARAP to control GABAAR trafficking30. Analysis of GABARAP knock-out mice did not reveal any alterations in synaptic γ2-containing GABAAR numbers31; however, this may reflect redundancy, given the existence of other GABARAP homologs that can interact with GABAARs32. Recently, it was demonstrated that GABARAP is necessary for increasing cell surface GABAAR expression after N-methyl-D-aspartate (NMDA) receptor activation33, suggesting that it may have a role in the regulated delivery of GABAARs to the surface after activity, rather than in the maintenance of basal receptor levels.
NSF has also been found to bind directly to GABAAR β subunits34. NSF and GABARAP may act together to promote the forward trafficking of GABAARs from the Golgi apparatus. Indeed, the subcellular distribution of both GABAARs and NSF is disturbed when the lipid modification of GABARAP is prevented in neurons, resulting in less GABAARs being trafficked to the plasma membrane30. However, another study found that overexpression of NSF significantly reduced GABAAR cell surface numbers in both heterologous systems and in neurons34. This is opposite to the effect observed on GABAARs when GABARAP is overexpressed27,28,29, and is also opposite to NSF’s role in enhancing α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor surface expression35,36. This may indicate that NSF has additional functions in the endocytic pathway, however further studies are required to understand the exact mechanism of how NSF regulates GABAAR numbers.
PRIP proteins
Phospholipase C-related catalytically inactive proteins (PRIP) are inositol 1,4,5-trisphosphate binding proteins37. PRIP-1 is expressed mainly in the brain, whereas PRIP-2 is expressed ubiquitously38. PRIP proteins bind to GABARAP as well as to the intracellular domains of GABAAR β subunits, and more weakly, γ2 subunits38,39. These findings prompted the hypothesis that PRIP proteins modulate GABAARs by competitively inhibiting GABARAP binding39. However, a more recent study40 suggests that PRIP molecules act as bridging proteins between GABARAP and GABAARs, facilitating the transport of γ2-containing receptors. This model was derived largely from studies of PRIP-1 and PRIP-2 double knock-out (PRIP-DKO) mice, in which the association between GABAARs and GABARAP in neurons was significantly reduced40. Furthermore, PRIP-DKO mice have reduced sensitivity to diazepam, suggesting an alteration in γ2-containing GABAARs40. PRIP-1 knockout mice showed a similar phenotype39. In a complementary approach, peptides were used to disrupt the binding of PRIP-1 to GABAAR subunits, resulting in a reduction in cell surface expression of γ2-containing GABAARs in cultured cell lines and neurons40. Thus, PRIP and GABARAP proteins may participate together in the trafficking of GABAARs to the synaptic membrane.
PRIP proteins may also regulate GABAAR function by regulating their phosphorylation. Phosphorylation has been shown to dynamically modulate GABAAR function, and β subunits are substrates for protein kinase C (PKC) and cAMP-dependent protein kinase A (PKA)41. Dephosphorylation of GABAARs by the protein phosphatase 1α (PP1α) terminates phosphorylation-dependent receptor modulation42, and PP1α has been shown to be inactivated by PRIP-143. PRIP-1 knock-out mice exhibited enhanced PP1α activity, resulting in diminished phosphorylation of GABAARs by PKA and subsequent changes in hippocampal neuronal inhibition42. Lastly, a recent study has implicated PRIP proteins in the constitutive internalization of recombinant GABAARs from the plasma membrane of non-neuronal cells44. Thus, PRIP proteins might have a central role in controlling GABAAR function via at least three distinct mechanisms: the trafficking of GABAARs, the modulation of GABAAR phosphorylation and the internalization of GABAARs.
Palmitoylation and GODZ
Palmitoylation involves the covalent attachment of the saturated fatty acid palmitate to a protein and has been shown to play a role in protein trafficking and function at both inhibitory and excitatory synapses45. Two groups have demonstrated that cysteine residues located within the intracellular domain of γ subunits are substrates for palmitoylation and that this modification is critical for the delivery of GABAARs to synapses46,47. The Golgi-specific DHHC zinc finger domain protein (GODZ) has been shown to mediate the palmitoyl acyl transfer to these subunits46. Of the twenty-three members of the Asp, His, His, Cys-cysteine-rich repeat domain (DHHC-CRD) protein family only GODZ and its close paralog Sertoli cell gene with a zinc finger domain-β (Serz-β) can efficiently palmitoylate the γ2 subunit48. Furthermore, studies using dominant-negative GODZ or GODZ-specific RNA interferance (RNAi) have demonstrated that GODZ is the principal palmitolytransferase for GABAARs48. GODZ is not found at inhibitory synapses, but is enriched in the trans-Golgi network, and is essential for the accumulation of γ2-containing GABAARs at synapses and for synaptic inhibitory function46,48. Therefore, GODZ presumably acts to control GABAAR trafficking in the secretory pathway and the delivery of these receptors to the plasma membrane46.
BIG2
Brefeldin A-inhibited GDP/ GTP exchange factor 2 (BIG2) plays an important role in the vesicular trafficking of GABAARs to the plasma membrane. A yeast two hybrid screen showed that it can bind to the intracellular domain of the β3 subunit, and it has since been shown to have high binding affinity for the intracellular loops of all β subunits49. In hippocampal neurons BIG2 is largely localized to the trans-Golgi network, but it is also found in trafficking vesicles and at the synaptic plasma membrane49. BIG2 has a known role in membrane budding and vesicular transport from the Golgi apparatus50. Taken together, this suggests that the main function of BIG2 is in the intracellular trafficking of GABAARs through the exocytic pathway to the plasma membrane.
GRIF/TRAK proteins
GABAAR-interacting factor 1 (GRIF-1) was first described as a protein that interacts with the β2 subunit of GABAARs51. It is a member of the TRAK family of coiled-coil domain proteins that have been implicated in the trafficking of intracellular vesicles. GRIF-1 (also known as TRAK2) and TRAK1 both interact with the microtubule-associated motor protein kinesin52,53. TRAK1 has also been shown to interact with GABAARs54, suggesting a role for these proteins in regulating the motor-dependent transport of GABAARs. Interestingly, deletion of TRAK1 in mice leads to hypertonia and reduced GABAAR expression in the brain and motor neurons54.
4. Clustering GABAARs at synapses
After navigating their way through the secretory pathway, GABAARs are inserted into the plasma membrane, where they are able to access inhibitory postsynaptic specializations or extrasynaptic sites, depending on subunit composition (Fig. 3). The mechanisms that facilitate these distinct subcellular fates are described below.
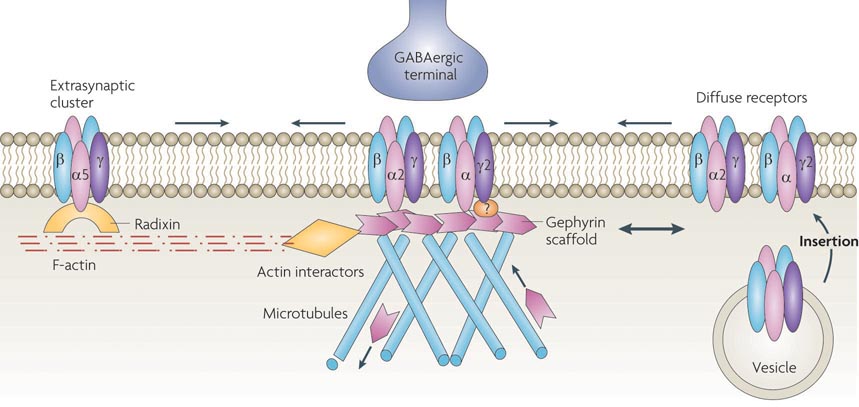
Figure 3. Dynamic regulation of receptor lateral mobility at the GABAergic synapse.
GABAA receptors are inserted into the plasma membrane at extrasynaptic sites, where they can then diffuse into synaptic sites. Lateral diffusion (black arrows) within the plasma membrane allows for continual exchange between diffuse receptor populations and synaptic or extrasynaptic receptor clusters, with anchoring molecules tethering or corralling moving receptors. The synaptic localization of α2-containing GABAA receptors is maintained by direct binding to gephyrin, which binds to microtubules and actin interactors such as the GDP/GTP exchange factor collybistin72, Mena/ VASP (vasodilator-stimulated phosphoprotein)144 and profilins 1 and 2144,145. No direct interaction between gephyrin and the γ2 subunit has been demonstrated. However, gephyrin depletion increases γ2 cluster mobility, and loss of the γ2 subunit results in post-synaptic sites devoid of gephyrin. This suggests an unidentified intermediary interactor or a post-translational modification that could link γ2-containing receptors and gephyrin. Alternatively, clustering of γ2-containing receptors might occur via an independent mechanism. Gephyrin also displays local lateral movements (red double arrow), and removal or addition by microtubule dependent trafficking, contributing additional mechanisms to regulate synaptic transmission. Extrasynaptic localization of α5-containing GABAA receptors is controlled by binding to activated radixin, which directly binds F-actin.
Synaptic vs. extrasynaptic GABAARs
GABAARs on the neuronal cell surface exist as diffuse receptor populations or synaptic or extrasynaptic clusters. Lateral diffusion within the plasma membrane allows for continual exchange between these receptor populations55,56. GABAARs that can bind bungarotoxin have been used to examine the subcellular sites of GABAAR insertion into the neuronal membrane. These studies have demonstrated that most receptors are delivered to extrasynaptic locations in the plasma membrane. Over time, diffusion and trapping increases the population of synaptic receptors57.
Heteromeric GABAARs retain distinct cell surface expression patterns dependent on subunit composition. Most surface receptor clusters of γ2 receptor subunits are synaptic, whereas β3-containing GABAARs have a higher proportion of diffuse and/or extrasynaptic receptors55,58. α5-containing receptors are predominantly extrasynaptic8,9. Other receptor subunits, such as δ, appear as diffuse populations on the neuronal surface59,60 and are exclusively located outside the synapse at perisynaptic and extrasynaptic locations57,61. These extrasynaptic α5- and δ- containing GABAARs are considered the main receptors mediating tonic inhibition.
Gephyrin-dependent clustering of GABAARs
One protein strongly implicated in regulating the clustering of GABAARs at inhibitory synapses is the multifunctional protein gephyrin, which was first identified by its association with glycine receptors62. Gephyrin binds directly with the intracellular domain of the β subunit of glycine receptors, which stabilizes these proteins at inhibitory synapses in the spinal cord63–67. Gephyrin is also widely expressed in non-neuronal tissues65. In the brain, it is found in neurons, and is enriched at postsynaptic specializations that contain GABAAR subtypes composed of α(1–3), β(2,3) and γ2 subunits68.
Reducing gephyrin expression compromises the accumulation of GABAAR subtypes containing α2 or γ2 subunits at inhibitory synapses55,66,69–71, although there is no change in overall levels of these subunits71 and only a modest reduction in the amplitude of miniature inhibitory postsynaptic currents (mIPSCs) or GABA-induced whole-cell currents66. In addition, surface clusters of GABAARs formed in the absence of gephyrin were three times more mobile than those in control neurons55, indicating that gephyrin plays a role in enhancing the confinement of GABAARs at synaptic sites. Furthermore, gene knockout of collybistin, an established binding partner for gephyrin72,73, also leads to loss of synaptic GABAAR clusters74. Together, these results support the concept that gephyrin may act to promote the stability of GABAAR clusters containing α2/γ2 subunits.
A loss of α3 and β(2/3) subunits were observed in spinal cord neurons of gephyrin knockout mice, whereas there were only minimal changes in α1 or α5 subunits in hippocampal and spinal cord neurons66,70. These observations suggested the existence of gephyrin-dependent and independent GABAAR clustering mechanisms. However, the development of compensatory clustering mechanisms in neurons devoid of gephyrin cannot be discounted.
The molecular mechanisms underlying gephyrin-dependent clustering of GABAARs remain poorly understood. Evidence suggests that a domain critical for clustering may exist within the γ2subunit, as cultured neurons from γ2 knock-out mice are devoid of both GABAARs and gephyrin at postsynaptic sites69,75. In order to identify such a domain, chimeric α2/γ2 and δ/γ2 receptors have been studied76,77. These suggest that the intracellular loop and/or transmembrane domain 4 of the γ2 subunit are critical for GABAAR synaptic clustering76,77, but whether these domains actually mediate their effects in a mechanism dependent upon gephyrin remains to be established.
Efforts to show gephyrin binding to native GABAARs have been unsuccessful63. Similarly, co-expression of gephyrin and α1–3, β1–3 and γ2 GABAAR subunits in HEK-293 cells revealed only a weak interaction with the β3 subunit78. Interestingly, a recent study79 identified a 10 amino acid hydrophobic motif within the major intracellular domain of the α2 subunit that is responsible for the targeting of GABAAR subunits to inhibitory synapses. Critically, this phenomenon is dependent upon gephyrin expression79. In addition, this motif was demonstrated to mediate the direct interaction of the intracellular domain of the α2 subunit with gephyrin in in vitro binding assays79. However, under the same conditions, minimal binding of gephyrin to the intracellular domains of the γ2 and β3 subunits was evident79. The interaction of the α2 intracellular domain with gephyrin was blocked by low concentrations of detergent79, thus providing a possible explanation as to why previous studies had not identified such a direct association between gephyrin and GABAARs.
In summary, these results provide strong evidence that gephyrin can bind directly to receptor subtypes containing α2 subunits and regulate their synaptic targeting, but the relevance of this mechanism for receptor subtypes containing other α subunit variants remains to be evaluated. Significantly, a large number of gephyrin splice variants have been identified80 and the synaptic localization and function of gephyrin can be regulated by both activity81,82 and phosphorylation83. It will therefore be of merit to examine the roles that these differing variants of gephyrin play in regulating the synaptic clustering of distinct GABAAR subtypes.
Gephyrin-independent clustering of GABAARs
Gephyrin-independent GABAAR clustering mechanisms are suggested by the presence of clustered receptors and mIPSCs in gephyrin knockout mice66,70,83. Recently, radixin, an ERM (ezrin, radixin, moesin)-family member protein, has been identified as a specific interactor for the intracellular domain of the α5 subunit84. ERM proteins exist in an inactive conformation and are activated by phosphatidylinositol-4,5-bisphosphate (PIP2) binding and subsequent phosphorylation of the C-terminus (for a review, see85). In neurons, depletion of radixin dramatically decreased α5-containing GABAAR clustering, although total cell surface levels of the α5 subunit remained unchanged84. Radixin appears to directly link the α5 subunit to the actin cytoskeleton, as activated radixin is capable of binding both the α5 subunit and F-actin84. The apparent radixin binding domain within the α5 subunit is a highly conserved region that is also found in α1–3 subunits, differing only in the last two amino acids in the α2 subunit. Further work clearly remains in elucidating the mechanism of radixin-dependent GABAAR anchoring.
5. Endocytosis and post-endocytic sorting of GABAARs
GABAAR endocytosis
GABAARs undergo extensive endocytosis in both heterologous and neuronal systems. Although a clathrin-independent endocytic pathway has been demonstrated in heterologous cells86, clathrin-dependent endocytosis appears to be the major internalization mechanism for neuronal GABAARs87 (Fig. 4), with approximately 25% of β3-containing cell surface GABAARs being internalized within 30 minutes88. Blocking clathrin-dependent endocytosis results in reduced GABAAR internalization87,89,90 and a large increase in mIPSC amplitude87, consistent with an increase in cell surface receptor levels87,89,90.
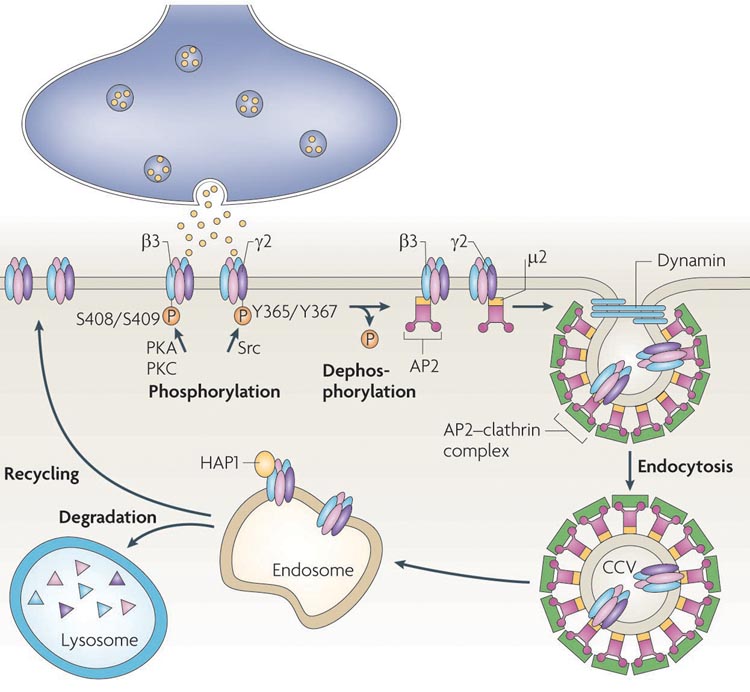
Figure 4. Regulation of GABAA receptor endocytosis and post-endocytic sorting.
Clathrin-dependent endocytosis is the major internalization mechanism for neuronal GABAA receptors. The intracellular loops of β and γ subunits interact with the clathrin adaptor protein 2 (AP2) complex. Binding of the μ2 subunit is inhibited by phosphorylation of the AP2-interacting motifs in GABAA receptor subunits, increasing cell surface receptor levels and enhancing the efficacy of inhibitory synaptic transmission. Once endocytosed in clathrin-coated vesicles (CCV), the vesicles are uncoated and fuse with early or sorting endosomes, resulting in GABAA receptors being subsequently recycled to the plasma membrane or degraded in lysosomes. Huntingtin associated protein-1 (HAP1) interacts with β subunits and promotes receptor recycling to the plasma membrane. PKA and PKC regulate phosphorylation of serine residues 408/9 in the AP2-binding motif of β3 subunits, while tyrosine residues 365/7 in the γ2 subunit are phosphorylated by Src kinase.
The clathrin adaptor protein 2 (AP2) complex plays a critical role in recruiting membrane-associated proteins into clathrin-coated pits. AP2 is composed of four distinct subunits (α, β2, μ2 and σ2 subunits; reviewed in91). GABAARs in the brain are intimately associated with AP2 via a direct binding of β1–3 and γ2 GABAAR subunits to the μ2 subunit of this complex87.
Within the β2 GABAAR subunit, a dileucine motif has been identified that is important for clathrin-dependent GABAAR internalization in heterologous cells89. In addition, an atypical AP2 binding motif within the intracellular domains of GABAAR β subunits has been identified92. Intriguingly, this binding motif contains the major sites of phosphorylation for PKA and PKC, and phosphorylation of these sites reduces binding to the μ2 subunit of AP292. A peptide corresponding to the AP2 binding motif in the β3 subunit binds to AP2 with high affinity only when dephosphorylated92. Furthermore, this peptide enhanced mIPSC amplitude and whole cell GABAAR currents.
More recently, another AP2 binding motif, centered around tyrosines 365/7 in the GABAAR γ2 subunit, has been identified93. These tyrosine residues are the principal sites for phosphorylation by Src kinase94. A peptide containing residues Y365/7 exhibits very high affinity for the μ2 subunit, and the affinity of this interaction is dramatically decreased via phosphorylation of these sites93. Introduction of the non-phosphorylated γ2 peptide into neurons produced a large increase in the mIPSC amplitude, and was observed to increase the number of cell surface GABAARs. Intriguingly, co-dialysis of neurons with both the non-phosphorylated β3 and γ2 subunit peptides produced an additive effect on mIPSC amplitudes93.
Together, these results provide direct evidence that phosphorylation of GABAAR subunits at distinct AP2 binding sites can regulate the cell surface stability of GABAARs and the strength of synaptic inhibition. Moreover they also provide a mechanism by which neurotransmitter and/or growth factor signaling pathways that regulate the activity of protein kinases/ phosphatases41,95–97; could influence the efficacy of synaptic inhibition by controlling the stoichiometry of GABAAR phosphorylation and, thus, their endocytosis.
GABAAR recycling and lysosomal degradation
Once endocytosed, most internalized GABAARs recycle back to the plasma membrane over short time frames; however over longer time periods they are targeted for lysosomal degradation88. Clearly the fate of internalized GABAARs will therefore play a critical role in controlling cell surface receptor levels and hence the efficacy of synaptic inhibition. Huntingtin associated protein-1 (HAP1)98 is a GABAAR associated protein that binds the intracellular loop of β subunits in vitro and in vivo88. HAP1 is a cytoplasmic protein with several central coil-coiled domains that are likely to regulate protein-protein interactions. Overexpression of HAP1 in neurons inhibits GABAAR degradation and consequently increases receptor recycling88. Furthermore, HAP1 overexpression increased steady state surface levels of GABAARs and produced a 63% increase in mIPSC amplitude, showing a dramatic functional effect of increased surface receptor number88. The mechanism underlying post-endocytic GABAAR sorting remains to be elucidated, and HAP1’s specific role in this process is also an area of active research. The impact of HAP1 regulation of GABAARs was recently shown in the hypothalamus, where down-regulation of HAP1 levels resulted in decreased GABAAR levels, causing decreased food intake and loss of body weight99. An unresolved issue is whether HAP1 acts to promote recycling of GABAARs or prevents their lysosomal degradation.
6. Compromised GABAAR trafficking in disease
The significance of the aforementioned mechanisms for maintaining homeostatic synaptic inhibition is highlighted by the multiple neurological and psychiatric diseases in which GABAAR dysfunction has been suggested. These include epilepsy100, anxiety disorders2, Huntington’s disease101, Angelman syndrome102, fragile X syndrome103, schizophrenia104, and drug abuse105. In this section, we highlight recent findings related to a few of these disorders.
Epilepsy
The epileptic state represents a dramatic change in balance between excitatory and inhibitory activity. Studies have shown that seizure activity results in altered GABAAR trafficking and/or subunit expression in animal models of status epilepticus (SE) and temporal lobe epilepsy (TLE), as well as in patients100,106. These changes involve both up- and down-regulation, depending on the particular GABAAR subunit in question, as well as on the time-point studied in relation to the evolution of the chronic seizure state.
SE is a life-threatening state in which seizures occur unremittingly107. Decreases in synaptic GABAARs, resulting from enhanced endocytosis, have been observed in animals in which SE has been experimentally induced108–110. The loss of these synaptic receptor populations, which are normally benzodiazepine-sensitive, may explain the rapid development of pharmacoresistance in patients with SE and also explain the non-terminating nature of these seizures. A recent study showed decreased phosphorylation of β3 GABAAR subunits during SE, resulting in an increased association of receptors with the clathrin adaptor AP2110 (Fig. 5A). Enhancing GABAAR subunit phosphorylation, or selectively blocking subunit binding to AP2, increased GABAAR surface expression levels and normalized synaptic inhibition in hippocampal slices derived from mice with SE110. Thus, novel therapeutic strategies for SE may one day be based on preventing or reversing this aberrant internalization of GABAARs.
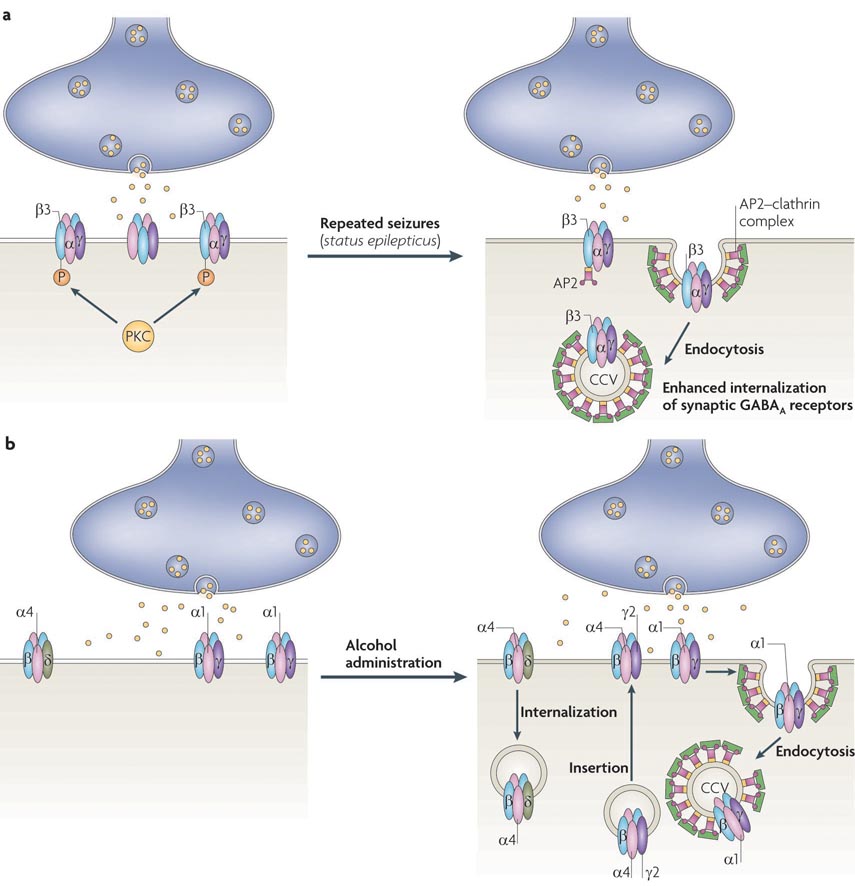
Figure 5. Dysregulation in GABAA receptor trafficking in neurological disease.
(A) Repetitive, non-abating seizures that lead to status epilepticus result in a decrease in the phosphorylation of GABAA receptor β subunits by PKC. This leads to an increased associated with the clathrin adaptor AP2, followed by increased internalization via clathrin-mediated endocytosis. Decreased numbers of synaptic GABAA receptors lead to reduced synaptic inhibition (ie. increased excitatory drive and a lower seizure threshold) as well as decreased benzodiazepine sensitivity. (B) Alcohol-induced plasticity in GABAA receptors involves changes in both synaptic and extrasynaptic GABAA receptor populations. After alcohol administration, there is an increased internalization of δ-containing extrasynaptic GABAA receptors. There is also an increased internalization of α1-containing synaptic GABAA receptors via clathrin-dependent internalization. Insertion of distinct GABAA receptor populations at synaptic sites (ie. α4βγ2) have been hypothesized to serve a compensatory role at inhibitory synapses, however, these receptors differ in their physiological functions from normal synaptic GABAA receptor populations, as well as being benzodiazepine-insensitive.
Altered GABAAR expression has also been observed in animal models of TLE, however these have generally shown increases in the expression of synaptic GABAARs, at least in the dentate gyrus111. Corresponding increases in the expression of GABAAR-associated proteins, such as gephyrin, and in the size and density of postsynaptic GABAAR clusters have also been demonstrated112. This suggests that novel GABAergic synapses form, which could occur as a result of the aberrant sprouting of GABAergic axons106. Extrasynaptic GABAAR subunits (α4 and δ) have also been reported to be increased in granule cells of the dentate gyrus in rat models of TLE113–115, however, one study in mice has observed decreases in δ subunit expression in these cells116. Analyses of hippocampal tissue from patients with TLE reveal alterations in GABAAR subunit expression patterns that are similar to those observed experimentally117,118.
Human genetic studies have provided further evidence that abnormal GABAAR function contributes to epilepsy disorders. Multiple distinct mutations in the γ2119–121, α1122,123 and δ subunits124 have been identified in patients with epilepsy. While the exact mechanisms by which each of these mutations contribute to seizure disorders remain to be fully elucidated, deficits in the assembly, trafficking and function of recombinant mutant receptors have been described119,120,125–127. For example, a missense mutation that occurs in the α1 subunit (A322D) leads to subunit retention in the ER, followed by ubiquitin-dependent degradation128, resulting in overall lower levels of α1-containing GABAARs at the cell surface.
Drug abuse
Considerable evidence exists to support a role for GABAARs in mediating the addictive properties of drugs of abuse105,129. In particular, chronic use of alcohol or benzodiazepines, which are both allosteric modulators of GABAARs, can lead to drug tolerance, dependence and withdrawal symptoms following drug cessation. Changes in the mRNA and protein expression of various GABAAR subunits have been documented after alcohol and benzodiazepine administration in both cultured neurons and animal models130,131. However, the mechanisms responsible for these alterations have only recently begun to be elucidated. Significant alterations in the surface expression and composition of both synaptic and extrasynaptic GABAAR populations have been observed after a single intoxicating dose of alcohol in rats132 (Fig. 5B). These changes were found to be persistent after chronic alcohol administration and withdrawal132,133. This long-term plasticity in GABAARs is likely to involve changes in the phosphorylation of GABAAR subunits and alterations in the endocytosis of specific GABAAR subtypes. For example, the association of PKC with GABAAR subunits is altered after chronic ethanol exposure134. Increased associations between clathrin adaptor proteins and α1 subunits have also been demonstrated135, suggesting that enhanced clathrin-mediated endocytosis of α1-containing GABAARs contributes to changes in GABAAR trafficking after chronic alcohol use (Fig. 5B). Interestingly, the well-documented phenomenon of cross-tolerance to benzodiazepines after chronic alcohol use136 suggests that similar mechanisms may be responsible for tolerance to both of these drugs. Thus, understanding tolerance-inducing alterations in GABAAR trafficking should not only advance our understanding of the disease process that leads to alcoholism, but also improve the development of drugs to treat insomnia and anxiety disorders without causing tolerance.
Schizophrenia
Altered expression of several proteins involved in GABAergic transmission have been reported in studies of postmortem tissue from subjects with schizophrenia. Significant reductions in the mRNA levels of GAD-67 (one of the major GABA synthesizing enzymes) and the GABA membrane transporter (GAT-1) have been observed in a subpopulation of interneurons in the prefrontal cortex (PFC) of schizophrenic subjects137,138. In addition, a compensatory upregulation of α2-containing GABAARs in the axon initial segment of pyramidal neurons has been demonstrated137. Reduced GABAergic signaling between these affected interneurons and pyramidal cells has been postulated to contribute to cognitive deficits associated with schizophrenia104.
The in vivo analysis of animal models will help to determine the extent to which aberrant GABAergic plasticity contributes to the pathophysiology of schizophrenia. For example, mice lacking the α3 GABAAR subunit showed select deficits in prepulse inhibition (PPI), which could be normalized by treatment with the antipsychotic haloperidol139. Deficits in PPI have been associated with a number of psychiatric disorders, including schizophrenia, and are a measure of a diminished ability for sensorimotor information processing140. There was a dramatic loss of synaptic GABAARs and gephyrin clusters in the thalamic reticular nucleus141 (one of the main regions in the brain where the α3 subunit is normally expressed142) of α3 subunit knockout mice, resulting in an absence of functional inhibitory receptors throughout development in this critical brain region. Deficits in PPI have also been observed in mutant mice in which there is a selective reduction in hippocampal α5-containing GABAARs143. Together, these findings suggest that sensorimotor gating is highly sensitive to an imbalance in inhibitory neurotransmission, and that hypofunction of select GABAAR populations can lead to a schizophrenia-related cognitive impairment. Pharmacological interventions to increase GABAAR function and/or trafficking of relevant GABAAR subpopulations may help to alleviate some of the symptoms of schizophrenia and other psychiatric disorders.
7. Conclusions and outlook
Fast inhibitory GABAergic synaptic transmission is a principal determinant of neuronal excitability. This process is dependent upon the delivery of individual GABAAR subtypes, which are endowed with unique physiological and pharmacological properties, to their appropriate synaptic or extrasynaptic sites, where they mediate phasic and tonic inhibition, respectively.
The synthesis and assembly of GABAARs in the ER is an important control point in determining receptor diversity on the plasma membrane. Results from knock-out mice have illustrated preferential receptor subunit partnerships, but how this process is orchestrated remains to be determined. It is becoming apparent that subunits within the ER are subject to activity-dependent ubiquitination, which decreases their stability and half-life and limits the rate of insertion of newly synthesized receptors into the plasma membrane. It will be exciting to determine whether the various GABAAR subunits are differentially ubiquitinated, as this may allow neuronal activity to shape the number and pharmacological properties of GABAARs on target cells. Modulating receptor palmitoylation, or their binding to accessory proteins as they passage through the Golgi apparatus, may further refine our understanding of how these processes shape the diversity of GABAARs on the plasma membrane.
GABAARs exhibit high rates of diffusion at the cell surface, which facilitates their delivery to synaptic sites, or entry into coated pits for removal via clathrin-dependent endocytosis. It is emerging that endocytosis is regulated via phosphorylation-dependent mechanisms; more specifically, receptor binding to clathrin-associated proteins can be negatively modulated via the phosphorylation of serine or tyrosine residues within specific GABAAR subunits. This could allow cell-signaling pathways that regulate GABAAR phosphorylation to also influence their cell surface stability. The relevance of these processes awaits the development of knock-in mouse lines in which the phosphorylation residues within individual AP2 binding motifs have been ablated. However, it is interesting to note that dephosphorylation of GABAARs and their enhanced endocytosis may be responsible for compromised synaptic inhibition during status epilepticus. Furthermore, the fate of endocytosed receptors is another determinant of steady-state cell surface expression levels. However our understanding of processes that control the recycling and lysosomal degradation of GABAARs remains rudimentary.
The stabilization of GABAARs on the plasma membrane is likely to be facilitated by multiple mechanisms. Extrasynaptic receptors mediate tonic inhibition, and the stabilization of α5-containing receptors at extrasynaptic specializations is facilitated by the actin binding protein radixin. For synaptic receptors, the multifunctional protein gephyrin is strongly implicated in stabilizing receptors containing α2 and γ2 subunits. There is also evidence that α1-containing GABAARs, although tightly co-localized with gephyrin, can be maintained at synaptic sites in the absence of gephyrin. Therefore, further studies are required to address the range of GABAAR subunits that are capable of binding specific gephyrin splice variants, and the roles that these binding motifs play in the accumulation of individual subtypes at inhibitory synapses.
Resolution of these issues will provide key insights into what controls inhibitory synaptic strength and how alterations in these processes result in the development of central nervous system pathologies, ranging from epilepsy to schizophrenia. This information is also likely to lead to the identification of novel therapeutic drug targets that will allow for the pharmacological modulation of individual GABAAR subtypes.
Acknowledgements
In memory of Professor Robert Eisenthal, the “master enzymologist”. SJM is supported by NIH grants NS046478, NS048045, NS051195, NS056359, P01NS054900, the MRC (UK) and the Wellcome Trust. We would like to thank Dr. Richard Olsen (University of California, Los Angeles) for communication of unpublished results.
Glossary
- BENZODIAZEPINES
Pharmacologically active molecules with sedative, anxiolytic, amnesic and anticonvulsant effects. They act by binding at the interface between α(1,2,3 or 5) and γ subunits of GABAA receptors to potentiate the response elicited by GABA.
- YEAST TWO-HYBRID SCREEN
System used to determine the existence of direct interactions between two proteins. It involves the expression of two proteins in yeast; the plasmids encoding these proteins are fused to GAL4 DNA-binding and GAL4 activation domains, respectively. If the proteins interact, the resulting complex will drive the expression of a reporter gene, commonly β-galactosidase.
- GABAergic PLASTICITY
Changes in local activity that lead to longer-term increases or decreases in inhibitory synaptic strength.
- UBIQUITIN-PROTEASOME SYSTEM
Ubiquitin is a 76 amino-acid protein that serves as a tag to mark proteins destined for degradation. Proteins tagged by a polyubiquitin chain are targeted to the proteasome, a large, multimeric barrel-like complex that acts by proteolysis to degrade proteins.
- PALMITOYLATION
The covalent attachment of a palmitate (16-carbon saturated fatty acid) to a cysteine residue through a thioester bond.
- CLATHRIN
One of the main protein components of the coats formed during membrane endocytosis.
- AP2 COMPLEX
Tetrameric complex composed of subunits called adaptins that play an important role in clathrin-dependent membrane endocytosis.
- RNA interference (RNAi)
A molecular method in which small interfering RNA sequences are introduced into cells or tissues, and subsequently decrease the expression of target genes.
- MINIATURE INHIBITORY POSTSYNAPTIC CURRENT (mIPSC)
The postsynaptic current that results from the activation of synaptic receptors by neurotransmitters (GABA or glycine) that are usually released from a single vesicle.
- TONIC INHIBITION
An inhibitory response that is mediated by the activation of extra- or peri-synaptic GABAA receptors through ambient concentrations of GABA.
Biographies
Tija C. Jacob
Tija C. Jacob obtained her Ph.D. degree from the University of California at Berkeley in the lab of Joshua M. Kaplan, studying neurotransmitter and neuropeptide modulation of behavior in the nematode C. elegans. She was a postdoctoral fellow with Steve J. Moss at University College London, UK, and is currently continuing her training in his lab at the University of Pennsylvania, Philadelphia, USA. Her research interests focus on regulation of GABAA receptor intracellular trafficking, cell surface mobility, and interactions with the inhibitory scaffold and how these processes are altered by disease or drug treatment.
Stephen J. Moss
Stephen J. Moss obtained his PhD in the laboratory of Eric Barnard at Imperial College London and the MRC-laboratory of Molecular Neurobiology in Cambridge. After postdoctoral training with British Biotechnology in Oxford, Richard Huganir at Johns Hopkins University and Martin Raff at University College London (UCL) he was appointed as a group leader in the MRC laboratory of Molecular Biology at UCL in 1994. In 2000 he was promoted to Professor of Molecular Pharmacology, in the Pharmacology Department at UCL. He joined the University of Pennsylvania as a Professor of Neuroscience in 2003. His research interests centre on the control of synaptic inhibition with particular emphasis on the functional modulation of metabotropic and ionotropic GABA receptors.
Rachel Jurd
Rachel Jurd obtained her Ph.D. at the Institute of Pharmacology and Toxicology, University of Zurich, Switzerland, in the laboratory of Dr. Uwe Rudolph, studying GABAA receptors and general anaesthetic mechanisms. She then pursued postdoctoral training at the Ernest Gallo Clinic and Research Center, University of California, San Francisco, USA, before joining Dr. Stephen J. Moss’s laboratory at the University of Pennsylvania, Philadelphia, USA, in 2007. Her research focuses on the regulation of GABAA receptor trafficking by phosphorylation, as well as on the molecular mechanisms underlying drug tolerance.
References
- 1.Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- 3.Bormann J, Feigenspan A. GABAC receptors. Trends Neurosci. 1995;18:515–519. doi: 10.1016/0166-2236(95)98370-e. [DOI] [PubMed] [Google Scholar]
- 4.Couve A, Moss SJ, Pangalos MN. GABAB receptors: a new paradigm in G protein signaling. Mol Cell Neurosci. 2000;16:296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- 5.Bettler B, Tiao JY. Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol Ther. 2006;110:533–543. doi: 10.1016/j.pharmthera.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Barnard EA, et al. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 7.Unwin N. The structure of ion channels in membranes of excitable cells. Neuron. 1989;3:665–676. doi: 10.1016/0896-6273(89)90235-3. [DOI] [PubMed] [Google Scholar]
- 8.Fritschy JM, Johnson DK, Mohler H, Rudolph U. Independent assembly and subcellular targeting of GABAA-receptor subtypes demonstrated in mouse hippocampal and olfactory neurons in vivo. Neurosci Lett. 1998;249:99–102. doi: 10.1016/s0304-3940(98)00397-8. [DOI] [PubMed] [Google Scholar]
- 9.Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of γ-aminobutyric acidA receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- 10.Draguhn A, Axmacher N, Kolbaev S. Presynaptic ionotropic GABA receptors. Results Probl Cell Differ. 2008;44:69–85. doi: 10.1007/400_2007_040. [DOI] [PubMed] [Google Scholar]
- 11.McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 12.Kittler JT, McAinsh K, Moss SJ. Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol Neurobiol. 2002;26:251–268. doi: 10.1385/MN:26:2-3:251. [DOI] [PubMed] [Google Scholar]
- 13.Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric γ-aminobutyric acidA receptors. J Biol Chem. 1996;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- 14.Gorrie GH, et al. Assembly of GABAA receptors composed α1 and β2 subunits in both cultured neurons and fibroblasts. J Neurosci. 1997;17:6587–6596. doi: 10.1523/JNEUROSCI.17-17-06587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nusser Z, et al. Alterations in the expression of GABAA receptor subunits in cerebellar granule cells after the disruption of the α6 subunit gene. Eur J Neurosci. 1999;11:1685–1697. doi: 10.1046/j.1460-9568.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- 16.Peng Z, et al. GABAA receptor changes in delta subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- 17.Korpi ER, et al. Altered receptor subtypes in the forebrain of GABAA receptor δ subunit-deficient mice: recruitment of γ2 subunits. Neuroscience. 2002;109:733–743. doi: 10.1016/s0306-4522(01)00527-9. [DOI] [PubMed] [Google Scholar]
- 18.Bedford FK, et al. GABAA receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat Neurosci. 2001;4:908–916. doi: 10.1038/nn0901-908.The first report demonstrating the stabilization of GABAARs by a direct interaction with the ubiquitin-like protein Plic-1.
- 19.Yi JJ, Ehlers MD. Emerging roles for ubiquitin and protein degradation in neuronal function. Pharmacol Rev. 2007;59:14–39. doi: 10.1124/pr.59.1.4. [DOI] [PubMed] [Google Scholar]
- 20.Saliba RS, Michels G, Jacob TC, Pangalos MN, Moss SJ. Activity-dependent ubiquitination of GABAA receptors regulates their accumulation at synaptic sites. J Neurosci. 2007;27:13341–13351. doi: 10.1523/JNEUROSCI.3277-07.2007.Activity-dependent ubiquitination of GABAARs and subsequent degradation by the proteasome was reported as a mechanism that regulates GABAAR accumulation at synaptic sites.
- 21.Kleijnen MF, et al. The hPLIC proteins may provide a link between the ubiquitination machinery and the proteasome. Mol Cell. 2000;6:409–419. doi: 10.1016/s1097-2765(00)00040-x. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABAA receptor-associated protein links GABAA receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264.First identification of GABARAP as a protein that interacts with the γ2 subunit of GABAARs.
- 23.Wang H, Olsen RW. Binding of the GABAA receptor-associated protein (GABARAP) to microtubules and microfilaments suggests involvement of the cytoskeleton in GABARAP-GABAA receptor interaction. J Neurochem. 2000;75:644–655. doi: 10.1046/j.1471-4159.2000.0750644.x. [DOI] [PubMed] [Google Scholar]
- 24.Kittler JT, et al. The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABAA receptors. Mol Cell Neurosci. 2001;18:13–25. doi: 10.1006/mcne.2001.1005. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Slevin JT, Whiteheart SW. Cellular functions of NSF: not just SNAPs and SNAREs. FEBS Lett. 2007;581:2140–2149. doi: 10.1016/j.febslet.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kneussel M, et al. The γ-aminobutyric acidA receptor (GABAAR)-associated protein GABARAP interacts with gephyrin but is not involved in receptor anchoring at the synapse. Proc Natl Acad Sci USA. 2000;97:8594–8599. doi: 10.1073/pnas.97.15.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Wang H, Vicini S, Olsen RW. The γ-aminobutyric acid type A (GABAA) receptor-associated protein (GABARAP) promotes GABAA receptor clustering and modulates the channel kinetics. Proc Natl Acad Sci USA. 2000;97:11557–15562. doi: 10.1073/pnas.190133497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen ZW, Chang CS, Leil TA, Olcese R, Olsen RW. GABAA Receptor-Associated Protein Regulates GABAA Receptor Cell-Surface Number in Xenopus laevis Oocytes. Mol Pharmacol. 2005;68:152–159. doi: 10.1124/mol.104.009878. [DOI] [PubMed] [Google Scholar]
- 29.Leil TA, Chen ZW, Chang CS, Olsen RW. GABAA receptor-associated protein traffics GABAA receptors to the plasma membrane in neurons. J Neurosci. 2004;24:11429–11438. doi: 10.1523/JNEUROSCI.3355-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen ZW, Chang CS, Leil TA, Olsen RW. C-terminal modification is required for GABARAP-mediated GABAA receptor trafficking. J Neurosci. 2007;27:6655–6663. doi: 10.1523/JNEUROSCI.0919-07.2007.Demonstrates that a post-translational lipid modification of GABARAP is essential for the proper localization of GABARAP and for its function as a trafficking protein of GABAARs.
- 31.O'Sullivan GA, Kneussel M, Elazar Z, Betz H. GABARAP is not essential for GABAA receptor targeting to the synapse. Eur J Neurosci. 2005;22:2644–2648. doi: 10.1111/j.1460-9568.2005.04448.x. [DOI] [PubMed] [Google Scholar]
- 32.Mansuy V, et al. GEC1, a protein related to GABARAP, interacts with tubulin and GABAA receptor. Biochem Biophys Res Commun. 2004;325:639–648. doi: 10.1016/j.bbrc.2004.10.072. [DOI] [PubMed] [Google Scholar]
- 33.Marsden KC, Beattie JB, Friedenthal J, Carroll RC. NMDA receptor activation potentiates inhibitory transmission through GABAA receptor-associated protein-dependent exocytosis of GABAA receptors. J Neurosci. 2007;27:14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goto H, et al. Direct interaction of N-ethylmaleimide-sensitive factor with GABAA receptor β subunits. Mol Cell Neurosci. 2005;30:197–206. doi: 10.1016/j.mcn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Nishimune A, et al. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- 36.Song I, et al. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- 37.Kanematsu T, et al. Domain organization of p130, PLC-related catalytically inactive protein, and structural basis for the lack of enzyme activity. Eur J Biochem. 2000;267:2731–2737. doi: 10.1046/j.1432-1327.2000.01291.x. [DOI] [PubMed] [Google Scholar]
- 38.Uji A, et al. Molecules interacting with PRIP-2, a novel Ins(1,4,5)P3 binding protein type 2: Comparison with PRIP-1. Life Sci. 2002;72:443–453. doi: 10.1016/s0024-3205(02)02275-0. [DOI] [PubMed] [Google Scholar]
- 39.Kanematsu T, et al. Role of the PLC-related, catalytically inactive protein p130 in GABAA receptor function. Embo J. 2002;21:1004–1011. doi: 10.1093/emboj/21.5.1004.First identification of PRIP-1 as a protein that interacts with GABAAR subunits. This study also reports electrophysiological and behavioral studies on PRIP-1 knockout mice that demonstrate an essential role for PRIP-1 in the normal functioning of GABAARs.
- 40.Mizokami A, et al. Phospholipase C-related inactive protein is involved in trafficking of γ2 subunit-containing GABAA receptors to the cell surface. J Neurosci. 2007;27:1692–1701. doi: 10.1523/JNEUROSCI.3155-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13:341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 42.Terunuma M, et al. GABAA receptor phospho-dependent modulation is regulated by phospholipase C-related inactive protein type 1, a novel protein phosphatase 1 anchoring protein. J Neurosci. 2004;24:7074–7084. doi: 10.1523/JNEUROSCI.1323-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshimura K, et al. Interaction of p130 with, and consequent inhibition of, the catalytic subunit of protein phosphatase 1α. J Biol Chem. 2001;276:17908–17913. doi: 10.1074/jbc.M009677200. [DOI] [PubMed] [Google Scholar]
- 44.Kanematsu T, et al. Phospholipase C-related inactive protein is implicated in the constitutive internalization of GABAA receptors mediated by clathrin and AP2 adaptor complex. J Neurochem. 2007;101:898–905. doi: 10.1111/j.1471-4159.2006.04399.x. [DOI] [PubMed] [Google Scholar]
- 45.Huang K, El-Husseini A. Modulation of neuronal protein trafficking and function by palmitoylation. Curr Opin Neurobiol. 2005;15:527–535. doi: 10.1016/j.conb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Keller CA, et al. The γ2 subunit of GABAA receptors is a substrate for palmitoylation by GODZ. J Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004.First identification of GODZ as a palmitoyltransferase that interacts with and palmitoylates the γ2 subunit of GABAARs.
- 47.Rathenberg J, Kittler JT, Moss SJ. Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Mol Cell Neurosci. 2004;26:251–257. doi: 10.1016/j.mcn.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Fang C, et al. GODZ-mediated palmitoylation of GABAA receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci. 2006;26:12758–12768. doi: 10.1523/JNEUROSCI.4214-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charych EI, et al. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the β subunits of the GABAA receptors. J Neurochem. 2004;90:173–189. doi: 10.1111/j.1471-4159.2004.02481.x. [DOI] [PubMed] [Google Scholar]
- 50.Moss J, Vaughan M. Structure and function of ARF proteins: activators of cholera toxin and critical components of intracellular vesicular transport processes. J Biol Chem. 1995;270:12327–12330. doi: 10.1074/jbc.270.21.12327. [DOI] [PubMed] [Google Scholar]
- 51.Beck M, et al. Identification, molecular cloning, and characterization of a novel GABAA receptor-associated protein, GRIF-1. J Biol Chem. 2002;277:30079–30090. doi: 10.1074/jbc.M200438200. [DOI] [PubMed] [Google Scholar]
- 52.Brickley K, Smith MJ, Beck M, Stephenson FA. GRIF-1 and OIP106, members of a novel gene family of coiled-coil domain proteins: association in vivo and in vitro with kinesin. J Biol Chem. 2005;280:14723–14732. doi: 10.1074/jbc.M409095200. [DOI] [PubMed] [Google Scholar]
- 53.Smith MJ, Pozo K, Brickley K, Stephenson FA. Mapping the GRIF-1 binding domain of the kinesin, KIF5C, substantiates a role for GRIF-1 as an adaptor protein in the anterograde trafficking of cargoes. J Biol Chem. 2006;281:27216–27228. doi: 10.1074/jbc.M600522200. [DOI] [PubMed] [Google Scholar]
- 54.Gilbert SL, et al. Trak1 mutation disrupts GABAA receptor homeostasis in hypertonic mice. Nat Genet. 2006;38:245–250. doi: 10.1038/ng1715. [DOI] [PubMed] [Google Scholar]
- 55.Jacob TC, et al. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas P, Mortensen M, Hosie AM, Smart TG. Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat Neurosci. 2005 doi: 10.1038/nn1483.The authors of this paper developed a novel electrophysiological tracking method to show that GABAAR lateral diffusion in the plasma membrane, not receptor insertion, results in rapid recovery from selective inhibition.
- 57.Bogdanov Y, et al. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. Embo J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danglot L, Triller A, Bessis A. Association of gephyrin with synaptic and extrasynaptic GABAA receptors varies during development in cultured hippocampal neurons. Mol Cell Neurosci. 2003;23:264–278. doi: 10.1016/s1044-7431(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 59.Sun C, Sieghart W, Kapur J. Distribution of α1, α4, γ2, and δ subunits of GABAA receptors in hippocampal granule cells. Brain Research. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mangan PS, et al. Cultured hippocampal pyramidal neurons express two kinds of GABAA receptors. Mol Pharmacol. 2005;67:775–788. doi: 10.1124/mol.104.007385. [DOI] [PubMed] [Google Scholar]
- 61.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfeiffer F, Graham D, Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982;257:9389–9393. [PubMed] [Google Scholar]
- 63.Meyer G, Kirsch J, Betz H, Langosch D. Identification of a gephyrin binding motif on the glycine receptor β subunit. Neuron. 1995;15:563–572. doi: 10.1016/0896-6273(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 64.Kneussel M, Hermann A, Kirsch J, Betz H. Hydrophobic interactions mediate binding of the glycine receptor β subunit to gephyrin. J Neurochem. 1999;72:1323–1326. doi: 10.1046/j.1471-4159.1999.0721323.x. [DOI] [PubMed] [Google Scholar]
- 65.Feng G, et al. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282:1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- 66.Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kirsch J, Wolters I, Triller A, Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993;366:745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- 68.Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 69.Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798.Analysis of mice that lack GABAAR γ2 subunits showed significant reductions in synaptic GABAAR and gephyrin clusters, indicating a γ2-dependent mechanism is involved in the formation of inhibitory synapses, and more recently, was also demonstrated to be required for the maintenance of mature synapses75.
- 70.Kneussel M, et al. Gephyrin-independent clustering of postsynaptic GABAA receptor subtypes. Mol Cell Neurosci. 2001;17:973–982. doi: 10.1006/mcne.2001.0983. [DOI] [PubMed] [Google Scholar]
- 71.Kneussel M, et al. Loss of postsynaptic GABAA receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kins S, Betz H, Kirsch J. Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nat Neurosci. 2000;3:22–29. doi: 10.1038/71096. [DOI] [PubMed] [Google Scholar]
- 73.Harvey K, et al. The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering. J Neurosci. 2004;24:5816–5826. doi: 10.1523/JNEUROSCI.1184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papadopoulos T, et al. Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. Embo J. 2007;26:3888–3899. doi: 10.1038/sj.emboj.7601819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schweizer C, et al. The γ2 subunit of GABAA receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 76.Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B. Distinct γ2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Christie SB, Li RW, Miralles CP, Yang BY, De Blas AL. Clustered and non-clustered GABAA receptors in cultured hippocampal neurons. Molecular and Cellular Neuroscience. 2006;31:1–14. doi: 10.1016/j.mcn.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 78.Kirsch J, Kuhse J, Betz H. Targeting of glycine receptor subunits to gephyrin-rich domains in transfected human embryonic kidney cells. Mol Cell Neurosci. 1995;6:450–461. doi: 10.1006/mcne.1995.1033. [DOI] [PubMed] [Google Scholar]
- 79.Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABAA receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor α2 subunits to gephyrin. J Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008.Describes the first evidence for the direct binding of GABAARs to gephyrin and that disruption of this process alters the synaptic targeting of receptor subtypes containing α2 subunits.
- 80.Prior P, et al. Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron. 1992;8:1161–1170. doi: 10.1016/0896-6273(92)90136-2. [DOI] [PubMed] [Google Scholar]
- 81.Maas C, et al. Neuronal cotransport of glycine receptor and the scaffold protein gephyrin. J Cell Biol. 2006;172:441–451. doi: 10.1083/jcb.200506066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hanus C, Ehrensperger MV, Triller A. Activity-dependent movements of postsynaptic scaffolds at inhibitory synapses. J Neurosci. 2006;26:4586–4595. doi: 10.1523/JNEUROSCI.5123-05.2006.This paper, along with81, employed live imaging of fluorescently tagged gephyrin to reveal constant synaptic movements of gephyrin which could be controlled by activity, revealing gephyrin as a significant dynamic force at inhibitory synapses.
- 83.Zita MM, et al. Post-phosphorylation prolyl isomerisation of gephyrin represents a mechanism to modulate glycine receptors function. Embo J. 2007;26:1761–1771. doi: 10.1038/sj.emboj.7601625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Loebrich S, Bahring R, Katsuno T, Tsukita S, Kneussel M. Activated radixin is essential for GABAA receptor α5 subunit anchoring at the actin cytoskeleton. Embo J. 2006;25:987–999. doi: 10.1038/sj.emboj.7600995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 86.Cinar H, Barnes EM., Jr Clathrin-independent endocytosis of GABAA receptors in HEK 293 cells. Biochemistry. 2001;40:14030–14036. doi: 10.1021/bi011025t. [DOI] [PubMed] [Google Scholar]
- 87.Kittler JT, et al. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7677. doi: 10.1523/JNEUROSCI.20-21-07972.2000.Provides the first evidence that GABAARs undergo constitutive endocytosis and describes the role that this process plays in regulating the efficacy of synaptic inhibition.
- 88.Kittler JT, et al. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating γ-aminobutyric acidA receptor membrane trafficking. Proc Natl Acad Sci USA. 2004;101:12736–12741. doi: 10.1073/pnas.0401860101.Demonstrates that GABAARs are internalized and either rapidly recycled to the cell surface membrane, or targeted for lysosomal degradation. This paper also demonstrates that this sorting decision can be regulated by a direct interaction of GABAARs with HAP1.
- 89.Herring D, et al. Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the β2 subunit of the receptor. J Biol Chem. 2003;278:24046–24052. doi: 10.1074/jbc.M301420200. [DOI] [PubMed] [Google Scholar]
- 90.van Rijnsoever C, Sidler C, Fritschy JM. Internalized GABAA receptor subunits are transferred to an intracellular pool associated with the postsynaptic density. Eur J Neurosci. 2005;21:327–338. doi: 10.1111/j.1460-9568.2005.03884.x. [DOI] [PubMed] [Google Scholar]
- 91.Pearse BMF, Smith CJ, Owen DJ. Clathrin coat construction in endocytosis. Current Opinion in Structural Biology. 2000;10:220–228. doi: 10.1016/s0959-440x(00)00071-3. [DOI] [PubMed] [Google Scholar]
- 92.Kittler JT, et al. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci USA. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102.This paper identified a novel AP2 binding motif within β3 GABAAR subunits. Furthermore, phosphorylation of this motif was demonstrated to decrease AP2 binding, showing that phospho-dependent modulation of AP2 binding to GABAARs can regulate endocytosis and receptor cell surface number.
- 93.Kittler JT, et al. Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor γ2 subunit. Proc Natl Acad Sci USA. 2008;105:3616–3621. doi: 10.1073/pnas.0707920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moss SJ, Gorrie GH, Amato A, Smart TG. Modulation of GABAA receptors by tyrosine phosphorylation. Nature. 1995;377:344–348. doi: 10.1038/377344a0. [DOI] [PubMed] [Google Scholar]
- 95.Chen G, Kittler JT, Moss SJ, Yan Z. Dopamine D3 receptors regulate GABAA receptor function through a phospho-dependent endocytosis mechanism in nucleus accumbens. J Neurosci. 2006;26:2513–2521. doi: 10.1523/JNEUROSCI.4712-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feng J, Cai X, Zhao J, Yan Z. Serotonin receptors modulate GABAA receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci. 2001;21:6502–6511. doi: 10.1523/JNEUROSCI.21-17-06502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan Z, Surmeier DJ. D5 dopamine receptors enhance Zn2+sensitive GABAA currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron. 1997;19:1115–1126. doi: 10.1016/s0896-6273(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 98.Li X-J, et al. A huntingtin-associated protein enriched in brain with implications for pathology. Nature. 1995;378:398–402. doi: 10.1038/378398a0. [DOI] [PubMed] [Google Scholar]
- 99.Sheng G, et al. Hypothalamic huntingtin-associated protein 1 as a mediator of feeding behavior. Nat Med. 2006;12:526–533. doi: 10.1038/nm1382.Elegant study demonstrating that decreases in HAP1 affect the activity of GABAARs in the hypothalamus and result in a functional change in food intake and body weight in rodents.
- 100.Benarroch EE. GABAA receptor heterogeneity, function, and implications for epilepsy. Neurology. 2007;68:612–614. doi: 10.1212/01.wnl.0000255669.83468.dd. [DOI] [PubMed] [Google Scholar]
- 101.Thompson-Vest NM, Waldvogel HJ, Rees MI, Faull RL. GABAA receptor subunit and gephyrin protein changes differ in the globus pallidus in Huntington's diseased brain. Brain Res. 2003;994:265–270. doi: 10.1016/j.brainres.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 102.DeLorey TM, Olsen RW. GABA and epileptogenesis: comparing gabrb3 gene-deficient mice with Angelman syndrome in man. Epilepsy Res. 1999;36:123–132. doi: 10.1016/s0920-1211(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 103.D'Hulst C, Kooy RF. The GABAA receptor: a novel target for treatment of fragile X? Trends Neurosci. 2007;30:425–431. doi: 10.1016/j.tins.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 104.Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- 105.Krystal JH, et al. γ-aminobutyric acidA receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry. 2006;63:957–968. doi: 10.1001/archpsyc.63.9.957. [DOI] [PubMed] [Google Scholar]
- 106.Coulter DA. Epilepsy-associated plasticity in gamma-aminobutyric acid receptor expression, function, and inhibitory synaptic properties. Int Rev Neurobiol. 2001;45:237–252. doi: 10.1016/s0074-7742(01)45013-6. [DOI] [PubMed] [Google Scholar]
- 107.Chen JW, Naylor DE, Wasterlain CG. Advances in the pathophysiology of status epilepticus. Acta Neurol Scand Suppl. 2007;186:7–15. [PubMed] [Google Scholar]
- 108.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005;25:5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Terunuma M, et al. Deficits in phosphorylation of GABAA receptors by intimately associated protein kinase C activity underlies compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28:37–84. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bouilleret V, Loup F, Kiener T, Marescaux C, Fritschy JM. Early loss of interneurons and delayed subunit-specific changes in GABAA receptor expression in a mouse model of mesial temporal lobe epilepsy. Hippocampus. 2000;10:305–324. doi: 10.1002/1098-1063(2000)10:3<305::AID-HIPO11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 112.Knuesel I, Zuellig RA, Schaub MC, Fritschy JM. Alterations in dystrophin and utrophin expression parallel the reorganization of GABAergic synapses in a mouse model of temporal lobe epilepsy. Eur J Neurosci. 2001;13:1113–1124. doi: 10.1046/j.0953-816x.2001.01476.x. [DOI] [PubMed] [Google Scholar]
- 113.Fritschy JM, Kiener T, Bouilleret V, Loup F. GABAergic neurons and GABAA receptors in temporal lobe epilepsy. Neurochem Int. 1999;34:435–445. doi: 10.1016/s0197-0186(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 114.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 115.Schwarzer C, et al. GABA(A) receptor subunits in the rat hippocampus II: altered distribution in kainic acid-induced temporal lobe epilepsy. Neuroscience. 1997;80:1001–1017. doi: 10.1016/s0306-4522(97)00145-0. [DOI] [PubMed] [Google Scholar]
- 116.Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pirker S, et al. Increased expression of GABAA receptor β subunits in the hippocampus of patients with temporal lobe epilepsy. J Neuropathol Exp Neurol. 2003;62:820–834. doi: 10.1093/jnen/62.8.820. [DOI] [PubMed] [Google Scholar]
- 118.Loup F, Wieser HG, Yonekawa Y, Aguzzi A, Fritschy JM. Selective alterations in GABAA receptor subtypes in human temporal lobe epilepsy. J Neurosci. 2000;20:5401–5419. doi: 10.1523/JNEUROSCI.20-14-05401.2000.Reports that there are marked changes in the expression of major GABAAR subtypes in the hippocampus of temporal lobe epilepsy patients.
- 119.Wallace RH, et al. Mutant GABAA receptor γ2 subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 120.Baulac S, et al. First genetic evidence of GABAA receptor dysfunction in epilepsy: a mutation in the γ2 subunit gene. Nat Genet. 2001;28:46–48. doi: 10.1038/ng0501-46.This paper, together with119, provided the first reports of mutations in genes encoding GABAAR subunits being associated with human epilepsy.
- 121.Kananura C, et al. A splice-site mutation in GABRG2 associated with childhood absence epilepsy and febrile convulsions. Arch Neurol. 2002;59:1137–1141. doi: 10.1001/archneur.59.7.1137. [DOI] [PubMed] [Google Scholar]
- 122.Cossette P, et al. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat Genet. 2002;31:184–189. doi: 10.1038/ng885. [DOI] [PubMed] [Google Scholar]
- 123.Maljevic S, et al. A mutation in the GABAA receptor α1 subunit is associated with absence epilepsy. Ann Neurol. 2006;59:983–987. doi: 10.1002/ana.20874. [DOI] [PubMed] [Google Scholar]
- 124.Feng HJ, et al. Delta subunit susceptibility variants E177A and R220H associated with complex epilepsy alter channel gating and surface expression of α4β2δ GABAA receptors. J Neurosci. 2006;26:1499–1506. doi: 10.1523/JNEUROSCI.2913-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hirose S. A new paradigm of channelopathy in epilepsy syndromes: intracellular trafficking abnormality of channel molecules. Epilepsy Res. 2006;70(Suppl 1):S206–S217. doi: 10.1016/j.eplepsyres.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 126.Bianchi MT, Song L, Zhang H, Macdonald RL. Two different mechanisms of disinhibition produced by GABAA receptor mutations linked to epilepsy in humans. J Neurosci. 2002;22:5321–5327. doi: 10.1523/JNEUROSCI.22-13-05321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eugene E, et al. GABAA Receptor γ2 Subunit Mutations Linked to Human Epileptic Syndromes Differentially Affect Phasic and Tonic Inhibition. J Neurosci. 2007;27:14108–14116. doi: 10.1523/JNEUROSCI.2618-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gallagher MJ, Ding L, Maheshwari A, Macdonald RL. The GABAA receptor α1 subunit epilepsy mutation A322D inhibits transmembrane helix formation and causes proteasomal degradation. Proc Natl Acad Sci USA. 2007;104:12999–13004. doi: 10.1073/pnas.0700163104.Presents a biochemical mechanism for how a mutation in the GABAAR α1 subunit may result in a form of human epilepsy. The mutation was demonstrated to lead to subunit misfolding, followed by endoplasmic reticulum-associated degradation, which resulted in reduced GABAAR cell surface expression.
- 129.Kalivas PW. Neurobiology of cocaine addiction: implications for new pharmacotherapy. Am J Addict. 2007;16:71–78. doi: 10.1080/10550490601184142. [DOI] [PubMed] [Google Scholar]
- 130.Kumar S, Fleming RL, Morrow AL. Ethanol regulation of γ-aminobutyric acidA receptors: genomic and nongenomic mechanisms. Pharmacol Ther. 2004;101:211–226. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 131.Wafford KA. GABAA receptor subtypes: any clues to the mechanism of benzodiazepine dependence? Curr Opin Pharmacol. 2005;5:47–52. doi: 10.1016/j.coph.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 132.Liang J, et al. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liang J, et al. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kumar S, Sieghart W, Morrow AL. Association of protein kinase C with GABAA receptors containing α1 and α4 subunits in the cerebral cortex: selective effects of chronic ethanol consumption. J Neurochem. 2002;82:110–117. doi: 10.1046/j.1471-4159.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- 135.Kumar S, Kralic JE, O'Buckley TK, Grobin AC, Morrow AL. Chronic ethanol consumption enhances internalization of α1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem. 2003;86:700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- 136.Khanna JM, Kalant H, Chau A, Shah G. Rapid tolerance and crosstolerance to motor impairment effects of benzodiazepines, barbiturates, and ethanol. Pharmacol Biochem Behav. 1998;59:511–519. doi: 10.1016/s0091-3057(97)00477-2. [DOI] [PubMed] [Google Scholar]
- 137.Volk DW, et al. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- 138.Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol. 2003;23:601–640. doi: 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- 139.Yee BK, et al. A schizophrenia-related sensorimotor deficit links α3-containing GABAA receptors to a dopamine hyperfunction. Proc Natl Acad Sci USA. 2005;102:17154–17159. doi: 10.1073/pnas.0508752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 141.Studer R, et al. Alteration of GABAergic synapses and gephyrin clusters in the thalamic reticular nucleus of GABAA receptor α3 subunit-null mice. Eur J Neurosci. 2006;24:1307–1315. doi: 10.1111/j.1460-9568.2006.05006.x. [DOI] [PubMed] [Google Scholar]
- 142.Fritschy JM, Mohler H. GABAA receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 143.Hauser J, et al. Hippocampal α5 subunit-containing GABAA receptors modulate the expression of prepulse inhibition. Mol Psychiatry. 2005;10:201–207. doi: 10.1038/sj.mp.4001554. [DOI] [PubMed] [Google Scholar]
- 144.Giesemann T, et al. Complex formation between the postsynaptic scaffolding protein gephyrin, profilin, and Mena: a possible link to the microfilament system. J Neurosci. 2003;23:8330–8339. doi: 10.1523/JNEUROSCI.23-23-08330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mammoto A, et al. Interactions of drebrin and gephyrin with profilin. Biochem Biophys Res Commun. 1998;243:86–89. doi: 10.1006/bbrc.1997.8068. [DOI] [PubMed] [Google Scholar]