Summary
Adipocyte differentiation consists of a complex series of events in which scores of cellular and extracellular factors interact to transform a fibroblast-like preadipocyte into a mature, lipid-filled adipocyte. Many of the pathways influencing this process have been identified using well-characterized preadipocyte culture systems and have subsequently been confirmed in animal models. Research conducted over the last decade has established the Wnt/β-catenin signaling pathway as an important regulator of adipocyte differentiation. While initial reports implicated activators of Wnt/β-catenin signaling as potent inhibitors of adipogenesis, recent investigations of mesenchymal cell fate, obesity, and type 2 diabetes highlight significant additional roles for Wnt signaling in metabolism and adipocyte biology.
Introduction
Adipogenesis is the process by which mesenchymal precursor cells differentiate into adipocytes, which store lipid and serve as central regulators of metabolism [1–3]. Identifying key factors that control adipocyte differentiation and metabolism is vital to understanding adipose tissue biology and pathology. The transcriptional cascade controlling adipogenesis has been well characterized over the past two decades and mechanisms by which master adipocyte regulators act are now beginning to be fully elucidated. Peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein α (C/EBPα) are the chief regulators thought to coordinately direct the adipogenic program. PPARγ is both necessary and sufficient for preadipocyte differentiation [1], while C/EBPα appears to be important for the acquisition of insulin sensitivity in adipocytes [4]. The current state of research on these important transcriptional regulators has been recently reviewed elsewhere [2,3].
Transcription factors that control the cascade of events leading to a fully differentiated adipocyte act downstream of complex signaling pathways that integrate signals from the surrounding microenvironment. Over the past several years, the field of adipogenesis has seen an upsurge in the number of reports implicating locally secreted or circulating extracellular factors as regulators of preadipocyte differentiation [3]. One of the extracellular signaling pathways now known to affect adipogenesis is the Wnt pathway. Wnts are an evolutionarily conserved family of secreted lipidated glycoproteins with well-established roles in cellular proliferation, differentiation, and polarity during embryogenesis [5,6]. More recently, Wnt signaling has been shown to modulate additional developmental and physiological processes, including aspects of adipocyte biology [7–11]. In this review, we provide an overview of the research revealing a principal role for Wnt signaling in adipogenesis. We present a brief chronology of the studies demonstrating Wnt inhibition of adipocyte differentiation in vitro and in vivo, culminating with the recent linkages of Wnt pathway members to human diseases including obesity and type 2 diabetes. Finally, we examine the latest reports providing mechanistic insight into how Wnt signaling functions to block adipogenesis and regulate mesenchymal cell fate.
Wnt signaling regulates adipogenesis in vitro and in vivo
Wnts are secreted proteins that act though autocrine and paracrine mechanisms to influence the development of many cell types [5,6]. Although Wnts can inhibit preadipocyte differentiation through both β-catenin-dependent and -independent mechanisms [12], current genetic evidence supports β-catenin as a particularly crucial regulator of adipogenesis [13]. In the canonical Wnt signaling pathway, β-catenin plays a central role as a transcriptional coactivator. Upon binding of Wnt ligands to frizzled receptors and low density lipoprotein receptor-related protein (LRP) coreceptors, cytoplasmic β-catenin is hypophosphorylated, stabilized, and translocated into the nucleus where it binds to and coactivates members of the T-cell factor/lymphoid-enhancing factor (TCF/LEF) family of transcription factors to direct target gene expression (Fig. 1).
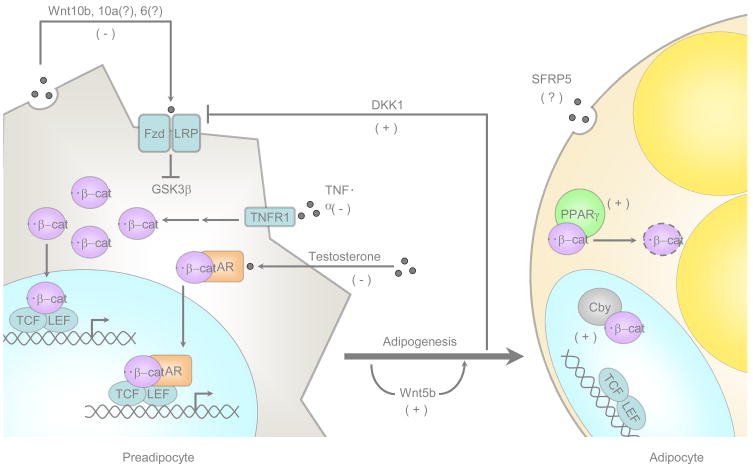
Figure 1. Wnt/β-catenin signaling is a central pathway regulating adipogenesis.
Wnt/β-catenin signaling in preadipocytes is initiated by expression of Wnt10b, Wnt10a, and Wnt6. Binding of these Wnts to transmembrane frizzled receptors and LRP coreceptors inhibits GSK3β leading to hypophosphorylation and stabilization of β-catenin in the cytoplasm. β-catenin is then translocated to the nucleus where it binds TCF/LEF transcription factors and activates downstream targets to inhibit preadipocyte differentiation. Factors from other inhibitory pathways converge on the Wnt/β-catenin pathway to block adipocyte conversion. TNFα signals through its receptor, TNFα receptor-1, to stabilize β-catenin and activate downstream pathways. Testosterone inhibits adipogenesis in part by stimulating interactions between androgen receptor and β-catenin. This complex travels to the nucleus to promote β-catenin-mediated gene transcription. DKK1 and Wnt5b are transiently induced during adipogenesis and stimulate preadipocyte differentiation. DKK1 prevents Wnt signaling by inhibiting LRP coreceptors, while Wnt5b promotes differentiation through an unknown mechanism. As adipogenesis proceeds, the expression of another Wnt inhibitor, Cby, is induced. Cby binds β-catenin in the nucleus and prevents coactivation of TCF/LEF transcription factors. Finally, binding of β-catenin to PPARγ leads to rapid degradation of β-catenin through a mechanism that involves the proteosome. (−) indicates factors that inhibit preadipocyte differentiation and (+) denotes factors that stimulate the process.
In vitro
Studies utilizing preadipocyte lines originally demonstrated that ectopic expression of Wnt1, an activator of Wnt/β-catenin signaling, potently represses adipogenesis (Fig. 1) [7,11]. Similarly, pharmacological agents that activate canonical Wnt signaling and stabilize free cytosolic β-catenin also block preadipocyte differentiation [7,8]. Conversely, inhibition of Wnt signaling in preadipocytes stimulates differentiation [7,8,14–16], suggesting that preadipocytes produce endogenous Wnts that strongly repress adipogenesis. One endogenous factor is Wnt10b, expression of which is high in dividing and confluent preadipocytes and is rapidly downregulated in response to elevated cAMP during induction of differentiation [7,8]. Furthermore, constitutive expression of Wnt10b stabilizes free cytosolic β-catenin and inhibits adipogenesis (Fig. 1) [7]. While considerable evidence suggests that Wnt10b is a prominent extracellular regulator of adipogenesis, other Wnt ligands are also expressed and likely contribute to the process. For example, Wnt6 and Wnt10a have been identified as endogenous regulators of brown adipocyte development [17,18]. Additionally, Wnt5b is transiently induced during adipogenesis and acts through an unknown mechanism to destabilize β-catenin and promote differentiation [19,20], indicating that preadipocytes integrate inputs from a variety of competing Wnt signals (Fig. 1). One of the mechanisms by which Wnt/β-catenin signaling inhibits adipogenesis is thought to involve dysregulated expression of cyclin dependent kinase inhibitors, p21 and p27 [21].
Adipogenesis is regulated not only by expression of specific Wnt ligands, but also by expression of factors that inhibit the Wnt/β-catenin pathway. For example, Li et al. recently reported that a nuclear β-catenin antagonist, chibby (Cby), is expressed in adipose tissue and is induced during differentiation of 3T3-L1 preadipocytes (Fig. 1) [14]. Cby binds the C-terminal portion of β-catenin and blocks interaction with TCF/LEF transcription factors, thus repressing β-catenin-mediated transcriptional activation [22]. Ectopic expression of Cby in 3T3-L1 cells induces spontaneous differentiation into mature adipocytes, while depletion of Cby stimulates β-catenin activity and blocks differentiation of both 3T3-L1 preadipocytes and mouse embryonic stem cells [14]. In harmony with these findings, another inhibitor of Wnt/β-catenin signaling, Dickkopf-1, is transiently expressed during human adipogenesis, and promotes differentiation of 3T3-L1 cells (Fig. 1) [16].
In vivo
In accordance with its expression during adipogenesis in vitro, Wnt10b is highly expressed in stromal vascular cells, which are enriched for preadipocytes, but not in mature adipocytes. While there is no evidence that a deficiency of Wnt10b in mice alters adipose tissue development, overexpression of Wnt10b in adipose tissues causes a 50% reduction in adiposity under standard laboratory conditions [9], and these mice resist expansion of adipose tissue under conditions of diet-induced and genetic obesity [9,10]. Mice expressing the Wnt10b transgene also show improved glucose homeostasis and increased insulin sensitivity [9,10]. Interestingly, expression of Wnt10b either blocks brown adipose tissue development, or stimulates its conversion to white adipose tissue, depending upon promoter usage [9,23].
Regulated expression of endogenous inhibitors of Wnt signaling may also be important for modulating Wnt activity in vivo. For example, expression of secreted frizzled-related protein (SFRP) 2, a putative extracellular Wnt inhibitor, is elevated in visceral adipose tissue compared to subcutaneous depots [24]. Two additional inhibitors of the Wnt pathway, SFRP5 and naked1, were recently found to be expressed in mature adipocytes and are positively correlated with increasing adiposity (Fig. 1) [25]. Furthermore, male mice deficient in SFRP1 show a 22% decrease in percent body fat [26] while elevated levels of SFRP1 are observed in individuals that display enhanced orbital adipogenesis associated with Graves’ ophthalmopathy [27]. More research is needed to determine mechanisms by which SFRPs function in adipose tissue biology as recent studies suggest that these factors may also have functions independent of Wnt inhibition [28–30].
Wnt/β-catenin signaling and human metabolic disorders
The importance of Wnt signaling in human health is illustrated by recent genetic research implicating members of the Wnt/β-catenin pathway in metabolic diseases. For example, polymorphisms of the Wnt10b and LRP5 genes may be associated with obesity in populations of European origin [31,32] while a mutation in LRP6 has been correlated with early coronary disease and multiple metabolic risk factors, including hyperlipidemia [33]. Furthermore, Wnt5b and transcription factor 7-like 2 (TCF7L2; formerly TCF4) variants have been linked to type 2 diabetes in ethnically diverse populations [19,34]. Following the initial report in which Grant et al. identified a link between TCF7L2 polymorphisms and susceptibility to type 2 diabetes in Icelandic, Danish, and U.S. cohorts [34], a number of studies were subsequently conducted that confirmed and extended this finding. Cohorts analyzed in the U.K., Finland, France, and Sweden demonstrated that variation in the TCF7L2 genomic region does indeed affect the risk for developing type 2 diabetes in these populations [35–38]. Within the U.S., polymorphisms in TCF7L2 were found to be associated with type 2 diabetes in large cohorts of both men and women across different ethnic backgrounds [39–41]. The mechanism by which the TCF7L2 gene is related to risk of type 2 diabetes remains unknown. However, because Wnt signals through TCF7L2 to activate glucagon-like peptide 1 [42], a putative mechanism in which altered levels of glucagon-like peptide 1 influence insulin secretion from pancreatic β-cells has been proposed [34]. Indeed, the reduction of insulin secretion observed in individuals harboring these polymorphisms is consistent with this hypothesis [43]. Additional studies will be required to determine the precise role of altered Wnt signaling in the pathogenesis of type 2 diabetes and other metabolic disorders, and to establish whether primary defects in adipose tissue are involved.
Recent advances in Wnt/β-catenin-mediated effects on adipogenesis
Regulation of mesenchymal cell fate
Current research is focused on delineating mechanisms by which Wnt signaling influences developmental and physiological processes, with considerable progress made on the role of Wnt signaling in mesenchymal stem cells. Multipotent precursors of the mesenchymal lineage possess the ability to differentiate into various cell types including osteoblasts and adipocytes [44]. In these cells, activation of Wnt/β-catenin signaling stimulates osteoblastogenesis and inhibits adipogenesis by modulating the relative levels of cell type specific transcription factors [45]. While early reports indicated that Wnt signaling prevents induction of the master adipogenic regulators C/EBPα and PPARγ during preadipocyte differentiation, recent research demonstrates that transient activation of Wnt/β-catenin signaling rapidly suppresses these factors in bipotential ST2 cells, and that this suppression precedes the Wnt-induced increase in osteoblastogenic transcription factors [46]. Thus, while expression of inhibitory Wnts does not influence induction of the early adipogenic factors C/EBPβ or C/EBPδ [7,45,46], repression of C/EBPα and PPARγ appears to be a primary mechanism by which Wnt signaling controls mesenchymal cell fate. Farmer and colleagues have observed an additional relationship between β-catenin and PPARγ in which these two factors functionally interact to negatively regulate each other’s activity (Fig. 1) [47,48].
Wnt/β-catenin signaling has also been implicated in the balance between adipogenesis and myogenesis. Specifically, loss of Wnt10b in vivo owing to aging or targeted deletion leads to increased adipogenic potential of myoblasts and the acquisition of adipocyte characteristics during muscle regeneration [49,50]. Furthermore, conditional deletion of β-catenin in the developing mouse myometrium results in its conversion to adipose tissue [13], providing compelling evidence that the Wnt/β-catenin pathway is an important regulator of adipogenesis and mesenchymal cell fate in vivo.
β-catenin as a central mechanism for inhibiting adipogenesis
Although β-catenin was initially thought to function exclusively as a Wnt effector, it is now clear that binding occurs between β-catenin and signaling factors in other pathways, and that these interactions are important for cellular processes including adipogenesis. For example, Cawthorn et al. demonstrated that suppression of C/EBPα and PPARγ by TNFα coincides with enhanced expression of several downstream targets of Wnt/β-catenin signaling (Fig. 1) [51]. Indeed, the authors reported that TCF7L2-dependent transcriptional activity is enhanced and β-catenin is stabilized during inhibition of adipogenesis by TNFα. Although stabilization of β-catenin still occurred, expression of dominant-negative TCF7L2 completely blocked the inhibition of adipogenesis by TNFα, providing further support for downstream effectors of the Wnt pathway mediating cytokine-induced inhibition of differentiation [51,52].
Additional evidence of a central role for the Wnt/β-catenin pathway in adipogenesis comes from studies on liganded nuclear receptors. In response to testosterone, androgen receptor binds β-catenin and shuttles it into the nucleus where it interacts with TCF/LEF transcription factors to inhibit adipogenesis (Fig. 1) [53]. A distinct mechanism exists for the vitamin D receptor, which inhibits adipogenesis in bone marrow stromal cells at least in part by suppressing the expression of dickkopf-1 and SFRP2, secreted inhibitors of Wnt/β-catenin signaling [54]. Thus diverse signaling mechanisms converge on the Wnt/β-catenin pathway directly through interaction with β-catenin, or indirectly through regulated expression of factors that modulate Wnt/β-catenin signaling. These data suggest that the Wnt/β-catenin pathway represents a major axis upon which various signals converge to influence preadipocyte differentiation.
Conclusions
Since the initial report that Wnt/β-catenin signaling potently inhibits adipogenesis in cell culture models, there have been numerous advances illustrating the importance of this regulatory pathway in directing the fate of mesenchymal precursors. While progress has been made with regard to the mechanisms underlying Wnt’s inhibition of adipogenesis and stimulation of osteoblastogenesis, questions remain that must be addressed to fully understand the roles of Wnt signaling in adipose tissue and bone biology. Of particular importance will be studies aimed at understanding the cascade of events that occurs following activation of TCF/LEF by β-catenin. Future experiments will provide insight into whether the Wnt/β-catenin pathway is a viable target to improve human health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 2.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 4.Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3:151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- 5.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 6.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 8.Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 9.Longo KA, Wright WS, Kang S, Gerin I, Chiang SH, Lucas PC, Opp MR, MacDougald OA. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279:35503–35509. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- 10.Wright WS, Longo KA, Dolinsky VW, Gerin I, Kang S, Bennett CN, Chiang SH, Prestwich TC, Gress C, Burant CF, et al. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes. 2007;56:295–303. doi: 10.2337/db06-1339. [DOI] [PubMed] [Google Scholar]
- 11.Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem J. 2003;376:607–613. doi: 10.1042/BJ20030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennell JA, MacDougald OA. Wnt signaling inhibits adipogenesis through beta-catenin-dependent and -independent mechanisms. J Biol Chem. 2005;280:24004–24010. doi: 10.1074/jbc.M501080200. [DOI] [PubMed] [Google Scholar]
- 13.Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol. 2005;288:276–283. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 14.Li FQ, Singh AM, Mofunanya A, Love D, Terada N, Moon RT, Takemaru K. Chibby promotes adipocyte differentiation through inhibition of beta-catenin signaling. Mol Cell Biol. 2007;27:4347–4354. doi: 10.1128/MCB.01640-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waki H, Park KW, Mitro N, Pei L, Damoiseaux R, Wilpitz DC, Reue K, Saez E, Tontonoz P. The small molecule harmine is an antidiabetic cell-type-specific regulator of PPARgamma expression. Cell Metab. 2007;5:357–370. doi: 10.1016/j.cmet.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Christodoulides C, Laudes M, Cawthorn WP, Schinner S, Soos M, O’Rahilly S, Sethi JK, Vidal-Puig A. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J Cell Sci. 2006;119:2613–2620. doi: 10.1242/jcs.02975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng YH, Kriauciunas KM, Kokkotou E, Kahn CR. Differential roles of insulin receptor substrates in brown adipocyte differentiation. Mol Cell Biol. 2004;24:1918–1929. doi: 10.1128/MCB.24.5.1918-1929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng YH, Butte AJ, Kokkotou E, Yechoor VK, Taniguchi CM, Kriauciunas KM, Cypess AM, Niinobe M, Yoshikawa K, Patti ME, et al. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nat Cell Biol. 2005;7:601–611. doi: 10.1038/ncb1259. [DOI] [PubMed] [Google Scholar]
- 19.Kanazawa A, Tsukada S, Sekine A, Tsunoda T, Takahashi A, Kashiwagi A, Tanaka Y, Babazono T, Matsuda M, Kaku K, et al. Association of the gene encoding wingless-type mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. Am J Hum Genet. 2004;75:832–843. doi: 10.1086/425340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanazawa A, Tsukada S, Kamiyama M, Yanagimoto T, Nakajima M, Maeda S. Wnt5b partially inhibits canonical Wnt/beta-catenin signaling pathway and promotes adipogenesis in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 2005;330:505–510. doi: 10.1016/j.bbrc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Ross SE, Erickson RL, Gerin I, DeRose PM, Bajnok L, Longo KA, Misek DE, Kuick R, Hanash SM, Atkins KB, et al. Microarray analyses during adipogenesis: understanding the effects of Wnt signaling on adipogenesis and the roles of liver X receptor alpha in adipocyte metabolism. Mol Cell Biol. 2002;22:5989–5999. doi: 10.1128/MCB.22.16.5989-5999.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takemaru K, Yamaguchi S, Lee YS, Zhang Y, Carthew RW, Moon RT. Chibby, a nuclear beta-catenin-associated antagonist of the Wnt/Wingless pathway. Nature. 2003;422:905–909. doi: 10.1038/nature01570. [DOI] [PubMed] [Google Scholar]
- 23.Kang S, Bajnok L, Longo KA, Petersen RK, Hansen JB, Kristiansen K, MacDougald OA. Effects of Wnt signaling on brown adipocyte differentiation and metabolism mediated by PGC-1alpha. Mol Cell Biol. 2005;25:1272–1282. doi: 10.1128/MCB.25.4.1272-1282.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koza RA, Nikonova L, Hogan J, Rim JS, Mendoza T, Faulk C, Skaf J, Kozak LP. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006;2:e81. doi: 10.1371/journal.pgen.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, Gaur T, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Leontovich A, Coenen MJ, Bahn RS. Gene expression profiling of orbital adipose tissue from patients with Graves’ ophthalmopathy: a potential role for secreted frizzled-related protein-1 in orbital adipogenesis. J Clin Endocrinol Metab. 2005;90:4730–4735. doi: 10.1210/jc.2004-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JL, Lin CT, Chueh LL, Chang CJ. Autocrine/paracrine secreted Frizzled-related protein 2 induces cellular resistance to apoptosis: a possible mechanism of mammary tumorigenesis. J Biol Chem. 2004;279:14602–14609. doi: 10.1074/jbc.M309008200. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez J, Esteve P, Weinl C, Ruiz JM, Fermin Y, Trousse F, Dwivedy A, Holt C, Bovolenta P. SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat Neurosci. 2005;8:1301–1309. doi: 10.1038/nn1547. [DOI] [PubMed] [Google Scholar]
- 30.Lee HX, Ambrosio AL, Reversade B, De Robertis EM. Embryonic dorsal-ventral signaling: secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell. 2006;124:147–159. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christodoulides C, Scarda A, Granzotto M, Milan G, Dalla Nora E, Keogh J, De Pergola G, Stirling H, Pannacciulli N, Sethi JK, et al. WNT10B mutations in human obesity. Diabetologia. 2006;49:678–684. doi: 10.1007/s00125-006-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo YF, Xiong DH, Shen H, Zhao LJ, Xiao P, Guo Y, Wang W, Yang TL, Recker RR, Deng HW. Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J Med Genet. 2006;43:798–803. doi: 10.1136/jmg.2006.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 35.Groves CJ, Zeggini E, Minton J, Frayling TM, Weedon MN, Rayner NW, Hitman GA, Walker M, Wiltshire S, Hattersley AT, et al. Association analysis of 6,736 U.K. subjects provides replication and confirms TCF7L2 as a type 2 diabetes susceptibility gene with a substantial effect on individual risk. Diabetes. 2006;55:2640–2644. doi: 10.2337/db06-0355. [DOI] [PubMed] [Google Scholar]
- 36.Scott LJ, Bonnycastle LL, Willer CJ, Sprau AG, Jackson AU, Narisu N, Duren WL, Chines PS, Stringham HM, Erdos MR, et al. Association of transcription factor 7-like 2 (TCF7L2) variants with type 2 diabetes in a Finnish sample. Diabetes. 2006;55:2649–2653. doi: 10.2337/db06-0341. [DOI] [PubMed] [Google Scholar]
- 37.Cauchi S, Meyre D, Dina C, Choquet H, Samson C, Gallina S, Balkau B, Charpentier G, Pattou F, Stetsyuk V, et al. Transcription factor TCF7L2 genetic study in the French population: expression in human beta-cells and adipose tissue and strong association with type 2 diabetes. Diabetes. 2006;55:2903–2908. doi: 10.2337/db06-0474. [DOI] [PubMed] [Google Scholar]
- 38.Mayans S, Lackovic K, Lindgren P, Ruikka K, Agren A, Eliasson M, Holmberg D. TCF7L2 polymorphisms are associated with type 2 diabetes in northern Sweden. Eur J Hum Genet. 2007;15:342–346. doi: 10.1038/sj.ejhg.5201773. [DOI] [PubMed] [Google Scholar]
- 39.Zhang C, Qi L, Hunter DJ, Meigs JB, Manson JE, van Dam RM, Hu FB. Variant of transcription factor 7-like 2 (TCF7L2) gene and the risk of type 2 diabetes in large cohorts of U.S. women and men. Diabetes. 2006;55:2645–2648. doi: 10.2337/db06-0643. [DOI] [PubMed] [Google Scholar]
- 40.Damcott CM, Pollin TI, Reinhart LJ, Ott SH, Shen H, Silver KD, Mitchell BD, Shuldiner AR. Polymorphisms in the transcription factor 7-like 2 (TCF7L2) gene are associated with type 2 diabetes in the Amish: replication and evidence for a role in both insulin secretion and insulin resistance. Diabetes. 2006;55:2654–2659. doi: 10.2337/db06-0338. [DOI] [PubMed] [Google Scholar]
- 41.Lehman DM, Hunt KJ, Leach RJ, Hamlington J, Arya R, Abboud HE, Duggirala R, Blangero J, Goring HH, Stern MP. Haplotypes of transcription factor 7-like 2 (TCF7L2) gene and its upstream region are associated with type 2 diabetes and age of onset in Mexican Americans. Diabetes. 2007;56:389–393. doi: 10.2337/db06-0860. [DOI] [PubMed] [Google Scholar]
- 42.Yi F, Brubaker PL, Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J Biol Chem. 2005;280:1457–1464. doi: 10.1074/jbc.M411487200. [DOI] [PubMed] [Google Scholar]
- 43.Saxena R, Gianniny L, Burtt NP, Lyssenko V, Giuducci C, Sjogren M, Florez JC, Almgren P, Isomaa B, Orho-Melander M, et al. Common single nucleotide polymorphisms in TCF7L2 are reproducibly associated with type 2 diabetes and reduce the insulin response to glucose in nondiabetic individuals. Diabetes. 2006;55:2890–2895. doi: 10.2337/db06-0381. [DOI] [PubMed] [Google Scholar]
- 44.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Farmer SR. Regulating the balance between peroxisome proliferator-activated receptor gamma and beta-catenin signaling during adipogenesis. A glycogen synthase kinase 3beta phosphorylation-defective mutant of beta-catenin inhibits expression of a subset of adipogenic genes. J Biol Chem. 2004;279:45020–45027. doi: 10.1074/jbc.M407050200. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol Cell Biol. 2006;26:5827–5837. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor-Jones JM, McGehee RE, Rando TA, Lecka-Czernik B, Lipschitz DA, Peterson CA. Activation of an adipogenic program in adult myoblasts with age. Mech Ageing Dev. 2002;123:649–661. doi: 10.1016/s0047-6374(01)00411-0. [DOI] [PubMed] [Google Scholar]
- 50.Vertino AM, Taylor-Jones JM, Longo KA, Bearden ED, Lane TF, McGehee RE, Jr, MacDougald OA, Peterson CA. Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Mol Biol Cell. 2005;16:2039–2048. doi: 10.1091/mbc.E04-08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cawthorn WP, Heyd F, Hegyi K, Sethi JK. Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ. 2007;14:1361–1373. doi: 10.1038/sj.cdd.4402127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gustafson B, Smith U. Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. J Biol Chem. 2006;281:9507–9516. doi: 10.1074/jbc.M512077200. [DOI] [PubMed] [Google Scholar]
- 53.Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid NF, Bhasin S. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147:141–154. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cianferotti L, Demay MB. VDR-mediated inhibition of DKK1 and SFRP2 suppresses adipogenic differentiation of murine bone marrow stromal cells. J Cell Biochem. 2007;101:80–88. doi: 10.1002/jcb.21151. [DOI] [PubMed] [Google Scholar]