Abstract
Lithium and valproic acid (VPA) are two primary drugs used to treat bipolar disorder, and have been shown to have neuroprotective properties in vivo and in vitro. A recent study demonstrated that combined treatment with lithium and VPA elicits synergistic neuroprotective effects against glutamate excitotoxicity in cultured brain neurons, and the synergy involves potentiated inhibition of glycogen synthase kinase-3 (GSK-3) activity through enhanced GSK-3 serine phosphorylation (Leng et al., J Neurosci 28: 2576–2588, 2008). We therefore investigated the effects of lithium and VPA cotreatment on the disease symptom onset, survival time and neurological deficits in cooper zinc superoxide dismutase (SOD-1) G93A mutant mice, a commonly used mouse model of amyotrophic lateral sclerosis (ALS). The G93A ALS mice received twice daily intraperitoneal injections with LiCl (60 mg/kg), VPA (300 mg/kg) or lithium plus VPA, starting from the 30th day after birth and continuing until death. We found that combined treatment with lithium and VPA produced a greater and more consistent effect in delaying the onset of disease symptoms, prolonging the life span and decreasing the neurological deficit scores, compared with the results of monotreatment with lithium or VPA. Moreover, lithium in conjunction with VPA was more effective than lithium or VPA alone in enhancing the immunostaining of phospho-GSK-3βSer9 in brain and lumbar spinal cord sections. To our knowledge, this is the first demonstration of enhanced neuroprotection by a combinatorial approach using mood stabilizers in a mouse ALS model. Our results suggest that clinical trials using lithium and VPA in combination for ALS patients are a rational strategy.
Keywords: lithium, valproic acid, GSK-3β, amyotrophic lateral sclerosis (ALS), G93A mice, behavioral deficits
Introduction
Mood stabilizing drugs, notably lithium and valproic acid (VPA), are used to treat bipolar mood disorder, a common, severe and chronic mental illness (Zarate et al., 2006). Both lithium and VPA have strong anti-manic effects, but are less efficacious against depressive episodes. Clinically, these agents are often co-administered when bipolar patients are resistant to monotherapy with either drug (Goodwin, 2003). Accumulating evidence supports the notion that lithium and VPA are neuroprotective against a variety of insults in both in vivo and in vitro experimental settings (Chuang, 2005). The neuroprotective mechanisms of mood stabilizers are complex, and likely involve activation of cell signaling pathways and changes in gene expression of proteins critically involved in neuronal survival. Lithium and VPA are known to be inhibitors of glycogen synthase kinase-3 (GSK-3) and histone deacetylases (HDACs), respectively (Göttlicher et al., 2001; Phiel et al., 2001). Inhibition of these enzymes likely causes a cascade of events, leading to neuroprotective and neurotrophic actions.
Treatment with lithium either in conjunction with an antioxidant or alone has been shown to improve motor function and slow the disease progress in the SOD-1 G93A amyotrophic lateral sclerosis (ALS) mouse model (Shin et al., 2007; Fornai et al., 2008). A clinical study showed that lithium and riluzole cotreatment markedly reduces the mortality of ALS patients during 15 months of follow-up, compared with matched control patients treated only with riluzole (Fornai et al., 2008). VPA treatment was also reported to exhibit variable effects on disease symptom onset and duration in G93A and G86R ALS mice (Sugai et al., 2004; Rouaux et al., 2007). It was recently reported that cotreatment with lithium and VPA elicits robust synergistic neuroprotective effects against glutamate-induced excitotoxicity in cultured rat brain neurons (Leng et al., 2008). The molecular target of the neuroprotective synergy likely involves a potentiation of the inhibition of GSK-3. The present study was undertaken to investigate whether lithium and VPA co-administration elicits greater effects in delaying the onset of motor dysfunction and disease progression, and prolonging the life span in G93A ALS mice.
EXPERIMENTAL PROCEDURES
Animals and drug treatments
Transgenic mice carrying high copy numbers of the transgene with the G93A human SOD1 mutation were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Male SOD1-G93A mice were crossed with B6SJLF1/J hybrid females as previously described (Gurney et al., 1994). All transgenic mice were genotyped by PCR amplification of DNA extracted from the tails to identify the SOD-1 mutation. Mice were randomly divided into vehicle, LiCl, VPA, LiCl plus VPA groups, and were matched for littermates. Drug treatments were initiated 30 days after birth and continued until the end stage with 6 animals (3 males + 3 females) in each group. LiCl (60 mg/kg), VPA (300 mg/kg) or LiCl (60 mg/kg) + VPA (300 mg/kg) were injected intraperitoneally (i.p.) twice daily with an interval of 10–12 hr. The vehicle control group received injections of the same volume of saline. Lithium chloride and VPA (sodium salt) were obtained from SIGMA (St. Louis, MO, USA). Mice were maintained on a 12-hr light/dark cycle and the behavior tests were performed during the light period. All experimental protocols were approved by the Animal Research Ethics Committee of Nanjing University Animal Model Research Center in China, and performed in accordance with international guidelines on the ethical use of animals.
Behavioral assessment and analysis
Starting from 12 weeks of age and until death, various behavioral tests were routinely performed to assess the effects of drugs on the onset and extent of neurological deficits. All tests were performed by an investigator who was blind to the experimental conditions of the animals.
(a) Rotarod performance test
After training sessions were conducted to acclimate the mice to the rotarod apparatus (Columbus Instruments, Columbus, OH, USA), motor coordination was assessed by measuring the length of time at which the mice remained on the rotating rod (16 rpm) as described (Azzouz et al., 2004). Three trials were given to each animal and the longest retention time was used as the measure of competence at this task. The evaluation scores were: grade 0, >180 sec; grade 1, 60–180 sec; grade 2, <60 sec; grade 3, falling off the rod before rotation.
(b) Postural reflex test
This was conducted essentially as described (Bederson et al., 1986) to examine the strength of the forelimbs. The deficits were defined and scored as follows: grade 0, no evidence of paralysis; grade 1, forelimb flexion upon tail suspension; grade 2, decreased resistance to lateral push (and forelimb flexion) without circling; grade 3, same as grade 2 but with circling; grade 4, unable to walk but maintaining upright body position; grade 5, complete paralysis.
(c) Balance beam test
This was performed essentially as described (Feeney et al., 1982) to measure body strength and equilibrium. The deficits were scored as follows: grade 0, able to lift itself onto the beam and walk without falling off; grade 1, same as grade 0 but with less than 50% chance of falling off; grade 2, same as grade 1 with more than 50% chance of falling off; grade 3, able to lift itself onto the beam with assistance but unable to move forward; grade 4, unable to lift onto the beam but able to stay in position; grade 5, falling off the beam instantaneously after placement.
(d) Screen test
This test serves as an indicator of general muscle strength (Combs and D’Alecy, 1987). The animal was placed on a horizontally positioned screen with grids. The screen was then rotated to the vertical position. The deficit scores are: grade 0, grasping the screen with forepaws for more than 5 sec; grade 1, temporarily holding the screen without falling off; grade 2, same as grade 1 but falling off within 5 sec; grade 3, falling off instantaneously.
(e) Tail suspension test
The mouse was suspended by its tail and extension of hindlimbs observed (Garbuzova-Davis et al., 2003). The deficits scores are: grade 0, normal; grade 1, partial hindlimb extension; grade 2, no hindlimb extension.
Data analysis
A total score of 18 from 5 different tests represents a complete loss of motor function, while a score of 0 means normal motor function. Results are quantified and expressed as mean ± SEM. SPSS13.0 software was used to determine statistical significance using Log-Rank test for the onset and survival time of the disease. A p<0.05 was considered statistically significance.
Immunohistochemistry
Mice were anesthetized and perfused via the ascending aorta with pre-cooled phosphate-buffered saline (PBS, pH 7.4) for 1 min and then with pre-cooled 0.1 M PBS (pH 7.4) containing 4% paraformaldehyde for 15 min. The brain and lumbar spinal cord were removed, and post-fixed in the same fixative for 6 hr and then transferred to a solution of 0.1 M PBS containing 20% sucrose for 24 hr. A series of coronal sections of the fixed tissues were prepared in paraffin-embebbed slides. The paraffin was removed from the tissue by xylene immersion. The endogenous peroxidase activity was blocked with 0.3% H2O2/methanol for 20 min. The sections were washed in 0.1 M PBS (pH 7.4), then boiled in 10 mM sodium citrate buffer (pH 6.0) for 20 min, followed by incubation overnight with primary antibody against phospho-GSK-3βSer9 (1:300, Abcam, Cambridge, MA, USA). Biotinylated secondary antibody (Vector, Burlingame, CA, USA) was then applied, and the immunohistochemical staining was revealed by the avidin-biotin complex using diaminobenzidine. The sections were finally dehydrated and covered.
Results
To assess whether combined treatment with lithium and VPA is superior to treatment with lithium or VPA alone, mutant SOD1-1 G93A ALS mice were twice daily i.p. injected with lithium (60 mg/kg or 1.41 mEq/kg), VPA (300 mg/kg or 1.80 mEq/kg) or lithium (60 mg/kg) plus VPA (300 mg/kg), starting from 30 days after birth and continuing till death. The onset of neurological symptoms was considered as the appearance of a subtle deficit as determined by any single behavioral test, namely, rotarod performance, postural reflex, balance beam performance, screen grasping or tail suspension behavior. Plots of cumulative probability of the symptom onset against the age of animals revealed a right shift of the curve by injections with lithium or VPA alone, and a further shift to the right by injections of lithium together with VPA (Fig. 1A). Quantified results showed that the onset times of motor deficits were 106 ± 3.0 days for the control group, 115.8 ± 1.5 days for the lithium group (p = 0.013), 114.5 ± 4.7 days for the VPA group (p = 0.067) and 121.0 ± 2.7 days for the lithium plus VPA group (p = 0.01), respectively (Fig. 1B).
Fig. 1.
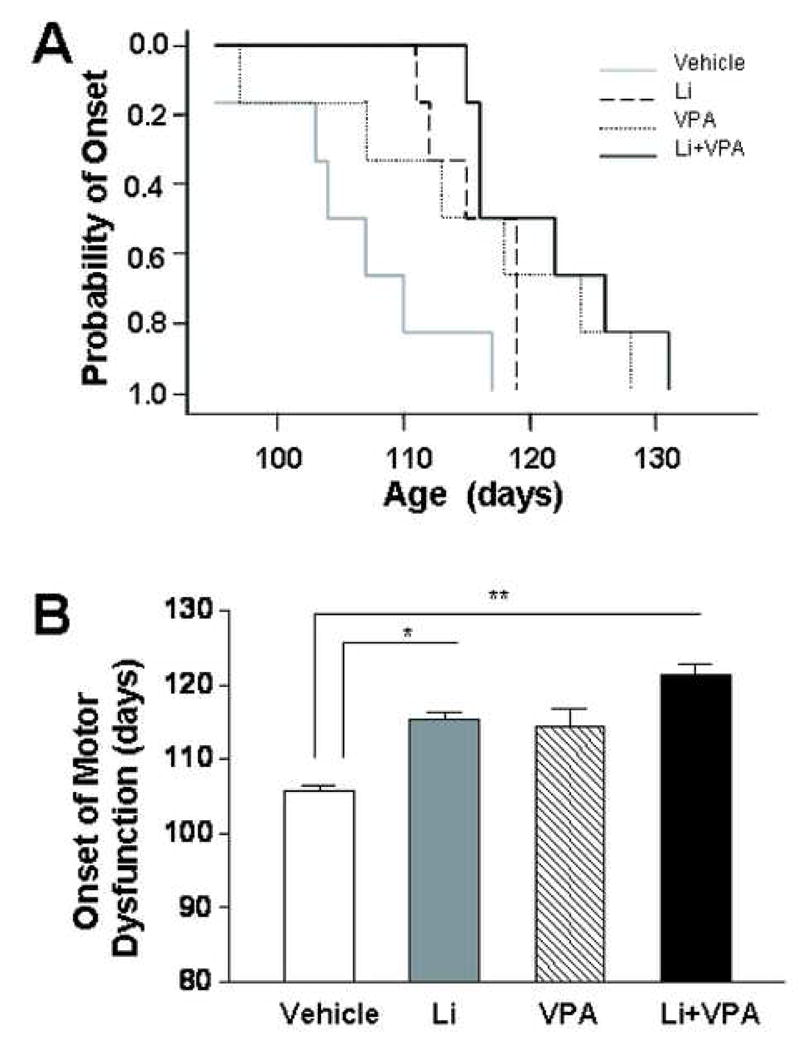
Effects of treatments with lithium and/or VPA on the onset of motor dysfunction in SOD-1 G93A ALS mice. Mutant G93A mice were twice daily i.p. injected with vehicle (normal saline), lithium (60 mg/kg), VPA (300 mg/kg), or lithium plus VPA, starting from 30 days after birth, as described in the Experimental Procedures. The onset of neurological symptoms was defined as the presence of a subtle deficit in any of the five behavioral tests employed. (A) Plots of cumulative probability of the symptom onset against the age of the mutant mice in four groups. (B) Quantified results of the onset times of motor deficits expressed as mean ± SEM (n = 6 in each group). *p<0.05, **p<0.01 between the indicated groups.
Next, we examined the effects of drug treatment on the survival time of the mutant G93A mice. We found that the curve of the survival time was similarly shifted to the right by lithium or VPA treatment, and further shifted by lithium plus VPA cotreatment (Fig 2A). The mean survival times were found to be 124.8 ± 3.2 days for the control group, 135.3 ± 2.1 days for the lithium group (p = 0.063), 137.7 ± 4.2 days for VPA group (p = 0.048) and 143.3 ± 4.1 days for the lithium plus VPA group (p = 0.005) (Fig 2B). There was also a statistical significance (p=0.045) between the groups of lithium plus VPA vs lithium alone.
Fig. 2.
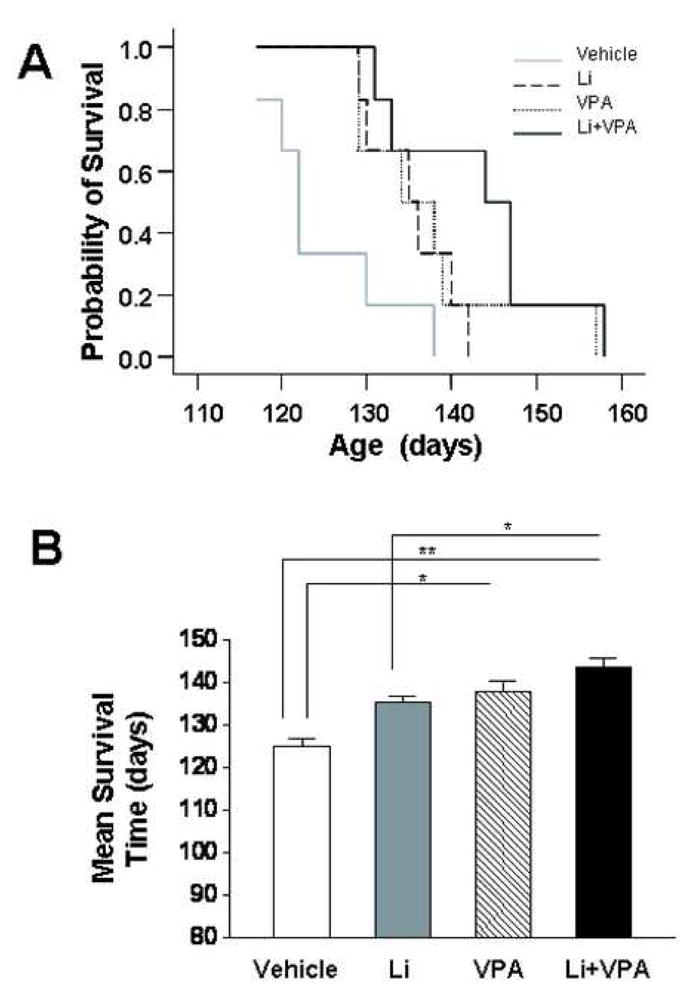
Effects of treatments with lithium and/or VPA on the survival time in SOD-1 G93A ALS mice. G93A ALS mice were treated with vehicle, lithium, VPA, or lithium plus VPA, as described in the legend to Fig. 1. (A) Plots of cumulative probability of death against the age of the mutant mice in the four groups. (B) Quantified results of the survival time expressed as mean ± SEM (n = 6 in each group). *p<0.05, **p<0.01 between the indicated groups.
The neurological deficits were then evaluated in the course of disease progression in G93A mice. Around 120 days of age, the vehicle-treated G93A mice showed severe body weakness and loss of hindlimb muscle mass, in comparison with the mice treated with lithium plus VPA (Fig. 3A). We also assessed the rotarod performance by measuring the retention time on the rotating rod in vehicle- and drug-treated animals. In the control group, the retention time decreased as age progressed in these G93A mutant mice with a near complete loss of competence around 125 days. The curve of the retention time was shifted to the right by monotreatment with lithium or VPA, and further shifted by cotreatment with lithium and VPA (Fig. 3B). Similar right shift phenomena by drug treatments were observed when the overall deficit scores of motor dysfunction were assessed by five independent behavioral tests and plotted against the age of the mutant mice (Fig. 3C).
Fig. 3.
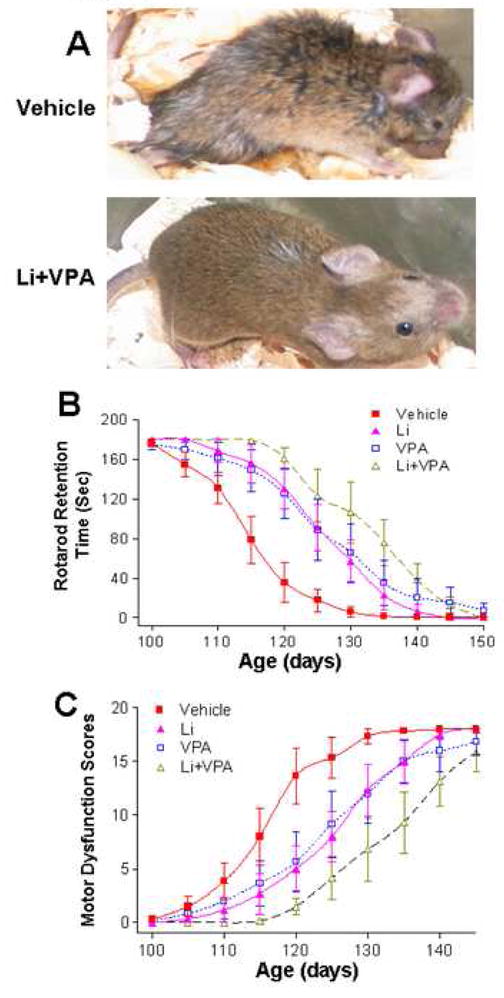
Effects of treatments with lithium and/or VPA on motor deficits in SOD-1 G93A ALS mice. G93A ALS mice were treated with vehicle, lithium, VPA, or lithium plus VPA, as described in the legend to Fig. 1. (A) Representative photographs of mice treated with vehicle, or lithium plus VPA around 120 days of age. Note the severe loss of hindlimb muscle mass in the vehicle-treated mouse, compared with the mouse treated with lithium plus VPA. (B) Rotarod performance was expressed as the retention time on the rotating rod vs the age of animals. Data are mean ± SEM (n = 6 in each of the groups of vehicle, lithium, VPA, and lithium plus VPA). (C) Overall neurological deficit scores vs the age of animals. The total neurological deficits were determined from five independent tests (rotarod performance, postural reflex, balance beam performance, screen grasping and tail suspension behavior). A total score of 18 represent a complete loss of motor function. Data are mean ± SEM (n = 6 in each of the four groups of mice).
Lithium and VPA cotreatment in cultured rat brain neurons and normal mice induces enhanced GSK-3 serine phosphorylation and hence inhibition of GSK-3 activity (Leng et al., 2008). Because the neuropathology of ALS affects the functions of not only spinal cord motor neurons, but also brain neurons (Phukan et al., 2007), we performed immunohistochemical staining of phospho-GSK-3βser9 using tissue sections derived from the brain and lumbar spinal cord of G93A mice. In both cases, the stainings were weak in the sections of the control mice, became detectable in the mice treated with lithium or VPA, and were markedly enhanced in the animals subjected to cotreatment with both drugs (Fig. 4A and B). Quantification of the number of phospho-GSK-3βSer9-positive cells revealed that lithium and VPA cotreatment induced a more than additive increase in the brain and spinal cord sections, compared with treatment with lithium or VPA alone (Fig. 4C and D).
Fig. 4.
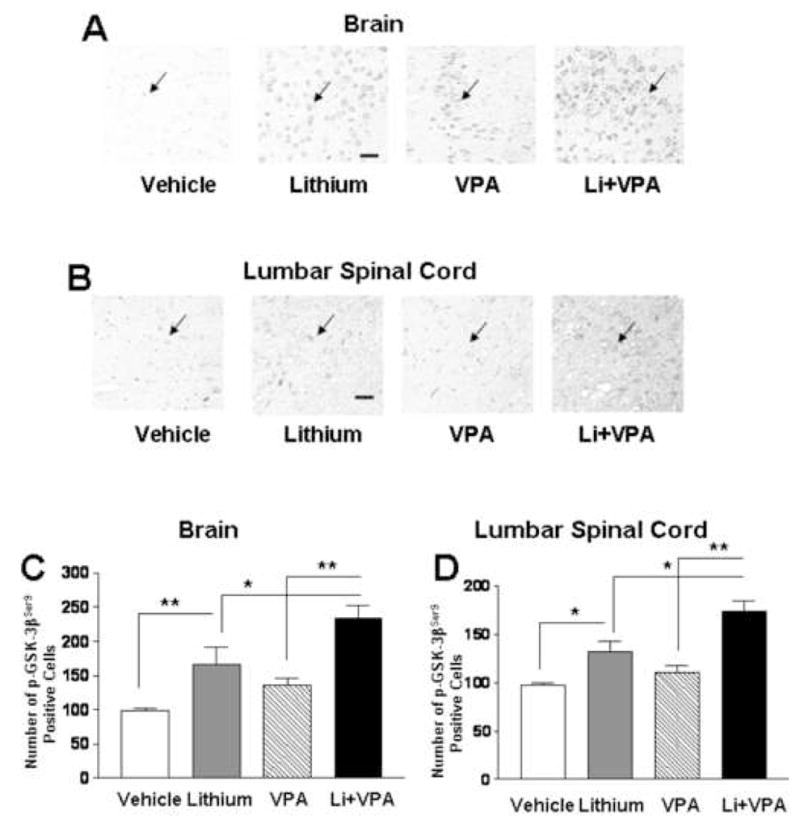
Immunohistochemical staining of phospho-GSK-3βSer9 in the frontal cortex and lumbar spinal cord sections of G93A ALS mice. ALS mice were treated with vehicle, lithium, VPA, or lithium plus VPA as described in the legend to Fig. 1. The animals were killed around 90 days of age and the brains and lumbar spinal cord were removed. Immunohistochemistry for phospho-GSK-3βSer9 was performed in sections from each group, as described in the methods. Shown are representative immunohistochemical stainings of phospho-GSK-3βSer9 in the corresponding areas in the frontal cortex (A) and lumbar spinal cord (B) of female mutant mice. Arrows, phospho-GSK-3βSer9 immunopositive cells. Bar, 30 μm. Quantifications of the number of phospho-GSK-3βSer9-positive cells were performed by counting the total cell number in 3 random fields of a given section. Data are mean ± SEM from sections of the brain (C) and lumbar spinal cord (D) derived from 6 G93A mutant mice in each group. *p<0.05, **p<0.01 between the groups, using one-way ANOVA and the Bonferroni’s post test.
Discussion
The present study compared the effects of combined treatment with two mood stabilizers, lithium and VPA, with those of monotreatment with either drug in the SOD1 G93A mouse model of ALS. We showed that combination of lithium and VPA was more consistent than lithium or VPA alone in delaying the onset of motor dysfunction, prolonging survival-time and reducing neurological deficits. Although lithium and VPA have been tested previously in SOD-1 transgenic ALS mice, this is the first combinatorial approach using mood stabilizers to study the beneficial effects in ALS mice.
Our results showed only marginal effects of lithium or VPA alone in the onset of disease symptoms, life span and degree of motor deficits. We also found that higher doses of LiCl (e.g., 120 mg/kg, i.p.) was toxic to the mutant mice, while lower doses of VPA (e.g., 200 or 100 mg/kg, i.p.) were less effective than the current dose (300 mg/kg, i.p.) used. Previous studies reported inconsistent effects of mood stabilizers on these parameters. For example, lithium was shown to have highly significant benefits on motor deficits and life span in one study (Fornai et al., 2008), but displayed little beneficial effects in another study (Shin et al., 2007). On the other hand, pre or post-symptomatic VPA treatment was reported to prolong disease duration (i.e. life span), but had no effect on the onset of motor symptoms in G93A mice containing low copies of the mutant gene (Sugai et al., 2004). A more recent study using SOD-1 G86R ALS mice reported that VPA treatment robustly restores the loss of levels of acetylated histone-H3 and cyclic AMP response element binding protein (CREB) binding protein (CBP), and rescues motor neurons from death (Rouaux et al., 2007). However, the mean survival time of the treated animal is not significantly prolonged, and the onset of motor decline is only slightly improved. The inconsistency among these independent studies likely arises from variations in the drug dosing, treatment time and methods of administrations (e.g. injection, needle feeding, dietary treatment, or drinking water delivery). The number of copies of mutant SOD-1 gene as well as the strain of the transgenic ALS mouse used may also contribute to the discrepancies in observations. Based on our data, we suggest that combined treatment with lithium and VPA is a rational strategy to obtain more consistent and robust beneficial effects in behavioral improvement and life span prolongation. Thus, clinical trials using lithium and VPA in combination seem to be warranted.
The potentiated beneficial effects of lithium and VPA cotreatment were accompanied by enhanced immunostaining of phospho-GSK-3βSer9 in brain and lumbar spinal sections. In this context, increasing evidence supports that non-motor systems are also affected in ALS, as characterized by cognitive impairment and neural frontotemporal dysfunction (Phukan et al., 2007). It has been shown that lithium and VPA through initial actions on GSK-3 and HDACs, respectively, potentiate GSK-3 serine phosphorylation, resulting in synergistic neuroprotective effects (Leng et al., 2008). GSK-3 inhibition, elicited by enhanced GSK-3 serine phosphorylation or other mechanisms, is expected to activate or inhibit an array of transcription factors, causing upregulation of neuroprotective and/or neurotrophic proteins but downregulation of pro-apoptotic proteins (Rowe et al., 2007). Among the critical proteins induced through GSK-3 inhibition are heat shock protein 70 (Ren et al., 2003), Bcl-2 (Liang and Chuang, 2006) and brain-derived neurotrophic factor (Yasuda et al., 2007). VPA and another HDAC inhibitor, phenylbutyrate, have also been shown to upregulate Bcl-2 in G86R and G93A ALS mice (Ryu et al., 2005; Rouaux et al., 2007). Recent microarray analysis revealed that the course of disease progression in G93A mice is associated with marked transcriptional repression of genes involved in transcriptional and metabolic functions, and increased expression of cyclins functioning in cell cycle regulation (Ferraiuolo et al., 2007). Clearly, further studies are necessary to identify the set of genes critically involved in the beneficial actions of combined treatment with lithium and VPA.
Acknowledgments
This research was supported by the grant from Natural Science Foundation of China (No. 30470584). The authors wish to thank Nanjing University Animal Model Research Center in China for assistance in the genotyping and breeding of the ALS transgenic mice. We also thank Peter Leeds of the Molecular Neurobiology Section, NIMH, NIH, USA for editorial work.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- Bcl-2
beta cell lymphoma-2
- CBP
CREB binding protein
- CREB
cyclic AMP response element binding protein
- GSK-3
glycogen synthase kinase-3
- HDAC
histone deacetylase
- i.p
intraperitoneally
- PBS
phosphate-buffered saline
- SOD1
superoxide dismutase-1
- VPA
valproic acid
References
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Chuang DM. The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann N Y Acad Sci. 2005;1053:195–204. doi: 10.1196/annals.1344.018. [DOI] [PubMed] [Google Scholar]
- Combs DJ, D’Alecy LG. Motor performance in rats exposed to severe forebrain ischemia: effect of fasting and 1,3-butanediol. Stroke. 1987;18:503–511. doi: 10.1161/01.str.18.2.503. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- Ferraiuolo L, Heath PR, Holden H, Kasher P, Kirby J, Shaw PJ. Microarray analysis of the cellular pathways involved in the adaptation to and progression of motor neuron injury in the SOD1 G93A mouse model of familial ALS. J Neurosci. 2007;27:9201–9219. doi: 10.1523/JNEUROSCI.1470-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornai F, Longone P, Cafaro L, Kastsiuchenka O, Ferrucci M, Manca ML, Lazzeri G, Spalloni A, Bellio N, Lenzi P, Modugno N, Siciliano G, Isidoro C, Murri L, Ruggieri S, Paparelli A. Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2008;105:2052–2057. doi: 10.1073/pnas.0708022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Willing AE, Zigova T, Saporta S, Justen EB, Lane JC, Hudson JE, Chen N, Davis CD, Sanberg PR. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: distribution, migration, and differentiation. J Hematother Stem Cell Res. 2003;12:255–270. doi: 10.1089/152581603322022990. [DOI] [PubMed] [Google Scholar]
- Goodwin FK. Rationale for using lithium in combination with other mood stabilizers in the management of bipolar disorder. J Clin Psychiatry. 2003;64(Suppl 5):18–24. [PubMed] [Google Scholar]
- Göttlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM. Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J Neurosci. 2008;28:2576–2588. doi: 10.1523/JNEUROSCI.5467-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang MH, Chuang DM. Differential roles of glycogen synthase kinase-3 isoforms in the regulation of transcriptional activation. J Biol Chem. 2006;281:30479–30484. doi: 10.1074/jbc.M607468200. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007;6:994–1003. doi: 10.1016/S1474-4422(07)70265-X. [DOI] [PubMed] [Google Scholar]
- Ren M, Senatorov VV, Chen RW, Chuang DM. Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. Proc Natl Acad Sci USA. 2003;100:6210–6215. doi: 10.1073/pnas.0937423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaux C, Panteleeva I, Rene F, Gonzalez de Aguilar JL, Echaniz-Laguna A, Dupuis L, Menger Y, Boutillier AL, Loeffler JP. Sodium valproate exerts neuroprotective effects in vivo through CREB-binding protein-dependent mechanisms but does not improve survival in an amyotrophic lateral sclerosis mouse model. J Neurosci. 2007;27:5535–5545. doi: 10.1523/JNEUROSCI.1139-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MK, Wiest C, Chuang DM. GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci Biobehav Rev. 2007;31:920–931. doi: 10.1016/j.neubiorev.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Smith K, Camelo SI, Carreras I, Lee J, Iglesias AH, Dangond F, Cormier KA, Cudkowicz ME, Brown RH, Jr, Ferrante RJ. Sodium phenylbutyrate prolongs survival and regulates expression of anti-apoptotic genes in transgenic amyotrophic lateral sclerosis mice. J Neurochem. 2005;93:1087–1098. doi: 10.1111/j.1471-4159.2005.03077.x. [DOI] [PubMed] [Google Scholar]
- Shin JH, Cho SI, Lim HR, Lee JK, Lee YA, Noh JS, Joo IS, Kim KW, Gwag BJ. Concurrent administration of Neu2000 and lithium produces marked improvement of motor neuron survival, motor function, and mortality in a mouse model of amyotrophic lateral sclerosis. Mol Pharmacol. 2007;71:965–975. doi: 10.1124/mol.106.030676. [DOI] [PubMed] [Google Scholar]
- Sugai F, Yamamoto Y, Miyaguchi K, Zhou Z, Sumi H, Hamasaki T, Goto M, Sakoda S. Benefit of valproic acid in suppressing disease progression of ALS model mice. Eur J Neurosci. 2004;20:3179–3183. doi: 10.1111/j.1460-9568.2004.03765.x. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002099. advance online, October 9. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh J, Manji HK. Cellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorder. Biol Psychiatry. 2006;59:1006–1020. doi: 10.1016/j.biopsych.2005.10.021. [DOI] [PubMed] [Google Scholar]


