Abstract
Intestinal electrical stimulation (IES) has been shown to produce inhibitory effects on gastric contractions, gastric emptying, food intake and body weight in rats and dogs, suggesting a therapeutic potential for obesity. The aims of this study were 1) to test the hypothesis that the neurons in the VMH are involved in the central mechanisms of IES treatment for obesity; 2) to compare the effects of IES at the duodenum and IES at the ileum on neuronal activities of the VMH; 3) to better understand if the neuronal activity modulated by IES was mediated via the vagal pathway.
Methods
Extracellular potentials of neurons in the VMH were recorded in 18 anesthetized rats. IES at the duodenum or ileum was performed in duodenal-distention responsive (DD-R) neurons with 3 sets of parameters (IES-1 with trains of short-pulses: 4mA, 2s-on, 3s-off, 2ms, 20Hz; IES-2 with long-pulses: 6mA, 20cpm, 100ms; IES-3, same as IES-1 but 40Hz).
Results
IES-1 at the duodenum and the ileum activated 70.6% and 73.3% of the DD-R neurons, respectively; Similar percentages of the neurons were activated with IES-3 at the duodenum and the ileum (70.6% vs. 66.7% P=0.91), respectively. IES-2 at these locations activated only 25% and 46.2% of the DD-R neurons, respectively (P>0.05). IES at the duodenum with parameter set, IES-1 or IES-3 was significantly more potent than the parameter set, IES-2 (neuronal activation: 70.6% vs. 25%, P<0.05). Bilateral vagotomy only partially blocked the effects of IES on the neuronal activity in the VMH, indicating that extra-vagal pathways can mediate these effects.
Conclusions
IES with different parameters activates 25–70.6% of the VMH neurons responsive to DD, and IES with trains of short-pulses seems more effective than IES with long-pulses. The vagal pathway and extra-vagal pathways are involved in the modulatory effects of IES on the central neurons in the satiety center.
Keywords: Obesity, intestinal electric stimulation, central nervous system, the ventromedial hypothalamus
Introduction
Electrical stimulation of the small intestine has been shown to reduce gastric tone or induce gastric relaxation, delay gastric emptying and inhibit antral contractions in dogs [10]. A significant decrease in food intake and increase in gastric volume were noted in dogs with acute duodenum electrical stimulations (DES). DES was found to delay gastric emptying and reduce water intake [5]. IES was also reported to accelerate intestinal transit and reduce fat absorption in rats [15]. One recent study reported acceleration of small intestinal transit with synchronized IES accompanied with enhanced intestinal contractions [17]. Similar inhibitory effects of IES on food intake and body weight were also reported in both regular and diet-induced obese rats [19].
Sun et al. evaluated the effects of IES of various parameters at the duodenum on the activity of neurons in the nucleus tractus solitarii (NTS), and reported that IES activated 39–72% of NTS neurons responsive to gastric distension, and that the effect was stimulation energy dependent [16]. These previous findings suggested that neuronal activation arising from IES might be integrated at the levels of the NTS and the hypothalamus. The hypothalamus is the key central nervous system region involved in the feedback regulation of appetite and food intake. It integrates neural and hormonal inputs to coordinate feeding and energy expenditure. It is well established that the VMH in the hypothalamus is closely related to the regulation of feeding behaviors and plays an important role in the mediation of satiety [2, 3, 9]. The previous study investigating the central mechanisms of IES focused on the effects of IES at one intestinal location - the duodenum, and the first order of the vagal afferent recipient-NTS [16]; it remains unknown whether the satiety center in the hypothalamus is also involved with IES. In addition, the small intestine is lengthy and it is unknown whether stimulation at the proximal intestine has a similar effect as stimulation at the distal intestine. Therefore, the aims of this study were to test the hypothesis that the neurons in the hypothalamus were involved in the central mechanisms of IES, to determine whether the central neuronal activity modulated by IES was mediated via the vagal pathway; and furthermore, to compare the effects of IES at the duodenum (the first part of the small intestine) and IES at the ileum (the final section of the small intestine) on neuronal activities in the VMH in rats.
Materials and methods
Animal preparation
Male Sprague-Dawley rats (n=18, 360.2±17.3 g, Charles River Laboratories, Greensboro, NC, USA) were used in this study. All of the following experimental protocols and procedures were approved by the Institutional Animal Care and Use Committee at the VA Medical Center, Oklahoma City, OK. The rats were anesthetized with urethane (1.4 g/kg, i.p.) with supplemental anesthetics administered intraperitoneally to maintain a deep level of surgical anesthesia and muscle relaxation. One polyethylene catheter (PE-60) was inserted into the right carotid artery for monitoring blood pressure. A plastic tube was inserted into the trachea for artificial ventilation using a volume-control pump (55–60 strokes/min, 3.0–5.0 ml stroke volume). A thermostatically controlled heating pad and an overhead infrared lamp were used to maintain rectal temperature between 36°C and 38°C. A separate group of rats received bilateral cervical vagotomy during the surgical preparation (n=4). After exposure of the bilateral vagal nerves and dissection of the surrounding connective tissues from the carotid artery at a high cervical level, both vagal nerves were isolated, ligated and cut.
Abdominal surgery
After midline laparotomy, a segment of the duodenum 2–3 cm distal to the pylorus of the stomach was isolated. The local duodenal contents were cleaned through a small incision in the duodenal wall using warm isotonic saline (37±1°C). A small latex balloon attached to a polyethylene tube (PE-240) with several small holes near the tip was inserted into the duodenal cavity and fixed well. Duodenal distension was produced by air inflation of 0.3–0.5 ml for 10–30 s. Two pairs of cardiac pacing wires (025–100, A&E Medical Corporation, Farmingdale, NJ, USA) were sutured onto the serosal surface of the small intestine: one at the duodenum, 2–4 cm below the pylorus and the other at the ileum, 3–4 cm above the ileocecal junction for delivering IES. The connecting wires were tunneled subcutaneously along the right truck and exited percutaneously on the back of the neck. Then, the rat’s abdominal cavity was closed with sutures.
Cranioaural surgery
After the abdominal surgery, the rat was positioned on a stereotaxic frame and the dorsal surface of the brain was exposed. Small holes were drilled in the skull to expose the cortex, and the dura was cleaned and cut. The stereotaxic coordinates were used in accordance with the atlas of Paxinos and Watson [11]. A one-barrel glass microelectrode filled with 0.5 M sodium acetate and 2% pontamine sky blue (tip diameter 3–10 μm, resistance 5–15 MΩ) was advanced with the aid of a hydraulic micro-positioner into the area of the VMH (2.3–2.8 mm posterior to the bregma, 0.5–1.0 mm right/left lateral to the midline and 8.8–10.0 mm below the outer surface of the skull). The open part of the brain was covered by 3% agar in saline to limit any displacement due to respiration or heartbeats.
Experimental protocol and electrophysiological recordings
Once the microelectrode was advanced into the area of the VMH, the extracelluar action potentials of the units were recorded via the glass microelectrode, amplified using a high input impedance amplifier, and displayed on an oscilloscope. All signals measured with or without duodenal distention or IES were recorded and stored in a computer for further analyses. When a neuron was identified and its firing pattern had become stable, DD of 0.3–0.5 ml for 10–30 s was performed to determine whether the neuron was responsive. The neuron was abandoned by the advancement of the microelectrode if it was not responsive to DD. The DD-responsive neuron was further classified into DD-excitatory (DD-E) or DD-inhibitory (DD-I) if their spontaneous discharges were transiently increased or decreased in frequency by at least 20%. For each identified and classified neuron, IES at the duodenum (DES) or the ileum (ImES) with 3 sets of parameters (IES-1: 4 mA, 2 s-on, 3 s-off, 2 ms, 20 Hz; IES-2: 6 mA, 20 cpm, 100 ms; IES-3: 4 mA, 2 s-on, 3 s-off, 2 ms, 40 Hz) were performed for one minute in a randomized order. The parameter sets were chosen because IES with these parameters were shown to reduce fat absorption in rats [15]. In another group of 4 rats, IES-3 at the ileum was performed for one minute before and after the bilateral cervical vagotomy. The interval between two consecutive IES periods was 5 min or longer allowing a complete recovery of spontaneous neuronal activity from IES.
IES was delivered by a pulse generator with a constant current output (Model A310, World Precision Instruments, Sarasota, FL, USA) via the stimulation electrodes placed in the duodenum and ileum.
Histology verification
To check the position of each neuronal recording, a direct current (10 mA, 20 min) was passed through the electrode to form an iron deposit of pontamine sky blue at the completion of testing in each neuron. At the completion of all tests in a rat, the rat was perfused transcardially with 0.9% saline followed by 10% buffered formalin. After decapitation, the brain was removed and post-fixed in the same formalin for 24 hrs. Coronal 50 μm sections were cut using a freezing microtome. The neuronal recording locations were examined under microscopy based on the cross sections from the rat brain atlas [11].
In the bilateral cervical vagotomy group, the complete cervical vagotomy was confirmed after the experiment by microscopic examination of the ligated nerve endings at the level of nerve dissection.
Data analysis
Spontaneous activity of each neuron was determined by counting the number of spikes for 10 seconds and was expressed as number of spikes per second. Neuronal responses (# of spikes/s) were defined as the difference in neuronal activity between the mean spontaneous activity at baseline and the activity during duodenal distention. An increase or decrease during DD of ≥ 20% of the mean spontaneous activity was considered an excitatory or inhibitory response to DD. The raw tracings of neuronal responses to the stimuli (DD or IES) were processed by the CED Spike 2 data acquisition software (Cambridge Electrical Design Limited, Cambridge, England) after the removal of the stimulation artifacts.
All data are presented as Mean±S.E. Statistical comparisons were made using ANOVA followed by the Tukey’s test and the Chi square test. The difference in each of the analyzed parameters was considered statistically significant if P<0.05.
Results
Fifty nine percent or 20 out of 34 tested neurons in the VMH were responsive to duodenum distension (DD with 0.3–0.5 ml, 10–30 s). The majority of these responsive neurons (14/20, 70%) were excited by DD, and the other 6 neurons are DD-inhibitory. IES-1 of pulse trains (4 mA, 2s-on, 3s-off, 2ms, 20Hz) at the duodenum activated 12/17 (70.6%) of the DD-R neurons with a latency of 20.9±4.1 s and a duration of 52.1±34.8 s. IES-1 at the ileum activated 11/15 (73.3%) of the DD-R neurons with a latency of 50.2±5.5 s and a duration of 62.3±21.2 s (Table 1 and Fig. 1). While no differences were noted in the percentage of neurons activated by IES between IES at the duodenum or DES and IES at the ileum or ImES (P=1.0). IES with parameter set IES-1 at the duodenum resulted in a shorter latency than that at the ileum (P<0.001, Fig. 1).
Table 1.
Effects of Intestinal Electrical Stimulation (IES) at the duodenum and ileum with 3 different parameters on the activity of VMH neurons responding to duodenum distension (DD) in rats
| DES-1 | DES-2 | DES-3 | ImES-1 | ImES-2 | ImES-3 | |
|---|---|---|---|---|---|---|
| Responded/tested | 12/17 | 5/20 | 12/17 | 11/15 | 6/13 | 8/12 |
| Percentage (%) | 70.6 | 25* | 70.6 | 73.3 | 46.2 | 66.7 |
P<0.05 compared to responsive neurons with DES-1 and DES-3.
Duodenum Electrical Stimulation: DES
Ileum Electrical Stimulation: ImES
DES/ImES-1: 4mA, 2s-on, 3s-off, 2ms, 20Hz
DES/ImES-2: 6mA, 20cpm, 100ms
DES/ImES-3: 4mA, 2s-on, 3s-off, 2ms, 40Hz
Fig. 1.
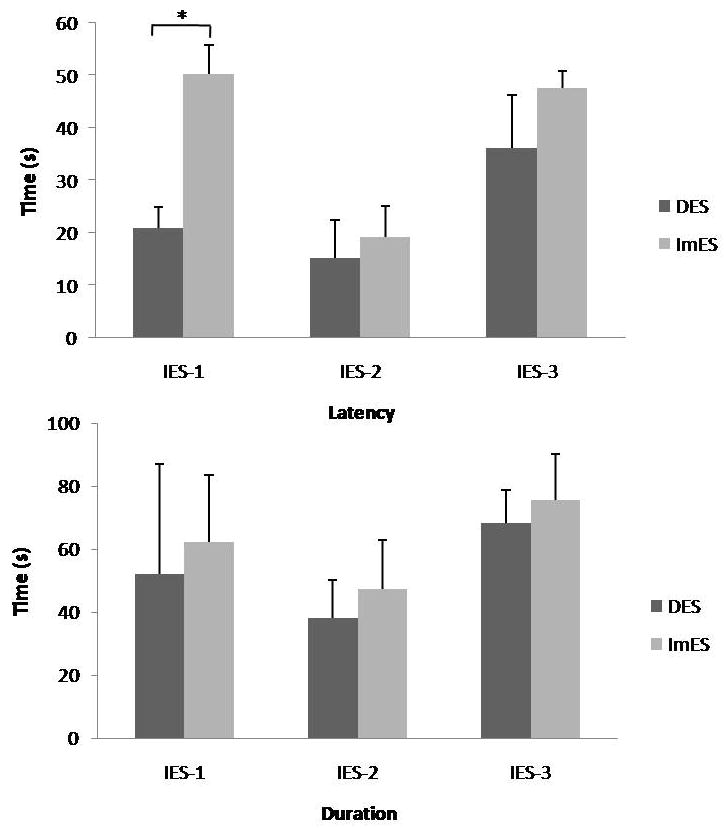
A summary (latency and duration) of the effects of Intestinal Electrical Stimulation with three different sets of parameters and two different locations on VMH neurons activated by duodenum distention. * P<0.001
DES: Duodenum Electrical Stimulation; ImES: Ileum Electrical stimulation.
IES-1: Intestinal Electrical Stimulation-Parameter 1: 4 mA, 2 s-on, 3 s-off, 2 ms, 20 Hz
IES-2: Intestinal Electrical Stimulation-Parameter 2: 6 mA, 20 cpm, 100 ms
IES-3: Intestinal Electrical Stimulation-Parameter 3: 4 mA, 2 s-on, 3 s-off, 2 ms, 40 Hz
Latency of the response was defined as the interval between the initiation of stimulation and the time when neuronal activity was increased or decreased by 20% compared with the baseline.
Duration of sustained effects was defined as the interval between the cessation of stimulation and the time when the neuronal activity returned to the baseline level.
IES-3 (pulse trains with increased frequency of 40 Hz) at the duodenum and the ileum activated 12/17 (70.6%) and 8/12 (66.7%) of the DD-R neurons, respectively (Table 1). No differences were noted between IES-3 at the duodenum and IES-3 at the ileum in the percentage of neurons being activated, the latency or the duration (see Fig. 1). Fig. 2 shows typical neuronal activities at baseline and during IES with parameter set IES-1 delivered at the duodenum or the ileum.
Fig. 2.

Effects of Intestinal Electrical Stimulation at the duodenum and the ileum with pulse trains (IES-1) on a DD-E VMH neuron
The A and B panels show the histograms (spikes/s) of the neuronal activity with or without stimulation artifacts; the C panel shows the original cell activity.
DD-E: Duodenum Distention-Excitatory
DES-1: Duodenum Electrical Stimulation-Parameter 1: 4 mA, 2 s-on, 3 s-off, 2 ms, 20 Hz.
ImES-1: Ileum Electrical Stimulation-Parameter 1: 4 mA, 2 s-on, 3 s-off, 2 ms, 20 Hz.
IES of long pulses (parameter set IES-2) at the duodenum activated only 5/20 (25%) of the DD-R neurons, and the same IES delivered at the ileum activated 6/13 (46.2%) of the DD-R neurons (P<0.05, vs. the same IES delivered at the duodenum, Table 1). The latencies of responses to IES-2 at the duodenum and IES-2 at the ileum were 15.2±7.2 s and 19.3±5.8 s, respectively (P>0.05, Fig. 1); and the response durations were 38.3±11.9 s and 47.2±15.8 s, respectively (P>0.05, Fig. 1). When IES was delivered at the duodenum, it activated a significantly higher percentage of DD-R neurons in the VMH with parameter set IES-1 or IES-3 (pulse trains) than with parameter set IES-2 (long pulses). This difference, however, became insignificant when IES was delivered at the ileum.
Bilateral cervical vagotomy partially attenuated the modulatory effects of IES-3 at the ileum on the neuronal activity of the VMH (Fig. 3), suggesting at least partial involvement of the vagal afferent pathway. In the 4 rats with cervical vagotomy, 5 neurons in the VMH responded to DD and activated by IES-3 at the ileum. Bilateral cervical vagotomy attenuated the effects of IES-3 at the ileum in 4 of the 5 neurons. Fig. 3 shows an example of the excitatory responses of a VMH neuron to DD and IES-3 at the ileum before and after vagotomy.
Fig. 3.

The effects of cervical vagotomy on the responses to DD-E and ImES-3 in one VMH neuron
DD-E: Duodenum Distention-Excitatory
ImES-3: Ileum Electrical stimulation-Parameter 3: 4mA, 2s-on, 3s-off, 2ms, 40Hz.
The A and B panels show the histograms of the neuronal activity (spikes/s) with or without stimulation artifacts; the C panel shows the original cell activity.
Discussion
The present study tested the hypothesis that intestinal electrical stimulation at the duodenum or the ileum modulates the neuronal activity in the satiety center of the hypothalamus. The results showed that 1) IES with long pulses at the duodenum was less effective in activating the neuronal activity in the VMH than IES with pulse trains; 2) IES with pulse trains at the proximal and distal intestine had similar effects in activating the neuronal activity in the VMH; whereas, IES with long pulses was more effective when the stimulation was delivered at the distal intestine than the proximal intestine. 3) IES with pulse trains at a higher pulse frequency (40 Hz) and at a lower pulse frequency (20 Hz) also showed similar effects; 4) bilateral cervical vagotomy partially attenuated the modulatory effects of IES on the central neurons.
Intestine electrical stimulation was previously shown to alter gastrointestinal motility in both animals and humans. In one previous canine study, IES with long pulses was shown to inhibit gastric tone and the inhibitory effect was mediated by both vagal and nitrergic pathways [18]. In another canine study, IES with long pulses was reported to inhibit antral contractions and delay gastric emptying [20]. Similarly, intestinal contractions in dogs were also attenuated with IES of long pulses and the inhibitory effect was mediated via the sympathetic pathway [6]. However, canine intestinal transit was observed to be accelerated when IES was performed in the proximal small intestine [1, 12, 14] but delayed when IES was performed in the distal small intestine [8]. In healthy volunteers, IES of long pulses was reported to delay gastric emptying and reduce gastric accommodation measured by a simple water-load test [5]. In another study in healthy volunteers, intestinal transit was significantly accelerated and absorption was reduced with IES of long pulses [4].
The potential of IES for the treatment of obesity was indicated in a number of previous studies involving food intake. In rats, IES with long pulses was reported to reduce food intake and body weight in normal lean rats as well as in diet induced obese rats [19]. In another rodent study, both IES with long pulses and IES with pulse trains were reported to reduce fat absorption [15]. In dogs, acute IES with long pulses was noted to reduce food intake and the reduced food intake was associated with the inhibition in gastric tone with IES [10]. The acceleration of intestinal transit and the reduction of absorption observed in healthy volunteers mentioned above also suggested the therapeutic potential of IES for obesity [4].
The VMH is a known satiety center and believed to receive inputs from both the vagal pathway and the sympathetic pathway. The findings of the current study showed that both DD and IES activated the VMH neurons. From these evidences, it is known that the vagal nerve receives meal-associated sensory inputs (DD) and electrical stimulation (IES), employs and integrates the information to the VMH of the hypothalamus for the metabolic coordination. This study helped us understand the central mechanism of IES involving the hypothalamic-vagal-visceral neural pathway. Qin et al. reported that spinal neuronal activation by DD and gastrointestinal electrical stimulation was also dependent on afferent impulses traveling in thoracic spinal (sympathetic) fibers [13]. In general, it is believed that the vagal afferents from the gastrointestinal organs play an important role in physiological functions, while the splanchnic sympathetic afferent nerves mediate noxious stimuli and project the information to the hypothalamus via the spinal cord. In this study, among the three parameter sets and the two stimulation locations, IES at the ileum with parameter set IES-3 seemed to be most effective and efficient in activating DD-R neurons in the VMH. Bilateral vagotomy partially blocked the effects of IES of pulse trains on the neuronal activity in the VMH. From the above data, we could not role out possible involvement of sympathetic or other pathways with IES.
The present study also indicated that IES at the ileum was more effective than IES at the duodenum in activating the VMH neurons when the stimulation was performed using long pulses; however, when the stimulation was performed using pulse trains, the efficacy was similar between IES at the duodenum and IES at the ileum. The difference might be explained according to the stimulation parameters. We hypothesize that the effects of IES on the VMH neurons were attributed to two factors: a) a direct neural activation from electrical current applied to the small intestine and b) an indirect effect due to a change in intestinal motility, such as tone, resulting from IES. For example, IES might induce intestinal relaxation and the relaxation of the intestine (stretch) activates VMH neurons. In IES with long pulses, the pulse width was set at 100 ms and the stimulation frequency was 20 cycles/min; this set of parameters (low frequency and long pulses) is known to have minimal effects on the neural system but capable of altering gastrointestinal motility such as gastric tone, gastric emptying and intestinal transit as discussed earlier. Accordingly, the neuronal activation in the VMH with IES of long pulses might be attributed to its effects on the motor functions of the stomach and small intestine, rather than its direct effect on the central nervous system. It might be possible that IES of long pulses was more effective in altering motility when it is applied in the ileum than when it is applied in the duodenum, resulting in an increased activation of the VMH neurons. In a recent unpublished study with electrical stimulation at various segments of the gut, it was found that gastric tone was reduced when electrical stimulation was applied to the stomach, intestine and colon and this inhibitory effect was more potent when electrical stimulation was applied at a more distal segment of the gut. In IES with pulse trains, the pulse width was set at 2 ms and the frequency at a much higher rate of 20 Hz or 40 Hz. This set of parameters is believed to be able to directly activate nerves (although it may also exert effects on gastrointestinal motility or other functions). The similar effects observed with IES of this set of parameters between IES at the duodenum and IES at the ileum might be attributed to similarities in the innovation of the intrinsic and extrinsic nerves in the duodenum and ileum.
The best stimulation location for IES should be determined by its application and all possible mechanisms involved in the particular application. Assuming IES is to be used to treat obesity, other factors such as motility mechanisms that are associated with food intake and nutrient absorption and hormonal mechanisms that control satiety and appetite must also be taken into consideration in addition to the activation of satiety neurons. A number of studies have suggested that ileal brake may be a potential long-term target to achieve sustainable reduction in food intake and weight management because of the combined effects influencing digestive process and ingestive behaviors. Dr. Maljaars and colleagues have reviewed and stated that the activation of the ileal brake can reduce food intake and increase satiety; surgical procedures that increase exposure of the ileum to nutrients produce weight loss; and the appetite-reducing effect of chronic ileal brake activation appears to be maintained over time [7]. Taken together, we may speculate that IES at a more distal segment of the small intestine, such as the ileum, may be more effective in reducing food intake by enhancing satiety signaling.
In addition to different stimulation locations, we also investigated the effects of different stimulation patterns (long pulses vs. pulse trains) and different parameters (20 Hz vs. 40 Hz). IES with pulse trains was more effective in activating the satiety neurons in the VMH than IES with long pulses. The enhanced effect with pulse trains was believed to be attributed to its narrow pulses and high frequency as IES with these parameters was able to directly activate the nervous system. It should be also noted that from the implementation point of view, it is easier and more practical to build a pulse generator to deliver pulse trains than long pulses. With regards to the stimulation frequencies that were tested in this study, it seemed that a stimulation frequency of 20 Hz was equivalent to the frequency of 40 Hz in activating the VMH neurons. It is obvious that a detailed procedure to optimize various stimulation parameters must be taken for any specific application of IES. We would acknowledge that this current experiment was rather limited in the optimization of stimulation parameters.
In conclusion, intestinal electric stimulation modulates neuronal activities in the satiety center of the hypothalamus, and IES with pulse trains seems more effective than IES with long-pulses in activating the neuronal activity in the VMH. This study helped us understand the central mechanism of IES.
Acknowledgments
This study was partially supported by a grant from Oklahoma Center for the Advancement of Science and Technology (HR07-114) and a grant from NIH (DK063733).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen JD, Lin HC. Electrical pacing accelerates intestinal transit slowed by fat-induced ileal brake. Dig Dis Sci. 2003;48:251–256. doi: 10.1023/a:1021911023155. [DOI] [PubMed] [Google Scholar]
- 2.Duggan JP, Booth DA. Obesity, overeating, and rapid gastric emptying in rats with ventromedial hypothalamic lesions. Science. 1986;231:609–611. doi: 10.1126/science.3511527. [DOI] [PubMed] [Google Scholar]
- 3.King BM. Ventromedial hypothalamic obesity: a reexamination of the irritative hypothesis. Neurosci Biobehav Rev. 1991;15:341–347. doi: 10.1016/s0149-7634(05)80027-2. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Qiao X, Hou X, Chen JD. Effect of intestinal pacing on small bowel transit and nutrient absorption in healthy volunteers. Obes Surg. 2009;19:196–201. doi: 10.1007/s11695-008-9533-8. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Hou X, Chen JD. Therapeutic potential of duodenal electrical stimulation for obesity: acute effects on gastric emptying and water intake. Am J Gastroenterol. 2005;100:792–796. doi: 10.1111/j.1572-0241.2005.40511.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Liu J, Chen JD. Neural mechanisms involved in the inhibition of intestinal motility induced by intestinal electrical stimulation in conscious dogs. Neurogastroenterol Motil. 2006;18:62–68. doi: 10.1111/j.1365-2982.2005.00739.x. [DOI] [PubMed] [Google Scholar]
- 7.Maljaars PW, Peters HP, Mela DJ, Masclee AA. Ileal brake: a sensible food target for appetite control. A review. Physiol Behav. 2008;95:271–281. doi: 10.1016/j.physbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 8.O’Connell PR, Kelly KA. Enteric transit and absorption after canine ileostomy. Effect of pacing. Arch Surg. 1987;122:1011–1017. doi: 10.1001/archsurg.1987.01400210049007. [DOI] [PubMed] [Google Scholar]
- 9.Oku J, Bray GA, Fisler JS, Schemmel R. Ventromedial hypothalamic knife-cut lesions in rats resistant to dietary obesity. Am J Physiol. 1984;246:R943–948. doi: 10.1152/ajpregu.1984.246.6.R943. [DOI] [PubMed] [Google Scholar]
- 10.Ouyang H, Yin J, Chen JD. Gastric or intestinal electrical stimulation-induced increase in gastric volume is correlated with reduced food intake. Scand J Gastroenterol. 2006;41:1261–1266. doi: 10.1080/00365520600708008. [DOI] [PubMed] [Google Scholar]
- 11.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 2005. [Google Scholar]
- 12.Qi H, Chen JD. Effects of intestinal electrical stimulation on postprandial small-bowel motility and transit in dogs. Am J Surg. 2007 doi: 10.1016/j.amjsurg.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Qin C, Chen JD, Zhang J, Foreman RD. Characterization of T9–T10 spinal neurons with duodenal input and modulation by gastric electrical stimulation in rats. Brain Res. 2007;1152:75–86. doi: 10.1016/j.brainres.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soper NJ, Geisler KL, Sarr MG, Kelly KA, Zinsmeister AR. Regulation of canine jejunal transit. Am J Physiol. 1990;259:G928–933. doi: 10.1152/ajpgi.1990.259.6.G928. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Chen J. Intestinal electric stimulation decreases fat absorption in rats: therapeutic potential for obesity. Obes Res. 2004;12:1235–1242. doi: 10.1038/oby.2004.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Qin C, Foreman RD, Chen JD. Intestinal electric stimulation modulates neuronal activity in the nucleus of the solitary tract in rats. Neurosci Lett. 2005;385:64–69. doi: 10.1016/j.neulet.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Yin J, Chen J. Excitatory effects of synchronized intestinal electrical stimulation on small intestinal motility in dogs. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1190–1195. doi: 10.1152/ajpgi.00092.2007. [DOI] [PubMed] [Google Scholar]
- 18.Yin J, Ouyang H, Chen JD. Potential of intestinal electrical stimulation for obesity: a preliminary canine study. Obesity (Silver Spring) 2007;15:1133–1138. doi: 10.1038/oby.2007.615. [DOI] [PubMed] [Google Scholar]
- 19.Yin J, Zhang J, Chen JD. Inhibitory effects of intestinal electrical stimulation on food intake, weight loss and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R78–82. doi: 10.1152/ajpregu.00318.2006. [DOI] [PubMed] [Google Scholar]
- 20.Zhao X, Yin J, Chen J, Song G, Wang L, Zhu H, Brining D, Chen JD. Inhibitory Effects and Mechanisms of Intestinal Electrical Stimulation on Gastric Tone, Antral Contractions, Pyloric Tone and Gastric Emptying in Dogs. Am J Physiol Regul Integr Comp Physiol. 2008 doi: 10.1152/ajpregu.90627.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


